Supplemental Digital Content is available in the text
Keywords: epidural blood patch, greater occipital nerve block, neuraxial anesthesia, postdural puncture headache
Abstract
Background:
This study aimed at assessing the therapeutic effectiveness of greater occipital nerve block (GONB) against postdural puncture headache (PDPH).
Methods:
Studies investigating analgesic effects of GONB against PDPH in adults were retrieved from the MEDLINE, EMBASE, Google scholar, and Cochrane central databases from their inception dates to May, 2021. Pain score at postprocedural 24 hours was the primary endpoint, while secondary endpoints were pain score at postprocedural 1 hour and 12 hours as well as the risk of intervention failure.
Results:
Of the 7 studies (randomized controlled trials [RCTs], n = 4; non-RCTs, n = 3) that recruited 275 patients, 2 investigated female patients undergoing cesarean section and the other 5 were conducted in both obstetric and nonobstetric settings. Pooled results showed a lower mean pain score at 24 hours (i.e., primary outcome) (mean difference [MD] = –2.66, 95%: CI: –3.98 to –1.33, P < .001; I2 = 97%, 6 studies), 1 hour (MD = –4.23, 95% confidence interval [CI]: –5.08 to –3.37, P < .00001; I2 = 86%, 5 studies), and 6 hours (MD = –2.78, 95% CI: –4.99 to –0.57, P = .01; I2 = 98%, 4 studies) in patients with GONB compared to those without. Trial sequential analysis supported the robustness of evidence at postprocedural 24 hours. The use of GONB also decreased the risk of intervention failure (relative ratio [RR] = 0.4, 95% CI: 0.19 to 0.82, P = .01; I2 = 96%, 6 studies, 277 patients).
Conclusion:
Our results suggested a therapeutic effect of greater occipital nerve block against postdural puncture headache up to postprocedural 24 hours. Further large-scale studies are warranted to evaluate its therapeutic benefit beyond the acute stage.
1. Introduction
Postdural puncture headache (PDPH) is a common neurologic complication and a cause of secondary headache following neuraxial anesthesia. For women suffering from dural puncture during labor epidural analgesia, 50% to 80% may develop PDPH.[1] The International Headache Society has defined PDPH as a bilateral headache that develops within 7 days and disappears within 14 days after dural puncture.[2] PDPH is characterized by a dull throbbing pain with a frontal-occipital distribution typically aggravated by an upright position and relieved by assuming a supine posture.[1] The headache may also be accompanied by neck stiffness, tinnitus, photophobia, and nausea.[1] Previous research has shown a negative impact of these symptoms on patient satisfaction, length of hospital stay, and cost of care.[3] Pathologically, PDPH is believed to be the result of a cerebrospinal fluid leakage through the site of dural puncture,[1] which causes a drop in intracranial pressure and traction of pain sensitive structures. The resulting dilatation of the intracranial vessels to maintain a constant intracranial volume triggers vascular headache.[1]
Due to a lack of guidelines on the treatment of PDPH,[4] various therapeutic strategies are being implemented at different institutes.[5] Conventional management of PDPH involves conservative measures including oral analgesics, hydration, bed rest, and the invasive strategy of epidural blood patch (EBP).[6] However, the evidence supporting the use of some conservative approaches such as bed rest, supine/prone positioning, and hydration is too weak to be recommended.[4] On the other hand, despite its being accepted as the definitive treatment for PDPH,[4] EBP is an invasive procedure with a potential for serious complications, such as chronic low back pain and spinal epidural hematoma.[7]
Previous studies have shown a therapeutic effect of greater occipital nerve block (GONB) against primary headache.[8–10] A previous meta-analysis has shown that GONB significantly reduced not only the frequency of migraine headaches but also the severity of headache compared with controls.[9] Moreover, several recent reports[11–13] have demonstrated the effectiveness of GONB in the management of PDPH. Some authors considered GONB to be a safe and technically simple procedure in an anesthesia clinic setting to achieve more rapid symptom relief compared with medications.[11] Nevertheless, its efficacy has never been fully studied in a systematic approach. We hypothesized that GONB could be an effective strategy for treating PDPH. Accordingly, the primary aim of the current meta-analysis is to investigate the therapeutic effectiveness of GONB against PDPH at post-procedural 24 hours, while the secondary outcomes included the severity of pain at 1- or 12-hour follow-ups as well as the risk of intervention failure.
2. Methods
2.1. Study design
Our meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO CRD42021232648). This study was reported in compliance with the Preferred Reporting Items for Systematic Review and Meta-analysis statement.
2.2. Search strategy
Two authors independently searched the Medline, Google scholar, Cochrane Library, and EMBASE databases from their inception dates till May 06, 2021. The Boolean operator “AND” was applied to intersect different concepts while “OR” was used to cover similar concepts. The keywords below were used to search for eligible records: “Post-dural puncture headache” or “Postdural puncture headache” or “PDPH” or “dural puncture” or “spinal tap headache” or “post-lumbar puncture headache” or “post-LP headache” or “post-spinal puncture headache” or “spinal puncture∗” or “spinal tap∗” or “lumbar puncture∗” or “Cerebrospinal Fluid Leak∗” or “Occipital nerve block∗” or “occipital nerve∗.” Subject headings (i.e., MeSH terms in PubMed) were also utilized for the literature search. The search strategy is demonstrated in Supplemental Digital Content Table S1, http://links.lww.com/MD/G555. Additional records were identified by reviewing the reference lists of the relevant studies.
2.3. Eligibility criteria
All eligible studies were examined by 2 reviewers according to the following PICO criteria: (a) Patient population: adult patients suffering from PDPH, (b) Intervention: use of GONB as the intervention measure for headache relief, (c) Comparison: the use of placebo or conventional treatment (e.g., bed rest) as a control, (d) Outcomes: postprocedural pain relief. A third reviewer was consulted for reaching consensus in case of a discrepancy in the process of study selection. Only studies published in English were included. For the current meta-analysis, we included randomized controlled trials (RCTs) or nonRCTs (e.g., case-control studies or before-and-after studies) that fulfilled the PICO criteria. To avoid duplicated data from different reports based on the same sample sets, we included only the articles with larger sample sizes and more information. On encountering missing data in the included studies, we contacted the authors for original information. Exclusion criteria were
-
1.
studies in which information regarding primary outcome was unavailable, and
-
2.
those published only as letters, case reports, reviews, or abstracts.
2.4. Outcomes and definitions
The primary outcome was the severity of pain at 24-hour follow-up after GONB, while the secondary outcomes included the severity of pain at 1- or 12-hour follow-ups as well as the risk of intervention failure. For the current meta-analysis, intervention failure was defined as repeated GONBs, the use of analgesics, or the requirement of EBP for pain relief after GONB.
2.5. Risk of bias assessment
The methodological quality (i.e., risks of bias) of the eligible RCTs was assessed with the Cochrane Collaboration risk of bias tool,[14] while that of comparative studies (e.g., cohorts and case-control studies) was evaluated with the tool of Risk of Bias in Non-Randomized studies-Intervention. The quality of the studies was assessed by 2 reviewers. Disagreements were settled through discussion with a third reviewer till a consensus was reached.
2.6. Data extraction
Two independent reviewers were responsible for data extraction from each study. The information extracted included: first author, publication year, patient characteristics, study setting, sample size, dosage or technique for GONB, country, and follow-up period. Disagreements were resolved through discussion with a third author.
2.7. Statistical analysis
For analysis of dichotomous outcomes, a random effects model was applied to calculate the risk ratios with 95% confidence intervals. The Mantel-Haenszel method was used to pool dichotomous data and to compute pooled risk ratios with 95% confidence intervals. For continuous data, the mean difference was adopted for grouping trials with the same outcome parameters, while the standardized mean difference was used to combine trials that utilized different parameters to measure the same outcome. The degree of statistical heterogeneity was assessed using I2 statistics (low: 0%–50%; moderate: 51%–75%, high: 75%–100%). The random effects model was utilized in the current study in view of the expected heterogeneity among different trials. Besides, subgroup analyses were conducted according to the study design (i.e., RCT vs Non-RCT subgroups). When 3 or more studies reported on a particular outcome, sensitivity analyses were performed by omitting the studies 1 at a time to explore the potential impact of a single trial on the overall results. If 10 or more studies reported on a particular outcome, we assessed the potential publication bias by visual inspection of the funnel plot produced by plotting the standard error against the log OR of the included studies. The significance level was set at 0.05 for all analyses. Cochrane Review Manager (RevMan 5.4; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used for data synthesis.
The strength and reliability of the cumulative evidence were examined by trial sequential analysis (TSA) (TSA viewer version 0.9.5.10 Beta, www.ctu.dk/tsa) that aimed at reducing false-negative or false-positive findings from multiple testing and sparse data.[15,16] For the primary outcome, we calculated the required information size as well as the trial sequential monitoring boundaries. The variance was obtained from the data of the included studies. The level of evidence for the anticipated intervention effect is deemed sufficient without the need for further studies when the cumulative Z curve crosses the TSA boundary or reaches the required information size.
3. Results
3.1. Study selection
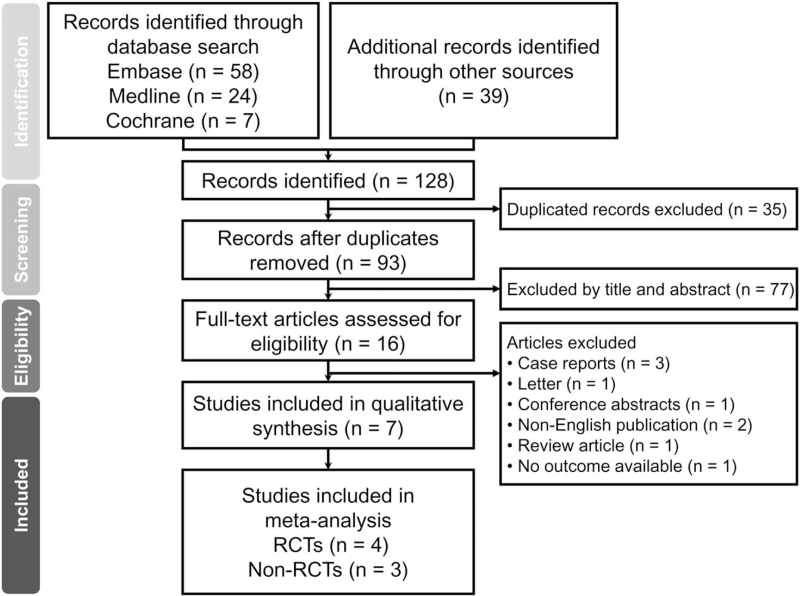
A total of 128 records were retrieved from the systematic search of the Embase, Medline, Google scholar, and Cochrane library databases (Fig. 1). After the removal of 35 duplicates, the titles and abstracts of the rest of 93 records were scrutinized for inappropriateness of their PICO questions, for which a further 77 reports were excluded. Of the remaining 16 records eligible for full-text appraisal, nine were excluded due to their natures of case report (n = 3), letter (n = 1), conference abstract (n = 1), non-English publication (n = 2), review article (n = 1), and unavailability of data on outcome (n = 1). Finally, a total of 7 studies comprising 4 RCTs[11–13,17] and 3 nonRCT studies[18–20] were included in the current meta-analysis.
Figure 1.
PRISMA flow diagram of study selection for the current meta-analysis. RCT = randomized controlled study.
3.2. Study characteristics
Table 1 presents the characteristics of the 7 studies published from 2009 to 2019, which were conducted on 275 adult patients. Four studies adopted a randomized controlled design,[11–13,17] while the other 3 used a non-RCT design.[18–20] Of the 7 studies, 2 investigated women undergoing a cesarean section[17,20] and the other 5 focused on patients presenting with PDPH in both the obstetric and nonobstetric settings.[11–13,18,19] Regarding the criteria of patient recruitment, 1 study adopted a set of diagnostic criteria by only enrolling patients with positional headache together with associated symptoms (i.e., nausea/vomiting, neck stiffness, or photophobia),[11] whilst the rest of the included studies recruited those with mere headaches. The local anesthetics used varied among the included studies with lidocaine being the most common (Table 1). For RCTs, the control group consisted of patients receiving physiological saline,[17] medications,[11] and conventional treatments.[12,13] Technically, GONB was performed under the guidance of ultrasound (n = 3),[11,12,18] nerve stimulator (n = 1),[13] and anatomical landmark (n = 3).[17,19,20] The time between PDPH and GONB varied across the included studies, ranging from 10 hours,[20] 12 hours,[11] 24 hours,[12,13,19] to 48 hours.[18] One study did not provide relevant information.[17] The sample size of the included trials ranged from 16 to 90. GONB-associated adverse events such as subcutaneous hematoma, dizziness, vasovagal attack were mentioned in 2 studies,[11,13] while 3 studies reported no adverse event.[12,19,20] No specific information regarding complications was described in the other 2 studies.[17,18] The follow-up period ranged from 1 to 8 days.
Table 1.
Characteristics of included studies (n = 7).
| Study type | Age (mean; years) Occipital vs control groups | Sample size | Female (%) | Population | Occipital block | Control group | Technique for Occipital block | Follow-up (days) | Country | |
| Abdelraouf 2019[17] | RCTs | 25.9 vs 27.8 | 90 | 100% | Females with PDPH following cesarean section under spinal anesthesia | Lidocaine 40 mg and dexamethasone 8 mg | Saline | Anatomical landmarks | 1 | Egypt |
| Akyol 2015[18] | Non-RCTs | 37∗ | 21 | NA | Patients who developed PDPH after spinal anesthesia, but did not respond to conservative medical treatment within 48 hours | 4 mL 0.25% levobupivacaine | NA | Ultrasound-guided | 1 | Turkey |
| Kamal 2014[11] | RCTs | 38 vs 33 | 30 | 53% | Patients (both gender) who developed PDPH either after a cesarian section or any operation under spinal anesthesia. | 1 mL of 1% lidocaine and 2 mL of 0.25% bupivacaine and 20 mg triamcinolone | Medication | Ultrasound-guided | 1 | Egypt |
| Mostafa Mohamed Stohy 2019[12] | RCTs | 37.4 vs 33.7 | 50 | 78% | Patients expressing PDPH after spinal anesthesia with 22G needle. | Lidocaine 2% 40 mg and dexamethasone 8 mg | Conventional treatment† | Ultrasound-guided | 1 | Egypt |
| Naja 2009[13] | RCTs | 37.3 vs 38.8 | 50 | 82% | Patients with PDPH following spinal anesthesia administered for surgical interventions such as fracture of the lower extremity. | 3 mL lidocaine (2%); 2.5 mL bupivacaine (0.5%); 25 μg fentanyl; 150 μg clonidine§ | Conventional treatment‡ | Nerve Stimulator-Guided | 8 | Lebanon |
| Niraj 2014[19] | Non-RCTs | NA | 18 | NA | Patients presenting with PDPH in both the obstetric and non-obstetric setting | 2 mL of dexamethasone and 2 mL of 1% lidocaine. | NA | Anatomical landmarks | 7 | UK |
| Türkyilmaz 2016[20] | Non-RCTs | 29.9∗ | 16 | 100% | Patients who had been diagnosed to have PDPH after cesarean operations | levobupivacaine 2.5 mg/mL and dexamethasone 1 mg/mL | NA | Anatomical landmarks | 7 | Turkey |
Overall patients; NA = not available.
Bed rest, hydration, Acetaminophen, Caffeine, NSAIDs and opioids.
Consisting of continued bed rest, adequate hydration, and analgesics; PDPH = postdural puncture headache.
Lesser occipital nerve blockade was also used for patients having pain extending to the frontal and temporal areas.
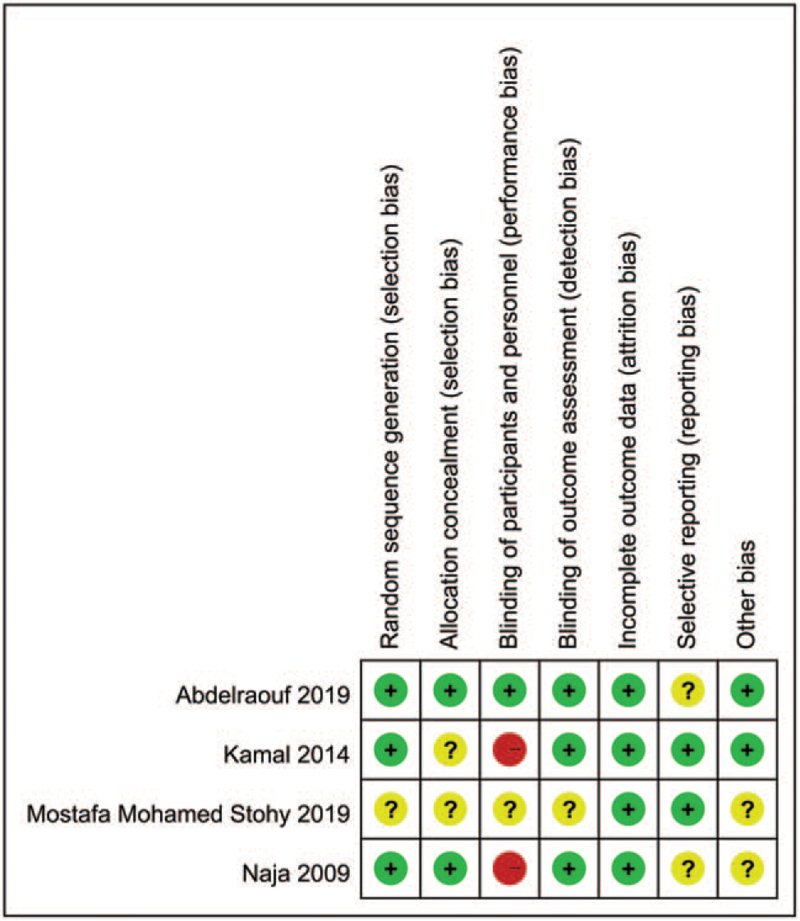
3.3. Risk of bias evaluation
For RCTs, the risks of bias of each trial are summarized in Figure 2. Details on bias assessment for each trial are shown in Supplemental Digital Content Table S2, http://links.lww.com/MD/G556. Appraisal of the nonRCT studies with Nonrandomized studies-intervention indicated a moderate risk of bias (Supplemental Digital Content Table S3, http://links.lww.com/MD/G557).
Figure 2.
Risks of bias of the included studies.
3.4. Primary and secondary outcomes
3.4.1. Primary outcome: impact of greater occipital nerve block on pain relief at postprocedural 24 hours
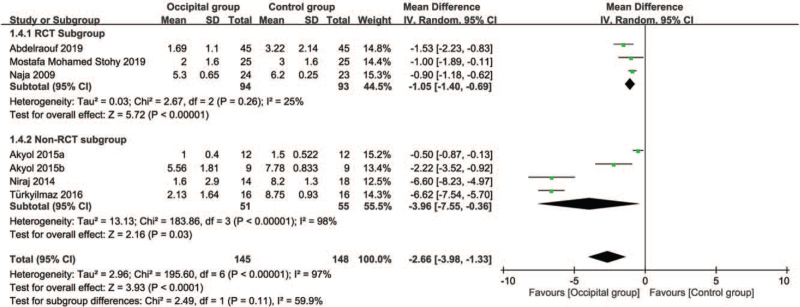
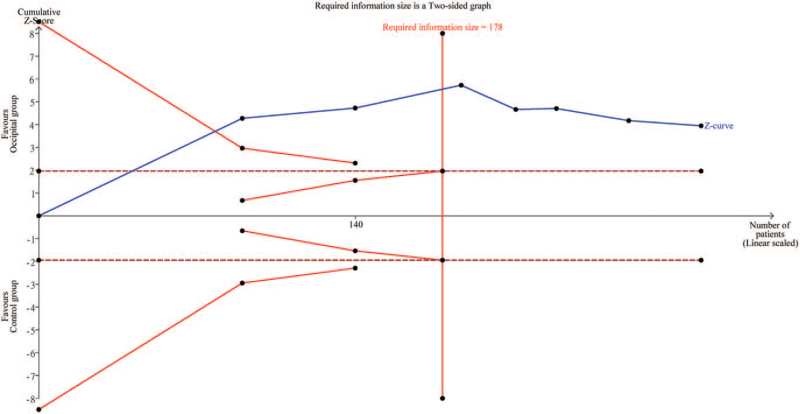
While 6 of the 7 included studies were available for the analysis,[12,13,17–20] 1 study only provided information on pain score at 12 hours.[11] Inspection of the forest plot revealed a lower severity of pain in the occipital group compared to that in the control group (MD = –2.66, 95%: CI: –3.98 to –1.33, P < .001; I2 = 97%) (Fig. 3). No significant difference between RCT- and NonRCT subgroups was noted on subgroup analysis (P = .11). In addition, sensitivity analysis did not show a significant impact on outcome by omitting certain trials. Crossing of the cumulative Z-curve through the required information size indicated sufficient evidence for a firm conclusion (Fig. 4).
Figure 3.
Forest plot for comparing pain score at post-procedural 24 hours between occipital and control groups. CI = confidence interval, IV = inverse variance.
Figure 4.
Trial sequential analysis on impact of greater occipital nerve block on pain relief at postprocedural 24 hours. Variance computed from data acquired from included trials with risk of type I error set at 5% with a power of 80%.
3.4.2. Secondary outcome: impact of greater occipital nerve block on pain relief at postprocedural 1 hour
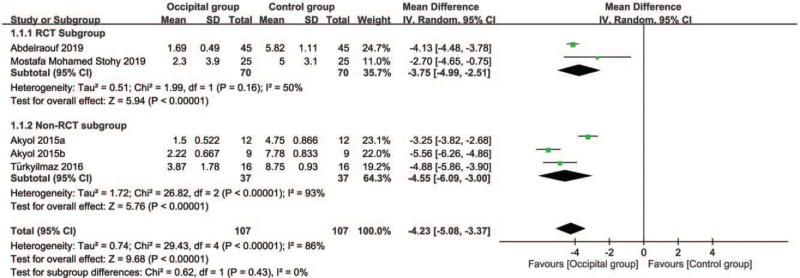
Five studies were available for the analysis.[12,17,18,20] Pooled results showed a lower mean pain score (MD = –4.23, 95% CI: –5.08 to –3.37, P < .00001; I2 = 86%) in the occipital group compared with that in the control group (Fig. 5). Subgroup analysis revealed no significant difference between the RCT and NonRCT subgroups (P = .43). Sensitivity analysis did not demonstrate a significant impact on outcome by omitting certain trials.
Figure 5.
Forest plot for comparing pain score at post-procedural 1 hour between occipital and control groups. CI = confidence interval, IV = inverse variance.
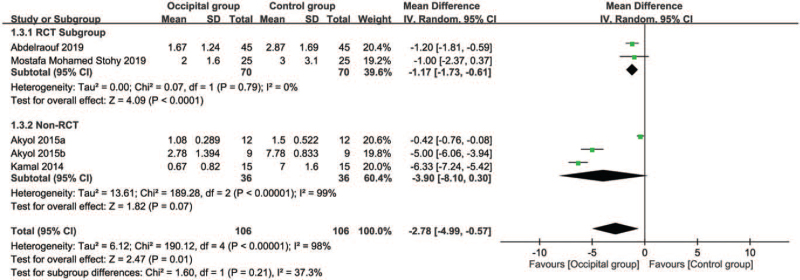
3.4.3. Secondary outcome: impact of greater occipital nerve block on pain relief at postprocedural 12 hours
Four studies reported the pain score at post-procedural 12 hours for analysis.[11,12,17,18] Based on the pooled results, there was a lower mean pain score (MD = –2.78, 95% CI: –4.99 to –0.57, P = .01; I2 = 98%) in the occipital group than that in the control group (Fig. 6). Comparison between the RCT- and Non-RCT subgroups demonstrated no significant difference (P = .21). However, sensitivity analysis showed an inconsistent outcome by omitting certain trials.[17,18]
Figure 6.
Forest plot for comparing pain score at post-procedural 12 hours between occipital and control groups. CI = confidence interval, IV = inverse variance.
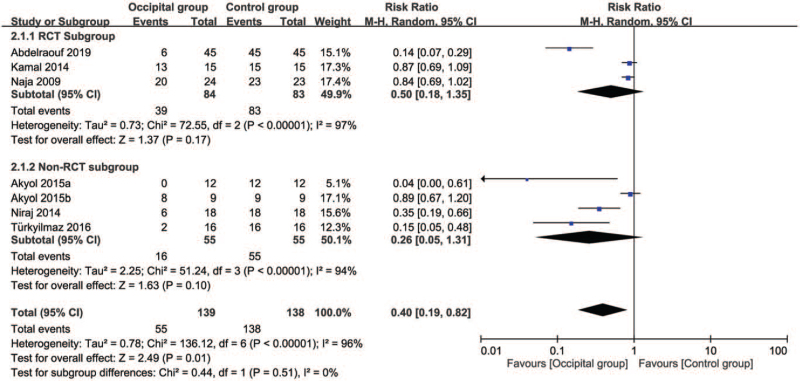
3.4.4. Secondary outcome: impact of greater occipital nerve block on risk of intervention failure
Six studies were eligible for the analysis.[11,13,17–20] A forest plot demonstrated an association between the use of GONB and a reduced risk of intervention failure (RR = 0.4, 95% CI: 0.19 to 0.82, P = .01; I2 = 96%) (Fig. 7). There was no significant difference in this outcome between the RCT and Non-RCT subgroups (P = .51). Sensitivity analysis did not reveal a significant impact on outcome by omitting certain trials.
Figure 7.
Forest plot for the comparison of risk of intervention failure between occipital and control groups. CI = confidence interval, M-H = Mantel-Haenszel.
4. Discussion
The current meta-analysis was the first to address the therapeutic impact of GONB on PDPH, which is a frequent neurologic complication and a contributor to secondary headache after neuraxial anesthesia. Our pooled results demonstrated a lower mean pain score at 24 hours, 1 hour, and 6 hours in patients with GONB compared to those without. Further TSA supported the robustness of our finding at postprocedural 24 hours. Besides, the use of GONB was found to reduce the risk of intervention failure.
Although PDPH appears to be a non-life-threatening clinical condition, rare complications (e.g., subdural hematoma)[21] and cortical venous thrombosis[22] have been reported to be related to untreated PDPH. However, there are no practice guidelines on its management.[4] Despite the previously reported promising outcome of using GONB in the treatment of patients with migraine (i.e., primary headache),[9] little evidence existed in the literature regarding its efficacy against PDPH. Through a systematic review of current evidence, this meta-analysis demonstrated the effectiveness of GONB for relieving the severity of PDPH from post-procedural 1 hour to 24 hours. Furthermore, the requirement for invasive EBP was also reduced with the application of GONB as the first treatment strategy. Therefore, our results support that GONB may be a feasible time-efficient treatment alternative to EBP for patients with PDPH refractory to conservative therapy.
The risk of developing PDPH is high in teenagers compared to those aged 20 to 45 years,[23] females, and patients with a relatively low body mass index.[24] As the symptoms of PDPH often resolve spontaneously within 1 to 2 weeks,[25,26] the first therapeutic strategies for PDPH are usually conservative. Nevertheless, a previous study reported an effectiveness of conservative interventions such as hydration, bed rest, weak oral pain killers, and caffeine administrations for pain relief in less than 14% of patients suffering from PDPH.[27] Indeed, some of these conservative treatments (e.g., hydration or bed rest) are not recommended because of a lack of supportive evidence.[4] Despite its short duration, unresolved PDPH may contribute to significant morbidities with impaired performance of daily activities for at least 1 week in up to 39% of patients, some of whom even required hospitalization.[2] Therefore, the findings underscore the need for definite effective interventions to prevent severe morbidities.
Sealing the site of dural puncture by injection of autologous blood into the epidural space through EBP is usually considered the last resort after the failure of conservative and pharmacological treatments. However, the indications for this technique remain an important concern in certain clinical scenarios such as potential seeding of malignant cells[28] or viral particles[29,30] in the central nervous system as well as the effects of anticoagulants used for the known hypercoagulable state in COVID-19-positive patients.[29,30] Other complications from EBP also included secondary low back pain, lumbar vertebral syndrome, intrathecal blood injection, subacute subdural hematoma, and adhesive arachnoiditis.[27,31] Besides, many patients may not be candidates for EBP such as those with local infection or those who refuse the invasive procedure.[4]
On the other hand, GONB is an accepted therapeutic option for relieving migraine headaches[9,32] through suppressing pain transmission from the cranial structures to the trigeminal ganglia.[33–35] Previous studies have shown a significant association of GONB with a reduction in the duration of migraine headache and pain intensity.[36–38] Furthermore, a previous meta-analysis involving 417 patients reported a significant decrease in pain score and frequency of migraine headaches with the use of GONB.[9] In terms of safety, very few adverse effects were demonstrated following the injection of local anesthetics into the greater occipital nerve[8,9] and many of the side effects were reported to be minimal, such as presyncope, vertigo, or pain at the injection site.[9] Because of the promising therapeutic effect of GONB against migraine headaches, several studies attempted to investigate its benefits in patients with PDPH.[11,12,17]
In the current meta-analysis on patients with PDPH, the severity of headache was reduced at 1, 12, and 24 hours after the procedure. The pooled mean difference at 24 hours was –2.66 (95% CI: –3.98 to –1.33), which was consistent with that in a previous meta-analysis focusing on patients with migraine receiving GONB.[9] In that study, a total of 341 patients were reviewed with a pooled mean difference in pain scores of –2.2 (95% CI: –1.56 to –2.84).[9] Furthermore, 2 of our included studies on PDPH reported a quicker relief of headache in the GONB group than that in the controls as well as an earlier hospital discharge of the former compared with that in the latter.[11,13] The findings of the current meta-analysis suggested that GONB may serve as a bridge intervention for patients with PDPH before EBP, especially in those with coagulopathy, bacteremia, or metastatic tumor who may benefit from GONB considering their risks associated with EBP.
To overcome the limited effective analgesic duration (i.e., a few hours) of nerve block,[39] perineural dexamethasone may be coadministered to prolong its analgesic effect.[40,41] For this purpose, 5 of our included trials used steroids (i.e., dexamethasone or triamcinolone) as an adjunct.[11,12,17,19,20] Our pooled results demonstrated an extended analgesic effect of GONB up to 24 hours with steroid coadministration, highlighting the feasibility of steroid application in this clinical setting.
Despite being the first meta-analytical study to address the positive impact of GONB on PDPH, the current investigation had its limitations. First, our results on both primary and secondary outcomes showed a high heterogeneity (i.e., 1 hour post injection: I2 = 86%, 12 hours: I2 = 98%, 24 hours: I2 = 97%), which may be attributed to variations in patient populations and pharmaceutical formulations. For instance, 2 studies[17,20] only included obstetric patients with PDPH following a cesarean section under spinal anesthesia, while the others included general population receiving spinal anesthesia.[11–13,18,19] Besides, the discrepancy in initial pain severity may affect treatment outcomes (e.g., strict recruitment criteria in 1 study[11]). In addition, the treatments differed widely in the control groups; while patients in the control group in 1 RCT received bilateral intramuscular injection of normal saline,[17] those in other studies underwent conventional pain management such as hydration, bed rest, and oral analgesics as monotherapies or combinations (e.g., paracetamol, non-steroidal anti-inflammatory agents).[11,12] Second, despite the robustness of our finding on the primary outcome as shown in TSA, the small number of RCTs available for analysis (i.e., only 4 of the 7 studies) not only may blemish the reliability of our results but also precluded our assessment of potential publication bias. Finally, although PDPH may last for over 1 week, our included studies only targeted the therapeutic efficacy of GONB for acute pain relief and could not shed light on its effectiveness beyond 24 hours. Further studies are required to elucidate its analgesic effect after the acute period.
5. Conclusion
The results of the current meta-analysis demonstrated that, compared with conservative treatments or placebos, greater occipital nerve block achieved significant pain relief for postdural puncture headache at 1, 12, and 24 hours after the procedure with a low risk of intervention failure. Because of the small number of included studies, further large-scale trials are warranted to support our findings and evaluate the therapeutic benefit of greater occipital nerve block beyond the acute phase of postdural puncture headache.
Author contributions
Conceptualization: Ying-Jen Chang, Ming Yew, Chung-Yi Wu.
Data curation: Ying-Jen Chang, Ming Yew, Shu-Wei Liao.
Formal analysis: Kuo-Chuan Hung, Chia-Hung Yu.
Investigation: I-Chia Teng, Kuo-Mao Lan.
Methodology: Kuo-Chuan Hung, I-Wen Chen, Chia-Hung Yu, Kuo-Mao Lan.
Software: I-Wen Chen, Chi-Lin Kuo, Chung-Yi Wu.
Validation: Chi-Lin Kuo, Ming-Chung Lin.
Visualization: I-Chia Teng, Ming-Chung Lin, Shu-Wei Liao.
Writing – original draft: Cheuk-Kwan Sun.
Writing – review & editing: Cheuk-Kwan Sun.
Footnotes
Abbreviations: EBP = epidural blood patch, GONB = occipital nerve block, PDPH = postdural puncture headache, RCTs = randomized controlled trials, TSA = trial sequential analysis.
How to cite this article: Chang YJ, Hung KC, Chen IW, Kuo CL, Teng IC, Lin MC, Yew M, Liao SW, Wu CY, Yu CH, Lan KM, Sun CK. Efficacy of greater occipital nerve block for pain relief in patients with postdural puncture headache: a meta-analysis. Medicine. 2021;100:51(e28438).
Y-JC and K-CH contributed equally to this work.
All analyses were based on previously published studies, thus no ethical approval and patient consent are required.
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].Buddeberg BS, Bandschapp O, Girard T. Post-dural puncture headache. Minerva Anestesiol 2019;85:543–53. [DOI] [PubMed] [Google Scholar]
- [2].Bezov D, Ashina S, Lipton R. Post-dural puncture headache: Part II--prevention, management, and prognosis. Headache 2010;50:1482–98. [DOI] [PubMed] [Google Scholar]
- [3].Smedstad KG. Dealing with post-dural puncture headache--is it different in obstetrics? Can J Anaesth 1998;45:06–9. [DOI] [PubMed] [Google Scholar]
- [4].Katz D, Beilin Y. Review of the alternatives to epidural blood patch for treatment of postdural puncture headache in the parturient. Anesth Analg 2017;124:1219–28. [DOI] [PubMed] [Google Scholar]
- [5].Harrington BE, Schmitt AM. Meningeal (postdural) puncture headache, unintentional dural puncture, and the epidural blood patch: a national survey of United States practice. Reg Anesth Pain Med 2009;34:430–7. [DOI] [PubMed] [Google Scholar]
- [6].Marr R, Kapoor A, Redfern N. Epidural blood patch is the gold standard treatment for dural puncture headache. Br J Anaesth 2012;109:288–9. [DOI] [PubMed] [Google Scholar]
- [7].Willner D, Weissman C, Shamir MY. Chronic back pain secondary to a calcified epidural blood patch. Anesthesiology 2008;108:535–7. [DOI] [PubMed] [Google Scholar]
- [8].Inan LE, Inan N, Unal-Artik HA, Atac C, Babaoglu G. Greater occipital nerve block in migraine prophylaxis: narrative review. Cephalalgia 2019;39:908–20. [DOI] [PubMed] [Google Scholar]
- [9].Shauly O, Gould DJ, Sahai-Srivastava S, Patel KM. Greater occipital nerve block for the treatment of chronic migraine headaches: a systematic review and meta-analysis. Plast Reconstr Surg 2019;144:943–52. [DOI] [PubMed] [Google Scholar]
- [10].Tang Y, Kang J, Zhang Y, Zhang X. Influence of greater occipital nerve block on pain severity in migraine patients: a systematic review and meta-analysis. Am J Emerg Med 2017;35:1750–4. [DOI] [PubMed] [Google Scholar]
- [11].Kamal SM, Hassan GA, Wahba SS. Management of postdural puncture headache: greater occipital nerve block technique. Ain-Shams J Anaesthesiol 2014;7:25–31. [Google Scholar]
- [12].Mostafa Mohamed Stohy E-S, Mohamed Mohamed El-Sayed M, Saeed Mohamed Bastawesy M. The effectiveness of bilateral greater occipital nerve block by ultrasound for treatment of post-dural puncture headache in comparison with other conventional treatment. Al-Azhar Med J 2019;48:479–88. [Google Scholar]
- [13].Naja Z, Al-Tannir M, El-Rajab M, Ziade F, Baraka A. Nerve stimulator-guided occipital nerve blockade for postdural puncture headache. Pain Pract 2009;9:51–8. [DOI] [PubMed] [Google Scholar]
- [14].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008;61:763–9. [DOI] [PubMed] [Google Scholar]
- [16].Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive--Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 2009;38:287–98. [DOI] [PubMed] [Google Scholar]
- [17].Abdelraouf M, Salah M, Waheb M, Elshall A. Suboccipital Muscles Injection for Management of Post-Dural Puncture Headache After Cesarean Delivery: A Randomized-Controlled Trial. Open Access Maced J Med Sci 2019;7:549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Akyol F, Binici O, Kuyrukluyildiz U, Karabakan G. Ultrasound-guided bilateral greater occipital nerve block for the treatment of post-dural puncture headache. Pak J Med Sci 2015;31:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Niraj G, Kelkar A, Girotra V. Greater occipital nerve block for postdural puncture headache (PDPH): a prospective audit of a modified guideline for the management of PDPH and review of the literature. J Clin Anesth 2014;26:539–44. [DOI] [PubMed] [Google Scholar]
- [20].Türkyilmaz EU, Eryilmaz NC, Güzey NA, Moraloğlu Ö. Bilateral greater occipital nerve block for treatment of post-dural puncture headache after caesarean operations. Rev Bras Anestesiol 2016;66:445–50. [DOI] [PubMed] [Google Scholar]
- [21].Zeidan A, Farhat O, Maaliki H, Baraka A. Does postdural puncture headache left untreated lead to subdural hematoma? Case report and review of the literature. Middle East J Anaesthesiol 2010;20:483–92. [PubMed] [Google Scholar]
- [22].Canhão P, Batista P, Falcão F. Lumbar puncture and dural sinus thrombosis--a causal or casual association? Cerebrovasc Dis 2005;19:53–6. [DOI] [PubMed] [Google Scholar]
- [23].DelPizzo K, Luu T, Fields KG, et al. Risk of postdural puncture headache in adolescents and adults. Anesth Analg 2020;131:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Peralta F, Higgins N, Lange E, Wong CA, McCarthy RJ. The relationship of body mass index with the incidence of postdural puncture headache in parturients. Anesth Analg 2015;121:451–6. [DOI] [PubMed] [Google Scholar]
- [25].Patel R, Urits I, Orhurhu V, et al. A comprehensive update on the treatment and management of postdural puncture headache. Curr Pain Headache Rep 2020;24:24. [DOI] [PubMed] [Google Scholar]
- [26].Turnbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth 2003;91:718–29. [DOI] [PubMed] [Google Scholar]
- [27].Uyar Türkyilmaz E, Camgöz Eryilmaz N, Aydin Güzey N, Moraloğlu Ö. Bilateral greater occipital nerve block for treatment of post-dural puncture headache after caesarean operations. Braz J Anesthesiol 2016;66:445–50. [DOI] [PubMed] [Google Scholar]
- [28].Morgan KJ, Mohan R, Karol SE, Flerlage J. Epidural blood patch for post-dural puncture headaches in adult and paediatric patients with malignancies: a review. Br J Anaesth 2021;126:1200–7. [DOI] [PubMed] [Google Scholar]
- [29].Ibrahim M, Darling R, Oaks N, Babazade R, Vadhera R. Epidural blood patch for a post-dural puncture headache in a COVID-19 positive patient following labor epidural analgesia. Int J Obstet Anesth 2021;46: 102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Scemama P, Farah F, Mann G, Margulis R, Gritsenko K, Shaparin N. Considerations for epidural blood patch and other postdural puncture headache treatments in patients with COVID-19. Pain Physician 2020;23:S305–10. [PubMed] [Google Scholar]
- [31].Seemiller J, Challagundla S, Taylor T, Zand R. Intrathecal blood injection: a case report of a rare complication of an epidural blood patch. BMC Neurol 2020;20:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ashkenazi A, Blumenfeld A, Napchan U, et al. Peripheral nerve blocks and trigger point injections in headache management - a systematic review and suggestions for future research. Headache 2010;50:943–52. [DOI] [PubMed] [Google Scholar]
- [33].Han DW, Koo BN, Chung WY, et al. Preoperative greater occipital nerve block in total thyroidectomy patients can reduce postoperative occipital headache and posterior neck pain. Thyroid 2006;16:599–603. [DOI] [PubMed] [Google Scholar]
- [34].Martelletti P, Giamberardino MA, Mitsikostas DD. Greater occipital nerve as target for refractory chronic headaches: from corticosteroid block to invasive neurostimulation and back. Expert Rev Neurother 2016;16:865–6. [DOI] [PubMed] [Google Scholar]
- [35].Okmen K, Dagistan Y, Dagistan E, Kaplan N, Cancan E. Efficacy of the greater occipital nerve block in recurrent migraine type headaches. Neurol Neurochir Pol 2016;50:151–4. [DOI] [PubMed] [Google Scholar]
- [36].Ashkenazi A, Matro R, Shaw JW, Abbas MA, Silberstein SD. Greater occipital nerve block using local anaesthetics alone or with triamcinolone for transformed migraine: a randomised comparative study. J Neurol Neurosurg Psychiatry 2008;79:415–7. [DOI] [PubMed] [Google Scholar]
- [37].Cuadrado ML, Aledo-Serrano Á, Navarro P, et al. Short-term effects of greater occipital nerve blocks in chronic migraine: a double-blind, randomised, placebo-controlled clinical trial. Cephalalgia 2017;37:864–72. [DOI] [PubMed] [Google Scholar]
- [38].Karadaş Ö, Özön A, Özçelik F, Özge A. Greater occipital nerve block in the treatment of triptan-overuse headache: a randomized comparative study. Acta Neurol Scand 2017;135:426–33. [DOI] [PubMed] [Google Scholar]
- [39].Pehora C, Pearson AM, Kaushal A, Crawford MW, Johnston B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev 2017;11: Cd011770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Albrecht E, Kern C, Kirkham KR. A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia 2015;70:71–83. [DOI] [PubMed] [Google Scholar]
- [41].Cummings KC, 3rd, Napierkowski DE, Parra-Sanchez I, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth 2011;107:446–53. [DOI] [PubMed] [Google Scholar]