Abstract
Rapid and accurate detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in dead bodies is essential to prevent infection among those working with dead bodies. This study focused on the Smart Amplification (SmartAmp) method, which has a short examination time (approximately an hour), is simple to perform, and demonstrates high specificity and sensitivity. This method has already been used for clinical specimens; however, its effectiveness in dead bodies has not been reported. This study examined the SmartAmp method using 11 autopsies or postmortem needle biopsies performed from January to May, 2021 (of these, five cases tested positive for SARS-CoV-2 by quantitative real-time polymerase chain reaction (qRT-PCR) and six cases tested negative). Swab samples were collected from the nasopharynx, oropharynx, or anus and the SmartAmp and qRT-PCR results were compared. For the nasopharynx and oropharynx samples, the same results were obtained for both methods in all cases; however, for the anal swabs, there was one case that was positive according to qRT-PCR but negative according to the SmartAmp method. The SmartAmp method may therefore be less sensitive than qRT-PCR and results may differ in specimens with a low viral load, such as anal swabs. However, in the nasopharynx and oropharynx specimens, which are normally used for testing, the results were the same using each method, suggesting that the SmartAmp method is useful in dead bodies. In the future, the SmartAmp method may be applied not only during autopsies, but also in various situations where dead bodies are handled.
Keywords: SmartAmp method, SARS-CoV-2, Swab test, Autopsy, Rapid test
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) and the first outbreak was reported in Wuhan, China, in December 2019. It has since rapidly spread worldwide, causing more than 4.5 million deaths as of September 2021 [1], and the number of fatalities continue to increase. In January 2021, Heinrich and colleagues revealed that subgenomic RNA of SARS-CoV-2 that was indicative of viral replication could be detected, and a viral culture was possible even 35.8 h after death in specimens collected from the nasopharynx of SARS-CoV-2-infected dead bodies, suggesting a risk of infection [2]. For this reason, the detection of SARS-CoV-2 during autopsies, for which the cause of death is unknown, is vital to not only determine the cause of death more reliably, but also to reduce the risk of infection for all personnel involved in the autopsy.
The most reliable detection method for SARS-CoV-2 is the quantitative real-time polymerase chain reaction (qRT-PCR) method. However, while it has high sensitivity and specificity, it requires relatively specialized facilities and technically-trained operators. qRT-PCR involves a number of complicated operations, such as RNA extraction and reverse transcriptase reactions, resulting in long testing times (about 2–3 h) and the risk of contamination. As a result, qRT-PCR is difficult to apply to onsite testing, such as autopsy or postmortem examinations at the time of a disaster. In recent years, isothermal nucleic acid amplification has gained attention as a new method for nucleic acid detection [3], [4], [5]. In contrast to the PCR method, which amplifies nucleic acids by cycling through denaturation, annealing, and elongation at different temperatures, the isothermal amplification method amplifies nucleic acids by reacting at a constant temperature. This offers a number of advantages, such as a faster reaction speed, the lack of specialized equipment such as a thermal cycler, and a simple operation that does not require specialized techniques. Therefore, in this study, we focused on the Smart Amplification (SmartAmp) method, which is one of the isothermal nucleic acid amplification methods [6].
The “SmartAmp™ 2019 New Coronavirus Detection Reagent” [7], co-developed by DNAFORM Corporation (Yokohama, Japan) and RIKEN (Yokohama, Japan), is an RT-SmartAmp assay that can perform both reverse transcriptase and isothermal DNA amplification reactions in a single reaction tube. When used in combination with the nucleic acid extraction reagent, “Smart Extract” [8], and the dedicated equipment “LifeCase Smart” and “LifeCase Amp,” the RNA extraction time is approximately 15 min for eight samples. Moreover, the reaction time is around 15 min when the viral load is high, and around 40 min when a sample is negative. The biggest advantage of SmartAmp is that the test time can be reduced to less than 1 h. Furthermore, as the necessary equipment is compactly stored in three attaché cases, it is easy to carry around. This enables rapid examinations where dead bodies are handled, such as at autopsies, external examinations, and general handling in morgues, and will lead to the prevention of infection among people who handle and work near dead bodies.
The usefulness of the SmartAmp method as a SARS-CoV-2 detection method has only been reported and tested using clinical specimens [9], [10] and there have been no reports using postmortem specimens. In this study, to investigate the effectiveness of the SmartAmp SARS-CoV-2 assay in postmortem specimens, we performed tests using the SmartAmp method and the qRT-PCR assay using swabs from dead bodies, and compared the operation times and the obtained results.
2. Materials and methods
2.1. Autopsy specimens
Swab samples were collected from the nasopharynx, oropharynx, and anus of 11 cadavers (five cases were determined to be SARS-CoV-2 positive and six were negative, based on qRT-PCR testing) that were autopsied or biopsied at postmortem at the Forensic Medicine Departments of three Japanese Universities, from January to May, 2021 ( Table 1). The postmortem time ranged from 2 to 11 days in the positive cases and 3–5 days in the negative cases. There were no dead bodies in advanced stages of decomposition.
Table 1.
List of cases.
| Age/sex | Condition | Time elapsed after death | Cause of death | PCR determination* | |
|---|---|---|---|---|---|
| 1 | 74/Male | Disappeared after recovering and being discharged from COVID-19 treatment in the hospital; found floating in a river | 7–11 days | Drowning | Positive |
| 2 | 78/Male | Infected with COVID-19 in the hospital while being treated for another disease; died 11 days after the infection was discovered | 5 days | COVID-19 | Positive |
| 3 | 58/Female | PCR positive after returning from abroad; died while recuperating in a hotel due to minor illness | 2 days | COVID-19 | Positive |
| 4 | 86/Female | Died at home | 3 days | Decubitus ulcer infection | Positive |
| 5 | 58/Male | PCR positive after arriving from overseas; died while recuperating in a hotel due to minor illness | 4 days | COVID-19 | Positive |
| 6 | 39/Female | Suicide by insulin overdose | 3 days | Insulin poisoning | Negative |
| 7 | 50/Male | Fell while working at a high altitude | 3 days | Traumatic shock | Negative |
| 8 | 48/Male | Died after collapsing unconscious in a sauna | 3 days | Heat stroke | Negative |
| 9 | 44/Male | Fell from a hotel window while intoxicated | 3 days | Traumatic shock | Negative |
| 10 | 35/Female | Suicide by using charcoal briquettes in a car | 5 days | Carbon monoxide poisoning | Negative |
| 11 | 69/Female | Stabbed to death with a kitchen knife after being strangled by her son | 4 days | Traumatic shock | Negative |
In qRT-PCR, amplification of at least two regions of ORF1ab, N-protein, and S-protein in at least one of the swabs collected from three locations was considered positive.
This study was conducted with the approval of the Institutional Review Boards of the three facilities.
2.2. qRT-PCR assays
2.2.1. RNA extraction for qRT-PCR
Viral RNA was extracted from two swab slices using an EZ1® Virus Mini kit and the EZ1® Advanced XL system (QIAGEN Inc., Hilden, Germany), following the manufacturer’s instructions. The RNA was eluted into 90 µL of TE buffer.
2.2.2. Quantification by qRT-PCR
The testing reagents used for qRT-PCR included the TaqMan™ 2019-nCoV Assay Kit v1, the TaqMan™ Fast Virus 1-Step Master Mix, and the TaqMan™ 2019-nCoV Control Kit v1 (all from Thermo Fisher Scientific, Franklin, MA, USA). As per the protocol, a 25-µL reaction mix was set up that comprised 6.25 µL of Master Mix, 1.25 µL of primers (to the ORF1ab, N-protein, and S-protein regions, respectively), 1.25 µL of standard internal primer, and 5 µL of the sample. qRT-PCR was performed using a StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific) at 50 °C for 5 min, 95 °C for 20 s, 95 °C for 3 s, and 60 °C for 30 s, for 45 cycles. In the qRT-PCR, amplification of at least two regions of ORF1ab, N-protein, or S-protein in at least one of the swabs collected from the three locations was considered positive.
Copy number quantification was performed using the same reagents and N-region primers with the same formulation, temperature, and cycling conditions. The calibration curve was prepared by diluting the positive control given by the National Institute of Infectious Diseases to seven serial dilutions (10, 102, 103, 104, 105, 106, 107). Both the calibration curve and the samples were measured at two of these dilutions.
2.3. SARS-CoV-2 detection by SmartAmp
2.3.1. RNA extraction for SmartAmp
RNA extraction was performed in accordance with the two protocols provided with the “Smart Extract” (DNAFORM), as described below.
2.3.2. Aspiration method
The sample swab was placed in 1 mL of Swab Suspension Buffer (SSB) and added to the 500-µL column before aspirating for 5 s. Then, wash solution 1 (WS1) was added to the 500-µL column before aspirating for 5 s. Subsequently, 99.5% ethanol was added to the 500-µL column before aspirating for 5 s and the RNA was collected in 100 µL of RNase-free water.
2.3.3. Simple centrifugation method
The sample swab was placed in 1 mL of SSB and added to the 500-µL column before centrifuging for 2 min in a small desktop centrifuge (Bio-Medical Science Co., Ltd., Tokyo, Japan). Then, 500 µL of WS1 was added and centrifuged for 1 min. Next, 500 µL of 99.5% ethanol was added, centrifuged for 1 min, and the RNA was collected in 100 µL of RNase-free water.
2.3.4. RNA amplification using SmartAmp
The SmartAmp™ 2019 novel coronavirus detection reagent (DNAFORM) was used for amplification. A 20-µL reaction solution was used that comprised 10 µL of RNA extraction solution, obtained using the simple centrifugation and aspiration methods for each sample, mixed with 10 µL of a mixture of "Reagent P" primer reagent and "Reagent E" enzyme reagent. For the modified simple centrifugation method, only 1 µL of RNA extraction solution was used per sample in the reaction mix.
Reactions were amplified under isothermal conditions at 67 °C using a “LifeCase Amp” device. A case was considered positive if the amplification curve increased with more than one sample.
3. Results
3.1. Determination results
Table 2 shows a comparison of the determination results obtained with the qRT-PCR and SmartAmp methods. Using the RNA obtained by the aspiration method, similar results were obtained with the SmartAmp method as with the qRT-PCR method for both positive and negative cases. Using 10 µL of RNA obtained by the simple centrifugation method, different results were obtained with the SmartAmp method from those obtained with the qRT-PCR method for all five positive cases. However, using the modified simple centrifugation method in which the amount of RNA added was reduced to 1 µL, the same determination results were obtained with the SmartAmp method as with the qRT-PCR method for all positive and negative cases.
Table 2.
Comparison of the qRT-PCR results with the SmartAmp results.
| qRT-PCR result | SmartAmp method | |||
|---|---|---|---|---|
| Aspiration method | Simple centrifugation method (10 µL RNA added) | Modified simple centrifugation method (1 µL RNA added) | ||
| 1 | Positive | Positive | Negative | Positive |
| 2 | Positive | Positive | Negative | Positive |
| 3 | Positive | Positive | Negative | Positive |
| 4 | Positive | Positive | Negative | Positive |
| 5 | Positive | Positive | Negative | Positive |
| 6 | Negative | Negative | Negative | Negative |
| 7 | Negative | Negative | Negative | Negative |
| 8 | Negative | Negative | Negative | Negative |
| 9 | Negative | Negative | Negative | Negative |
| 10 | Negative | Negative | Negative | Negative |
| 11 | Negative | Negative | Negative | Negative |
3.2. Results for each sample
The results obtained for each sample are listed in Table 3. The same determination results were obtained with the SmartAmp method as the qRT-PCR method for the nasopharyngeal and pharyngeal swabs using both the aspiration method and the modified simple centrifugation method with the addition of 1 µL of RNA. However, the SmartAmp method and the qRT-PCR method yielded different results for the anal swab of a positive case (Case 4).
Table 3.
Results for each sample.
| Nasopharyngeal |
Oropharyngeal |
Anal |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| qRT-PCR (copies/µL)* | SmartAmp |
qRT-PCR (copies/µL) | SmartAmp |
qRT-PCR (copies/µL) | SmartAmp |
||||
| a** | b*** | a | b | a | b | ||||
| 1 | +(3.99 ×10) | + | + | +(8.19 ×102) | + | + | – | – | – |
| 2 | +(4.12 ×104) | + | + | +(1.98 ×103) | + | + | + (1.98 ×102) | + | + |
| 3 | +(1.04 ×104) | + | + | +(1.04 ×104) | + | + | – | – | – |
| 4 | +(9.41 ×103) | + | + | +(7.49 ×103) | + | + | + (-) | – | – |
| 5 | +(1.91 ×106) | + | + | +(3.96 ×105) | + | + | – | – | – |
| 6 | – | – | – | – | – | – | No sample | ||
| 7 | – | – | – | – | – | – | No sample | ||
| 8 | – | – | – | – | – | – | No sample | ||
| 9 | – | – | – | – | – | – | No sample | ||
| 10 | – | – | – | – | – | – | No sample | ||
| 11 | – | – | – | – | – | – | No sample | ||
Number of copies in N region,
Aspiration method,
Modified simple centrifugation method
3.3. Influence of time elapsed after death
The drowning case (Case 1), whose postmortem interval was to be estimated 7–11 days, showed a similarly positive result with the SmartAmp method as with the qRT-PCR method.
4. Discussion
Testing for the presence of infection in dead bodies suspected of being infected with SARS-CoV-2 is important for the safety of those who may come into contact with the dead bodies and for controlling the spread of SARS-CoV-2 infection. In particular, because various bodily fluids and organs are handled during autopsy, it is necessary to obtain information on the infection status before autopsy so that necessary infection control measures can be taken. Therefore, a rapid and simple method of testing that can replace qRT-PCR is needed. In this study, we focused on the SmartAmp method, one of the isothermal amplification methods that can detect SARS-CoV-2 simply and rapidly, and reports its effectiveness in postmortem specimens for the first time.
The SmartAmp method has been developed for applications such as pathogen detection and the genotyping of single nucleotide polymorphisms [11], [12], [13]. It is also used in clinical practice as a rapid test for SARS-CoV-2 [9], [10]. In this study, we used SmartAmp™ 2019 SARS-CoV-2 detection reagents and Smart Extract for the extraction and amplification of RNA from samples. Smart Extract is capable of RNA extraction by two methods: aspiration and simple centrifugation. The first choice is the aspiration method, but in highly viscous samples that are difficult to aspirate, the simple centrifugation method can be used for extraction. However, the vacuum equipment required for the aspiration method is large and heavy, making it difficult to transport. By contrast, the simple centrifugation method can be performed using a desktop centrifuge, which is easy to transport. Furthermore, compared with clinical samples, samples swabbed from dead bodies may contain skin fragments, mucus, or other contaminants that make aspiration difficult, so both methods were examined in this study. Among the positive samples, the simple centrifugation method gave false negative results in all cases. Considering the possibility that impurities could not be sufficiently removed and PCR inhibitors remained, we reduced the volume of the extraction solution to reduce impurities, and obtained the correct results. This finding suggested that some impurities that inhibit PCR amplification were present in the postmortem specimens and that the simple centrifugation method was not able to remove them, resulting in false negative results. Although we could not identify this impurity, it is possible that an impurity may remain because the centrifugal force of the tabletop centrifuge supplied with this kit is weak (maximum 2000 × g). One of the reasons for insufficient centrifugation may be the effect of postmortem changes. The identification of impurities and an investigation into the cause of insufficient centrifugation will be the subject of a future study. However, because correct results can be obtained by reducing the amount of RNA added, it is possible to analyze postmortem specimens using a modified simple centrifugation method.
In one case (Case 4), the anal swab gave different results, testing positive by qRT-PCR and negative by the SmartAmp method. The RNA copy number determined by qRT-PCR of the anal swab from Case 4 was 1.31 × 102, suggesting a low viral load. The TaqMan™ 2019-nCoV Assay Kit v1 can detect as few as 10 copies, while the SmartAmp method can detect as many as 50 copies of artificially synthesized viruses, which is slightly less sensitive than qRT-PCR [4], [14]. In addition, the sensitivity of extracts from actual samples, especially those from cadavers, is expected to be even lower because they contain PCR inhibitors and other substances. Therefore, it is thought that the Case 4 anal swab, which had a low viral load, could not be detected by the SmartAmp method. However, in the nasopharynx and oropharynx specimens, which are normally used for testing, the results were the same using each method, suggesting that the SmartAmp method is useful in dead bodies. Furthermore, positive results were obtained using both the SmartAmp method and the qRT-PCR method on the sample from the drowned cadaver, which had an estimated postmortem interval of 7–11 days. This suggests that the SmartAmp method may be useful even after a long postmortem interval, provided a certain amount of virus remains.
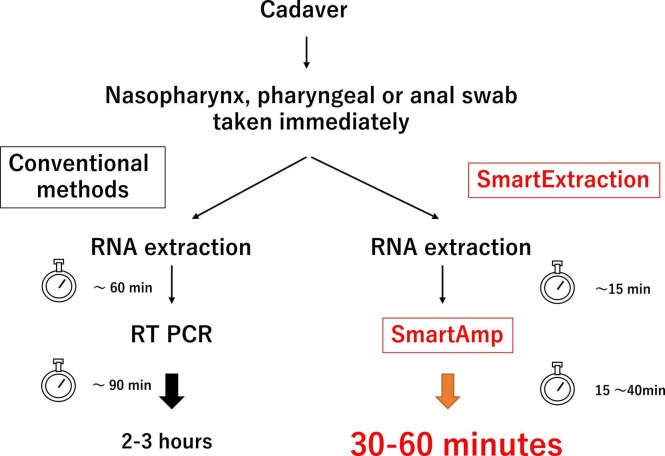
In this study, using SmartAmp™ 2019 SARS-CoV-2 detection reagents and Smart Extract for extraction and amplification, we were able to reduce the actual test time to approximately 1 h, which is significantly shorter than the time required for qRT-PCR ( Fig. 1). Additionally, the SmartAmp method uses exciton-controlled hybridization-sensitive fluorescent primers, which act as sequence-specific dyes and emit sequence-specific fluorescent signals after hybridization with complementary sequences, thus allowing visual detection [15], [16]. SmartAmp™ 2019, the SARS-CoV-2 detection reagent used in this study, is used along with equipment that can monitor these dye changes in real time, allowing us to make decisions using melting curve analysis in addition to visual confirmation. The equipment is packaged in an attaché case for easy transportation, enabling real-time monitoring at various locations. In fact, we were able to take the test to three facilities, including those that did not have qRT-PCR equipment. Consequently, the methods presented in this study and the use of these products will allow for rapid testing regardless of location or time. These advantages suggest that the methods described herein are useful not only during autopsies, but also in various situations where dead bodies are handled, such as during autopsies and in onsite morgues during disasters. Our findings may help prevent infection among those who handle dead bodies.
Fig. 1.
Using SmartExtract and Lifecase Smart to shorten testing time.
5. Conclusion
In this study, we conducted the first investigation of the effectiveness of the SmartAmp method for postmortem SARS-CoV-2 testing. Our findings clarified that the SmartAmp method is applicable for postmortem SARS-CoV-2 testing, in the same way as qRT-PCR. Furthermore, the SmartAmp method could be used for testing over a short time period regardless of the location, which would enable rapid testing prior to autopsy, thereby protecting those who come into contact with dead bodies from infection.
Funding
A portion of this study was conducted as a part of the project (20HA2008) of the Health and Labour Administration Promotion Research Project Japan (Research Project for Promotion of Policies for Emerging and Re-emerging Infectious Diseases and Immunization) in 2021.
Availability of data and material
The data that support the findings of this study are available from the corresponding author, SN, upon reasonable request.
Ethics approval
This study was conducted with the approval of the Institutional Review Boards of the Chiba University Graduate School of Medicine, the University of Tokyo Graduate School of Medicine, and the International University of Health and Welfare School of Medicine.
Conflict of interests
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgments
We would like to express our deepest gratitude to the Infectious Pathology Division of the National Institute of Infectious Diseases for providing us with RNA of SARS-CoV-2 and Japan Medical Association Research Institute and DNAFORM for their advice and cooperation in kinship with this study. We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Consent to participate
As no participants took part in this study, consent was waived.
Consent for publication
Not applicable.
References
- 1.World Health Organization (2021) Coronavirus (COVID-19) Dashboard. Available at: 〈https://covid19.who.int/〉.
- 2.Heinrich F., Meißner K., Langenwalder F., Püschel K., Nörz D., Hoffmann A., Lütgehetmann M., Aepfelbacher M., Bibiza-Freiwald E., Pfefferle S., Heinemann A. Postmortem stability of SARS-CoV-2 in nasopharyngeal mucosa. Emerg. Infect. Dis. 2021;27:329–331. doi: 10.3201/eid2701.203112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niemz A., Ferguson T.M., Boyle D.S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011;29:240–250. doi: 10.1016/j.tibtech.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill P., Ghaemi A. Nucleic acid isothermal amplification technologies: a review. Nucleosides Nucleotides Nucleic Acids. 2008;27:224–243. doi: 10.1080/15257770701845204. [DOI] [PubMed] [Google Scholar]
- 5.Jeong Y.J., Park K., Kim D.E. Isothermal DNA amplification in vitro: the helicase-dependent amplification system. Cell Mol. Life Sci. 2009;66:3325–3336. doi: 10.1007/s00018-009-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai Y., Kimura Y., Lezhava A., et al. One-step detection of the 2009 pandemic influenza A (H1N1) virus by the RT-SmartAmp assay and its clinical validation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DANAFORM®. 〈https://www.dnaform.jp/ja/products/snp_assay/smartamp_kit/〉.
- 8.DANAFORM® “Smart Extract”. 〈https://www.dnaform.jp/ja/about/news/〉.
- 9.Asai N., Nakamura A., Sakanashi D., Koita I., Ohashi W., et al. Comparative study of SmartAmp assay and reverse transcription-polymerase chain reaction by saliva specimen for the diagnosing COVID-19. J. Infect. Chemother. 2021;21:S1341. doi: 10.1016/j.jiac.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto K., Ohmagari N. Microbiological testing for cornavirus disease 2019. JMA J. 2021;4:67–75. doi: 10.31662/jmaj.2021-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitani Y., Lezhava A., Kawai Y., et al. Rapid SNP diagnostics using asymmetric isothermal amplification and a new mismatch-suppression technology. Nat. Methods. 2007;4:257–262. doi: 10.1038/nmeth1007. [DOI] [PubMed] [Google Scholar]
- 12.Lezhava A., Hayashizaki Y. Detection of SNP by the isothermal smart amplification method. Methods Mol. Biol. 2009;578:437–451. doi: 10.1007/978-1-60327-411-1_28. [DOI] [PubMed] [Google Scholar]
- 13.Mitani Y., Lezhava A., Sakurai A., Horikawa A., Nagakura M., Hayashizaki Y., Ishikawa T. Rapid and cost-effective SNP detection method: application of SmartAmp2 to pharmacogenomics research. Pharmacogenomics. 2009;10:1187–1197. doi: 10.2217/pgs.09.39. [DOI] [PubMed] [Google Scholar]
- 14.Thermo Scientific®. 〈https://www.thermofisher.com/order/catalog/product/A47532#/A47532〉.
- 15.Ikeda S., Kubota T., Yuki M., Okamoto A. Exciton-controlled hybridization-sensitive fluorescent probes: multicolor detection of nucleic acids. Angew. Chem. Int Ed. Engl. 2009;48:6480–6484. doi: 10.1002/anie.200902000. [DOI] [PubMed] [Google Scholar]
- 16.Lezhava A., Ishidao T., Ishizu Y., Naito K., Hanami T., Katayama A., Kogo Y., Soma T., Ikeda S., Murakami K., Nogawa C., Itoh M., Mitani Y., Harbers M., Okamoto A., Hayashizaki Y. Exciton primer-mediated SNP detection in SmartAmp2 reactions. Hum. Mutat. 2010;31:208–217. doi: 10.1002/humu.21177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, SN, upon reasonable request.