Abstract
Broad-range PCR primers were used to amplify part of the groESL operon of the canine pathogen Ehrlichia ewingii, recently recognized as a human pathogen, and the murine pathogen Ehrlichia muris. Phylogenetic analysis supported the relationships among Ehrlichia species previously determined by comparison of 16S rRNA gene sequences. These sequences provide additional PCR targets for species for which few gene sequences have been determined.
During the last 14 years, three Ehrlichia species have been newly recognized as human pathogens transmitted by ticks in the United States (1, 4, 5, 8, 9, 27). The most recently reported agent, Ehrlichia ewingii, is the etiologic agent of canine granulocytic ehrlichiosis (CGE). It was first recognized in 1971 (10) but was not considered a separate ehrlichial disease until 1985 (24). A tropism for granulocytes initially differentiated E. ewingii from E. canis, the etiologic agent of canine monocytic ehrlichiosis. However, antigenic cross-reactivity between E. canis (a monocytotropic species) and E. ewingii by Western immunoblot analysis was noted (21). E. ewingii was recognized as a separate species in 1992, when the 16S rRNA gene sequence was shown to be different from the corresponding sequences of the most closely related species, E. canis and E. chaffeensis (2). Subsequently, a number of reports that characterized the role of E. ewingii in CGE were published (3, 11, 13, 15). Recently, nucleotide sequences that matched the E. ewingii 16S rRNA gene sequence were amplified from blood samples of four human patients (4). These were the first documented cases of human ehrlichiosis caused by E. ewingii.
E. ewingii has not been propagated in cell culture, and antisera of infected dogs and human patients demonstrate extensive cross-reactivity with the closely related ehrlichiae, E. canis and the human pathogen E. chaffeensis, precluding definitive diagnosis by traditional indirect immunofluorescent-antibody assays (3, 4, 21). Molecular detection of E. ewingii by PCR remains the most practical method for confirmation of the diagnosis of this form of granulocytic ehrlichiosis. Amplification of ehrlichial groESL sequences by using broad-range PCR primers has provided valuable information for phylogenetic studies and a sensitive diagnostic assay when a nested PCR stage was added (25). Currently, researchers familiar with the ehrlichiae are interested in resolving the phylogenetic relationships among members of the genus and closely related bacteria. Ehrlichiae are similar in that they are gram-negative, obligate intracellular bacteria that typically infect leukocytes and grow within membrane-bound cytoplasmic compartments, which do not fuse with lysosomes. Molecular and antigenic analyses, particularly the comparison of 16S rRNA gene sequences, segregate Ehrlichia species into three monophyletic clades that are commonly referred to in the ehrlichial literature as genogroups and that bear the names of the prototype species, E. canis, E. phagocytophila, and E. sennetsu (1; for reviews, see references 9 and 27). However, each genogroup contains at least one species that is currently classified in another genus, indicating that the phylogenetic classification of the Ehrlichia species should be reevaluated. The species considered in this report, E. ewingii and E. muris, are members of the E. canis group, which also contains E. chaffeensis and Cowdria ruminantium (7), the etiologic agent of heartwater in ruminants of Africa and several islands in the Caribbean. All of these agents may establish infections in one or more species of wild or domesticated animals, and E. chaffeensis and E. ewingii also cause disease in humans in the United States (1, 4, 8, 18). E. muris is a recently characterized species isolated from a wild mouse in Japan (28). There are no reports of human ehrlichiosis caused by E. muris; however, antibodies reactive with E. muris have recently been detected in serum samples obtained from asymptomatic persons in Japan (12).
In this report we describe the amplification of partial groESL sequences from a blood sample from a human ehrlichiosis patient infected with E. ewingii (4), a blood sample from a Missouri dog naturally infected with E. ewingii (confirmed by detection of the 16S rRNA gene sequence), and from an E. muris type strain (AS145T)-infected cell culture. Our goals were to obtain new gene sequences to provide an additional PCR target and to add to the number of groESL sequences available for phylogenetic comparison. One of the advantages of having additional gene sequences for PCR targets is that the 16S rRNA gene sequences of members of the E. canis genogroup are very similar, limiting the choice of species-specific primers and making differentiation of amplicons from different species difficult.
QIAmp Blood or Tissue kits (Qiagen Inc., Valencia, Calif.) were used for extraction of DNA, according to the manufacturer's recommendations, from blood or cell culture samples, respectively. PCR primers HS1 and HS6 were used for primary amplification of groESL sequences (25). Nested PCR with primers EWNF1 (5′-AGTATATAGTCATGAAGGAG) and EWNR2 (5′-CTCAACAGCAGCCCTAGTTGC) was required for amplification of groESL sequences from the canine blood sample because the primary PCR did not provide enough product for nucleotide sequencing. EWNF1 and EWNR2 were selected from regions closely nested to the HS1 and HS6 sites, respectively, to provide for amplification of a large segment for comparison to DNA sequences amplified from the human patient. The specificities of EWNF1 and EWNR2 for amplification of groESL sequences from different species were not determined. PCR was performed by using PCR Ready-To-Go Beads in 0.2-ml tubes (Amersham Pharmacia Biotech, Piscataway, N.J.). Gamma-irradiated water was used as a negative control, and DNA extracted from E. chaffeensis infected DH82 cells was tested as a positive control. Duplicate reactions were conducted for samples other than the controls to produce adequate template for nucleotide sequencing. Two microliters of DNA extract was added to 23 μl of reaction mixture. The final concentrations in the reaction mixtures were 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, each deoxynucleoside triphosphate (dNTP), at a concentration of 200 μM, each primer at a concentration of 1 μM, and 1.5 U of Taq polymerase. A Perkin-Elmer 9600 thermal cycler (The Perkin-Elmer Corp., Norwalk, Conn.) was used with the following cycling parameters: a preliminary denaturation cycle of 95°C for 2 min, 40 cycles consisting of 94°C for 30 s, 52°C for 30 s, and 72°C for 1 min, followed by an extension cycle of 72°C for 5 min. The annealing temperature was raised to 55°C for nested PCR with primers EWNF1 and EWNR2, and 1 μl of the finished primary reaction was added to the nested reaction mixture. PCR products were detected by electrophoresis of 8-μl samples in 2% agarose gels containing ethidium bromide. Amplified products of the correct size were loaded into separate wells of a gel made with low-melting-point agarose (Boehringer Mannheim Biochemicals, Indianapolis, Ind.). Bands of the appropriate size were excised, and the DNA was purified by using Wizard PCR Preps (Promega, Madison, Wis.). The purified PCR products were sequenced by using the dRhodamine Terminator Cycle Sequencing Ready Reaction kits (Applied Biosystems, Foster City, Calif.) and a Perkin-Elmer 9600 thermocycler. The parameters used for sequencing with the thermocycler were 96°C for 1 min, 50°C for 15 s, and 60°C for 4 min for 25 cycles. Unincorporated fluorescence-labeled dNTPs were removed with Centri-Sep columns, according to the manufacturer's recommendations (Princeton Separations, Inc., Adelphia, N.J.). The samples were loaded onto 5% polyacrylamide gels for electrophoresis and detection on an Applied Biosystem model 377 automated sequencer. Both strands were sequenced by primer walking after the initial sequences were obtained by using the PCR primers. Nucleotide sequences were edited and assembled with the TED and XBAP programs of the STADEN sequence analysis package (23). Nucleotide sequence homology searches were made through the National Center for Biotechnology Information BLAST network service. Sequence homology comparisons were made by using the GAP and BestFit Programs, and multiple sequences were aligned by using the PileUp Program from the Wisconsin Sequence Analysis Package (Genetics Computer Group, Madison, Wis.) The GenBank accession numbers of previously determined groESL sequences used for phylogenetic analysis are as follows: E. chaffeensis, L10917; E. canis, U96732; E. phagocytophila, U96729; E. sennetsu, U88092; C. ruminantium, U13638; Anaplasma marginale, AF165812; and Rickettsia rickettsii, U96733.
Sequence analysis of the PCR products indicated that the correct sequences, partial groESL sequences of E. ewingii and E. muris, were obtained. PCR primers HS1 and HS6 span a region that includes the coding sequence for the last 20 amino acid residues of GroES, a spacer region that usually varies in length among different species, and a sequence that encodes approximately three-fourths of GroEL (25). A 1,431-bp product was amplified from the blood sample obtained from the human ehrlichiosis patient, and a 1,435-bp product was amplified from the E. muris-infected cell culture. A 1,416-bp product was amplified from the canine sample by using primers EWNF1 and EWNR2 in a nested PCR. The nucleotide sequences amplified from the human patient and from the dog with CGE were identical over the entire region represented in both taxa (1,375 bp, excluding the primer sequences). A comparison of the nucleotide sequences obtained from E. ewingii and E. muris to the sequences in the genetic databases showed that the groESL sequences from E. chaffeensis, E. canis, and C. ruminantium were most similar, with their identities ranging from 87 to 93%. Spacer lengths, including the number of nucleotides between the GroES translation termination codon and the putative translation initiation codon for GroEL, were 103 nucleotides for E. muris and 99 nucleotides for E. ewingii. For the E. ewingii sequence, there was a potential translation initiation codon (ATG) in the same reading frame with GroEL located 15 nucleotides upstream of the position expected by comparison to the groESL sequences from related bacteria. A putative ribosome binding site was located 9 nucleotides upstream of the ATG codon located in the more downstream position, however, indicating that it is the likely initiation codon. These spacer lengths are similar to those for other members of the group: 93 bp for E. canis, 100 bp for E. chaffeensis, and 96 bp for C. ruminantium. The spacer sequences were more divergent (74 to 91% identity) than the GroEL coding sequences (88 to 94% identity). In comparison, the spacer lengths (52 bp) and sequences of E. phagocytophila, E. equi, and the agent of human granulocytic ehrlichiosis are identical to one another (25), further illustrating that these three members of the E. phagocytophila group should be considered strains of E. phagocytophila rather than separate species.
Table 1 shows a comparison of the percentage of identical amino acids among the inferred GroEL sequences of E. ewingii, E. muris, and their close relatives. Sequence homologies among members of the E. canis group and C. ruminantium were 92.6 to 99.3%. Sequences from E. phagocytophila and A. marginale showed homologies of 84.6 to 87.3% to Ehrlichia species of the E. canis group. E. sennetsu represents the third group, which also includes E. risticii and Neorickettsia helminthoeca (20). Sequence homologies between E. sennetsu and the other species were no greater than 58.2%, showing a divergence comparable to the divergence between the sequences of R. rickettsii and the other species.
TABLE 1.
Percent amino acid sequence identity among inferred GroEL sequences from Ehrlichia species and related bacteria
| Species | % Identitya
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1b | 2b | 3b | 4b | 5b | 6c | 7c | 8d | 9 | |
| 1. Ehrlichia canis | 100 | 97.1 | 97.1 | 97.6 | 92.6 | 86.5 | 84.9 | 57.7 | 57.9 |
| 2. Ehrlichia muris | 100 | 99.3 | 97.1 | 93.4 | 87.3 | 84.6 | 58.2 | 58.4 | |
| 3. Ehrlichia chaffeensis | 100 | 96.8 | 93.6 | 86.8 | 84.6 | 58.2 | 58.1 | ||
| 4. Ehrlichia ewingii | 100 | 92.6 | 86.3 | 84.6 | 57.2 | 57.6 | |||
| 5. Cowdria ruminantium | 100 | 85.2 | 83.4 | 57.9 | 57.7 | ||||
| 6. Ehrlichia phagocytophilad | 100 | 91.5 | 54.6 | 54.3 | |||||
| 7. Anaplasma marginale | 100 | 54.1 | 59.9 | ||||||
| 8. Ehrlichia sennetsu | 100 | 58.4 | |||||||
| 9. Rickettsia rickettsii | 100 | ||||||||
The numbers in the subheads correspond to the species numbered in the left column. The letters b, c, and d denote species that have been segregated into three different genogroups with similar 16S rRNA gene sequences, respectively.
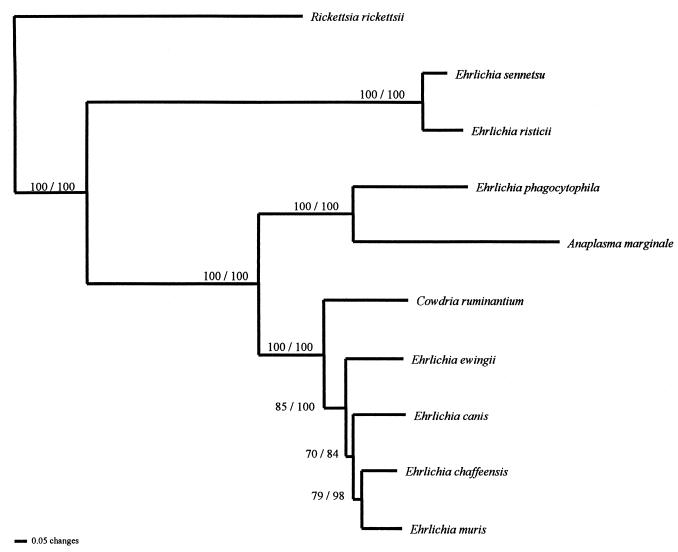
The phylogenetic relationships derived from comparisons of the partial groEL nucleotide sequences (ranging from 1,200 to 1,233 bp) are presented in the phylogram in Fig. 1. This comparison of the available groEL sequences produced a tree with a topology equivalent to those of trees derived from comparisons of 16S rRNA gene sequences, providing further evidence that the current phylogenetic classification should be restructured to reflect true evolutionary lineages (i.e., monophyletic clades). Considering the available sequence data and antigenic similarities, there is some divergence between the E. canis and the E. phagocytophila groups, suggesting that these two are independent lineages that should be placed in different genera that include their close relatives (e.g., C. ruminantium with members of the E. canis group). The data clearly distinguish members of the E. sennetsu lineage from members of both the E. canis and E. phagocytophila lineages, warranting the placement of the E. sennetsu lineage in a separate genus.
FIG. 1.
Phylogenetic relationships among Ehrlichia species and related bacteria derived from analysis of groESL gene sequences. Phylogenetic analyses used PAUP (version 4.0b2); (26) and both parsimony and neighbor-joining search algorithms. Parsimony analyses used the heuristic search option with tree bisection-reconnection branch swapping, MULPARS, and random addition of taxa (100 replicates). Neighbor-joining analyses used minimum evolution as the objective criterion, with maximum likelihood used to estimate the transition-to-transversion ratio and nucleotide base frequencies (settings correspond to the Hasegawa-Kishino-Yano [1985] model of nucleotide sequence evolution). Tree support was assessed by using the nonparametric bootstrap (1,000 replicates). The values adjacent to the branches are parsimony/neighbor-joining bootstrap proportions.
As the isolation of some Ehrlichia species (e.g., E. ewingii) in cell culture has not yet been achieved, the use of species-specific PCR for the detection of ehrlichiae in clinical samples is likely to remain a primary method for establishing which species are human and animal pathogens. Extensive serologic reactivity occurs across antigens derived from Ehrlichia species within the same lineage and occasionally across antigens derived from species in different lineages (6, 16, 22). The current limitation in available confirmatory diagnostic assays is well illustrated by the dilemma in diagnosing infections with E. ewingii, in which amplification and sequencing of the 16S rRNA gene was the only complement to serologic assays with surrogate antigens (e.g., Western blot analysis with preparations of E. canis and E. chaffeensis). In this context, the groESL operon provides a second genetic target that may be used for primary detection of ehrlichial infection and as a source of genetic information in addition to that available through 16S rRNA sequencing.
Nucleotide sequence accession numbers.
GenBank accession numbers for the groESL sequences determined in this study are AF195273 for E. ewingii and AF210459 for E. muris.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Green C E, Jones D C, Dawson J E. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int J Syst Bacteriol. 1992;42:299–302. doi: 10.1099/00207713-42-2-299. [DOI] [PubMed] [Google Scholar]
- 3.Breitschwerdt E B, Hegarty B C, Hancock S I. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645–2651. doi: 10.1128/jcm.36.9.2645-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buller R S, Arens M, Hmiel S P, Paddock C D, Sumner J W, Rikihisa Y, Unver A, Gaudreault-Keener M, Manian F A, Liddell A M, Schmulewitz N, Storch G A. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comer J A, Nicholson W L, Olson J G, Childs J E. Serologic testing for human granulocytic ehrlichiosis at a national referral center. J Clin Microbiol. 1999;37:558–564. doi: 10.1128/jcm.37.3.558-564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dame J B, Mahan S M, Yowell C A. Phylogenetic relationship of Cowdria ruminantium, agent of heartwater, to Anaplasma marginale and other members of the order Rickettsiales determined on the basis of 16S rRNA gene sequence. Int J Syst Bacteriol. 1992;42:270–274. doi: 10.1099/00207713-42-2-270. [DOI] [PubMed] [Google Scholar]
- 8.Dawson J E, Anderson B E, Fishbein D B, Sanchez J L, Goldsmith C S, Wilson K H, Duntley C W. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumler J S, Bakken J S. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu Rev Med. 1998;49:201–213. doi: 10.1146/annurev.med.49.1.201. [DOI] [PubMed] [Google Scholar]
- 10.Ewing S A, Roberson W R, Buckner R G, Hayat C S. A new strain of Ehrlichia canis. J Am Vet Med Assoc. 1971;159:1771–1774. [PubMed] [Google Scholar]
- 11.Goldman E E, Breitschwerdt E B, Grindem C B, Hegarty B C, Walls J J, Dumler J S. Granulocytic ehrlichiosis in dogs from North Carolina and Virginia. J Vet Intern Med. 1998;12:61–70. doi: 10.1111/j.1939-1676.1998.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawahara M, Ito T, Suto C, Shibata S, Rikihisa Y, Hata K, Hirai K. Comparison of Ehrlichia muris strains isolated from wild mice and ticks and serologic survey of humans and animals with E. muris antigen. J Clin Microbiol. 1999;37:1123–1129. doi: 10.1128/jcm.37.4.1123-1129.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kordick S K, Breitschwerdt E B, Hegarty B C, Southwick K L, Colitz C M, Hancock S I, Bradley J M, Rumbough R, Mcpherson J T, MacCormack J N. Coinfection with multiple tick-borne pathogens in a Walker hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotric-Furlan S, Petrovec M, Avsic-Zupanc T, Nicholson W L, Sumner J W, Childs J E, Strle F. Human granulocytic ehrlichiosis in Europe: clinical and laboratory findings for four patients from Slovenia. Clin Infect Dis. 1998;27:424–428. doi: 10.1086/514683. [DOI] [PubMed] [Google Scholar]
- 15.Murphy G I, Ewing S A, Whitworth L C, Fox J C, Kocan A A. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol. 1998;79:325–339. doi: 10.1016/s0304-4017(98)00179-4. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson W L, Comer J A, Sumner J W, Gingrich-Baker C, Coughlin R T, Magnarelli L A, Olson J G, Childs J E. An indirect immunofluorescence assay using a cell culture-derived antigen for detection of antibodies to the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:1510–1516. doi: 10.1128/jcm.35.6.1510-1516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson W L, Castro M B, Kramer V L, Sumner J W, Childs J E. Dusky-footed wood rats (Neotoma fuscipes) as reservoirs of granulocytic ehrlichiae (Rickettsiales: Ehrlichieae) in northern California. J Clin Microbiol. 1999;37:3323–3327. doi: 10.1128/jcm.37.10.3323-3327.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paddock C D, Sumner J W, Shore G M, Bartley D C, Elie R C, McQuade J G, Martin C R, Goldsmith C S, Childs J E. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol. 1997;35:2496–2502. doi: 10.1128/jcm.35.10.2496-2502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrovec M, Sumner J W, Nicholson W L, Childs J E, Strle F, Barlic J, Lotric-Furlan S, Avsic Zupanc T. Identity of ehrlichial DNA sequences derived from Ixodes ricinus ticks with those obtained from patients with human granulocytic ehrlichiosis in Slovenia. J Clin Microbiol. 1999;37:209–210. doi: 10.1128/jcm.37.1.209-210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pretzman C, Ralph D, Stothard D R, Fuerst P A, Rikihisa Y. 16S rRNA gene sequence of Neorickettsia helminthoeca: phylogenetic alignment with members of the genus Ehrlichia. Int J Syst Bacteriol. 1995;45:207–211. doi: 10.1099/00207713-45-2-207. [DOI] [PubMed] [Google Scholar]
- 21.Rikihisa Y, Ewing S A, Fox J C, Siregar A G, Pasariba F H, Malole M B. Analyses of Ehrlichia canis and a canine granulocytic Ehrlichia infection. J Clin Microbiol. 1992;30:143–148. doi: 10.1128/jcm.30.1.143-148.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rikihisa Y, Ewing S A, Fox J C. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J Clin Microbiol. 1994;32:2107–2112. doi: 10.1128/jcm.32.9.2107-2112.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staden R. The STADEN package. Methods Mol Biol. 1994;25:9–170. doi: 10.1385/0-89603-276-0:9. [DOI] [PubMed] [Google Scholar]
- 24.Stockham S L, Schmidt D A, Tyler J W. Canine granulocytic ehrlichiosis in dogs from central Missouri: a possible cause of polyarthritis. Vet Med Review. 1985;6:3–5. [Google Scholar]
- 25.Sumner J W, Nicholson W L, Massung R F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swofford D L. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. Sunderland, Mass: Sinauer Associates; 1999. [Google Scholar]
- 27.Walker D H, Dumler J S. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen B, Rikihisa Y, Mott J, Fuerst P A, Kawahara M, Suto C. Ehrlichia muris sp. nov., identified on the basis of 16S rRNA base sequences and serological, morphological, and biological characteristics. Int J Syst Bacteriol. 1995;45:250–254. doi: 10.1099/00207713-45-2-250. [DOI] [PubMed] [Google Scholar]