Abstract
As a result of population growth, an emerging middle-class, and a more health-conscious society concerned with overconsumption of fats and carbohydrates, dietary protein intake is on the rise. To address this rapid change in the food market, and the subsequent high demand for protein products, agriculture, aquaculture, and the food industry have been working actively in recent years to increase protein product output from both production and processing aspects. Dietary proteins derived from animal sources are of the highest quality, containing well-balanced profiles of essential amino acids that generally exceed those of other food sources. However, as a result of studies highlighting low production efficiency (e.g., feed to food conversion) and significant environmental impacts, together with the negative health impacts associated with the dietary intake of some animal products, especially red meats, the consumption of animal proteins has been remaining steady or even declining over the past few decades. To fill this gap, researchers and product development specialists at all levels have been working closely to discover new sources of protein, such as plant-based ingredients. In this regard, microalgae have been recognized as strategic crops, which, due to their vast biological diversity, have distinctive phenotypic traits and interactions with the environment in the production of biomass and protein, offering possibilities of production of large quantities of microalgal protein through manipulating growing systems and conditions and bioengineering technologies. Despite this, microalgae remain underexploited crops and research into their nutritional values and health benefits is in its infancy. In fact, only a small handful of microalgal species are being produced at a commercial scale for use as human food or protein supplements. This review is intended to provide an overview on microalgal protein content, its impact by environmental factors, its protein quality, and its associated evaluation methods. We also attempt to present the current challenges and future research directions, with a hope to enhance the research, product development, and commercialization, and ultimately meet the rapidly increasing market demand for high-quality protein products.
Keywords: microalgae, protein content, protein quality, protein quality assessment, environmental factors
1. Introduction
Over the last half century, factors including improved agricultural technology, improved food production, improved processing and supply chains, along with the increase in income per capita, have resulted in substantial reductions in hunger worldwide, even though the world population has almost doubled during the same period [1,2]. However, the global population is still increasing at a rapid pace and is estimated to reach 9.7 billion by 2050: an increase of more than 25% from 7.7 billion in 2019 (United Nations, 2019). To feed this growing population, an increase of 70% in food production is required [2]. Improved land clearing and the more efficient use of existing croplands have certainly contributed to the increased demand for crop production; however, the environmental impacts and trade-offs of these alternative means of agricultural expansion are under scrutiny [2,3]. Recent studies suggest that global food production is having huge environmental impacts on the planet and generates 30–34% of global greenhouse gas (GHG) emissions [4,5]. The methods and technologies conventionally used for intensifying agriculture have been very successful throughout the green revolution but are predicted to no longer be sustainable solutions soon. These strategies come with high-impact trade-offs on the environment, such as the disruption of natural habitats, threats to biodiversity, climate changing GHG emissions, deforestation, desertification for livestock production, and polluting nutrient run-off from chemical fertilizers, which are damaging aquatic and terrestrial ecosystems [3,6,7]. In addition to the growing population, many other factors have placed higher demands on finite global resources to provide more food and different types of food [8]. Significant changes globally, including rising incomes, growing cities and urbanization, aging populations, and the heightened awareness of the health impacts of what we consume, have been driving a shift in consumption patterns, of which the increasing dietary protein intake is of particular interest [9]. New technologies and products, along with changes in dietary patterns, such as a growing vegan population, can potentially help to reduce the environmental impact of food production [10,11,12]; this change has stimulated the evolution of agricultural policy and investment in research and product development from various governmental levels to the private sectors.
Consumers are increasingly aware of the impact of specific nutrients on health and disease and this trend has, in turn, been driving food market growth, in particular the sector of functional foods, dietary supplements, and nutraceuticals. It is emerging that consumption of fat and carbohydrates is associated with the onset and progression of metabolic diseases, while protein intake, generally, is not [13,14,15]. Consistently, the global market for protein ingredients was USD 38.5 billion in 2020 and is anticipated to experience a compound annual growth rate (CAGR) of 10.5% from 2021 to 2028 (https://www.grandviewresearch.com/industry-analysis/protein-ingredients-market (accessed on 28 November 2021)). Animal-derived proteins have long provided significant human food security by supplying high-quality essential amino acids, dietary calories, and critical micronutrients, such as vitamins and minerals, which are often lacking in many terrestrial plant-based foods [16,17,18]. The impacts that particular foods (and their production systems) have on regional and global food security, and overall ecological sustainability, are measured by numerous factors [19]. Many human food resources are also in demand for formulated livestock feeds, and most terrestrial farmed animals convert raw feedstuffs into food products with relatively low efficiency [16]. As a consequence, the required higher production volumes of terrestrial foods must strike a balance with its ecological footprint and nutrient conversion efficacy. Thus, protein-rich products derived from terrestrial livestock production are now under particularly strong scrutiny [20]. In this regard, high-quality plant-based proteins, derived from novel sources, such as pulses (e.g., peas, lentils, etc.), have become increasingly popular and have been recently taken as one of the key strategies to meet the fast-growing food protein market. Nevertheless, due to limited resources of arable land and fresh water, a strong demand for alternative plant proteins has arisen, resulting in the increased development and use of algal proteins.
In recent years, microalgae have become important crops for global food and beverage industries, aqua-farming, and animal and human nutrition. The reasons include the following: (1) high content of protein, essential amino acids, and other healthy nutrients such as vitamins, antioxidants, omega-3 PUFAs, and minerals; (2) long-term sustainability, because microalgae have the lowest carbon, water, and arable land footprints of any crops; (3) environmental pollution remediation (e.g., ecological services); (4) high productivity compared with terrestrial crops and animal foods [1,21,22,23]. Algal proteins have already been used as food items, animal and aquaculture feeds, dietary supplements, pharmaceuticals, and cosmetics. The global algal protein market is expected to grow at the same rate as the total protein market, to reach USD 0.84 billion by 2023, from USD 0.6 billion in 2018 [9]. However, the development and use of microalgae for human foods are still at very early stages [1]. Algal cultivation techniques are first developed at laboratory conditions, with a controlled environment, and then moved to outdoor conditions for large-scale production of biomass. Cultivation of microalgae at large scales for commercial production is complicated. In addition to skilled and experienced personnel, methods and protocols must be developed, targeting specific species, culture systems, production plant locations, and the assessment of product quantity and quality [24,25]. This review focuses on the use of microalgae as human food, microalgal protein quantity, the impact of analytical methods on microalgal protein content, influence of environmental factors on microalgae protein yield, protein quality, and methods for protein quality assessment.
2. Historical Use of Microalgae as Human Food
Microalgae consumption is not foreign to humans, as their first use dates back over 2000 years, when Chinese people used Nostoc to survive during famine; additionally, the use of microalgae by indigenous peoples has occurred for centuries [26]. Despite this long history, however, only a handful of wild microalgae species, such as Arthrospira platensis (Spirulina), Chlorella vulgaris (Chlorella), and Aphanizomenon, have been domesticated and grown for human consumption and/or use [1,10,27]. Indeed, technologies for intensive outdoor cultivation of microalgae has only been developed since the 1950s [28]. The earliest efforts to mass cultivate microalgae with what can be considered modern technological approaches were carried out in Japan during the period of economic recovery following World War II [29]. With some strains accumulating up to ~65–70% (w/w) protein, microalgae were considered a promising source at a time when the supply of plant and animal protein was limited and therefore expensive [7,30,31,32,33]. In photosynthetic microalgae, proteins accumulate to such high levels during periods of rapid cell division and growth, when the synthesis of large protein complexes that are required for light harvesting and carbon fixation functions are at or near maximum capacity [34]. Research into the mass cultivation of microalgae began in the late 1940s and early 1950s in the United States and Europe, in particular, in Germany [35,36]. Interestingly, most of the theoretical constraints which limit the growth and yields of microalgae in outdoor tank or pond cultures were understood by this time. Similarly, it was during this period that all of the practical barriers to mass cultivation of algae—related to, for instance, the supply of CO2 and essential nutrients and the requirement for turbulent mixing—were first encountered and solved [35]. Aside from a few incremental advances in some of the finer technical details, the basic design and operation of open-pond cultures of protein-rich microalgae has changed very little since the 1970s [36,37]. However, despite continued research on optimizing microalgae growth and biomass yields in open-pond cultures, and as a result of a variety of technical and economic reasons, to date, microalgae have not been used widely as a source of protein, but rather have mainly been propagated as a source of whole biomass [24].
Several microalgal species are currently exploited for a variety of biological and industrial applications, including human foods, functional ingredients, cosmeceuticals, pharmaceuticals, animal and aquaculture feeds, fatty acids, alginates, carotenoids, wastewater treatment, and biofuels [38,39,40,41,42,43,44,45]. The cyanobacteria Arthrospira sp. and Chlorella vulgaris are sold not only as protein-rich food ingredients and supplements, but also as functional foods due to their high vitamin, mineral, and carotenoid contents, and they are generally regarded as safe (GRAS) [39]. While annual global microalgae production is presently rather modest (5.0 × 104 tonnes dry matter) compared with macroalgae (seaweeds) (7.5 × 106 tonnes dry matter), microalgae biomass and bioactives extracted from it are of great nutritional and economic importance (values at USD 1.25 × 109 annually) [38]. Importantly, microalgae represent a large, polyphyletic group, numbering tens of thousands of different species, the majority of which have not been studied, let alone commercialized. Therefore, a huge potential exists in exploring and developing microalgae as sources of high-quality, sustainable protein for human food and dietary supplements.
3. Protein Quantity and Difference among Microalgae Species
Microalgae are a very diverse group of microorganisms and are estimated to comprise approximately 200,000 species [46]. The most familiar and arguably more ecologically important microalgal classes/divisions are Cyanophyceae (blue-green algae), Chlorophyceae (green algae), Bacillariophyceae (including the diatoms), and Chrysophyceae (including the golden algae) [39]. Protein contents can be vastly different among microalgal species and strains (Table 1) and is substantially affected by the environments in which they are grown. Under cultivation, many species may contain high levels of protein, typically 40–60% of dry matter, while some species have relatively low levels of protein, especially those selected for oil and biodiesel production [34], similar to the values of many species that were reviewed by others [47]. It was reported in a review that the crude protein content in microalgae biomass ranges from 6 to 63%, where most species have over 40% crude protein content, on the basis of dry mass [39]. The analysis of protein content in 16 microalgae revealed that protein content (% of dry cell matter) ranged from 12% (Chaetoceros gracilis, a diatom) to 35% (Nannochloropsis oculata, a eustigmatophyte) [48]. In 2007, Becker provided an overview on the major constituents of 13 microalgae [34]. There was a large range of protein content, 6–71% of dry matter, with over 50% in most of the species. An exception existed in Spirogyra sp., which had a lower, but larger, range of protein content (6–20% of dry matter), in line with the values of 12–24% reported by Tipnee et al., (2015) [49] and 18% by Saragih et al., (2019) [50]. A similar range (10–71%) of protein content was reported in 2013 by Becker in 33 microalgal species [51]. In 2020, Acquah et al. reported a range of protein content of 6–58% in 17 microalgal species [47]. Another review presented the protein content of 22 microalgal species, studied in different laboratories. Except Spirogyra sp. (6–20%) and Scenedesmus dimorphus (8–18%), a range of 28–71% of protein in dry biomass was reported and most species had over 50% protein [52]. It is evident that most microalgal species contain a high content of protein and some of them have been developed and used for food proteins or dietary supplements, such as the chlorophyceae Chlorella sp. and Scenedesmus obliquus and the cyanobacteria Arthrospira sp. [34]. It appears that Chlorella vulgaris and Arthrospira sp. are the most commonly exploited industrial species due to their high protein contents (51–58% of dry matter) and favorable essential amino acid profiles [34,53].
Table 1.
Protein content of different microalgal species.
| Species | Protein Content (% Dry Matter) | Reference |
|---|---|---|
| Acutodesmus dimorphus | 28 | [54] |
| Anabaena cylindrica | 43–56 | [34] |
| Aphanizomenon flos-aquae | 62 | [34] |
| Arthrospira fusiformis | 62 | [55] |
| Arthrospira maxima | 60–71 | [31,34] |
| 65 | [32] | |
| Arthrospira platensis (Bangladesh) | 60 | [33] |
| Arthrospira platensis (France) | 65 | [33] |
| Arthrospira platensis (Malaysia) | 61 | [33] |
| Arthrospira platensis (Thailand) | 55–70 | [33] |
| Arthrospira platensis | 63 | [34] |
| 53–70 | [7] | |
| 45–62 | [8] | |
| 22–38 | [9] | |
| 61 | [56] | |
| 64 | [55] | |
| 17–32 | [57] | |
| 26–72 | [58] | |
| 22–51 | [59] | |
| 57–70 | [60] | |
| 45–62 | [61] | |
| 56 | [54] | |
| Botryococcus braunii | 22 | [62] |
| 39–40 | [54] | |
| Chaetoceros calcitrans | 34 | [48] |
| Chaetoceros gracilis | 12 | [48] |
| Chlamydomonas rheinhardii | 48 | [34] |
| Chlorella 71105 | 56 | [63] |
| Chlorella pyrenoidosa | 57 | [34] |
| 60 | [64] | |
| Chlorella pyrenoidosa and Chlorella vulgaris | 53 | [54] |
| Chlorella vulgaris | 51–58 | [34] |
| 48 | [56] | |
| Chroomonas salina | 29 | [48] |
| Diacronema vlkianum | 57 | [1] |
| Dunaliella hardawil | 10 | [62] |
| Dunaliella salina | 57 | [34] |
| 29 | [62] | |
| Dunaliella tertiolecta | 20 | [48] |
| Euglena gracilis | 39–61 | [34] |
| Haematococcus pluvialis | 48 | [65] |
| Isochrisis aff.galbana (T-iso) | 23 | [48] |
| Isochrysis galbana | 29 | [48] |
| 27 | [56] | |
| Nannochloris atomus | 30 | [48] |
| Nannochloropsis granulata | 18–34 | [54] |
| Nannochloropsis oculata | 35 | [48] |
| Nannochloropsis spp. | 29 | [66] |
| Neochloris oleoabundans | 30 | [54] |
| Nitzschia closterium | 26 | [48] |
| Nitzschia sp. | 17 | [62] |
| Nochloris oleoabundans | 30 | [1] |
| Pavlova lutheri | 29 | [48] |
| Pavlova salina | 26 | [48] |
| Phaeodactylum tricornutum | 30 | [48] |
| 40 | [54] | |
| Porphyridium aerugineum | 32 | [54] |
| Porphyridium cruentum | 28–39 | [34] |
| 34 | [67] | |
| Scenedesmus almeriensis | 47 | [68] |
| Scenedesmus obliquus | 50–56 | [34] |
| Skeletonema costatum | 25 | [48] |
| Spirogyra sp. | 6–20 | [34] |
| Spirogyra varians | 17 | [49] |
| Spongiococcum excentricum | 32 | [64] |
| Synechococcus sp. | 46–63 | [34] |
| Tetraselmis chuii | 31 | [48] |
| 47 | [54] | |
| Tetraselmis suecica | 31 | [48] |
| Thalassiosira pseudonana | 34 | [48] |
| Tisochrysis lutea | 37–42 | [69] |
It is understood that the differences in protein content between microalgal species are primarily attributed to their different genetic traits. However, the reason for different protein contents of a given species is multifaceted, including the method employed for protein analysis, the environmental conditions under which they were cultivated, and the growth phase at which the microalgal biomass was harvested.
4. Influence of Analytical Methods on the Protein Content of Microalgal Biomass
Several methods have been used to measure protein content, including nitrogen analysis, colorimetric assays, and summation of anhydroamino acids [48]. These methods generate different values of protein content for the same samples [48,70,71]. In 2010, Lopez et al. measured the protein content in the dry biomass of five microalgal species by analyzing total nitrogen, using the Kjeldahl method and elemental analysis [72]. It was found that the nitrogen-to-protein (N-to-P) conversion factors for biomass obtained from the exponential phase were 5.95 for nitrogen measured by Kjeldahl and 4.44 for nitrogen measured by elemental analysis, and the protein content in the dry biomass ranged from 30% to 50%. An N-to-P conversion factor range of 3.00–6.35 has been reported for various microalgal species that were analyzed using different methods and in different research laboratories [47]. Apparently, elemental analysis of total nitrogen generated a higher value of total nitrogen and thus a lower N-to-P conversion factor compared with the Kjeldahl method. A different N-to-P conversion factor of 4.22 was established by others, who also used the method of elemental analysis to measure the total nitrogen [73]. In this study, nitrogen content was analyzed in 12 microalgal species/strains cultivated under mixotrophic and autotrophic conditions, and the protein content ranged from 73.9–76.5% [73]. Another study reported the protein content of microalgae ranging from 7 to 40% and changing dramatically over the course of their growth cycles [74]. In this study, 100 samples of 3 species were analyzed for protein content, using elemental analysis, and an N-to-P conversion factor of 4.78, established by others [73], was used for the calculation of protein content. The elemental analysis has generated a narrow range (4.22–4.78) of N-to-P conversion factors. An average of 4.78 was recommended for estimating protein content when elemental analysis is employed [73], and was later confirmed to be appropriate when the high non-protein nitrogen (NPN) was taken into account [75]. However, it was emphasized that differences exist between species and strains and even between samples collected from a given species or strain under different growing conditions or harvested at different growth phases [75]. The difference in N-to-P conversion factors is considered to be a result of different concentrations of NPN-containing molecules, particularly nucleic acids. It was reported that the median composition of nutrient-sufficient, exponentially growing microalgae was 5.7% RNA and 1% DNA, and varied between samples cultured under different conditions and collected at different growth phases [76]. Thus, validation and optimization of the N-to-P conversion factor are important for accurately measuring the protein content of microalgal biomass. This can be implemented by referring to the results by other methods that are able to measure the “true” protein concentrations, such as the summation of anhydroamino acids [75,77].
The Lowry method is a colorimetric assay, based on both the Biuret reaction and the Folin–Ciocalteau reaction, results in a strong blue color, which depends partly on the tyrosine and tryptophan content [78]. Although standards are used for calibration, the standard protein may not match that of the protein of interest and many substances interfere with the reaction; in addition, Folin reagents are reactive for a short period of time after addition [79]. Although this method can be used for quantifying proteins [80], the accuracy largely relies on the accessibility to intracellular proteins, or in other words, on the efficacy of cell wall disruption and protein extraction from the raw biomass. As such, the accuracy of this method is highly dependent on the cell wall structure, its rigidity, and its protein extraction efficiency, which can be quite different between microalgal species. Therefore, this method may be used for the measurement of protein content in extracted or purified protein products but may not be suitable for measuring protein content in the raw biomass of microalgae. On the other hand, the colorimetric methods may be used for high throughput screening of multiple algal species and strains, and when an accurate analysis is required, a more reliable method must be employed.
Accurately determining protein content in the raw material of microalgae is critical to the valorization of algal biomass for food and other forms of application, as microalgae are currently used predominantly in the form of raw biomass, while increased use of the purer forms of microalgal protein products may be achieved in the near future. In this regard, the method of elemental analysis is well-accepted and widely used for its multiple advantages, including, but not limited to, its simplicity, rapidity, and inexpensiveness, while the accuracy depends on the use of an appropriate N-to-P conversion factor [81]. Therefore, there exists a demand to establish an N-to-P conversation factor in reference to the value generated with a more reliable method, such as the summation of anhydroamino acids [48]. As amino acids are the building blocks or constituents of a protein, the summation of anhydroamino acids can be applied across different microalgal species, on the condition that proteins are completely released and fully hydrolyzed to single amino acids in the amino acid analysis. Due to differences in the ratio of protein nitrogen to NPN and differences in contents in different microalgae species and strains, dedicated N-to-P conversion factors may be required for the accurate measurement of protein content when elemental analysis is employed [82]. This idea may also apply to the samples collected from the same species or strain grown under different conditions or harvested at different growth phases. More research is required to determine dedicated N-to-P conversion factors in the biomass of different microalgae species or strains and also for samples of a given species or strain that are grown under different conditions and harvested at different growth phases. A quality control sample, which refers to a designated sample available in sufficient quantities such that replicate data can be obtained over a long period of time, should be used to validate the analysis [82]. In addition, to meet the requirement for comparing the results between different laboratories, analytical performance should be calibrated using a globally accepted, standard protein, produced by an internationally certified reference material laboratory or supplier.
5. Influence of Growing Conditions on Microalgae Protein Content
Many studies have been conducted to evaluate the impact of environmental factors on microalgal growth and biochemical profiles, including protein. A recent study showed significant effects of culture conditions on the growth rate, biomass production, nutrient composition, and the content of protein and other bioactive compounds in five microalgae species [83]. In addition to genetic traits, protein content in microalgal biomass is affected by a number of factors in the culture system, including light intensity and spectrum, temperature, CO2, pH, and nutrient media composition; moreover, the effect of each factor varies greatly among different species [83,84].
Light is the energy source of phototrophic microalgae for growth and synthesis of biomolecules. In some species, for example, Botryococcus braunii, the increase in light intensity was negatively related to protein content [85], while in other species, such as Nannochloropsis sp. [86] and Ankistrodesmus falcatus [87], a positive relationship was observed. In an experiment with Chlorella vulgaris, Desmodesmus sp., Ettlia pseudoalveolaris, and Scenedesmus obliquus, a clear pattern of increase in biomass yield or cell growth rate, with increasing light intensity from 50 µE to 500 µE m−2 s−1, was observed [88]. Greenhouse bioreactor trials, conducted throughout changing seasons, demonstrated that protein content remained relatively constant with rising light intensity and temperature. Protein content in Isochrysis sp. and Rhodomonas sp. decreased when they were cultivated at high temperatures in a range of 25–35 °C, while protein content increased in Prymnesiophyte sp., Cryptomonas sp., and Chaetoceros sp., with the largest change occurring in Isochrysis sp. [89], in line with the results reported by others [90]. A study with 8 marine microalgae species showed a U-shape relationship between protein content and temperature from 10 to 25 °C in P. pseudonana, P. tricornutum, and P. lutheri, and a bell shape in C. gracilis, while protein content decreased in C. simplex and I. aff. Galbana and remained slightly increased in D. tertiolecta and C. calcitrans with the increase in temperature [91]. Response of the protein content of microalgae to temperature and light intensity of culture systems varies from species to species, without a consistent trend; therefore, the determination of species-specific responses to light density and temperature is vital for the maximal rate of protein production.
Microalgae can grow in a wide range of non-ideal CO2 concentrations and their CO2 fixation rates are 5–8 times higher than terrestrial plants, of which, 70–80% goes to biomass production [92,93]. There exists an interaction between biomass productivity and CO2 concentration in the culture medium [94,95]. For example, Scenedesmus bajacalifornicus was investigated for CO2 fixation capability and biomass chemical composition, where cultivation was carried out at CO2 concentrations ranging from 5 to 25%, while the temperature and light intensity were kept constant. The CO2 concentration had a significant impact on growth and biochemical profile, with maximal biomass productivity and protein content achieved at 15% CO2 [96]. In a non-axenic polyculture of native microalgae of 16 species, CO2 concentration in culture media showed a significant effect on the biomass yield, and the maximal protein content was observed at 400 mg L−1 [97]. A review of 17 studies showed a substantial effect of CO2 supply on the biomass productivity and protein content, and an interaction between CO2 concentration and microalgal species [98]. For maximal protein production, a dedicated CO2 concentration in the culture medium must be established for a given species.
Nitrogen represents a critical macronutrient that regulates the metabolism and consequently, the growth and biochemical composition of microalgae [99]. In Chaetoceros sp., grown in batch cultures, nitrogen from 0.5 to 1.0 g/L led to an increase in biomass yield from 1 to 2 g/L and protein content from 38 to 46%, while no further increases were observed when the nitrogen concentration exceeded 1.0 g/L [100]. In contrast, it was observed in Isochrysis galbana that cell growth and protein content decreased with diminishing nitrogen concentration when cultured at nitrogen concentrations ranging from 0 to 0.29 mg/L [101]. Similarly, nitrogen limitation or starvation changes photosynthetic activity and reduces the protein content of microalgae in Parietochloris incise [102], I. galbana [101], and many other microalgal species [103]. Microalgae are generally able to utilize various forms of nitrogen, including nitrate, nitrite, ammonium, and organic nitrogen sources, for example, urea [104]. Each nitrogen source is first reduced to the ammonium form and assimilated into amino acids through a variety of pathways [103]. Although the source of nitrogen can affect the gross biochemical composition, the protein content of microalgae is more strongly controlled by the growth phase than by the particular source of nitrogen used for growth [105].
The pH of the nutrient media is also an important consideration for microalgae cultivation as it directly affects solubility of nutrients and minerals in the media, injected CO2, and photosynthetic activity of the algal cells [106,107]. In flask cultivation, the optimal pH for Chlorella sorokiniana was reported to be approximately 6.0 when only accounting for cell growth and not considering the CO2 fixation efficiency [106]. In the same study, a flat panel airlift photobioreactor was used for scale-up cultivation at five different pH levels. It was found that biomass productivity decreased while protein content in the biomass increased with the increase in pH [106]. It was demonstrated that microalgae Euglena gracilis did not survive at pH < 4 and >8, while the highest growth rate was detected at pH 7 and the photosynthesis was the most effective at pH 6 [107]. Each microalgal species has an optimal pH range for biomass production and biochemical composition, which is narrow, and species and strain specific, based on studies in 10 microalgal species [108,109,110,111]. Some key factors that can influence the pH of the cultivation media include its nutrient composition and their respective buffering capacities, CO2 concentration, cultivation temperature, and metabolic activity of the algal cells themselves. The methods for controlling pH include CO2 injection, buffer addition, and acid/base adjustment, with the first two approaches being used commonly in algae cultivation [26,108,112]. In cultures, buffer solutions are used to reduce pH fluctuations. However, the use of large volumes of buffers may be impossible in a large scale or industrial production settings because of cost. Instead, in aerate cultures, pH control is achieved by pumping atmospheric air or CO2-enriched air through the medium [113].
6. Protein Content of Microalgae Collected at Different Growth Phases
During different growth phases, microalgal cells undergo changes in cell structure, composition, and nutrient content [114]. It was reported that protein content in the extracellular polymeric substances fraction, extracted from the surface of E. texensis cells at the end of the stationary phase, was eight times as much as that at the end of the exponential phase [115]. An experiment on the effect of the growth phase on biochemical composition of two strains of Tisochrysis lutea revealed that protein content per cell of each strain was significantly higher at the exponential phase than at the stationary phase, regardless of whether nitrogen was replete or reduced [116]. In another study with Isochrysis sp. (clone T. ISO), Pavlova lutheri, and Nannochloropsis oculata, the protein accumulation was enhanced during the exponential phase but started to decline during the stationary phase [117]. Protein accumulation in the biomasses of C. vulgaris and N. gaditana was maximized during exponential growth but declined for C. vulgaris four days into the stationary growth phase [118]; this reaffirms the important effect of the growth phase on final biomass protein content. An optimal harvest time in the exponential phase should be determined by assessing both the accumulation rate and total yield to achieve the maximal protein production rate.
7. Protein Quality of Microalgae Biomass
Protein quality is vital when evaluating what nutritional value a protein product can provide. Several methods have been developed and used to assess the protein quality of a given product, such as amino acid composition, amino acid score (AAS, Equation (1)), essential amino acid index (EAAI), chemical score (CS), biological value (BV), protein digestibility (either in vitro or in vivo), net protein utilization (NPU), protein efficiency ratio (PER), protein digestibility corrected amino acid score (PDCAAS, Equation (2)), and digestible indispensable amino acid score (DIAAS) [47,119]. AAS and PDCAAS are two of the most commonly used parameters to date in protein quality assessment, with DIAAS being adopted slowly in recent years to replace PDCAAS in some countries [120]. The FAO/WHO expert panel concluded, in 1989, that AAS was a suitable measure of protein quality [121], defined as follows:
| AAS (amino acid score) = mg of amino acid per g test protein/mg of the same amino acid per g reference protein | (1) |
Subsequently, PDCAAS was adopted to correct AAS for the true fecal protein digestibility of the test protein as measured in a rat assay, calculated as follows:
| PDCAAS (protein digestibility corrected AAS) = (mg of limiting amino acid per g of test protein/mg of same amino acid per g of reference protein) × fecal true digestibility percentage | (2) |
This reference protein had earlier been recommended by FAO/WHO in 1985 and was based on the essential amino acid requirements of infants and young children [122], which have been periodically updated since that time [123,124]. AAS reflects the balance of essential amino acids and the abundance of the first limiting amino acid in a protein product, relative to the reference protein; therefore, a protein with a higher AAS value should yield a higher protein utilization rate or bio-efficiency in the body. A protein-rich ingredient, having an AAS equal to or higher than one, indicates that all of its constituent essential amino acids meet or exceed dietary requirements of the target age group. In this regard, animal-derived proteins are generally the highest rated, due to their well-balanced essential amino acid contents, relative to human and animal dietary requirements [19,119]. For example, casein has the AAS values of 1.03–1.32 based on several studies [19,125,126]. Microalgae Chlorella vulgaris and Chlorella sorokiniana had AAS values of 1.10 and 1.16, respectively, while Acutodesmus obliquus had an AAS value of 0.86 [81]. Kent et al. (2015) reported that the microalgae Nannochloropsis sp., Scenedesmus sp., Dunaliella sp., and Chlorophyta sp. had AAS values of 0.98–1.05, and two microalgae products derived from Spirulina and Chlorella had AAS values of 0.81 and 0.92, respectively [127]. The essential amino acids profile and AAS of some microalgae are summarized in Table 2. EAAI is similar to AAS and has also been used to evaluate the balance of essential amino acids in microalgae proteins [128,129]. Information on the AAS of microalgal proteins is limited and more research should be conducted in the future.
Table 2.
Amino acid profile and score of different microalgal species.
| Microalgal Species | His | ISO | Leu | Lys | SAA | AAA | Thr | Trp | Val | AAS # | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference protein | 16 | 30 | 61 | 48 | 23 | 41 | 25 | 6.6 | 40 | [123] | |
| Acutodesmus obliquus | 14 & | 36 | 85 | 41 | 33 | 92 | 59 | 20 | 60 | 0.86 | [81] |
| Acutodesmus obliquus * | 12 | 38 | 89 | 36 | 34 | 90 | 61 | 22 | 62 | 0.76 | [81] |
| Arthrospira maxima | 18 | 60 | 80 | 46 | 18 | 88 | 46 | 14 | 65 | 0.78 | [34] |
| Arthrospira platensis | 22 | 45 | 98 | 71 | 39 | 157 | 46 | 12 | 78 | 1.37 | [130] |
| Botryococcus braunii (A) | 15 | 34 | 71 | 47 | 39 | 72 | 37 | 22 | 44 | 0.94 | [54] |
| Chaetoceros calcitrans | 19 | 55 | 82 | 63 | 30 | 112 | 45 | 14 | 59 | 1.19 | [48] |
| Chaetoceros gracilis | 24 | 58 | 72 | 51 | 29 | 125 | 59 | 16 | 62 | 1.06 | [48] |
| Chlorella pyrenoidosa | 16 | 62 | 34 | 81 | 61 | 51 | 35 | 52 | 0.56 | [128] | |
| Chlorella sorokiniana | 20 | 35 | 84 | 57 | 27 | 87 | 53 | 20 | 59 | 1.16 | [81] |
| Chlorella sorokiniana * | 19 | 35 | 83 | 58 | 28 | 86 | 50 | 22 | 59 | 1.16 | [81] |
| Chlorella vulgaris | 18 | 36 | 92 | 52 | 29 | 98 | 43 | 23 | 58 | 1.10 | [81] |
| Chlorella vulgaris | 20 | 38 | 88 | 84 | 36 | 84 | 48 | 21 | 55 | 1.25 | [34] |
| Chlorella vulgaris * | 18 | 37 | 93 | 48 | 25 | 95 | 45 | 23 | 60 | 1.10 | [81] |
| Chroomonas salina | 18 | 41 | 78 | 61 | 30 | 111 | 54 | 13 | 61 | 1.13 | [48] |
| Dunaliella bardawil | 18 | 42 | 110 | 70 | 35 | 95 | 54 | 7 | 58 | 1.06 | [34] |
| Dunaliella tertiolecta | 21 | 48 | 84 | 60 | 18 | 117 | 47 | 15 | 62 | 0.80 | [48] |
| Isochrisis aff.galbana (T-iso) | 20 | 46 | 87 | 60 | 31 | 105 | 45 | 16 | 61 | 1.25 | [48] |
| Isochrysis galbana | 21 | 48 | 87 | 62 | 27 | 108 | 52 | 13 | 62 | 1.15 | [48] |
| Nannochloris atomus | 18 | 34 | 75 | 52 | 25 | 94 | 40 | 11 | 59 | 1.07 | [48] |
| Nannochloropsis granulata (A) | 23 | 56 | 110 | 85 | 51 | 104 | 54 | 28 | 71 | 1.44 | [54] |
| Nannochloropsis oculata | 21 | 48 | 78 | 61 | 20 | 104 | 55 | 16 | 65 | 0.87 | [48] |
| Nitzschia closterium | 14 | 50 | 81 | 57 | 22 | 108 | 55 | 14 | 62 | 0.88 | [48] |
| Nostoc sp. | 20 | 37 | 95 | 65 | 38 | 140 | 53 | 10 | 72 | 1.23 | [130] |
| Pavlova lutheri | 50 | 49 | 81 | 56 | 37 | 111 | 43 | 15 | 67 | 1.17 | [48] |
| Pavlova salina | 15 | 44 | 90 | 62 | 20 | 92 | 52 | 9 | 61 | 0.94 | [48] |
| Phaeodactylum tricornutum | 15 | 46 | 70 | 64 | 42 | 82 | 48 | 26 | 51 | 0.94 | [54] |
| Phaeodactylum tricornutum | 17 | 49 | 77 | 56 | 23 | 107 | 54 | 16 | 59 | 1.06 | [48] |
| Pleurochrysis carterae | 19 | 42 | 99 | 72 | 44 | 154 | 57 | 11 | 76 | 1.18 | [130] |
| Porphyridium aerugineum | 19 | 71 | 119 | 80 | 59 | 121 | 58 | 33 | 73 | 1.19 | [54] |
| Scenedesmus obliquus | 21 | 36 | 73 | 56 | 21 | 80 | 51 | 3 | 60 | 0.45 | [34] |
| Skeletonema costatum | 16 | 52 | 83 | 57 | 26 | 109 | 51 | 13 | 63 | 1.00 | [48] |
| Spirulina platensis | 22 | 67 | 98 | 48 | 34 | 106 | 62 | 3 | 71 | 0.45 | [34] |
| Tetraselmis chuii | 18 | 35 | 75 | 57 | 25 | 91 | 42 | 10 | 58 | 1.07 | [48] |
| Tetraselmis chuii | 16 | 34 | 73 | 56 | 52 | 77 | 40 | 23 | 48 | 1.00 | [54] |
| Tetraselmis suecica | 18 | 35 | 80 | 60 | 30 | 97 | 41 | 12 | 57 | 1.13 | [48] |
| Thalassiosira pseudonana | 16 | 55 | 84 | 59 | 27 | 110 | 52 | 8.7 | 61 | 1.00 | [48] |
SAA—methionine and cystein; AAA—phenylananine and tyrosine. *—disrupted using microfluidizer. #—calculated by the authors of this review mathmatically, against the reference pattern of the essential amino acids for 3–10-year-old children (FAO/WHO/UNU, 2013). &—the first limiting amino acid.
Although AAS reflects the amino acid composition of a protein, it does not tell the true biological value that a given protein can provide after it is ingested due to the differences in protein digestibility of various protein products. To adjust this, more comprehensive methods, including PDCAAS, have been developed to assess the overall quality of proteins in a product, which involves the use of AAS and in vivo protein digestibility [131]. Two types of in vivo protein digestibility have been used for over a century, depending on whether the endogenous protein contribution to the fecal protein output is corrected for or not. Apparent protein digestibility (ADP) does not correct for the amount of endogenous protein contributed to the total of undigested fecal proteins and thus underestimates the digestibility of proteins in a given product. Therefore, true protein digestibility (TPD) has been developed to correct for the contribution of endogenous proteins to the total fecal protein output. As significant fermentation and metabolism of proteins, peptides, and amino acids by bacteria occurs in the large intestine, ileal APD and TPD have also been established by collecting and analyzing ileal samples for the amounts of undigested proteins. However, sample collection in this method has some difficulties and thus not been widely practiced to date, even although it has been highly recommended. Although APD has been used to assess protein quality in some countries, TPD becomes mandatory in many countries where research technologies and instruments are available to carry out the required experiments and analysis. PDCAAS is a method for assessing the quality of a protein, based on both the amino acid requirements of humans and their ability to digest it and has been used commonly worldwide [120]. In Canada, PER is still the official method for protein quality assessment while PDCAAS is also accepted [132]. Most protein products have PDCAAS values of lower than one, especially the plant [125] and microalgal proteins [81,133].
Wang et al., (2020) evaluated the protein quality of Chlorella vulgaris, Chlorella sorokiniana, and Acutodesmus obliquus using a rat bioassay [81]. The raw biomass of these 3 species had PDCAAS values of 0.63, 0.64, and 0.29, respectively, which are lower than that of animal proteins, such as egg, milk, and meat proteins, but the value of the first 2 species were comparable to, or better than, wheat and pulse proteins [134,135]. The same group also investigated the effect of mechanical cell wall disruption using micro-fluidization and found the PDCAAS of the disrupted biomass of these 3 species increased to 0.77, 0.81, and 0.46. The PDCAAS values of Chlorella vulgaris and Chlorella sorokiniana are higher than that of cooked pulses [135]. The observed low PDCAAS of microalgal protein is primarily a result of low TPD, as Chlorella vulgaris and Chlorella sorokiniana had AAS values of over 1.0 [81]. Indeed, while ADP values for Chlorella vulgaris, Chlorella sorokiniana, and Acutodesmus obliquus were 60.6%, 54.8%, and 32.6%, TPD values were all higher, at 64.7%, 59.3%, and 37.9%, respectively. Furthermore, mechanical cell wall disruption significantly increased both ADP values (72.9%, 70.5%, and 62.4%, respectively) and TPD values (77.5%, 74.9%, and 67.2%, respectively). This study reported, for the first time, on the protein quality marker PDCAAS of microalgae biomass for human food using the standard rat assay, the impact of mechanical cell wall disruption, differences between microalgal species, and the elemental analysis of nitrogen in dietary and fecal samples for the calculation of ingested and undigested proteins. Recently, Tessier et al. [133] evaluated the protein quality of biomass produced from the blue-green algae Spirulina using a rat model. These researchers quantified 15N concentrations in caecal contents 6 h post-prandial and obtained a TPD of 86.0% and a PDCAAS of 0.84. These values are higher than those obtained for Chlorella vulgaris, Chlorella sorokiniana, and Acutodesmus obliquus, using the fecal protein digestibility method [81] and may be indicative of the different cell wall recalcitrance between the various species studied. A range of 51–90% of in vivo protein digestibility was observed in rats, mice, and humans for the biomass of several different microalgal species and strains (e.g., Arthrospira, Chlorella, Coelastrum, Nannochloropsis, Scenedesmus, Uronema) over the past decades by different research groups [47]. As different species were assessed for protein quality using different methods in the two aforementioned studies, it is not fully possible to elucidate whether the differences in TPD and PDCAAS between these two studies are attributed to the nature of microalgal species, function and products of gut bacteria, and/or a result of the different model and assay protocols employed. However, differences in cell wall recalcitrance are already well documented between Spirulina and Chlorella. The DIAAS values are corrected for the standardized ileal digestibility of individual amino acids, rather than simply protein as a whole. The difference between PDCAAS and DIAAS lies in the fact that fecal digestibility, used in PDCAAS, may be affected by microbial degradation, while true ileal digestibility used in DIAAS more accurately represents the amount of amino acids absorbed in the gastrointestinal tract [136]. There is no information available about the DIAAS values of microalgal biomass or more pure forms of microalgal protein products for human foods; however, high in vivo APD of essential amino acids (>92%) and DIAAS values (>1) were recently reported for Pavlova sp. microalgae when measured in Atlantic salmon [137].
As discussed previously, there is a rapidly growing interest in finding new protein alternatives to animal-derived sources. Of particular interest in recent years have been plant-based resources such as pulses and microalgae [10,138]. The protein quality of microalgae such as Chlorella vulgaris and Chlorella sorokiniana, as determined by PDCAAS values, appears to be higher than that of pulses such as lentils, beans, peas, and chickpeas [125]. Microalgae have been traditionally consumed as dried whole cells for their health benefits in addition to their abundant supply of nutrients [139]. However, digestibility can be low for some species, so cell wall disruption using a variety of techniques and the selection of species with inherently low recalcitrance is critical for those consumed as whole cells. Although more costly to produce, digestibility and ultimate protein quality of microalgae can be greatly enhanced if consumed in more refined forms, such as protein concentrates or protein isolates [140]. As the protein of some microalgae species has good essential amino acid profiles or amino acid scores, increasing protein digestibility is critical to the enhancement of microalgal protein quality and nutritional and commercial values. Extraction and purification are options when new processing technologies and equipment are developed by adapting to the industry needs and become affordable and economically attractive.
Another important application of microalgal proteins in human nutrition is to balance the essential amino acids of plant and pulse proteins as they have different first limiting amino acids. Methionine and cysteine or tryptophan are the limiting amino acids in most pulses, such as beans, peas, and lentils [135], while histidine or isoleucine are the first limiting amino acids in microalgae such as Chlorella vulgaris, Chlorella sorokiniana, and Acutodesmus obliquus [81]. When they are combined or consumed together, a more balanced amino acid profile may be achieved and thus improve the overall bio-efficiency of amino acids of the pooled protein product.
8. Current Challenges and Future Research Directions
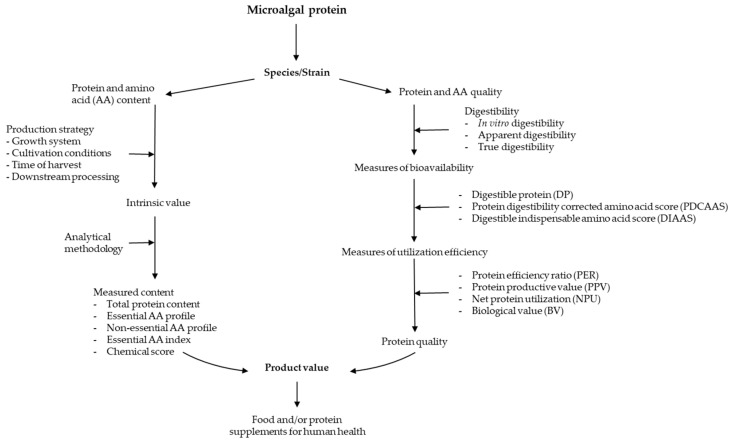
Microalgae are a source of renewable nutrition and there is a fast-growing interest in algae-based functional foods and dietary supplements in the form of whole biomass, such as Chlorella and Arthrospira or purified protein products. The quality of food protein products is determined mainly by protein content, amino acid profile, and digestibility (Figure 1). Elemental analysis appears to be suitable for the estimation of protein content of microalgal biomass, while more research needs to be performed in terms of developing and standardizing N-to-P conversion factors. Different methods are used to measure protein quality, of which, AAS and TPD are critical, and determine the endpoint protein quality markers of PDCAAS and DIASS. Although microalgae have been well recognized as green and sustainable sources of high-quality protein—compared with other sources of protein, especially terrestrial plant proteins—and are increasingly used globally as dietary supplements or food protein ingredients, information on the protein yield and its interaction with growing conditions, protein quality assessment, and processing technologies for protein quality improvement falls far behind those of animal and other plant-based proteins. There is a strong need to increase investment and efforts in the research and product development of microalgae.
Figure 1.
Factors affecting microalgal protein content and quality and common assessment criteria.
Microalgal proteins can be used to balance dietary proteins in countries where animal proteins are insufficient, or where the balance of dietary essential amino acids is a big challenge. Microalgal proteins are also valuable for human health promotion, particularly in those who are vegans or vegetarians [16,17,18]. Microalgal proteins have many other applications as well, such as cosmeceutical, pharmaceutical, and animal/aquaculture feed aspects, which are beyond the scope of this review and should be addressed separately. The wide use of microalgae protein for food remains a challenge, largely due to a lack of scalable and cost-effective cultivation and knowledge gaps regarding harvesting and downstream processing [10]. The important cost factors are the capital costs of algal ponds and associated operational costs including those of mixing processes, the photosynthetic efficiency of systems, the growth media, and CO2. An optimization study on these cost factors sheds a light as a future direction to improve economic output [141]. As low-trophic aquatic organisms, microalgae can synthesize and/or bioaccumulate other metabolites, contaminants, and impurities that have the potential to cause harm upon ingestion [142,143]. As such, significant research and product monitoring efforts are required to fully characterize the microalgae of interest and to improve production methods in order to ensure the nutritional value and safety of the final consumer products and this may be achieved by production of concentrated or purified forms (e.g., protein concentrates or isolates) for human food and supplements. Continued advancements in microalgae research and technology should result in increased biomass production and assist society in meeting our ever-growing demand for high-quality sustainable protein-rich foods.
Author Contributions
Conceptualization, Y.W.; resources, Y.W.; data curation, Y.W., S.M.T. and P.J.M.; writing—original draft preparation, Y.W.; writing—review and editing, S.M.T., P.J.M. and Y.W.; supervision, Y.W.; project administration, Y.W.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Aquatic and Crop Resource Development Research Center, National Research Council of Canada.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bleakley S., Hayes M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods. 2017;6:33. doi: 10.3390/foods6050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godfray H.C.J., Beddington J.R., Crute I.R., Haddad L., Lawrence D., Muir J.F., Pretty J., Robinson S., Thomas S.M., Toulmin C. Food Security: The Challenge of Feeding 9 Billion People. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- 3.Tilman D., Balzer C., Hill J., Befort B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA. 2011;108:20260–20264. doi: 10.1073/pnas.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tukker A., Jansen B. Environmental Impacts of Products: A Detailed Review of Studies. J. Ind. Ecol. 2006;10:159–182. doi: 10.1162/jiec.2006.10.3.159. [DOI] [Google Scholar]
- 5.Vermeulen S.J., Campbell B.M., Ingram J.S.I. Climate Change and Food Systems. Annu. Rev. Environ. Resour. 2012;37:195–222. doi: 10.1146/annurev-environ-020411-130608. [DOI] [Google Scholar]
- 6.Crippa M., Solazzo E., Guizzardi D., Monforti-Ferrario F., Tubiello F.N., Leip A. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food. 2021;2:198–209. doi: 10.1038/s43016-021-00225-9. [DOI] [PubMed] [Google Scholar]
- 7.Tester M., Langridge P. Breeding Technologies to Increase Crop Production in a Changing World. Science. 2010;327:818–822. doi: 10.1126/science.1183700. [DOI] [PubMed] [Google Scholar]
- 8.Henchion M., Hayes M., Mullen A.M., Fenelon M., Tiwari B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods. 2017;6:53. doi: 10.3390/foods6070053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgado C.L. Rising Consumption of Meat and Milk in Developing Countries Has Created a New Food Revolution. J. Nutr. 2003;133:3907S–3910S. doi: 10.1093/jn/133.11.3907S. [DOI] [PubMed] [Google Scholar]
- 10.Caporgno M.P., Mathys A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018;5:58. doi: 10.3389/fnut.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smetana S., Mathys A., Knoch A., Heinz V. Meat alternatives: Life cycle assessment of most known meat substitutes. Int. J. Life Cycle Assess. 2015;20:1254–1267. doi: 10.1007/s11367-015-0931-6. [DOI] [Google Scholar]
- 12.Pimentel D., Pimentel M. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 2003;78:660S–663S. doi: 10.1093/ajcn/78.3.660S. [DOI] [PubMed] [Google Scholar]
- 13.Storlien L.H., Higgins J.A., Thomas T.C., Brown M.A., Wang H.Q., Huang X.F., Else P.L. Diet composition and insulin action in animal models. Br. J. Nutr. 2000;83:S85–S90. doi: 10.1017/S0007114500001008. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Gagnon J., Nair S., Sha S. Herring Milt Protein Hydrolysate Improves Insulin Resistance in High-Fat-Diet-Induced Obese Male C57BL/6J Mice. Mar. Drugs. 2019;17:456. doi: 10.3390/md17080456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Nair S., Gagnon J. Herring Milt and Herring Milt Protein Hydrolysate Are Equally Effective in Improving Insulin Sensitivity and Pancreatic Beta-Cell Function in Diet-Induced Obese- and Insulin-Resistant Mice. Mar. Drugs. 2020;18:635. doi: 10.3390/md18120635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLeod M.J., Vellinga T., Opio C., Falcucci A., Tempio G., Henderson B., Makkar H., Mottet A., Robinson T., Steinfeld H., et al. Invited review: A position on the Global Livestock Environmental Assessment Model (GLEAM) Animal. 2018;12:383–397. doi: 10.1017/S1751731117001847. [DOI] [PubMed] [Google Scholar]
- 17.Murphy S.P., Allen L.H. Nutritional Importance of Animal Source Foods. J. Nutr. 2003;133:3932S–3935S. doi: 10.1093/jn/133.11.3932S. [DOI] [PubMed] [Google Scholar]
- 18.Randolph T.F., Schelling E., Grace D., Nicholson C.F., Leroy J.L., Cole D., Demment M.W., Omore A., Zinsstag J., Ruel M. Invited Review: Role of livestock in human nutrition and health for poverty reduction in developing countries. J. Anim. Sci. 2007;85:2788–2800. doi: 10.2527/jas.2007-0467. [DOI] [PubMed] [Google Scholar]
- 19.Gerber P.J., Mottet A., Opio C.I., Falcucci A., Teillard F. Environmental impacts of beef production: Review of challenges and perspectives for durability. Meat Sci. 2015;109:2–12. doi: 10.1016/j.meatsci.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Herrero M., Havlik P., Valin H., Notenbaert A.M.O., Rufino M., Thornton P.K., Blümmel M., Weiss F., Grace D., Obersteiner M. Biomass use, production, feed efficiencies, and greenhouse gas emissions from global livestock systems. Proc. Natl. Acad. Sci. USA. 2013;110:20888–20893. doi: 10.1073/pnas.1308149110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tredici M.R., Rodolfi L., Biondi N., Bassi N., Sampietro G. Techno-economic analysis of microalgal biomass production in a 1-ha Green Wall Panel (GWP®) plant. Algal Res. 2016;19:253–263. doi: 10.1016/j.algal.2016.09.005. [DOI] [Google Scholar]
- 22.Ferreira A.F., Ferreira A., Dias A.P.S., Gouveia L. Pyrolysis of Scenedesmus obliquus Biomass Following the Treatment of Different Wastewaters. BioEnergy Res. 2020;13:896–906. doi: 10.1007/s12155-020-10102-1. [DOI] [Google Scholar]
- 23.Navarro-López E., Ruíz-Nieto A., Ferreira A., Acién F.G., Gouveia L. Biostimulant Potential of Scenedesmus obliquus Grown in Brewery Wastewater. Molecules. 2020;25:664. doi: 10.3390/molecules25030664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borowitzka M.A., Vonshak A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017;52:407–418. doi: 10.1080/09670262.2017.1365177. [DOI] [Google Scholar]
- 25.Vonshak A. Chapter 15—Micro-algae: Laboratory growth techniques and outdoor biomass production. In: Coombs J., Hall D.O., Long S.P., Scurlock J.M.O., editors. Techniques in Bioproductivity and Photosynthesis. 2nd ed. Pergamon Press; Oxford, UK: 1985. pp. 188–200. [Google Scholar]
- 26.Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Optimization of Nannochloropsis oculata growth using the response surface method. J. Chem. Technol. Biotechnol. 2006;81:1049–1056. doi: 10.1002/jctb.1529. [DOI] [Google Scholar]
- 27.Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006;101:87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- 28.Richmond A. CRC Handbook of Microalgal Mass Culture. 1st ed. CRC Press; Boca Raton, FL, USA: 1986. [Google Scholar]
- 29.Burlew J.S. Algal Culture from Laboratory to Pilot Plant. Carnegie Institution of Washington Publication; Washington, DC, USA: 1953. [Google Scholar]
- 30.Ismail B.P., Senaratne-Lenagala L., Stube A., Brackenridge A. Protein demand: Review of plant and animal proteins used in alternative protein product development and production. Anim. Front. 2020;10:53–63. doi: 10.1093/af/vfaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milledge J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Bio/Technol. 2011;10:31–41. doi: 10.1007/s11157-010-9214-7. [DOI] [Google Scholar]
- 32.Aaronson S., Berner T., Dubinsky Z. Microalgae as a source of chemicals and natural products. In: Shelef G., Soeder C.J., editors. Algae Biomass: Production and Use/[Sponsored by the National Council for Research and Development, Israel and the Gesellschaft fur Strahlen-und Umweltforschung (GSF), Munich, Germany] Elsevier/North-Holland Biomedical Press; Amsterdam, The Netherlands: 1980. pp. 595–601. [Google Scholar]
- 33.Habib M.A.B. Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish. Food and Agriculture Organization of the United Nations; Rome, Italy: 2008. [Google Scholar]
- 34.Becker E. Micro-algae as a source of protein. Biotechnol. Adv. 2007;25:207–210. doi: 10.1016/j.biotechadv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Burlew J.S. Algal Culture from Laboratory to Pilot Plant. AIBS Bull. 1953;3:11 [Google Scholar]
- 36.Goldman J.C. Outdoor algal mass cultures—I. Applications. Water Res. 1979;13:1–19. doi: 10.1016/0043-1354(79)90249-5. [DOI] [Google Scholar]
- 37.Goldman J.C. Outdoor algal mass cultures—II. Photosynthetic yield limitations. Water Res. 1979;13:119–136. doi: 10.1016/0043-1354(79)90083-6. [DOI] [Google Scholar]
- 38.Pulz O., Gross W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 39.Chacón-Lee T., González-Mariño G. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010;9:655–675. doi: 10.1111/j.1541-4337.2010.00132.x. [DOI] [PubMed] [Google Scholar]
- 40.Brennan L., Owende P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010;14:557–577. doi: 10.1016/j.rser.2009.10.009. [DOI] [Google Scholar]
- 41.Khan M.I., Shin J.H., Kim J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories. 2018;17:36. doi: 10.1186/s12934-018-0879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batista A.P., Niccolai A., Bursic I., Sousa I., Raymundo A., Rodolfi L., Biondi N., Tredici M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods. 2019;8:611. doi: 10.3390/foods8120611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niccolai A., Bažec K., Rodolfi L., Biondi N., Zlatić E., Jamnik P., Tredici M.R. Lactic Acid Fermentation of Arthrospira platensis (Spirulina) in a Vegetal Soybean Drink for Developing New Functional Lactose-Free Beverages. Front. Microbiol. 2020;11:560684. doi: 10.3389/fmicb.2020.560684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bleakley S., Hayes M. Functional and Bioactive Properties of Protein Extracts Generated from Spirulina platensis and Isochrysis galbana T-Iso. Appl. Sci. 2021;11:3964. doi: 10.3390/app11093964. [DOI] [Google Scholar]
- 45.Sousa I., Gouveia L., Batista A.P., Raymundo A., Bandarra N.M. Microalgae in novel food products. Food Chem. Res. Dev. 2008:75–112. [Google Scholar]
- 46.Norton T.A., Melkonian M., Andersen R.A. Algal biodiversity. Phycologia. 1996;35:308–326. doi: 10.2216/i0031-8884-35-4-308.1. [DOI] [Google Scholar]
- 47.Acquah C., Tibbetts S.M., Pan S., Udenigwe C. Chapter 19—Nutritional quality and bioactive properties of proteins and peptides from microalgae. In: Jacob-Lopes E., Maroneze M.M., Queiroz M.I., Zepka L.Q., editors. Handbook of Microalgae-Based Processes and Products. Academic Press; Cambridge, MA, USA: 2020. pp. 493–531. [Google Scholar]
- 48.Brown M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991;145:79–99. doi: 10.1016/0022-0981(91)90007-J. [DOI] [Google Scholar]
- 49.Tipnee S., Ramaraj R., Unpaprom Y. Nutritional evaluation of edible freshwater green macroalga Spirogyra varians. Emergent Life Sci. Res. 2015;1:1–7. [Google Scholar]
- 50.Saragih H.T., Muhamad A.A.K., Alfianto A., Viniwidihastuti F., Untari L.F., Lesmana I., Widyatmoko H., Rohmah Z. Effects of Spirogyra jaoensis as a dietary supplement on growth, pectoralis muscle performance, and small intestine morphology of broiler chickens. Vet. World. 2019;12:1233–1239. doi: 10.14202/vetworld.2019.1233-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becker E.W. Microalgae for Human and Animal Nutrition. In: Richmong A., Hu Q., editors. Handbook of Microalgal Culture: Applied Phycology and Biotechnology. 2nd ed. Wiley-Blackwell; Hoboken, NJ, USA: 2013. pp. 461–503. [Google Scholar]
- 52.Koyande A.K., Chew K.W., Rambabu K., Tao Y., Chu D.-T., Show P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness. 2019;8:16–24. doi: 10.1016/j.fshw.2019.03.001. [DOI] [Google Scholar]
- 53.Ismail I., Hwang Y.-H., Joo S.-T. Meat analog as future food: A review. J. Anim. Sci. Technol. 2020;62:111–120. doi: 10.5187/jast.2020.62.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tibbetts S.M., Milley J.E., Lall S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. Environ. Boil. Fishes. 2015;27:1109–1119. doi: 10.1007/s10811-014-0428-x. [DOI] [Google Scholar]
- 55.Islam R., Hassan A., Sulebele G., Orosco C., Roustaian P. Influence of temperature on growth and biochemical composition of Spirulina platensis and S. fusiformis. Ital. Int. J. Sci. 2003;4:97–106. [Google Scholar]
- 56.Tokuşoglu O., Uunal M. Biomass Nutrient Profiles of Three Microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003;68:1144–1148. doi: 10.1111/j.1365-2621.2003.tb09615.x. [DOI] [Google Scholar]
- 57.Ferreira L., Rodrigues M., Converti A., Sato S., Carvalho J. Arthrospira (Spirulina) platensis cultivation in tubular photobioreactor: Use of no-cost CO2 from ethanol fermentation. Appl. Energy. 2012;92:379–385. doi: 10.1016/j.apenergy.2011.11.019. [DOI] [Google Scholar]
- 58.Coca M., Barrocal V.M., Lucas S., Benito G.G., García-Cubero M.T. Protein production in Spirulina platensis biomass using beet vinasse-supplemented culture media. Food Bioprod. Process. 2015;94:306–312. doi: 10.1016/j.fbp.2014.03.012. [DOI] [Google Scholar]
- 59.Salla A.C.V., Margarites A.C.F., Seibel F.I., Holz L.C., Brião V.B., Bertolin T.E., Colla L., Costa J.A.V. Increase in the carbohydrate content of the microalgae Spirulina in culture by nutrient starvation and the addition of residues of whey protein concentrate. Bioresour. Technol. 2016;209:133–141. doi: 10.1016/j.biortech.2016.02.069. [DOI] [PubMed] [Google Scholar]
- 60.Colla L., Reinehr C., Reichert C., Costa J.A.V. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007;98:1489–1493. doi: 10.1016/j.biortech.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 61.Matsudo M.C., Bezerra R.P., Sato S., Perego P., Converti A., Carvalho J.C.M. Repeated fed-batch cultivation of Arthrospira (Spirulina) platensis using urea as nitrogen source. Biochem. Eng. J. 2009;43:52–57. doi: 10.1016/j.bej.2008.08.009. [DOI] [Google Scholar]
- 62.Ben-Amotz A., Tornabene T.G., Thomas W.H. Chemical profile of selected species of microalgae with emphasis on lipids1. J. Phycol. 1985;21:72–81. doi: 10.1111/j.0022-3646.1985.00072.x. [DOI] [Google Scholar]
- 63.Lubitz J.A. The Protein Quality, Digestibility, and Composition of Algae, Chlorella 71105. J. Food Sci. 1963;28:229–232. doi: 10.1111/j.1365-2621.1963.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 64.Leveille G.A., Sauberlich H.E., Shockley J.W. Protein Value and the Amino Acid Deficiencies of Various Algae for Growth of Rats and Chicks. J. Nutr. 1962;76:423–428. doi: 10.1093/jn/76.4.423. [DOI] [PubMed] [Google Scholar]
- 65.Sousa I., Gouveia L., Batista A., Raymundo A., Bandarra N. Microalgae in novel food products. In: Papadopoulos K.N., editor. Food Chemistry Research Developments. Nova Science Publishers Inc.; New York, NY, USA: 2008. [Google Scholar]
- 66.Rebolloso-Fuentes M.M., Navarro-Pérez A., García-Camacho F., Ramos-Miras J.J., Guil-Guerrero J.L. Biomass Nutrient Profiles of the Microalga Nannochloropsis. J. Agric. Food Chem. 2001;49:2966–2972. doi: 10.1021/jf0010376. [DOI] [PubMed] [Google Scholar]
- 67.Fuentes M.R., Fernández G.A., Pérez J.S., Guerrero J.G. Biomass nutrient profiles of the microalga Porphyridium cruentum. Food Chem. 2000;70:345–353. doi: 10.1016/S0308-8146(00)00101-1. [DOI] [Google Scholar]
- 68.Tomás-Almenar C., Larrán A., de Mercado E., Sanz-Calvo M., Hernández D., Riaño B., González M.C.G. Scenedesmus almeriensis from an integrated system waste-nutrient, as sustainable protein source for feed to rainbow trout (Oncorhynchus mykiss) Aquaculture. 2018;497:422–430. doi: 10.1016/j.aquaculture.2018.08.011. [DOI] [Google Scholar]
- 69.Alkhamis Y., Qin J.G. Comparison of pigment and proximate compositions of Tisochrysis lutea in phototrophic and mixotrophic cultures. Environ. Boil. Fishes. 2016;28:35–42. doi: 10.1007/s10811-015-0599-0. [DOI] [Google Scholar]
- 70.Clayton J.R., Dortch Q., Thoresen S.S., Ahmed S. Evaluation of methods for the separation and analysis of proteins and free amino acids in phytoplankton samples. J. Plankton Res. 1988;10:341–358. doi: 10.1093/plankt/10.3.341. [DOI] [Google Scholar]
- 71.Lohrenz S., Taylor C.D. Inorganic 14C as a probe of growth rate-dependent variations in intracellular free amino acid and protein composition of NH+4 -limited continuous cultures of Nannochloris atomis Butcher. J. Exp. Mar. Biol. Ecol. 1987;106:31–55. doi: 10.1016/0022-0981(87)90146-8. [DOI] [Google Scholar]
- 72.López C.V.G., del Carmen Cerón García M., Fernandez F.G.A., Bustos C.S., Chisti Y., Sevilla J.M.F. Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 2010;101:7587–7591. doi: 10.1016/j.biortech.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 73.Lourenço S.O., Barbarino E., Lavin P., Marquez U.M.L., Aidar E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004;39:17–32. doi: 10.1080/0967026032000157156. [DOI] [Google Scholar]
- 74.Laurens L.M., Van Wychen S., McAllister J.P., Arrowsmith S., Dempster T.A., McGowen J., Pienkos P.T. Strain, biochemistry, and cultivation-dependent measurement variability of algal biomass composition. Anal. Biochem. 2014;452:86–95. doi: 10.1016/j.ab.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 75.Templeton D., Laurens L.M. Nitrogen-to-protein conversion factors revisited for applications of microalgal biomass conversion to food, feed and fuel. Algal Res. 2015;11:359–367. doi: 10.1016/j.algal.2015.07.013. [DOI] [Google Scholar]
- 76.Finkel Z.V., Follows M.J., Liefer J., Brown C.M., Benner I., Irwin A.J. Phylogenetic Diversity in the Macromolecular Composition of Microalgae. PLoS ONE. 2016;11:e0155977. doi: 10.1371/journal.pone.0155977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Slocombe S.P., Ross M., Thomas N., McNeill S., Stanley M.S. A rapid and general method for measurement of protein in micro-algal biomass. Bioresour. Technol. 2013;129:51–57. doi: 10.1016/j.biortech.2012.10.163. [DOI] [PubMed] [Google Scholar]
- 78.Waterborg J. The Lowry Method for Protein Quantitation. Humana Press; Totowa, NJ, USA: 2009. pp. 7–10. [Google Scholar]
- 79.Shen C.-H. Chapter 8—Quantification and Analysis of Proteins. In: Shen C.-H., editor. Diagnostic Molecular Biology. Academic Press; Cambridge, MA, USA: 2019. pp. 187–214. [Google Scholar]
- 80.Peterson G.L. Review of the folin phenol protein quantitation method of lowry, rosebrough, farr and randall. Anal. Biochem. 1979;100:201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y., Tibbetts S.M., Berrue F., McGinn P.J., MacQuarrie S.P., Puttaswamy A., Patelakis S., Schmidt D., Melanson R., MacKenzie S.E. A Rat Study to Evaluate the Protein Quality of Three Green Microalgal Species and the Impact of Mechanical Cell Wall Disruption. Foods. 2020;9:1531. doi: 10.3390/foods9111531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laurens L.M.L., Olstad J.L., Templeton D.W. Total Protein Content Determination of Microalgal Biomass by Elemental Nitrogen Analysis and a Dedicated Nitrogen-to-Protein Conversion Factor. In: Spilling K., editor. Biofuels from Algae: Methods and Protocols. Springer New York; New York, NY, USA: 2020. pp. 233–242. [DOI] [PubMed] [Google Scholar]
- 83.Metsoviti M.N., Katsoulas N., Karapanagiotidis I.T., Papapolymerou G. Effect of nitrogen concentration, two-stage and prolonged cultivation on growth rate, lipid and protein content of Chlorella vulgaris. J. Chem. Technol. Biotechnol. 2019;94:1466–1473. doi: 10.1002/jctb.5899. [DOI] [Google Scholar]
- 84.Zhu S., Wang Y., Shang C., Wang Z., Xu J., Yuan Z. Characterization of lipid and fatty acids composition of Chlorella zofingiensis in response to nitrogen starvation. J. Biosci. Bioeng. 2015;120:205–209. doi: 10.1016/j.jbiosc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 85.Ruangsomboon S. Effect of light, nutrient, cultivation time and salinity on lipid production of newly isolated strain of the green microalga, Botryococcus braunii KMITL 2. Bioresour. Technol. 2012;109:261–265. doi: 10.1016/j.biortech.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 86.Sukenik A., Carmeli Y., Berner T. Regulation of fatty acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. J. Phycol. 1989;25:686–692. doi: 10.1111/j.0022-3646.1989.00686.x. [DOI] [Google Scholar]
- 87.George B., Pancha I., Desai C., Chokshi K., Paliwal C., Ghosh T., Mishra S. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus—A potential strain for bio-fuel production. Bioresour. Technol. 2014;171:367–374. doi: 10.1016/j.biortech.2014.08.086. [DOI] [PubMed] [Google Scholar]
- 88.Nzayisenga J.C., Farge X., Groll S.L., Sellstedt A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels. 2020;13:1–8. doi: 10.1186/s13068-019-1646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Renaud S.M., Thinh L.-V., Lambrinidis G., Parry D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture. 2002;211:195–214. doi: 10.1016/S0044-8486(01)00875-4. [DOI] [Google Scholar]
- 90.De Oliveira M., Monteiro M., Robbs P., Leite S. Growth and Chemical Composition of Spirulina maxima and Spirulina platensis Biomass at Different Temperatures. Aquac. Int. 1999;7:261–275. doi: 10.1023/A:1009233230706. [DOI] [Google Scholar]
- 91.Thompson P., Guo M.-X., Harrison P.J. Effects of variation in temperature. I. On the biochemical composition of eight species of marine phytoplankton. J. Phycol. 1992;28:481–488. doi: 10.1111/j.0022-3646.1992.00481.x. [DOI] [Google Scholar]
- 92.Anderson S.L., Vacek J.R., Macharg M.A., Holtkamp D.J. Occurrence of Incisional Complications and Associated Risk Factors Using a Right Ventral Paramedian Celiotomy Incision in 159 Horses. Vet. Surg. 2011;40:82–89. doi: 10.1111/j.1532-950X.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- 93.Sydney E.B., Sturm W., de Carvalho J.C., Thomaz-Soccol V., Larroche C., Pandey A., Soccol C.R. Potential carbon dioxide fixation by industrially important microalgae. Bioresour. Technol. 2010;101:5892–5896. doi: 10.1016/j.biortech.2010.02.088. [DOI] [PubMed] [Google Scholar]
- 94.Tang D., Han W., Li P., Miao X., Zhong J.-J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011;102:3071–3076. doi: 10.1016/j.biortech.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 95.Silva H.J., Pirt S.J. Carbon Dioxide Inhibition of Photosynthetic Growth of Chlorella. Microbiology. 1984;130:2833–2838. doi: 10.1099/00221287-130-11-2833. [DOI] [Google Scholar]
- 96.Patil L., Kaliwal B. Effect of CO2 Concentration on Growth and Biochemical Composition of Newly Isolated Indigenous Microalga Scenedesmus bajacalifornicus BBKLP-07. Appl. Biochem. Biotechnol. 2016;182:335–348. doi: 10.1007/s12010-016-2330-2. [DOI] [PubMed] [Google Scholar]
- 97.Kandasamy L., Neves M., Demura M., Nakajima M. The Effects of Total Dissolved Carbon Dioxide on the Growth Rate, Biochemical Composition, and Biomass Productivity of Nonaxenic Microalgal Polyculture. Sustainability. 2021;13:2267. doi: 10.3390/su13042267. [DOI] [Google Scholar]
- 98.Singh S.P., Singh P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014;38:172–179. doi: 10.1016/j.rser.2014.05.043. [DOI] [Google Scholar]
- 99.Wang J., Sommerfeld M.R., Lu C., Hu Q. Combined effect of initial biomass density and nitrogen concentration on growth and astaxanthin production of Haematococcus pluvialis (Chlorophyta) in outdoor cultivation. Algae. 2013;28:193–202. doi: 10.4490/algae.2013.28.2.193. [DOI] [Google Scholar]
- 100.AnanadhiPadmanabhan M.R., Renita A., Stanley S.A. Studies on the effect of nitrogen source and the growth of Marine microalgae algae; Proceedings of the Recent Advances in Space Technology Services and Climate Change 2010 (RSTS & CC-2010); Chennai, India. 13–15 November 2010; Piscataway, NJ, USA: IEEE; 2010. pp. 350–352. [Google Scholar]
- 101.Zarrinmehr M.J., Farhadian O., Heyrati F.P., Keramat J., Koutra E., Kornaros M., Daneshvar E. Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt. J. Aquat. Res. 2020;46:153–158. doi: 10.1016/j.ejar.2019.11.003. [DOI] [Google Scholar]
- 102.Solovchenko A., Solovchenko O., Khozin-Goldberg I., Didi-Cohen S., Pal D., Cohen Z., Boussiba S. Probing the effects of high-light stress on pigment and lipid metabolism in nitrogen-starving microalgae by measuring chlorophyll fluorescence transients: Studies with a Δ5 desaturase mutant of Parietochloris incisa (Chlorophyta, Trebouxiophyceae) Algal Res. 2013;2:175–182. doi: 10.1016/j.algal.2013.01.010. [DOI] [Google Scholar]
- 103.Ho S.-H., Ye X., Hasunuma T., Chang J.-S., Kondo A. Perspectives on engineering strategies for improving biofuel production from microalgae—A critical review. Biotechnol. Adv. 2014;32:1448–1459. doi: 10.1016/j.biotechadv.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 104.Kim G., Mujtaba G., Lee K. Effects of nitrogen sources on cell growth and biochemical composition of marine chlorophyte Tetraselmis sp. for lipid production. Algae. 2016;31:257–266. doi: 10.4490/algae.2016.31.8.18. [DOI] [Google Scholar]
- 105.Fidalgo J., Cid A., Torres E., Sukenik A., Herrero C. Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture. 1998;166:105–116. doi: 10.1016/S0044-8486(98)00278-6. [DOI] [Google Scholar]
- 106.Qiu R., Gao S., Lopez P.A., Ogden K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017;28:192–199. doi: 10.1016/j.algal.2017.11.004. [DOI] [Google Scholar]
- 107.Danilov R.A., Ekelund N.G.A. Effects of pH on the growth rate, motility and photosynthesis in Euglena gracilis. Folia Microbiol. 2001;46:549–554. doi: 10.1007/BF02818001. [DOI] [PubMed] [Google Scholar]
- 108.Moheimani N.R. Inorganic carbon and pH effect on growth and lipid productivity of Tetraselmis suecica and Chlorella sp. (Chlorophyta) grown outdoors in bag photobioreactors. Environ. Boil. Fishes. 2012;25:387–398. doi: 10.1007/s10811-012-9873-6. [DOI] [Google Scholar]
- 109.Moheimani N.R., Borowitzka M.A. Increased CO2 and the effect of pH on growth and calcification of Pleurochrysis carterae and Emiliania huxleyi (Haptophyta) in semicontinuous cultures. Appl. Microbiol. Biotechnol. 2011;90:1399–1407. doi: 10.1007/s00253-011-3174-x. [DOI] [PubMed] [Google Scholar]
- 110.Khalil Z.I., Asker M.M.S., El-Sayed S., Kobbia I.A. Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J. Microbiol. Biotechnol. 2009;26:1225–1231. doi: 10.1007/s11274-009-0292-z. [DOI] [PubMed] [Google Scholar]
- 111.Goldman J.C., Azov Y., Riley C.B., Dennett M.R. The effect of pH in intensive microalgal cultures. I. Biomass regulation. J. Exp. Mar. Biol. Ecol. 1982;57:1–13. doi: 10.1016/0022-0981(82)90140-X. [DOI] [Google Scholar]
- 112.Bartley M., Boeing W.J., Dungan B.N., Holguin F.O., Schaub T. pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. Environ. Boil. Fishes. 2013;26:1431–1437. doi: 10.1007/s10811-013-0177-2. [DOI] [Google Scholar]
- 113.Valdés F., Hernández M., Catalá L., Marcilla A. Estimation of CO2 stripping/CO2 microalgae consumption ratios in a bubble column photobioreactor using the analysis of the pH profiles. Application to Nannochloropsis oculata microalgae culture. Bioresour. Technol. 2012;119:1–6. doi: 10.1016/j.biortech.2012.05.120. [DOI] [PubMed] [Google Scholar]
- 114.Danquah M.K., Gladman B., Moheimani N., Forde G.M. Microalgal growth characteristics and subsequent influence on dewatering efficiency. Chem. Eng. J. 2009;151:73–78. doi: 10.1016/j.cej.2009.01.047. [DOI] [Google Scholar]
- 115.Salim S., Shi Z., Vermuë M., Wijffels R. Effect of growth phase on harvesting characteristics, autoflocculation and lipid content of Ettlia texensis for microalgal biodiesel production. Bioresour. Technol. 2013;138:214–221. doi: 10.1016/j.biortech.2013.03.173. [DOI] [PubMed] [Google Scholar]
- 116.Da Costa F., Le Grand F., Quéré C., Bougaran G., Cadoret J.-P., Robert R., Soudant P. Effects of growth phase and nitrogen limitation on biochemical composition of two strains of Tisochrysis lutea. Algal Res. 2017;27:177–189. doi: 10.1016/j.algal.2017.09.003. [DOI] [Google Scholar]
- 117.Brown M.R., Garland C.D., Jeffrey S.W., Jameson I.D., Leroi J.M. The gross and amino acid compositions of batch and semi-continuous cultures OfIsochrysis sp. (clone T.ISO), Pavlova lutheri and Nannochloropsis oculata. Environ. Boil. Fishes. 1993;5:285–296. doi: 10.1007/bf02186231. [DOI] [Google Scholar]
- 118.Sánchez-Bayo A., Morales V., Rodríguez R., Vicente G., Bautista L.F. Cultivation of Microalgae and Cyanobacteria: Effect of Operating Conditions on Growth and Biomass Composition. Molecules. 2020;25:2834. doi: 10.3390/molecules25122834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoffman J.R., Falvo M.J. Protein—Which is Best? J. Sports Sci. Med. 2004;3:118–130. [PMC free article] [PubMed] [Google Scholar]
- 120.Rutherfurd S.M., Fanning A., Miller B.J., Moughan P.J. Protein Digestibility-Corrected Amino Acid Scores and Digestible Indispensable Amino Acid Scores Differentially Describe Protein Quality in Growing Male Rats. J. Nutr. 2015;145:372–379. doi: 10.3945/jn.114.195438. [DOI] [PubMed] [Google Scholar]
- 121.FAO. WHO . Protein Quality Evaluation: Report of Joint FAO/WHO Expert Consultation. Food and Agriculture Organization; Rome, Italy: 1991. p. 51. [Google Scholar]
- 122.FAO. WHO . Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. World Health Organization; Geneva, Switzerland: 1985. (WHO Technical Report Series 724). [PubMed] [Google Scholar]
- 123.FAO . Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation. Food and Agriculture Organization of the United Nations; Rome, Italy: 2013. p. 92. [PubMed] [Google Scholar]
- 124.FAO . Protein Quality Assessment in Follow-Up Formula for Young Children and Ready to Use Therapeutic Foods. Food and Agriculture Organization of the United Nations; Rome, Italy: 2017. [Google Scholar]
- 125.Nosworthy M.G., Franczyk A.J., Medina G., Neufeld J., Appah P., Utioh A., Frohlich P., House J.D. Effect of Processing on the in Vitro and in Vivo Protein Quality of Yellow and Green Split Peas (Pisum sativum) J. Agric. Food Chem. 2017;65:7790–7796. doi: 10.1021/acs.jafc.7b03597. [DOI] [PubMed] [Google Scholar]
- 126.Sarwar G. The Protein Digestibility—Corrected Amino Acid Score Method Overestimates Quality of Proteins Containing Antinutritional Factors and of Poorly Digestible Proteins Supplemented with Limiting Amino Acids in Rats. J. Nutr. 1997;127:758–764. doi: 10.1093/jn/127.5.758. [DOI] [PubMed] [Google Scholar]
- 127.Kent M., Welladsen H.M., Mangott A., Li Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE. 2015;10:e0118985. doi: 10.1371/journal.pone.0118985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Waghmare A.G., Salve M.K., Leblanc J.G., Arya S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour. Bioprocess. 2016;3:16. doi: 10.1186/s40643-016-0094-8. [DOI] [Google Scholar]
- 129.Machado M., Machado S., Pimentel F.B., Freitas V., Alves R.C., Oliveira M.B.P.P. Amino Acid Profile and Protein Quality Assessment of Macroalgae Produced in an Integrated Multi-Trophic Aquaculture System. Foods. 2020;9:1382. doi: 10.3390/foods9101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Andreeva A., Budenkova E., Babich O., Sukhikh S., Ulrikh E., Ivanova S., Prosekov A., Dolganyuk V. Production, Purification, and Study of the Amino Acid Composition of Microalgae Proteins. Molecules. 2021;26:2767. doi: 10.3390/molecules26092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hendriks W.H., Van Baal J., Bosch G. Ileal and faecal protein digestibility measurement in humans and other non-ruminants—A comparative species view. Br. J. Nutr. 2012;108:S247–S257. doi: 10.1017/S0007114512002395. [DOI] [PubMed] [Google Scholar]
- 132.Health Canada. 1981. [(accessed on 15 July 2021)]. Available online: https://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/res-rech/fo-1-eng.pdf.
- 133.Tessier R., Calvez J., Khodorova N., Gaudichon C. Protein and amino acid digestibility of 15N Spirulina in rats. Eur. J. Nutr. 2021;60:2263–2269. doi: 10.1007/s00394-020-02368-0. [DOI] [PubMed] [Google Scholar]
- 134.Amorim M.L., Soares J., Coimbra J.S.D.R., Leite M.D.O., Albino L.F.T., Martins M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2021;61:1976–2002. doi: 10.1080/10408398.2020.1768046. [DOI] [PubMed] [Google Scholar]
- 135.Nosworthy M., Neufeld J., Frohlich P., Young G., Malcolmson L., House J.D. Determination of the protein quality of cooked Canadian pulses. Food Sci. Nutr. 2017;5:896–903. doi: 10.1002/fsn3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jiménez-Munoz L.M., Tavares G.M., Corredig M. Design future foods using plant protein blends for best nutritional and technological functionality. Trends Food Sci. Technol. 2021;113:139–150. doi: 10.1016/j.tifs.2021.04.049. [DOI] [Google Scholar]
- 137.Tibbetts S.M., Patelakis S.J. Apparent digestibility coefficients (ADCs) of intact-cell marine microalgae meal (Pavlova sp. 459) for juvenile Atlantic salmon (Salmo salar L.) Aquaculture. 2022;546:737236. doi: 10.1016/j.aquaculture.2021.737236. [DOI] [Google Scholar]
- 138.Shevkani K., Singh N., Chen Y., Kaur A., Yu L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019;56:2787–2798. doi: 10.1007/s13197-019-03723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wells M.L., Potin P., Craigie J.S., Raven J.A., Merchant S.S., Helliwell K.E., Smith A.G., Camire M.E., Brawley S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017;29:949–982. doi: 10.1007/s10811-016-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Banaszek A., Townsend J.R., Bender D., Vantrease W.C., Marshall A.C., Johnson K.D. The Effects of Whey vs. Pea Protein on Physical Adaptations Following 8-Weeks of High-Intensity Functional Training (HIFT): A Pilot Study. Sports. 2019;7:12. doi: 10.3390/sports7010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Norsker N.-H., Barbosa M., Vermuë M.H., Wijffels R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011;29:24–27. doi: 10.1016/j.biotechadv.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 142.Van der Spiegel M., Noordam M., van der Fels-Klerx H. Safety of Novel Protein Sources (Insects, Microalgae, Seaweed, Duckweed, and Rapeseed) and Legislative Aspects for Their Application in Food and Feed Production. Compr. Rev. Food Sci. Food Saf. 2013;12:662–678. doi: 10.1111/1541-4337.12032. [DOI] [PubMed] [Google Scholar]
- 143.Barkia I., Saari N., Manning S.R. Microalgae for High-Value Products towards Human Health and Nutrition. Mar. Drugs. 2019;17:304. doi: 10.3390/md17050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.