Abstract
The polymorphism of the thymidine kinase (TK) gene of herpes simplex virus type 1 (HSV-1) was analyzed and was compared with the restriction fragment length polymorphism (RFLP) of the whole genome to evaluate the relative efficiency of the TK gene as a potential probe for identification and discrimination of HSV-1. The effectiveness of using the polymorphism of the TK gene in classifying HSV-1 strains was comparable to that of RFLP analysis of 66 sites, suggesting that TK gene sequencing may have important applications in epidemiological studies of HSV-1.
Several studies have examined genomic variation in herpes simplex virus (HSV) type 1 (HSV-1) species by restriction fragment length polymorphism (RFLP) analysis (1, 11). The results of such genomic analysis demonstrated various epidemiological applications, including identification of the infectious route in some clinical cases (6, 12; B. Roizman and M. Tognon, Letter, Lancet i:677, 1982). However, the RFLP assay is relatively difficult and troublesome, as it requires the identification of numerous DNA fragments that range in size from a few hundred base pairs to over 10 kbp and that must be analyzed on a single gel. We considered DNA sequencing to be an easier method of studying the genomic variation of HSV-1, since recent developments in PCR and with automatic sequencers have vastly improved DNA sequencing techniques in terms of the ease of use as well as efficiency and cost-effectiveness.
The thymidine kinase (TK) of HSV-1 is one of the key enzymes in the determination of susceptibility to acyclovir (ACV), which is widely used for the treatment of HSV infections (4, 5, 7, 17). The frequency of nucleotide substitutions per 1 kb of the TK gene was 2.5 to 4.3 times higher than those of the genes for three other enzymes, DNase, protein kinase (UL13), and virion host shutoff protein (UL41), of HSV-1 (2); and the average number of nucleotide differences in the TK genes of Japanese isolates was 3.3 per 1,131 bp (9). This value was comparable to that estimated from the results of RFLP assay of the whole genome, including open reading frames and noncoding regions (14), suggesting that the HSV-1 TK gene is a relatively highly polymorphic gene. On the basis of these results, we compared the nucleotide sequence polymorphism of the TK gene and the RFLP of the genomic DNA of HSV-1 to evaluate the relative efficiency of the TK gene as a target for detailed epidemiological study of HSV-1.
Virus strains.
For this study, we used 63 clinical isolates of HSV-1 from epidemiologically unrelated Japanese patients, comprising 41 patients with herpetic keratitis, 19 patients with herpetic dermatitis, 2 patients with genital herpes infection, and 1 patient with herpes simplex encephalomeningitis. In order to exclude TK mutants, which are inappropriate for polymorphism analysis of TK genes, the susceptibilities of the HSV-1 strains to ACV were determined by a plaque reduction assay with Vero cells. Plaque formation by the 63 isolates tested was completely inhibited by 5 μg of ACV per ml; therefore, we concluded that all isolates were susceptible to ACV and encoded wild-type TK (3).
Sequences of the TK genes of the isolates.
The nucleotide sequences of the TK genes of the 63 HSV-1 isolates were determined by a PCR-directed sequencing method with primers that have been described previously (15). While there were no deletions or insertions, nucleotide substitutions at 38 positions that resulted in amino acid substitutions at 19 codons were observed in the TK genes analyzed. The 63 isolates were classified into 25 groups in accordance with the nucleotide sequences of their TK genes. Of the 25 groups, 17 groups included only 1 isolate, and the most common sequence was observed in 15 isolates and the second most common sequence was observed in 11 isolates. The average number of nucleotide substitutions in the TK gene was 4.3 per 1,131 bp for the Japanese isolates, and the value of nucleotide diversity was 0.0038 (4.3 of 1,131 bp). This value was very close to the nucleotide diversity value for the whole HSV-1 genome (0.0037), estimated from the results of an RFLP assay with Japanese isolates (9, 14).
Comparison of TK gene polymorphism and RFLP.
The effectiveness of the polymorphism of the TK gene for its use as a probe for the identification and discrimination of HSV-1 strains was evaluated by comparison of the TK gene polymorphism with the RFLP of the whole genome. Eleven pairs of primers were designed (Table 1) and were then used for PCR amplification of about 85% of a long unique region. Each amplified fragment was digested with BamHI, KpnI, and SalI and was electrophoretically analyzed on a 1.0% agarose gel. The numbers of restriction enzyme cleavage sites and groups classified by RFLP analysis are summarized in Table 2. Use of a combination of the three restriction enzymes classified 63 isolates into 33 groups, with the largest group consisting of 13 isolates (21% of 63 isolates), the second largest group consisting of 7 isolates (11%), and the third largest group consisting of 6 isolates (10%). The largest group showed an RFLP pattern identical to that of genotype F1, which is one of the two predominant genotypes in Japan (22%), and the RFLP pattern of the third largest group was similar to that of genotype F35, which is the other major genotype (9.4%) (16). This indicated that the results obtained for the 63 isolates might reflect the average for strains from Japanese individuals.
TABLE 1.
Primers used in RFLP assay of the whole HSV-1 genome
| Primer name | Position and directiona | Sequence |
|---|---|---|
| f1-5′ | 13681→13700 | 5′-GCTGCTGTTTGCTGTGCACC-3′ |
| f1-3′ | 24720→24700 | 5′-GGACGGTGATAGTAACGGGAT-3′ |
| f2-5′ | 21426→21445 | 5′-GTAGTGGTCATAGATCCGCC-3′ |
| f2-3′ | 30073→30052 | 5′-CCATGCGAAAGAAAAGAGGACT-3′ |
| f3-5′ | 26198→26220 | 5′-CATAGCTGGCAGTCGGCCTGGTT-3′ |
| f3-3′ | 36063→36041 | 5′-TGGATCGGCGCCATGCTGGCGGA-3′ |
| f4-5′ | 40249→40269 | 5′-GCCCCTCGTTCATGTAGGCCA-3′ |
| f4-3′ | 50543→50514 | 5′-GGAATGAACCCCAGACATAAAAAGTACAAC-3′ |
| f5-5′ | 48804→48825 | 5′-CTCTCGCATATGGACCCGTACT-3′ |
| f5-3′ | 61519→61496 | 5′-GGAGGCTCGGACAAGGTAACCATA-3′ |
| f6-5′ | 58080→58100 | 5′-AACACATAGGTCTGCACCTGC-3′ |
| f6-3′ | 66515→66494 | 5′-CTCATGCTAGAGTATCAAAGGC-3′ |
| f7-5′ | 67087→67106 | 5′-AGACAGAAGGGCAGCGAGAC-3′ |
| f7-3′ | 77198→77179 | 5′-CAGTACCGAGATGCCCTGGA-3′ |
| f8-5′ | 79157→79179 | 5′-TGGATTTCTTCTTGGTTTTGGCA-3′ |
| f8-3′ | 90829→90808 | 5′-ACAGTGGCTTCATGTGGATAAG-3′ |
| f9-5′ | 91990→92013 | 5′-GAGGTCAGTGTCCGTGGTGTACAC-3′ |
| f9-3′ | 97966→97944 | 5′-GATCGACCAAGGATGACCTGAGG-3′ |
| f10-5′ | 96300→96319 | 5′-AGGCGTCGGCATGGCCCCGG-3′ |
| f10-3′ | 104170→104147 | 5′-CTCCCGCTGGTGCGCAGCGCGGCT-3′ |
| f11-5′ | 106465→106484 | 5′-GTGGAGAGGTCGAGCGATAG-3′ |
| f11-3′ | 116848→116827 | 5′-TACGATAGCTTGTCTGGTAGGA-3′ |
TABLE 2.
Classification of 63 HSV-1 isolates by RFLP analysis with BamHI, KpnI, and SalI
| Enzyme analyzed by RFLP analysis | No. of restriction sites tested | No. of groups classified by:
|
|
|---|---|---|---|
| RFLP | RFLP with TK gene polymorphism | ||
| BamHI | 29 | 10 | 31 |
| KpnI | 25 | 9 | 31 |
| SalI | 38 | 18 | 35 |
| BamHI + KpnI | 54 | 17 | 36 |
| BamHI + SalI | 67 | 27 | 39 |
| KpnI + SalI | 63 | 26 | 38 |
| Combination of all three restriction enzymes (REs) | 92 | 33 | 41 |
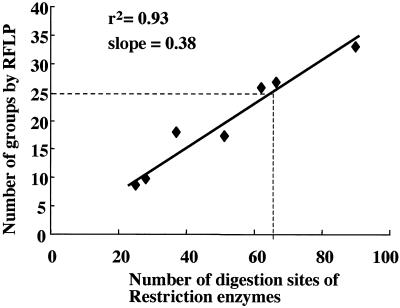
The numbers of sites analyzed and groups classified by RFLP analysis showed a good linear relationship (r2 = 0.93, slope = 0.38; Fig. 1). According to this regression curve, the classification of 63 isolates into 25 groups, which was the number of groups obtained by use of the TK gene sequence, required the analysis of 66 sites by RFLP analysis. The combination of TK gene polymorphism and RFLP allowed a more detailed classification of the 63 isolates, such as their further subclassification into 41 groups by use of the three restriction enzymes and the TK gene sequence (Table 2). Analysis of the correlation between the TK gene polymorphism and RFLP of the whole genome revealed a low level of correlation between these two parameters (correlation coefficient, 0.48), which explains the effective classification of strains according to their combination described above.
FIG. 1.
Correlation between the number of tested restriction enzyme cleavage sites and groups classified by RFLP assay of 63 Japanese isolates. The broken line indicates the classification capability of the polymorphism in the TK gene sequence that corresponds to that of the RFLP of the whole genome.
Epidemiological analysis by the use of RFLP is a common and established method for the analysis of microorganisms. However, a study on the interlaboratory reproducibility of typing of Mycobacterium tuberculosis strains by RFLP analysis reported large discrepancies in the quality of RFLP assays among laboratories due to differences in resolution, use of reference markers, and use of computer-assisted analysis (8). In contrast, DNA sequencing is a more objective and accurate method, and the sequence data can be registered in a database like GenBank. By consideration of these merits, DNA sequencing of useful target genes could become a convenient method of epidemiological analysis.
We chose the TK gene as a target for epidemiological testing of HSV-1, after considering not only the polymorphism of the gene but also the importance of screening for clinical TK mutants. Analysis of the nucleotide sequences of the TK gene in serial isolates from a patient could reveal the appearance of an ACV-resistant mutant and its origin (13). Moreover, in order to monitor the transmission of ACV-resistant mutants between individuals, large-scale sequence analysis of the TK genes from clinical isolates is required.
Conclusion.
We have shown that sequence analysis of the HSV-1 TK gene, on the basis of its polymorphism, is an easy-to-use and effective method that is comparable to RFLP analysis for the identification and discrimination of HSV-1 strains.
Nucleotide sequence accession numbers.
The nucleotide sequences of the HSV-1 TK genes have been submitted to the DDBJ database and have been assigned accession no. AB032866 to AB032890.
Acknowledgments
This work was supported by a grant from the Charitable Trust Clinical Pathology Research Foundation of Japan in 1999.
REFERENCES
- 1.Buchman T G, Simpson T, Nosal C, Roizman B, Nahmias A J. The structure of herpes simplex virus DNA and its application to molecular epidemiology. Ann N Y Acad Sci. 1980;354:279–290. doi: 10.1111/j.1749-6632.1980.tb27972.x. [DOI] [PubMed] [Google Scholar]
- 2.Chiba A, Suzutani T, Saijo M, Koyano S, Azuma M. Analysis of nucleotide sequence variations in herpes simplex virus types 1 and 2, and varicella-zoster virus. Acta Virol. 1998;42:401–407. [PubMed] [Google Scholar]
- 3.Collins P, Ellis M N. Sensitivity monitoring of clinical isolates of herpes simplex virus to acyclovir. J Med Virol. 1993;Suppl. 1:58–66. doi: 10.1002/jmv.1890410512. [DOI] [PubMed] [Google Scholar]
- 4.Elion G B, Furman P A, Fyfe J A, de Miranda P, Beauchamp L, Schaeffer H J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine. Proc Natl Acad Sci USA. 1977;74:5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fyfe J A, Keller P M, Furman P A, Miller R L, Elion G B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978;253:8721–8727. [PubMed] [Google Scholar]
- 6.Hammerberg O, Watts J, Chernesky M, Luchsinger I, Rawls W. An outbreak of herpes simplex virus type 1 in an intensive care nursery. Pediatr Infect Dis. 1983;2:290–294. doi: 10.1097/00006454-198307000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Keller P M, Fyfe J A, Beauchamp L, Lubbers C M, Furman P A, Schaeffer H J, Elion G B. Enzymatic phosphorylation of acyclic nucleoside analogs and correlations with antiherpetic activities. Biochem Pharmacol. 1981;30:3071–3077. doi: 10.1016/0006-2952(81)90495-0. [DOI] [PubMed] [Google Scholar]
- 8.Kremer K, van Soolingen D, Frothingham R, Haas W H, Hermans P W, Martin C, Palittapongarnpim P, Plikaytis B B, Riley L W, Yakrus M A, Musser J M, van Embden J D. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol. 1999;37:2607–2618. doi: 10.1128/jcm.37.8.2607-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudo E, Shiota H, Naito T, Satake K, Itakura M. Polymorphisms of thymidine kinase gene in herpes simplex virus type 1: analysis of clinical isolates from herpetic keratitis patients and laboratory strains. J Med Virol. 1998;56:151–158. doi: 10.1002/(sici)1096-9071(199810)56:2<151::aid-jmv9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 11.Roizman B, Buchman T. The molecular epidemiology of herpes simplex viruses. Hosp Pract. 1979;14:95–104. doi: 10.1080/21548331.1979.11707470. [DOI] [PubMed] [Google Scholar]
- 12.Roizman B, Tognon M. Restriction endonuclease patterns of herpes simplex virus DNA: application to diagnosis and molecular epidemiology. Curr Top Microbiol Immunol. 1983;104:273–286. doi: 10.1007/978-3-642-68949-9_17. [DOI] [PubMed] [Google Scholar]
- 13.Saijo M, Suzutani T, Itoh K, Hirano Y, Murono K, Nagamine M, Mizuta K, Niikura M, Morikawa S. Nucleotide sequence of thymidine kinase gene of sequential acyclovir-resistant herpes simplex virus type 1 isolates recovered from a child with Wiskott-Aldrich syndrome: evidence for reactivation of acyclovir-resistant herpes simplex virus y. J Med Virol. 1999;58:387–393. [PubMed] [Google Scholar]
- 14.Sakaoka H, Kurita K, Iida Y, Takada S, Umene K, Kim Y T, Ren C S, Nahmias A J. Quantitative analysis of genomic polymorphism of herpes simplex virus type 1 strains from six countries: studies of molecular evolution and molecular epidemiology of the virus. J Gen Virol. 1994;75:513–527. doi: 10.1099/0022-1317-75-3-513. [DOI] [PubMed] [Google Scholar]
- 15.Suzutani T, Koyano S, Takada M, Yoshida I, Azuma M. Analysis of the relationship between cellular thymidine kinase activity and virulence of thymidine kinase-negative herpes simplex virus types 1 and 2. Microbiol Immunol. 1995;39:787–794. doi: 10.1111/j.1348-0421.1995.tb03271.x. [DOI] [PubMed] [Google Scholar]
- 16.Umene K, Yoshida M. Genomic characterization of two predominant genotypes of herpes simplex virus type 1. Arch Virol. 1993;131:29–46. doi: 10.1007/BF01379078. [DOI] [PubMed] [Google Scholar]
- 17.Whitley R J, Gnann J W., Jr Acyclovir: a decade later. N Engl J Med. 1992;327:782–789. doi: 10.1056/NEJM199209103271108. . (Errata, 328:671, 1993; 337:1703, 1997.) [DOI] [PubMed] [Google Scholar]