Abstract
TC21 is a member of the Ras superfamily of small GTP-binding proteins that, like Ras, has been implicated in the regulation of growth-stimulating pathways. We have previously identified the Raf/mitogen-activated protein kinase pathway as a direct TC21 effector pathway required for TC21-induced transformation (M. Rosário, H. F. Paterson, and C. J. Marshall, EMBO J. 18:1270–1279, 1999). In this study we have identified two further effector pathways for TC21, which contribute to TC21-stimulated transformation: the phosphatidylinositol 3′ kinase (PI-3K) and Ral signaling pathways. Expression of constitutively active TC21 leads to the activation of Ral A and the PI-3K-dependent activation of Akt/protein kinase B. Strong activation of the PI-3K/Akt pathway is seen even with very low levels of TC21 expression, suggesting that TC21 may be a key small GTPase-regulator of PI-3K. TC21-induced alterations in cellular morphology in NIH 3T3 and PC12 cells are also PI-3K dependent. On the other hand, activation of the Ral pathway by TC21 is required for TC21-stimulated DNA synthesis but not transformed morphology. We show that inhibition of Ral signaling blocks DNA synthesis in human tumor cell lines containing activating mutations in TC21, demonstrating for the first time that this pathway is required for the proliferation of human tumor cells. Finally, we provide mechanisms for the activation of these pathways, namely, the direct in vivo interaction of TC21 with guanine nucleotide exchange factors for Ral, resulting in their translocation to the plasma membrane, and the direct interaction of TC21 with PI-3K. In both cases, the effector domain region of TC21 is required since point mutations in this region can interfere with activation of downstream signaling.
The regulation of several cellular processes including proliferation, differentiation, and the modulation of the cytoskeleton has been ascribed to the Ras subfamily of small GTP-binding proteins. This family includes the classical Ras proteins (H-Ras, N-Ras, K4A-Ras, and K4B-Ras), the R-Ras-like proteins (R-Ras, TC21, and M-Ras/R-Ras 3), the Rap proteins (Rap 1A, Rap 1B, Rap 2A, and Rap 2B) and the Ral proteins (Ral A and Ral B) (3). While the role of the classical Ras proteins is becoming clearer, the role of other members of the subfamily is still unclear. The classical Ras proteins have been of particular interest, given the high incidence of mutation of these genes in human cancers. Apart from the classical Ras proteins, the only other member of the Ras subfamily of GTPases found to be mutated in human cancers is TC21 (also called R-Ras 2) (2, 5, 19). Constitutively active TC21 will transform a wide variety of fibroblast and epithelial cell lines, and injection of TC21-transformed fibroblasts into nude mice results in the formation of highly aggressive tumors (5, 6, 14, 19). In addition, up-regulation of the wild-type protein has been observed in seven of nine breast tumor cell lines (6). These observations have suggested that TC21, like the classical Ras proteins, may be involved in the regulation of growth.
TC21 has 55% amino acid identity to the classical Ras proteins but has an absolutely conserved core effector domain (residues 32 to 40 in H-Ras and 43 to 51 in TC21), a region that is required for the interaction of all identified Ras effectors (10). However, other family members such as R-Ras, which is 70% identical to TC21, also share conserved effector domains but do not activate the same effector pathways and are not transforming (8, 17, 29, 35).
We have previously demonstrated that TC21 will directly interact with the Raf serine/threonine kinases and therefore lead to the activation of the Raf/mitogen-activated protein (MAP) kinase pathway, a step that is crucial to TC21-stimulated cellular transformation (39). Here we identify two further effector pathways of TC21: the phosphatidylinositol 3′ kinase (PI-3K) and Ral signaling pathways. We demonstrate that TC21 leads to a strong PI-3K-dependent activation of the serine/threonine kinase Akt/protein kinase B (PKB). Activation of this pathway is probably due to the direct interaction of TC21 with PI-3K and is required for TC21-induced transformation of NIH 3T3 cells and for TC21-induced morphological alteration of PC12 cells. In addition, we show that TC21 interacts directly with several exchange factors for Ral, leading to their membrane localization and to the subsequent activation of Ral A. Activation of this pathway by TC21 is crucial for TC21-induced DNA synthesis both in human tumor cell lines harboring activating mutations in TC21 and in fibroblasts expressing active TC21, but it does not appear to be involved in the morphological alterations stimulated by TC21 expression. These results define a novel role for the Ral pathway in the stimulation of DNA synthesis in human tumor cell lines. The effector domain of TC21 is shown to be required for the activation of both PI-3K and Ral signaling pathways by TC21, since point mutations in this region specifically interfere with signaling.
MATERIALS AND METHODS
Plasmids.
The Ral binding domain of RalBP1 (residues 397 to 518) (51) was subcloned by PCR into the pEF Plink HA.6 vector that incorporates a HA tag at the N terminus of the protein. The Ral binding domain of RalBP1 and full-length wild-type RalGDS were subcloned into the pEGFP-C1 mammalian expression vector (Clontech) that fuses the green fluorescent protein (GFP) at the N terminus of RalGDS. Mutations in the effector domain of Myc-tagged V23 TC21 were generated using PCR-directed mutagenesis. All other constructs have been described previously (39).
Cell culture, transfection, and microinjection.
Untransformed and transformed NIH 3T3 cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 or 5% calf serum (GibcoBRL Life Technologies), respectively. Madin-Darby canine kidney (MDCK) cells and all human tumor cell lines were maintained in DMEM supplemented with 10% fetal calf serum. PC12 cells were grown in DMEM supplemented with 10% horse serum and 5% fetal calf serum. DNA transfections were performed using LipofectAMINE (GibcoBRL Life Technologies), and microinjections were performed on a Zeiss Microinjection Workstation (Carl Zeiss, Oberkochen), both as previously described (39). Cells undergoing DNA synthesis were identified by incorporation into newly synthesized DNA of bromodeoxyuridine (BrdU; Amersham Life Sciences) present at 10 mM in the culture medium. Microinjected samples were fixed and stained as previously described (39) using the A14 rabbit polyclonal anti-Myc antibody (Santa Cruz), 9E10 mouse monoclonal antibody, anti-HA antibody (3F10, Boehringer Mannheim), rat anti-BrdU monoclonal antibody, or Texas red-conjugated phalloidin (Molecular Probes) to detect polymerized actin. All immunofluorescence samples were analyzed with a Bio-Rad MRC 1024 confocal imaging system equipped with a Nikon Eclipse 400 microscope.
Preparation of cell lysates for immunoprecipitation, pull-down, and Western blotting.
Samples for detection of phosphorylated Akt or extracellular signal-regulated kinase (ERK) or for Ral pull-down assays were prepared in NP-40 buffer (50 mM Tris [pH 7.4], 1% NP-40, 15% glycerol, 200 mM NaCl, 5 mM MgCl2) containing protease inhibitors (10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg of pepstatin A per ml) on ice. Lysates were cleared by centrifugation, and protein concentrations were determined. A 100-μg sample of cleared whole-cell lysate was analyzed by Western blotting with anti-phosphorylated Ser 473 or Thr 308 Akt polyclonal antibodies (NEB), anti-Akt (NEB), anti-ERK2 122 rabbit polyclonal antibody (7), and anti-phosphorylated ERK monoclonal antibody (Sigma).
For analysis of Ral-GTP levels, equal levels of protein were incubated for 1 h at 4°C with glutathione-Sepharose beads that had been precoupled to recombinant glutathione S-transferase (GST)-RalBP1 RalBD as described by Wolthuis et al. (51). The beads were washed three times in large volumes of NP-40 buffer, and precipitated endogenous Ral A was analyzed by Western blotting with an anti-Ral A antibody (Transduction Laboratories).
Cell lysates for the analysis of coimmunoprecipitated Ras-related proteins and endogenous p110β were prepared in modified Ras lysis buffer (20 mM Tris [pH 7.5], 1% [vol/vol] Triton X-100, 10% glycerol, 100 mM KCl, 5 mM MgCl2, 5 mM NaF, 1 mM EGTA) supplemented with inhibitors (1 mM Na3VO4, 10 μg of leupeptin per ml 10 μg of aprotinin per ml, 10 μg of pepstatin A per ml, 10 mM benzamidine, 0.5 μg of microcystin LR per ml). Cleared lysates equilibrated for levels of protein were used immediately after preparation in immunoprecipitations with anti-Myc antibodies (mouse 9E10 monoclonal antibody and A14 rabbit anti-Myc polyclonal antibody; Santa Cruz). Coimmunoprecipitates were washed three times in modified Ras lysis buffer before being analyzed for coimmunoprecipitating endogenous p110β by Western blotting with anti-p110β antibody (sc-602; Santa Cruz). Cell lysates for the analysis of coimmunoprecipitated Rlf and TC21 proteins were prepared in Ras lysis buffer as previously described (39). TC21 proteins were immunoprecipitated with the 9E10 anti-Myc monoclonal antibody, and coimmunoprecipitated Rlf was detected by Western blotting with an anti-HA monoclonal antibody (3F10; Boehringer Mannheim).
RESULTS
Constitutive activation of the ERK/MAP kinase and Akt/PI-3K signaling pathways in TC21-transformed cells.
Activating mutations in TC21 transform a variety of different cell types and appear to transform NIH 3T3 cells with an efficiency as high as or even higher than that of H-Ras (5, 6, 14, 19). Other R-Ras-like GTPases have a very low transforming efficiency (8, 25, 33).
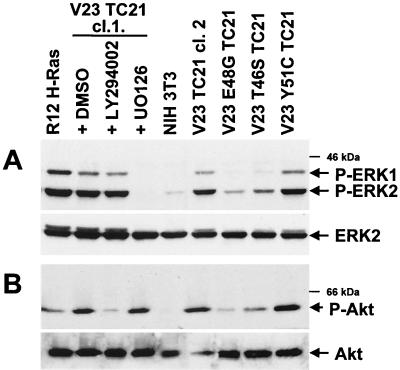
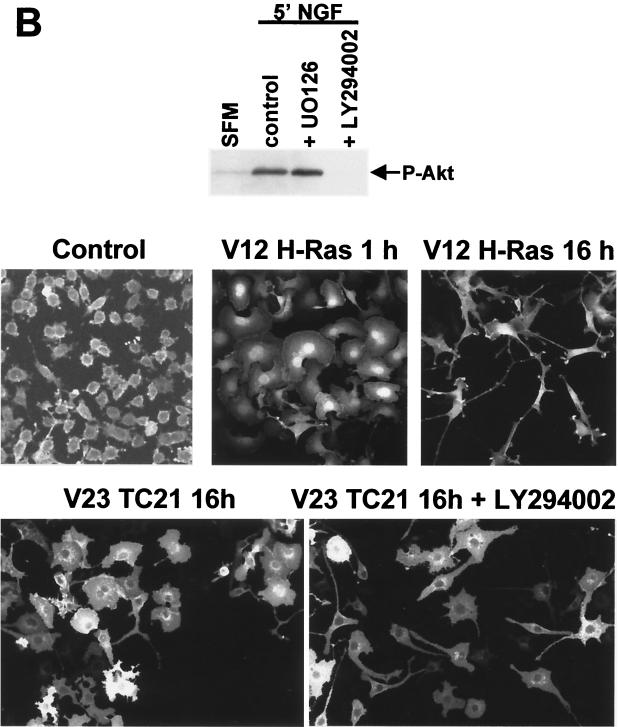
As we have previously reported, NIH 3T3 fibroblasts stably transformed with constitutively active TC21 or H-Ras show elevated levels of phosphorylated active ERK1 and ERK2 as compared to the parental untransformed NIH 3T3 cells (39) (Fig. 1A). This activity is completely inhibited by the MEK inhibitor UO126. Since activation of the PI-3K pathway is associated with signaling by Ras, we have investigated whether this pathway is also involved in TC21-stimulated transformation. TC21 transformed cells showed elevated levels of phosphorylated active Akt/PKB, a downstream effector of PI-3K (Fig. 1B). Activation of Akt/PKB in these cells is absolutely dependent on the activation of PI-3K, as indicated by the strong inhibitory effect of the PI-3K inhibitor LY294002. H-Ras-transformed NIH 3T3 cells had elevated levels of Akt/PKB phosphorylation, in agreement with previous work (9, 36, 38); however, we consistently found that activation of the Akt/PI-3K pathway in H-Ras-transformed cell lines was significantly lower than that observed in TC21-transformed cell lines (Fig. 1).
FIG. 1.
Activation of the ERK/MAP kinase and Akt/PI-3K pathways in TC21-transformed cells. (A) NIH 3T3 cell lines stably expressing oncogenic TC21, H-Ras, or TC21 proteins containing mutations in the effector domain and untransformed NIH 3T3 were transferred to serum-free medium for 4 h. TC21-transformed cells were also exposed either to 25 μM LY294002, 20 μM UO126 (a MEK inhibitor), or the equivalent volume of ethanol carrier for 2 h prior harvesting. Whole-cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and specific antibodies were used to detect phosphorylated ERK1 and ERK2 as well as total ERK2. Two independently isolated clones of TC21-transformed cells (cl.1 and cl.2) are shown. (B) The transformed NIH 3T3 cell lines were also analyzed for levels of phosphorylated Akt/PKB and total Akt/PKB in the presence of 50 μM LY294002, 20 μM UO126, or the equivalent volume of dimethyl sulfoxide (DMSO) carrier added for 2 h prior to harvesting as described above. Antibodies against the phosphorylated Ser 473 or Thr 308 sites in Akt/PKB gave similar results.
We generated point mutations in the effector domain of TC21 based on effector mutations in H-Ras that preferentially affect the binding and activation of specific effectors (39, 47, 50). Like the cognate mutations in H-Ras, the point mutations in the effector domain of TC21 E48G and T46S but not Y51C interfere with the constitutive activation of Akt in stably expressing cells, further suggesting that TC21-directed signaling is responsible for Akt activation (Fig. 1B). We have also observed elevated levels of activated Akt in the human tumor cell lines that contain activating mutations in TC21 (data not shown).
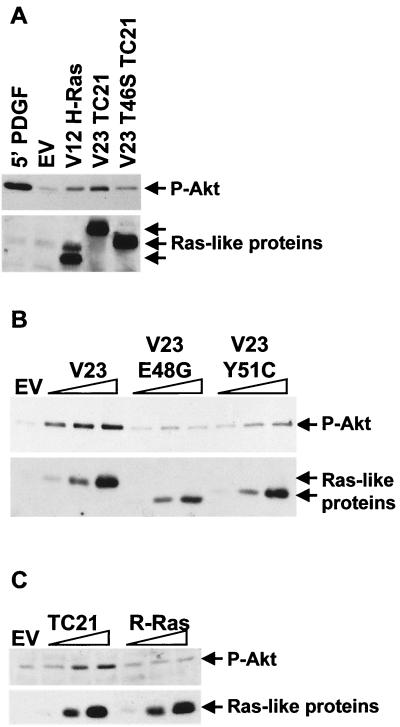
We confirmed that activation of the Akt/PI-3K pathway in the transformed NIH 3T3 cells was due to the expression of the TC21 oncogene, rather than being acquired as a result of clonal selection procedures, by determining the effect of transient transfection of activated forms of H-Ras, TC21, or R-Ras on the phosphorylation state of endogenous Akt/PKB. A 5-min stimulation with platelet-derived growth factor (PDGF) was included for comparison (Fig. 2). In accordance with our observations in stable cell lines (Fig. 1), Fig. 2 shows that expression of constitutively active TC21 results in strong activation of the Akt/PI-3K pathway. Point mutations in the effector domain of TC21 partially or completely inhibit this activation, indicating that direct signaling by TC21 is required. The Y51C mutation partially interferes with the activation of Akt/PKB, since higher expression levels are required for activation to be observed (Fig. 2B). The equivalent V12 Y40C H-Ras mutant has been reported to induce a three- to fourfold-lower activation of the Akt/PI-3K pathway than does the wild-type oncogenic H-Ras (21, 37, 50). Longer-term expression of the Y51C mutant does, however, allow activation of the Akt/PKB pathway comparable to that seen by V23 TC21 (Fig. 1B and data not shown), indicating that several mechanisms may be responsible for Akt/PKB activation by TC21.
FIG. 2.
Activation of endogenous Akt/PKB by TC21. NIH 3T3 cells were transiently transfected with fixed or increasing amounts of expression vectors for Myc-tagged constitutively active forms of H-Ras, TC21, the TC21 effector mutants, R-Ras, or an empty-vector control (EV). The cells were harvested 20 h posttransfection following a 4-h serum starvation. One of the empty-vector transfections was stimulated with 25 ng of platelet-derived growth factor (PDGF) per ml for 5 min prior to harvesting. Whole-cell lysates equilibrated for protein levels were separated by SDS-PAGE. Specific anti-phosphorylated Akt/PKB and anti-Myc antibodies were used to detect the active Akt/PKB and the Ras-like proteins, respectively.
R-Ras has also been proposed to lead to a strong activation of the Akt/PI-3K pathway (29). However, side-by-side titrations of oncogenic TC21 and R-Ras indicate that activation of this pathway by TC21 is considerably stronger than activation by R-Ras (Fig. 2C).
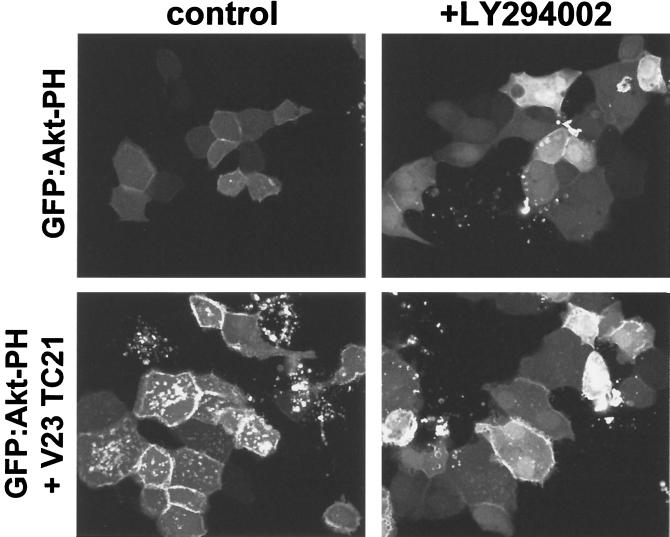
The notion that the effect of TC21 expression on the phosphorylation of Akt/PKB is due to the stimulation of 3′-phosphorylated inositide production was confirmed using the pleckstrin homology (PH) domain of Akt/PKB as a specific probe for these lipids (45) (Fig. 3). Expression of the GFP:Akt PH domain fusion protein in MDCK epithelial cells resulted in a diffuse cytoplasmic and nuclear staining pattern, with some protein also visible at cell-cell junctions as previously reported (45) (Fig. 3). Coexpression of active TC21 resulted in a strong translocation of the GFP:Akt PH domain fusion protein to the plasma membrane and internal vesicular membranes, presumably as a result of 3′-phosphoinositide generation, since incubation with LY294002 prevents this translocation, with the fusion protein again accumulating in the nucleus and cytoplasm (Fig. 3).
FIG. 3.
Translocation of the PH domain of Akt/PKB to the plasma membrane by TC21. Confluent MDCK cells were microinjected with 10 μg of an expression vector for the GFP:Akt PH domain fusion protein per ml alone or in conjunction with 50 μg of an expression vector for the Myc-tagged V23 TC21 per ml. LY294002 (25 μM) or the equivalent volume of ethanol was added to the medium just prior to microinjection. Cells were fixed 8 h after microinjection. GFP fluorescence is shown.
Direct interaction of PI-3K with TC21.
H-Ras interacts directly with the catalytic subunit (p110) of PI-3K α, PI-3K β, and PI-3K γ through the Ras effector domain (36, 38, 40). This region is completely conserved in TC21, although it is likely that other regions outside the effector domain are also involved in binding or in conferring specificity (44). Therefore it is possible that the strong activation of this pathway by TC21 is mediated through the direct interaction of TC21 with PI-3K.
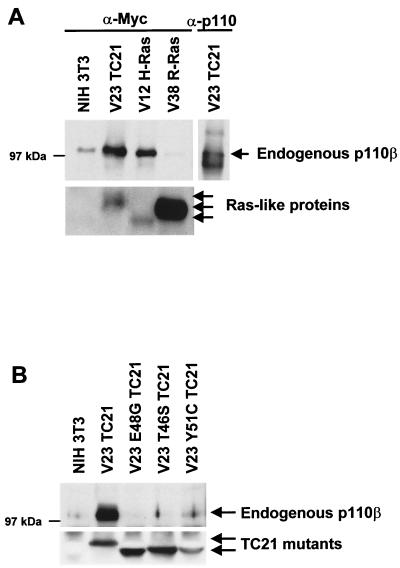
To demonstrate the interaction between TC21 and PI-3K, we sought to coimmunoprecipitate endogenous PI-3K with the Ras-related proteins from NIH 3T3 stable cell lines that express Myc-tagged versions of these proteins (Fig. 4). Endogenous p110 could be coimmunoprecipitated with TC21 and H-Ras but not with R-Ras, consistent with the observed activation of Akt/PKB by H-Ras and TC21 but not by R-Ras (Fig. 4A). Point mutations in the effector domain of TC21, all of which affect activation of Akt/PKB by TC21, interfered with binding to p110, indicating that this region is required for the interaction (Fig. 4B). Despite a weak activation of Akt by the Y51C mutant in transient transfections, only very little or no p110 was found associated with this protein by coimmunoprecipitation. This failure to coimmunoprecipitate p110 with Y51C may be a consequence of low levels of expression of this mutant in NIH 3T3 cells, making it technically difficult to immunoprecipitate sufficient Y51C protein to reveal the interaction.
FIG. 4.
Interaction of TC21 with PI-3K. Myc-tagged oncogenic TC21, R-Ras, and H-Ras (A) or Myc-tagged constitutively active TC21 proteins containing specific mutations in the effector domain (B) were immunoprecipitated from NIH 3T3 cell lines stably expressing these proteins and grown in medium supplemented with 5% serum. Coimmunoprecipitating endogenous p110 was detected after SDS-PAGE by immunoblotting with specific antibodies.
The role of TC21-induced PI-3K activation in TC21-stimulated signaling.
Several biological functions have been ascribed to the PI-3K pathway, including effects on cell proliferation (11, 12), actin polymerization and thus cell morphology (27, 32, 34, 37, 46), and cell survival after exposure to a number of apoptotic stimuli (21, 23, 52).
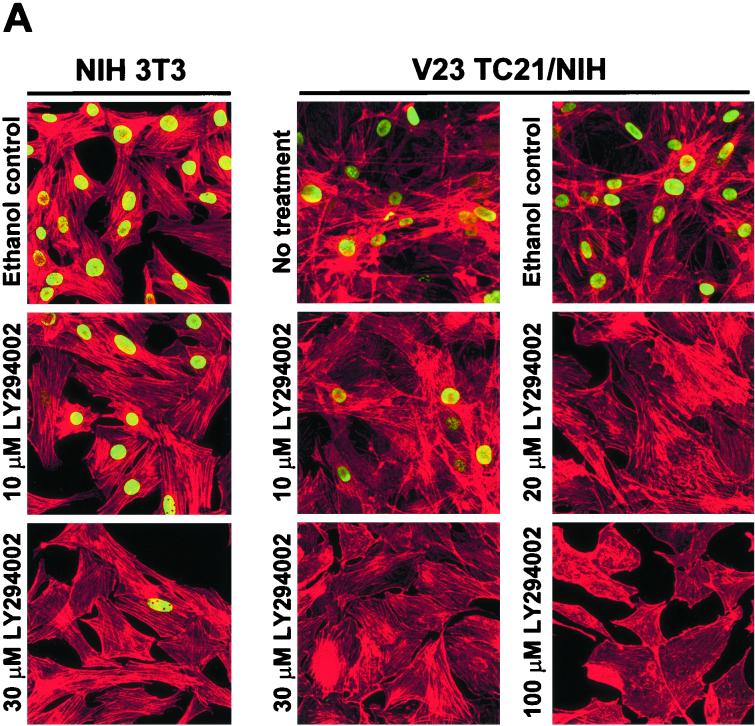
Treatment of TC21-transformed NIH 3T3 with LY294002 resulted in inhibition of DNA synthesis and morphological reversion of these cells, as seen by an inhibition in the incorporation of bromodeoxyuridine (BrdU) into newly synthesized DNA and the loss of spindle shape and cellular flattening (Fig. 5A). LY294002 affected the ability of parental NIH 3T3 cells to incorporate BrdU without affecting their cellular morphology, indicating that basal PI-3K levels are required for cell cycling of untransformed NIH 3T3 cells. The PI-3K pathway is thus crucial to TC21-induced morphological transformation.
FIG. 5.
TC21-induced transformation of NIH 3T3 cells (A) and morphological alteration of PC12 cells (B) are dependent on PI-3K activation. (A) V23 TC21-transformed NIH 3T3 or parental untransformed NIH 3T3 cells were seeded on several plastic dishes in DMEM supplemented with 5% serum. The cells were exposed to various concentrations of LY294002 or to the equivalent volume of the ethanol carrier for 42 h and allowed to incorporate BrdU during the last 15 h. Samples were then fixed and stained for the incorporated BrdU (shown in green) and for polymerized actin (shown in red). (B) PC12 cells were seeded on laminin-coated plates in medium containing 5% fetal calf serum and 10% horse serum. The medium was diluted 1:4 with serum-free medium 24 h after seeding. At 48 h after seeding, the cells were microinjected with 25 μg of an expression vector for Myc-tagged oncogenic TC21 or H-Ras per ml. LY294002 (25 μM) was added to a second duplicate plate of cells after injection. The cells were fixed 15 h after injection and stained for TC21 or H-Ras expression using anti-Myc antibodies. Levels of phosphorylated Akt/PKB in duplicate plates exposed to 25 μM LY294002, 20 μM UO126, or the equivalent volume of dimethyl sulfoxide carrier for 4 h prior to stimulation with 100 ng of NGF per ml for 5 min are shown.
Nerve growth factor (NGF) stimulation or the expression of active H-Ras results in a two-stage differentiation process of PC12 neuronal precursor cells, involving transient rapid spreading of lamellipodia followed by cell cycle arrest and neurite outgrowth (1, 16). Expression of constitutively active forms of the MAP kinase kinase MEK1 is sufficient to induce neurite outgrowth in these cells with little or no initial cellular flattening (7; H. Paterson, unpublished data). Expression of active TC21 in PC12 cells resulted in a distinctive morphology (Fig. 5B). The cells exhibited intense lamellipodia formation (reminiscent of the first stages of NGF-induced differentiation) with peripheral filopodia. There was no inhibition of DNA synthesis (data not shown) or induction of neurites. Unlike Movilla et al. (31) and Graham et al. (15), we did not observe neurite outgrowth on TC21 expression (Fig. 5B). However, in their experiments, cells were analyzed 14 to 20 days after introduction of activated TC21, whereas NGF stimulation or introduction of activated Ras or MEK1 induces neurite outgrowth within 24 h (7, 16).
Since PI-3K activation is required for the differentiation of the neuronal precursor PC12 rat pheochromocytoma cell line in response to NGF (20), we tested whether the TC21-induced morphology was a result of the activation of the PI-3K pathway by using LY294002. Inhibition of PI-3K did not cause these cells to revert to the original morphology but did partly inhibit flattening and resulted in the formation of elongated processes (Fig. 5B). Surrounding uninjected PC12 cells did not exhibit any significant morphological alterations on exposure to LY294002 (data not shown).
Interaction of TC21 with RalGEFs.
Guanine nucleotide exchange factors for Ral proteins (RalGEFs) have been proposed to be effectors of TC21, based on the identification of one of the members of this family, RalGDS, in yeast two-hybrid screens with activated TC21 (28).
RalGEFs are cytoplasmic proteins, and several lines of evidence suggest that translocation of these proteins to the plasma membrane is a crucial step in activation of the membrane-bound Ral proteins (18, 26, 50). Like other Ras proteins, TC21 is localized to the plasma membrane through C-terminal posttranslation lipid modifications (4). We examined whether activated TC21 could recruit RalGEFs to the plasma membrane.
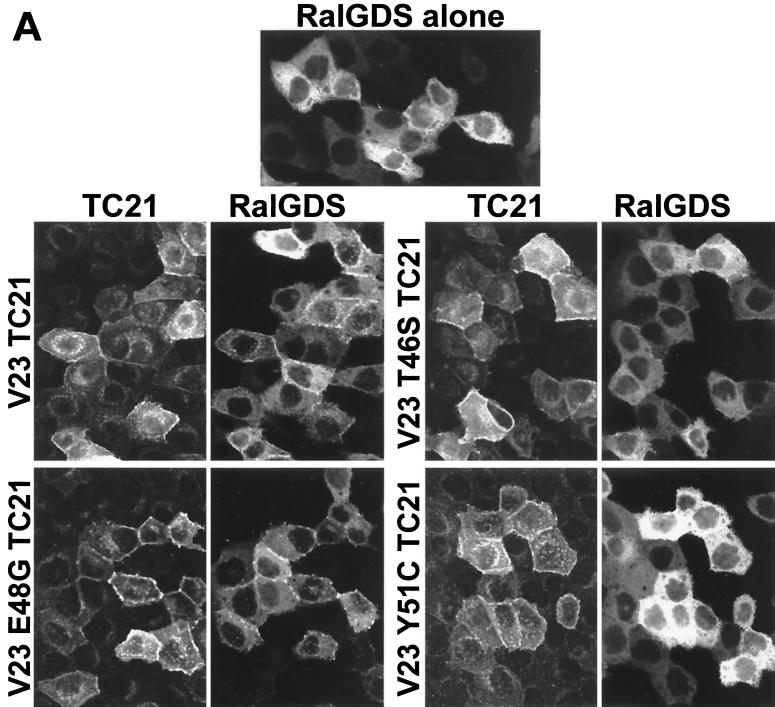
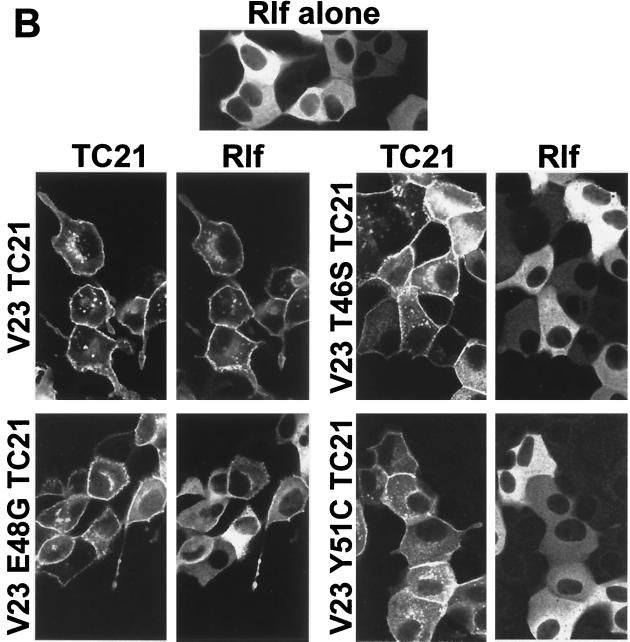
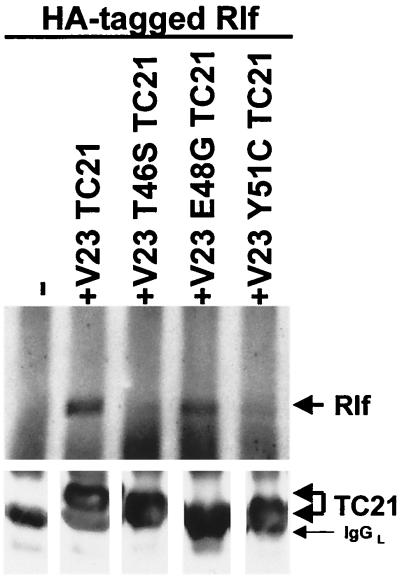
Figure 6 shows that two RalGEF family members, RalGDS and Rlf, translocate from the cytoplasm to the plasma membrane on coexpression of active TC21. The translocation of Rlf by TC21 is particularly complete. Further confirmation of the interaction of TC21 with Rlf was obtained by coimmunoprecipitation of transiently coexpressed wild-type Rlf with oncogenic TC21 (Fig. 7). Insertion of the T46S and Y51C point mutations in the effector domain region of TC21 completely abolished both TC21-induced plasma membrane translocation and coimmunoprecipitation of the RalGEFs with TC21. However, like the cognate E37G mutation in H-Ras, the E48G mutation in TC21 did not inhibit the interaction with the RalGEFs (Fig. 6 and 7) (37, 50).
FIG. 6.
Plasma membrane translocation of RalGDS and Rlf by V23 and V23 E48G TC21. Confluent MDCK cells were microinjected with 20 μg of an expression vector for a GFP:wild-type RalGDS fusion protein per ml (A) or 50 μg of an expression vector for HA-tagged wild-type Rlf per ml (B) alone or in conjunction with expression vectors for Myc-tagged V23 TC21 wild-type or effector mutant proteins (25 and 50 μg/ml, respectively). Cells were fixed and stained 14 h after microinjection for Myc-tagged TC21 or HA-tagged Rlf using specific antibodies. GFP fluorescence is shown for the GFP:RalGDS fusion protein. Samples were analyzed by confocal microscopy.
FIG. 7.
Coimmunoprecipitation of Rlf with V23 and V23 E48G TC21. NIH 3T3 cells were transiently transfected with expression vectors for HA-tagged Rlf alone or in conjunction with expression vectors for Myc-tagged V23, V23 T46S, V23 E48G, or V23 Y51C TC21. Myc-tagged TC21 proteins were immunoprecipitated from whole-cell lysates, and coimmunoprecipitated Rlf was detected after SDS-PAGE by immunoblotting with anti-HA antibodies.
Activation of Ral A by TC21.
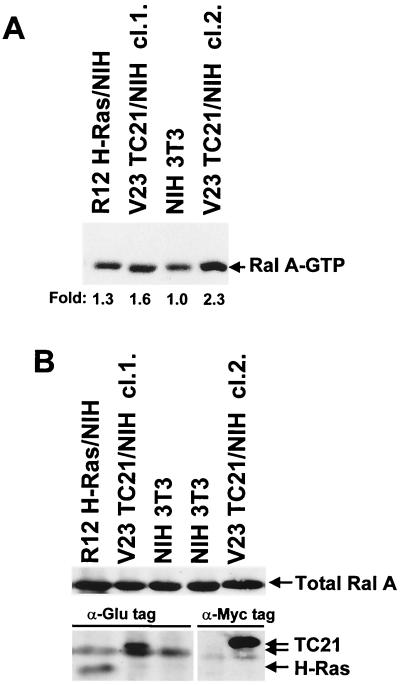
Several members of the Ras family of GTPases interact with RalGEFs (28, 41, 43, 48, 49). However, interaction has not always correlated with the ability of these GTPases to activate the Ral pathway. Indeed, R-Ras and Rap have been reported to bind RalGDS in vitro but do not activate this pathway in vivo (17, 26, 41, 43, 49). Therefore we tested whether the interaction of TC21 with the RalGEFs results in the activation of Ral A in vivo, using the Ral binding domain of the Ral effector RalBP1 (RalBP1 RalBD) to selectively pull down GTP-bound Ral (51). First we determined the constitutive levels of Ral-GTP in stably transformed cell lines (Fig. 8). TC21-transformed NIH 3T3 clones consistently exhibited elevated levels of Ral-GTP compared to parental untransformed NIH 3T3 cells. A smaller elevation of Ral-GTP levels was also seen in Ras-transformed cells. Figure 8B shows that total levels of endogenous Ral A are equivalent in the transformed and untransformed cell lines.
FIG. 8.
Constitutive activation of Ral A in TC21-transformed cells. H-Ras-transformed, TC21-transformed, and untransformed NIH 3T3 cells were transferred to serum-free medium for 4 h and then lysed. GST-RalBP1-RalBD-conjugated glutathione-Sepharose beads were used to pull down endogenous Ral-GTP from whole cell lysates. Precipitated Ral A-GTP (A) and total Ral A and Glu-tagged and Myc-tagged H-Ras and TC21 proteins in whole-cell lysates (B) were detected after SDS-PAGE by immunoblotting with specific antibodies. Two independently isolated TC21 clones (cl.1. and cl.2.) are shown. Fold increases in the levels of Ral A-GTP are indicated below panel A.
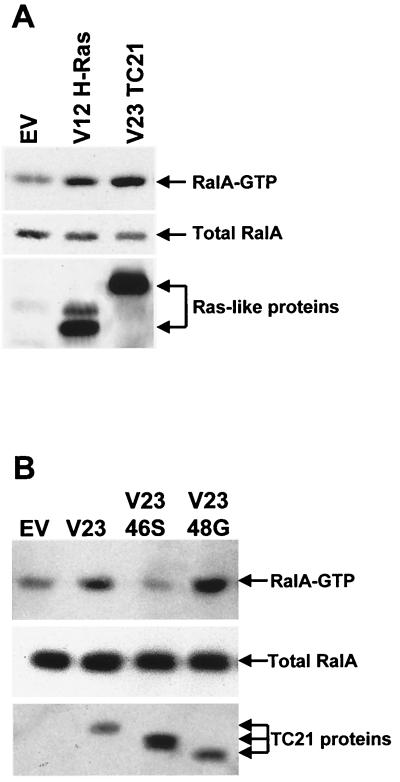
We confirmed these results by investigating the activation state of endogenous RalA on transient expression of TC21 and H-Ras in NIH 3T3 cells (Fig. 9). Similar to our observations in stably transformed cells, expression of active TC21 resulted in an increase in the level of GTP-bound Ral A, as did expression of H-Ras (Fig. 9A). Insertion of the T46S point mutation in the effector domain of TC21 interfered with RalGEF binding to TC21 and also inhibited the activation of Ral by TC21, while the E48G mutation, which has no effect on binding, also does not prevent the activation of Ral by TC21 (Fig. 9B). This indicates that the ability of TC21 to bind RalGEFs is crucial to the activation of Ral by TC21.
FIG. 9.
Activation of endogenous Ral A by V23 and V23 E48G TC21. Expression vectors for Myc-tagged V12 H-Ras and V23 TC21 (A) as well as for V23 T46S and V23 E48G TC21 (B) were transiently transfected into NIH 3T3 cells. The cells were transferred to medium containing 1.5% serum after transfection and harvested 20 h later. Ral A-GTP was specifically precipitated using GST-RalBP1-RalBD-conjugated glutathione-Sepharose beads. Ral A and Myc-tagged H-Ras and TC21 proteins were detected by immunoblotting with specific antibodies as described in Materials and Methods.
Requirement for Ral activation for TC21-stimulated DNA synthesis.
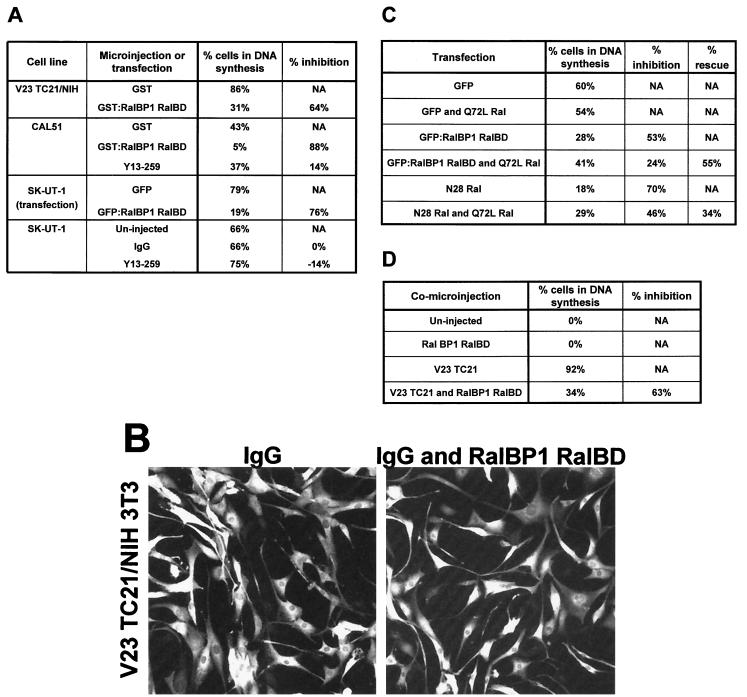
The direct interaction of TC21 with RalGEFs and the activation of endogenous Ral by constitutively active TC21 strongly suggest that the Ral pathway is a TC21 effector pathway. It has been previously reported that this pathway cooperates with other Ras effector pathways, such as the Raf/MAP kinase pathway, in cellular transformation (43, 47). To assess the function of the Ral pathway in TC21-induced transformation, we used overexpression of the Ral-GTP binding domain of the Ral effector RalBP1 (RalBP1 RalBD) as a way of selectively inhibiting Ral signaling (Fig. 10). Overexpression of this domain prevents the signaling of Ral to its effectors by forming nonproductive complexes with GTP-bound Ral.
FIG. 10.
Requirement of Ral activation for TC21-stimulated DNA synthesis. (A) TC21-transformed NIH 3T3 and the human tumor cell lines CAL51 and SK-UT-1 were seeded on collagen-coated dishes for microinjection. The cells were transferred to serum-free medium containing ITS 10 h after seeding, with the exception of SK-UT-1 cells, which were kept in low-serum medium. The cells were comicroinjected the next day with 6 mg of Y13-259 or whole immunoglobulin G (IgG) per ml alone or in conjunction with 0.2 mg of recombinant GST-RalBP1-RalBD per ml or 0.2 mg of GST protein per ml. The culture medium of injected TC21-transformed NIH 3T3, CAL51, and SK-UT-1 cells was supplemented with 10 mM BrdU 8 or 20 h (for the human tumor cell lines) after injection, and the cells were fixed 21, 26, or 44 h after injection, respectively, and stained for the microinjected proteins and for incorporated BrdU using specific antibodies. SK-UT-1 cells were also transfected with an expression vector for GFP or with an expression vector for the GFP:RalBP1 RalBD fusion protein and then changed to serum-free medium. Transfected SK-UT-1 cells were allowed to incorporate BrdU for 7 h at 17 h after transfection and were then fixed and stained as described above. The percentage of BrdU-positive cells is shown. Under these conditions, untransformed NIH 3T3 cells do not synthesize DNA. The experiments were repeated at least three times with similar results. Similar results were obtained using the dominant negative N28 Ral mutant protein. (B) The morphology of injected TC21-transformed NIH 3T3 cells injected with IgG alone or together with GST-RalBP1-RalBD is shown after staining for the rabbit IgG injection marker. (C) SK-UT 1 cells were transfected with low levels (10 ng) of an expression vector for GFP, for the GFP:RalBP1 RalBD fusion protein, or for N28 Ral in the absence or presence of low levels (5 ng) of an expression vector for the constitutively active form of Ral A, Q72L Ral. The cells were kept in 1% serum and allowed to incorporate BrdU for 7 h at 24 h after transfection and were then fixed and analyzed as before. The percentage of cells in DNA synthesis, the percent inhibition in DNA synthesis after expressing the RalBD or N28 Ral, and the percent rescue after coexpressing Q72L Ral are shown. The experiment was repeated three times with similar results. (D) Swiss 3T3 cells were seeded on glass coverslips and allowed to reach contact inhibition. They were then transferred to serum-free medium for 24 h. The cells were microinjected with 12.5 μg of an expression vector for Myc-tagged V23 TC21 per ml and 25 μg of an expression vector for HA-tagged RalBP1 RalBD per ml in the combinations indicated. The culture medium was supplemented with 10 mM BrdU 20 h postinjection, and the cells were fixed 44 h postinjection and stained for Myc-tagged TC21, HA-tagged RalBP1 RalBD, and incorporated BrdU using specific antibodies. NA, not applicable.
Expression of the RalBD in serum-starved TC21-transformed NIH 3T3 resulted in a strong inhibition of DNA synthesis with no significant effect on cellular morphology (Fig. 10A and B). Significantly, we also found that inhibition of Ral signaling in the breast adenocarcinoma-derived CAL51 cell line and the SK-UT-1 uterine leiomyosarcoma cell line, which are human tumor cell lines that harbour activating mutations in TC21 (2, 13, 19), inhibited DNA synthesis (Fig. 10A). This is the first demonstration to our knowledge of the involvement of the Ral pathway in human tumor cell line growth. CAL51 and SK-UT-1 cells have activating mutations in TC21 and are independent of Ras function, as demonstrated by their insensitivity to the Ras-neutralizing antibody, Y13-259 (Fig. 10A).
Inhibition of Ral activation by expression of the dominant negative Ral form, N28 Ral, also leads to an inhibition in DNA synthesis in these cells (Fig. 10C and data not shown). Furthermore, inhibition of DNA synthesis in SK-UT-1 cells due to expression of either the RalBD or N28Ral can be partially reversed by coexpression of constitutively active Ral, demonstrating that these proteins act by having specific effects on signaling by endogenous Ral proteins (Fig. 10C).
To confirm that activation of the Ral pathway was required for TC21-induced DNA synthesis, we coinjected an expression vector for RalBP1 RalBD with an expression vector for constitutively active TC21 into quiescent Swiss 3T3 fibroblasts. Expression of active TC21 in these cells resulted in a strong stimulation of DNA synthesis that was partially inhibited by expression of the RalBD (Fig. 10D). Again, no effect of the RalBD on TC21-induced cellular morphology was observed (data not shown).
DISCUSSION
Constitutively active TC21 will transform NIH 3T3 and other fibroblast and epithelial cell lines with high efficiency (5, 6, 14, 19, 24, 33, 39). We have previously shown that the high transformation potential of TC21 in NIH 3T3 cells is mediated by direct activation of the Raf/MAP kinase pathway by TC21 (39). Despite the aggressive transformation and strong stimulation of DNA synthesis by TC21, activation of the Raf/MAP kinase pathway by TC21 is less potent than by the classical Ras proteins (39), suggesting that the activation of other signaling pathways is involved in TC21-induced transformation. We now show that TC21-transformed cells exhibit constitutive activation not only of the Raf/MAP kinase pathway but also of the PI-3K and the Ral pathways, both of which contribute to several aspects of TC21-induced transformation. Unlike the Raf/MAP kinase pathway, constitutive activation of these pathways in TC21-transformed NIH 3T3 cells appears to be higher than in H-Ras-transformed cells. We have also found that the human tumor cell lines which have activating mutations in TC21 and do not require classical Ras function have very high levels of constitutively active Akt/PKB and require Ral activation for DNA synthesis.
Activation of the Akt/PI-3K pathway by TC21.
Not only do TC21-transformed NIH 3T3 and tumor cell lines containing activating mutations in TC21 show a strong constitutive PI-3K-dependent activation of Akt/PKB, but TC21 expression in transiently transfected NIH 3T3 cells also leads to a parallel increase in Akt/PKB activation. Microinjection of oncogenic TC21 together with a GFP:Akt PH domain fusion protein expression vector results in PI-3K-dependent recruitment of the PH domain to the plasma membrane, demonstrating that TC21 can stimulate the generation of 3′-phosphorylated lipids. Furthermore, microinjection of the Y13-259 Ras-neutralizing antibody into TC21-transformed cells does not inhibit the activation of Akt/PKB, suggesting that TC21-stimulated activation of PI-3K is not mediated by classical Ras proteins (data not shown). In transient-transfection assays and in assays with stably transformed cell lines, activation of Akt/PKB by TC21 is comparable to or higher than that by other Ras family members, suggesting that TC21 may be a key regulator of PI-3K.
H-Ras has been previously found to interact directly with PI-3K, suggesting a mechanism for the activation of this pathway by H-Ras (36). From the crystal structure of p110γ, it has been predicted that Glu 37, Asp 38, Tyr 40, and Tyr 64 in H-Ras lie at the PI-3K:Ras interface (44). Residues equivalent to Glu 37, Asp 38, and Tyr 40 are conserved in TC21. The equivalent residue to Tyr 64 in H-Ras is a phenylalanine in TC21, which would interfere with the proposed hydrogen bond formation at this site (44). However, endogenous p110 is readily coimmunoprecipitated with oncogenic TC21. Other Ras-related proteins such as R-Ras and M-Ras/R-Ras 3 also have a Phe residue at this site and have been reported to activate the Akt/PI-3K pathway (25, 29).
We have observed that in vivo activation of the PI-3K pathway by TC21 can be inhibited by mutations in the effector domain of TC21. The T46S and E48G point mutations in the effector domain of TC21 interfere with binding of TC21 to PI-3K, the generation of 3′ phosphoinositides, and the activation of Akt/PKB in vivo, further indicating the requirement for the direct interaction of TC21 with PI-3K for the activation of this pathway by TC21. Equivalent mutations in H-Ras also interfere with activation of the PI-3K pathway by this oncogene (37).
Activation of the PI-3K pathway appears to be central to various facets of cellular transformation by TC21 or H-Ras oncogenes in NIH 3T3 cells, including DNA synthesis, cellular morphology, and protection from cell death resulting from loss of adherence, known as anoikis (Fig. 5A and data not shown). The TC21-induced morphological alteration of PC12 pheochromocytoma cells is also dependent on activation of PI-3K (Fig. 5B). This unique phenotype does not appear to be dependent on activation of the Ral or the MAP kinase pathway since inhibition of either pathway (by expression of the RalBD or by exposure to the MEK inhibitor UO126) has no effect on the TC21-induced morphology (data not shown). Previous work has demonstrated that activation of the MAP kinase pathway is central to the induction of neurites in these cells (7). However, the failure of TC21-expressing cells to produce neurites is not due to a lower activation of the Raf/MAP kinase pathway (39), since coexpression of activated MEK1 with V23 TC21 in PC12 cells does not induce neurites (data not shown).
Association of TC21 with RalGEFs and the role of TC21-induced Ral activation in transformation.
Apart from the Akt/PI-3K pathway, we also observed the constitutive activation of the Ral pathway in TC21-transformed cells and the activation of endogenous Ral A upon transient transfection of TC21. This activation requires an intact effector domain since certain mutations in this region of TC21 abolish activation of Ral A by TC21.
A possible mechanism for this activation was uncovered after identification in a yeast two-hybrid screen of RalGDS as a GTP-dependent binding partner for TC21 (28). We have expanded these initial observations to show that TC21 will interact not only with the prototypical member, RalGDS, but also with another Ral nucleotide exchange factor, Rlf. Rlf has only approximately 30% homology to other RalGEFs, with the homology lying for the most part in the catalytic and Ras binding domains (49). We show that interaction with TC21 results in the translocation of these cytosolic exchange factors to the plasma membrane. Consistent with these observations, previous work has shown that the posttranslational modification, and therefore plasma membrane localization of Ras and Ral proteins, is essential for the Ras-dependent activation of Ral (18, 26). Targeting RalGEFs to the plasma membrane by fusion to a Ras lipid modification CAAX box is sufficient for constitutive activation of this pathway (50). These observations suggest that the recruitment of cytosolic RalGEFs to the plasma membrane by Ras GTPases is a key event in Ral activation. Interaction with RalGEFs and plasma membrane translocation of RalGEFs are both inhibited by the T46S and Y51C mutations in the effector domain of TC21, mutations that also interfere with the ability of TC21 to activate endogenous Ral A, again arguing that activation of Ral by TC21 is achieved through the direct recruitment of RalGEFs to the plasma membrane. A further mutation in the effector domain, E48G, has no effect on the interaction of TC21 with RalGEFs, on the TC21-stimulated plasma membrane translocation of RalGEFs, or on the activation of Ral A by TC21. Equivalent mutations in H-Ras have similar effects on the ability of Ras to interact with RalGEFs (22, 37, 50), suggesting that TC21 and H-Ras may interact with RalGEFs in a similar fashion.
We have found that activation of the Ral pathway is required for TC21-induced stimulation of DNA synthesis but not for morphological alterations in human tumor cell lines harboring activating mutations in TC21 or in TC21-transformed NIH 3T3 cells. Earlier suggestions that the Ral pathway may be involved in the stimulation of DNA synthesis came from the observation that the RalGEF-binding H-Ras effector mutant (E37G) could stimulate DNA synthesis when expressed in thyrocytes (30). However, we found that activation of the Ral pathway is not sufficient for the induction of DNA synthesis or for transformation, since the E48G effector mutant of TC21 does not stimulate DNA synthesis in quiescent fibroblasts and alone is unable to transform cells (reference 39 and data not shown).
These studies, together with previous work (14, 15, 28, 31, 39), demonstrate that TC21 activates the same signal transduction pathways as the classical Ras proteins, although there may be quantitative differences in the magnitude of activation that could give rise to different biological outcomes. This is clearly seen in the PC12 cell system, where activated Ras induces neurite differentiation through a MAP kinase-dependent pathway whereas TC21 induces cell flattening through a PI-3K-dependent pathway. On the other hand, the ability of TC21 to activate similar pathways to those activated by classical Ras proteins also suggests that TC21 can perform many of the same functions as Ras. This conclusion is supported by the observation that while microinjection of the Ras-neutralizing antibody, Y13-259, into 14 of the 15 human tumor cell lines so far tested, blocks DNA synthesis (42; R. Wilson, H. Paterson, and C. J. Marshall, unpublished observations), microinjection of Y13-259 into two tumor cell lines containing activated TC21 has no effect (Fig. 10). Furthermore, Ras mutations are rare in the tumor types in which oncogenic mutations in TC21 have so far been found.
ACKNOWLEDGMENTS
We thank Mark Crompton for the CAL51 cell line and Andrew Chan (Mount Sinai) for the SK-UT-1 cell line. The PH domain Akt pEGFP C1 plasmid was a kind gift of Matilda Katan. We also thank Johannes Bos for the kind gifts of the full-length RalGDS cDNA, RalBP1 RalBD pGEX4T3, N28 Ral A pMT2-HA and full-length Rlf pMT2-HA plasmids, as well as for advice on the Ral pull-down assays.
This work was funded jointly by a Wellcome Prize Fellowship (M.R.) and a CRC project grant (C.J.M. and M.R.). C.J.M. is a Gibb life fellow of the Cancer Research Campaign.
REFERENCES
- 1.Aletta J M, Greene L A. Growth cone configuration and advance: a time-lapse study using video-enhanced differential interference contrast microscopy. J Neurosci. 1988;8:1425–1435. doi: 10.1523/JNEUROSCI.08-04-01425.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker K T, Crompton M R. Ras-related TC21 is activated by mutation in breast cancer cell line, but infrequently in breast carcinomas in vivo. Br J Cancer. 1998;78:296–300. doi: 10.1038/bjc.1998.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos J L. Ras-like GTPases. Biochim Biophys Acta. 1997;1333:M19–M31. doi: 10.1016/s0304-419x(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 4.Carboni J M, Yan N, Cox A D, Bustelo X R, Graham S M, Lynch M J, Weinmann R, Seizinger B R, Der C J, Barbacid M, Manne V. Farnesyltransferase inhibitors are inhibitors of Ras but not R-Ras2/TC21, transformation. Oncogene. 1995;10:1905–1913. [PubMed] [Google Scholar]
- 5.Chan A M L, Miki T, Kimberly A M, Aaronson S A. A human oncogene of the RAS superfamily unmasked by expression cDNA cloning. Proc Natl Acad Sci USA. 1994;91:77558–7562. doi: 10.1073/pnas.91.16.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark G J, Kinch M S, Gilmer T M, Burridge K, Der C J. Overexpression of the Ras-related TC21/R-Ras2 protein may contribute to the development of human breast cancers. Oncogene. 1996;12:169–176. [PubMed] [Google Scholar]
- 7.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 8.Cox A D, Brtva T R, Lowe D G, Der C J. R-Ras induces malignant, but not morphologic transformation of NIH 3T3 cells. Oncogene. 1994;9:3281–3288. [PubMed] [Google Scholar]
- 9.Datta K, Bellacosa A, Chan T O, Tsichlis P N. Akt is a direct target of the phosphatidylinositol 3-kinase: activation by growth factors, v-Src and v-Ha-Ras, in Sf9 and mammalian cells. J Biol Chem. 1996;271:30835–30839. doi: 10.1074/jbc.271.48.30835. [DOI] [PubMed] [Google Scholar]
- 10.Drivas G T, Shih A, Coutavas E, Rush M G, D'Eutsachio P. Characterization of four novel ras-like genes expressed in a human teratocarcinoma cell line. Mol Cell Biol. 1990;10:1793–1798. doi: 10.1128/mcb.10.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufourny B, Alblas J, van Teeffelen H A, van Schaik F M, van der Burg B, Steenbergh P H, Sussenbach J S. Mitogenic signalling of insulin-like growth factor I in MCF-7 human breast cancer cells requires phosphatidylinositol 3-kinase and is independent of mitogen-activated protein kinase. J Biol Chem. 1997;272:31163–31171. doi: 10.1074/jbc.272.49.31163. [DOI] [PubMed] [Google Scholar]
- 12.Gille H, Downward J. Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem. 1999;274:22033–22040. doi: 10.1074/jbc.274.31.22033. [DOI] [PubMed] [Google Scholar]
- 13.Gioanni J, Le François D, Zanghellini E, Mazeau C, Ettore F, Lambert J-C, Scneider M, Dutrillaux B. Establishment and characterisation of a new tumorigenic cell line with a normal karyotype derived from a human breast adenocarcinoma. Br J Cancer. 1990;62:8–13. doi: 10.1038/bjc.1990.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham S M, Cox A D, Drivas G, Rush M G, D'Eustachio P, Der C J. Aberrant function of the Ras-related protein TC21/R-Ras2 triggers malignant transformation. Mol Cell Biol. 1994;14:4108–4115. doi: 10.1128/mcb.14.6.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham S M, Oldham S M, Martin C B, Drugan J K, Zohn I E, Campbell S, Der C J. TC21 and Ras share indistinguishable transforming and differentiating activities. Oncogene. 1999;18:2107–2116. doi: 10.1038/sj.onc.1202517. [DOI] [PubMed] [Google Scholar]
- 16.Guerrero I, Wong H, Pellicer A, Burstein D E. Activated N-ras gene induces neuronal differentiation of PC12 rat pheochromocytoma cells. J Cell Physiol. 1986;129:71–76. doi: 10.1002/jcp.1041290111. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann C, Horn G, Spaargaren M, Wittinghofer A. Differential interaction of the ras family GTP-binding proteins H-Ras, RaplA, and R-Ras with the putative effector molecules Raf kinase and Ral-guanine nucleotide exchange factor. J Biol Chem. 1996;271:6794–6800. doi: 10.1074/jbc.271.12.6794. [DOI] [PubMed] [Google Scholar]
- 18.Hinoi T, Kishida S, Koyama S, Ikeda M, Matsuura Y, Kikuchi A. Posttranslational modifications of Ras and Ral are important for the action of Ral GDP dissociation stimulator. J Biol Chem. 1996;271:19710–19716. doi: 10.1074/jbc.271.33.19710. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Saez R, Chao L, Santos E, Aaronson S A, Chan A M-L. A novel insertional mutation in the TC21 gene activates its transforming activity in a human leiomyosarcoma cell line. Oncogene. 1995;11:1255–1260. [PubMed] [Google Scholar]
- 20.Jackson T R, Blader I J, Hammondsodie L P, Burga c R, Cooke F, Hawkins P T, Wolf A G, Heldman K A, Theibert A B. Initiation and maintenance of NGF-stimulated neurite outgrowth requires activation of a phosphoinositide 3-kinase. J Cell Sci. 1996;109:289–300. doi: 10.1242/jcs.109.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kauffman-Zeh A, RodriguezViciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 22.Khosravi Far R, White M A, Westwick J K, Solski P A, Chrzanowska Wodnicka M, Van Aeist L, Wigler M H, Der C J. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khwaja A, RodriguezViciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimmelman A, Tolkacheva T, Lorenzi M V, Osada M, Chan A M L. Identification and characterization of R-ras3: a novel member of the RAS gene family with a non-ubiquitous pattern of tissue distribution. Oncogene. 1997;15:2675–2685. doi: 10.1038/sj.onc.1201674. [DOI] [PubMed] [Google Scholar]
- 25.Kimmelman A C, Osada M, Chan A M. R-Ras3, a brain-specific Ras-related protein, activates Akt and promotes cell survival in PC12 cells. Oncogene. 2000;19:2014–2022. doi: 10.1038/sj.onc.1203530. [DOI] [PubMed] [Google Scholar]
- 26.Kishida S, Koyama S, Matsubara K, Kishida M, Matsuura Y, Kikuchi A. Colocalization of Ras and Ral on the membrane is required for Ras-dependent Ral activation through Ral GDP dissociation stimulator. Oncogene. 1997;15:2899–2907. doi: 10.1038/sj.onc.1201473. [DOI] [PubMed] [Google Scholar]
- 27.Kotani K, Yonezawa K, Hara K, Ueda H, Kitamura Y, Sakaue H, Ando A, Chavanieu A, Calas B, Grigorescu F, et al. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994;13:2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez Barahona M, Bustelo X, Barbacid M. The TC21 oncoprotein interacts with the Ral guanosine nucleotide dissociation factor. Oncogene. 1996;12:463–470. [PubMed] [Google Scholar]
- 29.Marte B M, RodriguezViciana P, Wennstrom S, Warne P H, Downward J. R-Ras can activate the phosphoinositide 3-kinase but not the MAP kinase arm of the Ras effector pathways. Curr Biol. 1997;7:63–70. doi: 10.1016/s0960-9822(06)00028-5. [DOI] [PubMed] [Google Scholar]
- 30.Miller M J, Prigent S, Kupperman E, Rioux L, Park S H, Feramisco J R, White M A, Rutkowski J L, Meinkoth J L. RalGDS functions in Ras- and cAMP-mediated growth stimulation. J Biol Chem. 1997;272:5600–5605. doi: 10.1074/jbc.272.9.5600. [DOI] [PubMed] [Google Scholar]
- 31.Movilla N, Crespo P, Bustelo X R. Signal transduction elements of TC21, an oncogenic member of the R-Ras subfamily of GTP-binding proteins. Oncogene. 1999;18:5860–5869. doi: 10.1038/sj.onc.1202968. [DOI] [PubMed] [Google Scholar]
- 32.Nobes C D, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- 33.Quilliam L A, Castro A F, Rogers-Graham K S, Martin C B, Der C J, Bi C. M-Ras/R-Ras3, a transforming ras protein regulated by Sos1, GRF1, and p120 Ras GTPase-activating protein, interacts with the putative Ras effector AF6. J Biol Chem. 1999;274:23850–23857. doi: 10.1074/jbc.274.34.23850. [DOI] [PubMed] [Google Scholar]
- 34.Reif K, Nobes C D, Thomas G, Hall A, Cantrell D A. Phosphatidylinositol 3-kinase signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- 35.Rey I, Taylor-Harris P, van Erp H, Hall A. R-ras interacts with rasGAP, neurofibromin and c-raf but does not regulate cell growth or differentiation. Oncogene. 1994;9:685–692. [PubMed] [Google Scholar]
- 36.RodriguezViciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 37.RodriguezViciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 38.RodriguezViciana P, Warne P H, Vanhaesebroeck B, Waterfield M D, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- 39.Rosário M, Paterson H F, Marshall C J. Activation of the Raf/MAP kinase cascade by the Ras-related protein TC21 is required for the TC21-mediated transformation of NIH 3T3 cells. EMBO J. 1999;18:1270–1279. doi: 10.1093/emboj/18.5.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubio I, RodriguezViciana P, Downward J, Wetzker R. Interaction of Ras with phosphoinositide 3-kinase gamma. Biochem J. 1997;326:891–895. doi: 10.1042/bj3260891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spaargaren M, Bischof J R. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras and Rap. Proc Natl Acad Sci USA. 1994;91:12609–12613. doi: 10.1073/pnas.91.26.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stacey D W, De Gudicibus S R, Smith M R. Cellular ras activity and tumor cell proliferation. Exp Cell Res. 1987;171:232–242. doi: 10.1016/0014-4827(87)90266-7. [DOI] [PubMed] [Google Scholar]
- 43.Urano T, Emkey R, Feig L A. Ral-GTPases mediate a distinct downstream signalling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15:810–816. [PMC free article] [PubMed] [Google Scholar]
- 44.Walker E H, Perisic O, Ried C, Stephens L, Williams R L. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature. 1999;402:313–320. doi: 10.1038/46319. [DOI] [PubMed] [Google Scholar]
- 45.Watton S J, Downward J. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr Biol. 1999;9:433–436. doi: 10.1016/s0960-9822(99)80192-4. [DOI] [PubMed] [Google Scholar]
- 46.Wennstrom S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 47.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M. Multiple ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 48.White M A, Vale T, Camonis J H, Schaefer E, Wigler M H. A role for the Ral guanine-nucleotide dissociation stimulator in mediating Ras-induced transformation. J Biol Chem. 1996;271:16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- 49.Wolthuis R M, Bauer B, van't Veer L J, de Vries Smits A M, Cool R H, Spaargaren M, Wittinghofer A, Burgering B M, Bos J L. RalGDS-like factor (Rlf) is a novel Ras and Rap 1A-associating protein. Oncogene. 1996;13:353–362. [PubMed] [Google Scholar]
- 50.Wolthuis R M, de Ruiter N D, Cool R H, Bos J L. Stimulation of gene induction and cell growth by the Ras effector Rlf. EMBO J. 1997;16:6748–6761. doi: 10.1093/emboj/16.22.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolthuis R M, Franke B, van Triest M, Bauer B, Cool R H, Camonis J H, Akkerman J W, Bos J L. Activation of the small GTPase Ral in platelets. Mol Cell Biol. 1998;18:2486–2491. doi: 10.1128/mcb.18.5.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao R J, Cooper G M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]