Abstract
Background. The classic Ph-negative myeloproliferative neoplasms (MPN) are a group of clonal haematopoietic disorders, including polycythemia vera (PV), essential thrombocythemia (ET) and myelofibrosis (MF), whose shared and diverse phenotypic signatures are caused by a dysregulated JAK/STAT signal transduction because of acquired somatic mutations. It has been demonstrated that autoimmune diseases and MPN can be associated (Kristinsson et al., Haematologica. 2010 Jul;95(7):1216-20.), suggesting a common background of immune dysregulation (Barosi, Curr Hematol Malig Rep. 2014 Dec;9(4):331-9). SARS-CoV-2 infection displays extreme inter-individual clinical variability, ranging from silent infection to lethal disease. It has been described that at least 10% of patients with life-threatening coronavirus disease 2019 (COVID-19) pneumonia have neutralizing autoantibodies (AAbs) against type I IFNs, that precede SARS-CoV-2 infection (Bastard et al., Science. 2020 Oct 23;370(6515):eabd4585). In this study we searched for AAbs against type I IFNs in a cohort of MPN patients to evaluate the prevalence of these AAbs in the MPN population and to check for clinical correlations, including severity of COVID-19.
Methods. Plasma samples from consecutively referred MPN patients were prospectively collected between November 2020 and June 2021 and frozen at -30°C immediately after collection. Levels of AAbs against type I IFN subtypes including IFNs alpha, beta and omega were measured using the enzyme-linked immunosorbent assay (ELISA) and a neutralization assay, as previously reported (Bastard et al., Science. 2020 Oct 23;370(6515):eabd4585; Moreews et al., Sci Immunol. 2021 May 25;6(59):eabh1516).
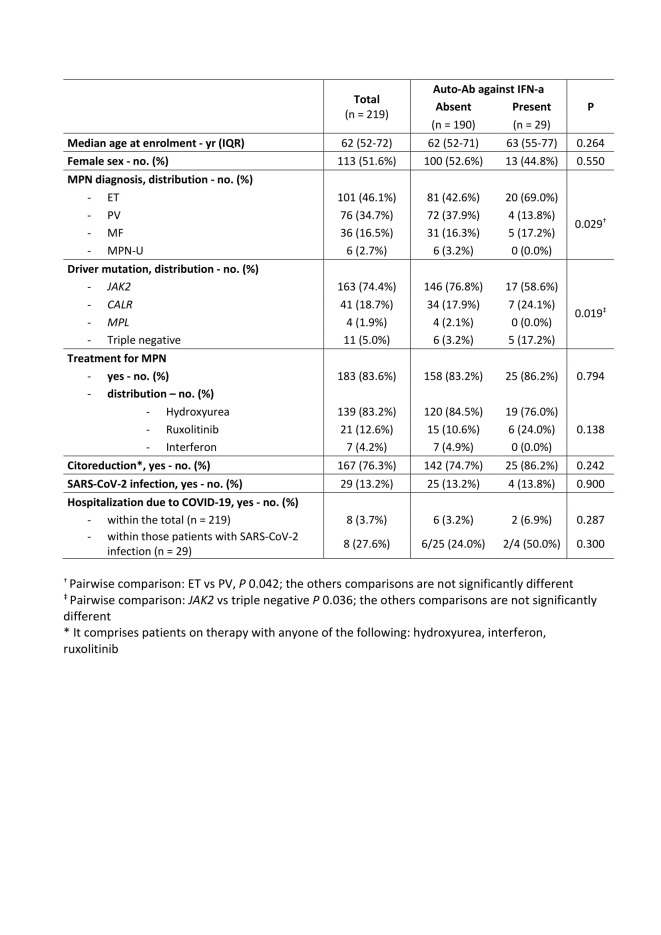
Results. We included a total of 219 MPN patients (101 ET, 76 PV, 36 MF and 6 MPN unclassificable). Neutralizing AAbs to type I IFNs were detected in 29 patients (13.2%, 95%CI: 9.1% - 18.5%). Comparing patients with and without AAbs we observed a significant difference in terms of distribution of MPN diagnosis (P = 0.029) and driver mutations (P = 0.019), while we did not observe a difference in terms of age, sex, and treatment (Table 1). Overall, 29 patients (13%) got SARS-CoV-2 infection and 8 of them (28%) required hospitalization due to severe COVID-19. AAbs against type I IFNs were detected in 4 of the 29 SARS-CoV-2 infected patients. A higher rate of hospitalization for severe COVID-19 was observed in patients with AAbs to type I IFNs (2 of 4 patients, 50%) compared to those without these AAbs (6 of 25 patients, 24%), although the difference did not reach a statistical significance (P = 0.300).
Conclusions. In this study, we detected a prevalence of AAbs against type I IFNs which is much higher in our MPN cohort (13%) than in the general population (2-3%). We also found a correlation between the presence of AAbs to type I IFNs and both the hematological diagnosis and the driver mutation. Despite a comparable prevalence of SARS-CoV-2 infection between MPN patients with or without AAbs to type I IFNs, we observed a different rate of hospitalization due to severe COVID-19 which is almost twice in those with AAbs against type I IFNs compared to those without these AAbs. However, this difference did not reach a statistical significance, probably because of the low number of SARS-CoV-2 infection in the subgroup of patients with AAbs against type I IFNs. Thus, further studies to analyse the prevalence of AAbs against type I IFNs in patients with MPN, their association with other forms of auto-immunity and severe COVID-19 are warranted.
Figure 1.
Disclosures
Arcaini: Gilead Sciences: Research Funding; Bayer, Celgene, Gilead Sciences, Roche, Sandoz, Janssen-Cilag, VERASTEM: Consultancy; Celgene, Roche, Janssen-Cilag, Gilead: Other: Travel expenses; Celgene: Speakers Bureau. Rumi: Novartis, Abbvie: Consultancy.