Abstract
Chronic rhinosinusitis (CRS) is a significant health problem. It affects 5–12% of the general population. The causes that underlie the onset of CRS are not yet well known. However, many factors may contribute to its onset, such as environmental factors and the host’s general condition. Medical treatment mainly uses local corticosteroids, nasal irrigation, and antibiotics. In recent years, a new therapeutic approach that employs the use of probiotics emerged. Probiotics have been extensively studied as a therapy for dysbiosis and inflammatory pathologies of various parts of the body. We aimed to examine the studies in vivo and in vitro and clinicals reports in the existing literature to update probiotics’ role in rhinosinusitis chronic medical treatment.
Keywords: chronic rhinosinusitis, probiotics, microbiome, nasal microbiota, microbiome therapy
1. Introduction
Chronic rhinosinusitis (CRS) is a significant health problem affecting 5–12% of the general population [1]. Furthermore, it is referred to as chronic when the inflammatory process persists for more than 12 consecutive weeks [2]. The investigation conducted by the Global Allergy and Asthma European Network (GALEN) in 2011 concluded that the prevalence of CRS in Europe amounts to 10.9%—between 6.9% and 27.1% in different European cities [3]. This pathology negatively impacts patients’ quality of life. Therefore, it must be correctly identified and treated.
The causes that underlie the onset of CRS are not yet well known. However, many factors may contribute to its onset, such as environmental factors (temperature, humidity, and air pollution) and the host’s general condition (anatomical variants, allergies, local or systemic immune system imbalance, and genetic predisposition) [4,5]. Medical treatment mainly uses local corticosteroids, nasal irrigation, and antibiotics; however, there is a scarcity of information in the literature about the use of nasal spray medical formulations for local treatment of CRS [6]. If medical treatment is insufficient, endoscopic sinus surgery is proposed. In recent years, a new therapeutic approach that employs the use of probiotics has emerged [7]. The World Health Organization (WHO) defines probiotics as products containing live microorganisms that, when administered in the right amounts, have a beneficial effect on the host’s health [8]. Probiotics have been extensively studied as a therapy for dysbiosis (imbalance of the microbial flora of a given habitat, or alteration of its composition or function) and inflammatory pathologies of various parts of the body (the digestive system and upper and lower airways) [9,10,11,12,13]. Studies conducted to evaluate the effect of probiotics on CRS are still few in number. Furthermore, there are concerns regarding experimental studies on animal models, including in vitro and clinical studies. We aimed to examine the studies in the existing literature to update the role of probiotics in rhinosinusitis chronic medical treatment.
2. Microbiota
A microbiota is described as a community of microorganisms that resides in a distinct environment, and the collection of entire genomic elements of a distinct microbiota is the microbiome [14]. In the various microenvironments of our organism, 10–100 trillion colonies of microorganisms coexist synergistically, constituting a commensal microbiome capable of coexisting within our organism and defending it from insults coming from the external environment. Since childhood, the microbiota composition tends to change to adapt to various internal and external stimuli during the normal physiological growth of tissues [15]. Humans acquire significant quantities of microbiota from the mother during birth by natural means [16]. This microbiota changes within the first three years of life and then becomes more stable, but is always subject to minor changes in the later stages of life [17].
Each site of the organism is colonized by various microorganisms (microbiota), characterizing the inhabited site, which is not constant but variable according to the subject’s age. With their complex and tortuous anatomy, the nasal cavities define anatomical spaces where we find different microbiota. Consequently, in the nasal vestibule—the nasal cavities and the paranasal sinuses—there exist diverse microbiota residents.
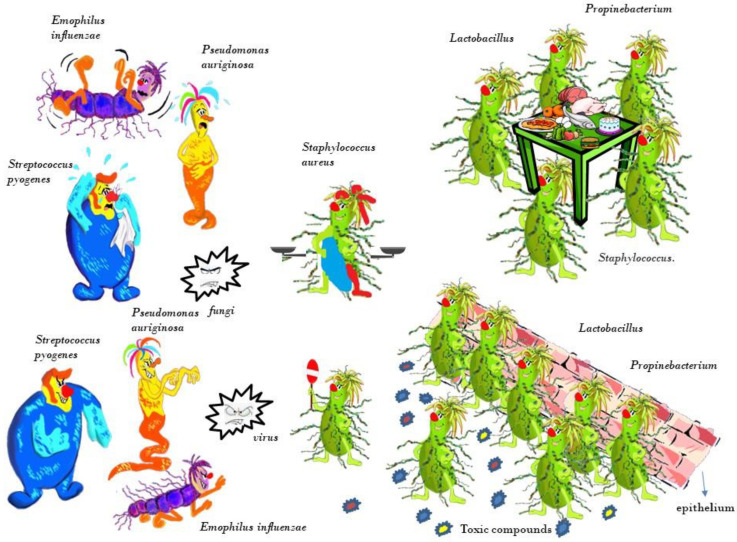
Resident commensal bacteria are involved in the homeostasis of the nasal microbiota, controlling and suppressing opportunistic pathogens, through a competition mechanism for spaces and nutrients. Additionally, products with toxic compounds inhibit or directly kill competing microorganisms (lactic acid, antibacterial peptides, and hydrogen peroxide; Figure 1).
Figure 1.
Resident commensal bacteria are involved in the homeostasis of the nasal microbiota, controlling and suppressing opportunistic pathogens, through a competition mechanism for spaces and nutrients. Additionally, products with toxic compounds inhibit or directly kill competing microorganisms (lactic acid, antibacterial peptides, and hydrogen peroxide). Opportunistic bacteria (see Staphylococcus aureus) retain the ability to act as commensals or pathogens.
The commensal bacteria are also an essential part of the nasal flora, and contribute to maintaining this district’s physiological and immune functions. Several studies show that failure balancing and dysbiosis [18] between the microorganisms (due to the use of antimicrobials, lifestyle factors, and factors dietary) determine an increase in opportunistic pathogens, favoring an alteration of the immune functions and increased infectious processes. The dysmicrobism of the upper respiratory tract can cause chronic inflammation of the airways, resulting in the spread of microorganisms in the various sections of the airways, thus generating pathological conditions, such as rhinosinusitis, chronic otitis media, asthma, and the worsening of the course of allergic rhinitis [19,20].
3. Nasal Microbiota
Recent studies have shown that microorganisms that make up the microbiota of the nasopharynx are different to those present in paranasal sinuses. Therefore, it is evident that there are several varieties of microbiota in the various sections of the upper respiratory tract [15].
As evidenced by Yan M. et al. [21], the nasal cavities constitute a transition zone between the external environment exposed to constant insults and the protected internal environment. The nose is an environment deficient in nutrients which is acidic and salty. The higher the acidity and salinity (nostrils), the greater the difficulty of microbial colonization. The nasal mucus, also containing small amounts of nutrients, restricts microbial growth, and, as such, only particular species and microbes have adapted to this environment. In nasal microbiota, phyla Actinobacteria (Corynebacterium and Cutibacterium), Firmicutes (Staphylococcus, Streptococcus, Dolosigranulum, and Lactobacillus), and Proteobacteria (Moraxella and Haemophilus), whose abundance varies depending on the portion of the nose, are commonly identified (Table 1). Among the phyla present in the nasal cavities, Actinobacteria are the predominant phyla, and are present at all stages of life [22]. Furthermore, they display patterns of microorganisms that tend to change over time. The growth of the individual (typically Proteobacteria) and more stable patterns (Staphylococcus epidermidis of the species Firmicutes) tend to be maintained over time.
Table 1.
Commensal bacteria, represented genera in the different sites of the nose.
| Sites of the Nose | Commensal Bacteria |
|---|---|
| Vestibules | Corynebacterium, Propionibavterium, Staphylococcus |
| Nasal cavities | Staphylococcus, Corynebacterium, Dolosigranulum |
| Nasopharynx | Moraxella, Streptococcus, Fusobacterium, Haemophilus |
The commensal bacteria represent the majority of the bacteria present in the nasal cavities. Additionally, there are opportunistic bacteria. Opportunistic bacteria retain the ability to act as commensals or pathogens. However, this depends on the integrity of the bacterial flora of the environment where they are found. For example, Staphylococcus aureus can become pathogenic following an alteration to the microbiome due to antibiotic therapies, pharmacological immunosuppression, or radiant therapies.
Staphylococcus aureus [23] colonizes approximately 30% of the human population asymptomatically in the nostrils, transiently or persistently. Therefore, it can be considered a human commensal [24].
4. Microbiota and Rhinosinusitis
CRS is a common and widespread inflammatory disease of the upper respiratory tract that significantly impacts the social aspect of life. It worsens the quality of life in everyday life and society, as well as negatively affecting public health costs.
The nasal microbiome related to CRS has been analyzed in different studies, which revealed a frequent presence of coagulase-negative Staphylococcus, Pseudomonas, and Staphylococcus aureus [25]. A recent study [26,27] analyzed the microbiome of patients with nasal polyps by comparing it to those of patients without nasal polyposis. The study established a prevalence of Streptococcus, Haemophilus, and Fusobacterium in patients without nasal polyps versus a predominance of Staphylococcus, Alloiococcus, and Corynebacterium in patients with nasal polyposis.
In a recent study by De Boeck et al. [28], Dolosigranulum pigrum was clearly more associated with upper respiratory tract (URT) in healthy subjects, while Corynebacterium tuberculostearicum, Haemophilus influenzae/H. aegyptius, and Staphylococcus taxa were found to be more present in CRS patients. Understanding the mechanisms underlying the dysregulation of the nasal microbiome can be instrumental in both the clinical and post-operative evolution of patients with CRS. In patients with CRS, the microbiome has a reduced bacterial diversity, but a higher bacterial load.
Furthermore, less stable bacterial species replace more stable bacterial species (Propionibacterium acnes), favoring the colonization of potentially pathogenic bacteria [29,30]. The resulting dysbiosis could cause an alteration of the epithelial barrier, increasing its permeability to pathogens, followed by the release of inflammatory factors (cytokines and chemokines) with a consequent compromise of the immune system and chronic inflammation [31]. Moreover, some studies have demonstrated the presence of viruses and fungi in the mucosa of patients with CRS, which would contribute to increased adhesion of bacterial pathogens to the damaged mucosa [29,32,33,34,35]. Of great importance are the bacterial biofilms that are detected on the mucosa of patients with CRS. The development of a microbial biofilm is a complex process. Initially, sessile planktonic bacteria adhere to the mucosal surface and form microcolonies. Once they have taken root, the bacteria begin to proliferate and secrete an extracellular matrix composed of polysaccharides, nucleic acids, and proteins. This matrix protects the biofilm from harmful factors present in the environment. When bacterial density reaches a critical point, interbacterial cross-talk occurs, triggering a phenomenon known as “quorum sensing” or the “communication capacity of bacterial cells.” This phenomenon determines the biofilm phenotype that allows bacteria to communicate through small signal molecules to adapt to any change in the environment. The biofilm phenotype is morphologically characterized by the formation of microbial towers, composed of layers of live bacteria embedded within intermediate water channels. Bacteria in biofilms are more resistant to host defenses. The extracellular matrix that makes up most biofilm protects bacteria from antibodies, immune system phagocytosis, antibiotic penetration, and complement binding [36,37].
The biofilm may be pro-inflammatory through different mechanisms, including the release of planktonic organisms and the production of superantigens, which can cause ciliary dysfunction and the inhibition of mucociliary clearance [38].
The mucociliary clearance system represents a defense mechanism against inhaled particles. Therefore, its dysfunction favors the colonization of pathogenic bacteria and the establishment of inflammatory processes [39], contributing to the pathogenesis of CRS [30,40].
5. Probiotics
The WHO defines probiotics as products that contain living microorganisms that, when administered at the correct quantity, benefit the host’s health [8].
Probiotics should not be confused with prebiotics. Prebiotics are substances derived from foods that cannot be digested, whose beneficial effect on the host is their contribution to the growth, activity, or both of bacteria.
The products containing prebiotics and probiotics are referred to as symbiotic [41]. The mechanism of action of probiotics has been described mainly in the gastrointestinal system, and includes several strategies through which they inhibit the action of pathogenic microorganisms. Probiotics may induce the inhibition of adhesion of pathogens to the mucous membranes, the stabilization of tight junctions in the epithelial layer with a reduction in the permeability of the mucosa, the competitive inhibition of pathogens, modulation of the immune system, and the production of various substances toxic to pathogenic microorganisms [42]. In a 2018 review, Martens et al. [43] described the possible mechanisms of action of probiotics in the respiratory tract, focusing on the positive effect of probiotics on the epithelial barrier and the immune system. They described the action of probiotics in restoring the epithelial barrier through the modulation of tight junctions and adherence junctions and their role in modulating the host’s immune response through their interaction with dendritic cells. This promotes regulatory T-cells (Tregs) and downregulates T-helper 1 and T-helper 2. In studies evaluating potential probiotics, the ability to adhere to the nasal epithelium, that is, the ability to survive in aerobic conditions and at low temperatures, must be considered [44]. These conditions are necessary for the probiotics to compete with opportunistic bacteria such as Staphylococcus aureus.
6. Clinical Studies
6.1. Clinical Studies That Used Oral Administration of Probiotics
Habermann et al., in 2002 [45], conducted a double-blind, placebo-controlled, multicenter study on the efficacy of human Enterococcus faecalis (Symbioflor®1) in reducing the frequency of exacerbations of CRS in a sample of 157 patients. Half of the patients were treated with the oral administration of drops containing the probiotic for six months. The other half of the patients were treated with placebo also for six months. After eight months of follow-up, there were approximately half of the exacerbations in patients treated with human Enterococcus faecalis compared to patients treated with placebo.
In another prospective, randomized, double-blind, placebo-controlled trial, Mukerji et al. in 2009 [46] used the oral administration of a probiotic strain of Lactobacillus rhamnosus R0011 (500 million active cells in tablets, twice a day) for four weeks in a group of 38 patients with CRS; 39 CRS patients represented the placebo-treated control group. The authors used the SNOT-20 quality of life test to assess the effectiveness of the treatment. After four weeks, patients treated with the Lactobacillus probiotic reported a better quality of life than the control group. However, this benefit was not confirmed over time, as after eight weeks, there were no significant differences in the responses to the quality-of-life test between the two groups (Table 2).
Table 2.
Clinical studies on the treatment of CRS with probiotics—Oral administration.
| Author | Type of Study | Probiotic | N. Patients | Results |
|---|---|---|---|---|
| Habermann et al., 2002 | Multicenter, randomized, double blind, placebo controlled trial | Enterococcus faecalis | 157 | Reduction of CRS flare-ups |
| Mukerji et al., 2009 | prospective, randomized, double-blind, placebo-controlled trial | Lactobacillus rhamnosus | 77 | Transient improvement in the quality of life |
6.2. Clinical Studies That Used Local Administration of Probiotics
Martensson et al. in 2017 [47], in a randomized, double-blinded, crossover, and sham-controlled study, evaluated the effects of administration through a nasal spray of Honeybee lactic acid bacteria (LAB). Honeybee LAB, consisting of various Lactobacilli and Bifidus bacteria, was administered to 20 patients with CRSsNP for two weeks. The efficacy of the treatment was assessed by considering the trend of the symptoms through the use of the SNOT-22 questionnaire. The impact of the treatment on the microbiome and inflammation products (IL-6, IL-8, and TNF-9) was evaluated using the nasal wash fluid. The treatment proved to be well tolerated. However, it was not effective in reducing symptoms, nor did it affect the microbiota composition. There was no change in the inflammation processes.
Endam et al. [48] conducted a prospective open-label pilot trial of the safety and feasibility study. The authors aimed to verify if topical administration of Lactococcus lactis W 136 for 14 days to the nasal and sinus cavities would be safe for patients with CRS refractory to medical and surgical treatment. The evaluation of symptoms was performed with the SNOT-22 test.
Simultaneously, an endoscopy nasal was carried out to evaluate the conditions of the mucosa nasal and the UPSIT-40 test to detect the olfactory function. The treatment turned out to be well tolerated by all 24 patients, and was found to improve symptoms that remained 14 days after the end of the course of treatment with the probiotic, while the sense of smell remained stable (Table 3).
Table 3.
Clinical studies on the treatment of CRS with probiotics—Local administration.
| Author | Type of Study | Probiotic | N. Patients | Results |
|---|---|---|---|---|
| Martensson et al., 2017 | randomized, double-blinded, crossover, and sham-controlled trial | Honeybee lactic acid bacteria | 20 | Not effective |
| Endam et al., 2020 | Prospective open-label pilot trial of safety and feasibility | Lactococcus lactis | 24 | Transient improvement in CRS symptoms |
7. In Vivo and In Vitro Experimental Studies
In 2016, Schwartz et al. [49] evaluated the capacity of the two Gram-positive probiotics strains of Lactococcus lactis to stimulate the production of IL-10 and TNF on preparations of peripheral blood monocytes (PBMC). Furthermore, the authors assessed the safety of applying Lactococcus lactis on the mucosal cells of the paranasal sinuses of patients with and without rhinosinusitis. These in vitro studies have supported the safety and immunomodulatory capacities of Lactococcus lactis for intranasal use. The cultures of cells of the mucosa of the paranasal sinuses of patients with and without CRS showed no evidence of toxicity when exposed to the supernatant of this strain. Conversely, the preparations of peripheral blood monocytes showed the induction of IL-10 and TNF without evidence of toxicity or excessive Th1-type inflammation. The authors concluded by stating that topical nasal therapy could represent a new therapeutic strategy for patients with CRS. In an in vitro study, Cho et al. [50], in 2020, assessed the growth of six strains of Pseudomonas aeruginosa derived from patients with CRS (three patients with cystic fibrosis, and one patient with ciliary dyskinesia)—the first strain of Pseudomonas aeruginosa from the laboratory. These strains were co-cultured with Lactococcus lactis (obtained from commercial probiotic nasal washes) in the presence of mucin.
Many Pseudomonas aeruginosa strains were grown without Lactococcus lactis (control cases). No influence on the growth of Pseudomonas aeruginosa colonies was observed in cultures where Lactococcus lactis was present. The growth inhibition of Pseudomonas aeruginosa was observed only in one culture found to be contaminated with Stenotrophomonas maltophilia. The authors concluded that nasal lavage with probiotics (Lactococcus lactis) may not be helpful for all patients. Therefore, further experiments are needed to evaluate the interactions between Pseudomonas aeruginosa and Lactococcus lactis.
In 2012, Abreu et al. [51] conducted a study to determine whether Corynebacterium tuberculostearicum exhibited pathogenic potential and whether this could be affected by the resident microbiota. They developed a mouse model of sinus infection using goblet cell hyperplasia and mucin hypersecretion as markers of pathology.
Nasal inoculation of large numbers of Corynebacterium tuberculostearicum in the presence of a complete (healthy) sinus microbiota resulted in an increase in the number of mucin-secreting goblet cells. Animals treated with both an antibiotic (to reduce the bacterial load in the microbiota) and Corynebacterium tuberculostearicum showed profound goblet cell hyperplasia. To demonstrate that goblet cell hyperplasia and mucin hypersecretion were explicitly induced by Corynebacterium tuberculostearicum, they repeated the experiment by adding a group of antibiotic-treated murine models before nasal inoculation of Lactobacillus sakei, which is present in abundance in healthy mucosal samples and significantly reduced in CRS patients. Sinus mucosal histology demonstrated that the group treated with antibiotics and inoculated with Corynebacterium tuberculostearicum showed significant increases in goblet cell hyperplasia and mucin hypersecretion. However, mice that received identical numbers of Lactobacillus sakei demonstrated epithelial physiology comparable to that of control animals (no significant difference in the number of goblet cells), thus confirming that the observed sinus histopathology was explicitly due to Corynebacterium tuberculostearicum. Furthermore, species such as Lactobacillus sakei protect the epithelium of the rhino-sinus mucosa through competitive inhibition of Corynebacterium tuberculostearicum. In 2014, Cleland et al. [52] investigated the probiotic properties of Staphylococcus epidermidis against Staphylococcus aureus in murine sinusitis models.
They demonstrated that Staphylococcus epidermidis exerts a probiotic effect by producing a serine protease that inhibits biofilm production and Staphylococcus aureus colonization. Even in this case, the hypertrophy of muciparous cells and their hypersecretion as well as the characteristics of the CRS were considered markers of inflammation. This study showed that Staphylococcus epidermidis can be a potential probiotic, having induced, in a murine sinusitis model, reduced counts of goblet cells in a group of mice co-inoculated with Staphylococcus epidermidis + Staphylococcus aureus compared to those who receive only Staphylococcus aureus (Table 4).
Table 4.
In vitro and in vivo experimental studies on probiotics activity.
| Author | Type of Study | Probiotic | Conclusions |
|---|---|---|---|
| Schwartz et al., 2016 | In vitro study | Lactococcus lactis | Absence of cellular toxicity, induction of IL-10 and TNF |
| Cho et al., 2020 | In vitro study | Lactococcus lactis | Lactis nasal washes may not be helpful for all CRS patients |
| Abreu et al., 2012 | In vivo study (mouse) | Lactobacillus sakei | Treatment with L.sakei could counteract the action of C. tuberculostearicum |
| Cleland et al., 2014 | In vivo study (mouse) | Staphylococcus epidermidis | S. epidermidis inhibits the colonization of S. aureus |
8. Conclusions
In the literature, there are still few and conflicting studies on the efficacy of probiotics in acute inflammatory diseases of the upper airways and, in particular, in CRS. The studies available to date are also based on small sample sizes. Only three of the studies described above, of which two are in vivo and one in vitro, described a beneficial effect of treatment with probiotics on CRS. Animal studies highlight the ability of some probiotics to reduce the inflammatory phenomena of CRS on the mucosa. To the best of our knowledge, no other data in the literature can illustrate the long-term effect of probiotics on CRS. Further efforts will undoubtedly have to be made to evaluate the potential of probiotics on CRS. Indeed, the study of the microbiota of affected patients and bacterial biofilm will have to continue.
Author Contributions
M.R.B. writing, review editing; M.R. improved the manuscript; D.M.M. resources; M.A. carried out the data interpretation; S.P. and G.M. data curation; E.A. supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fokkens W.J., Lund V.J., Hopkins C., Hellings P.W., Kern R., Reitsma S., Toppila-Salmi S., Bernal-Sprekelsen M., Mullol J., Alobid I., et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020;58((Suppl. S29)):1–464. doi: 10.4193/Rhin20.401. [DOI] [PubMed] [Google Scholar]
- 2.Benninger M.S., Ferguson B.J., Hadley J.A., Hamilos D.L., Jacobs M., Kennedy D.W., Lanza D.C., Marple B.F., Osguthorpe J.D., Stankiewicz J.A., et al. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol. Head Neck Surg. 2003;129:S1–S32. doi: 10.1053/hn.2003.v128.amhn0312811. [DOI] [PubMed] [Google Scholar]
- 3.Hastan D., Fokkens W.J., Bachert C., Newson R.B., Bislimovska J., Bockelbrink A., Bousquet P.J., Brozek G., Bruno A., Dahlén S.E., et al. Chronic rhinosinusitis in Europe—An underestimated disease. A GA²LEN study. Allergy. 2011;66:1216–1223. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 4.Lam K., Schleimer R., Kern R.C. The etiology and pathogenesis of chronic rhinosinusitis: A review of current hypotheses. Curr. Allergy Asthma Rep. 2015;15:41. doi: 10.1007/s11882-015-0540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianco M.R., Sabatini U., Alessio C., Chimento I., Russo E., Allegra E. Role of anatomical variations in chronic non polipoid rhinosinusitis. Acta Med. Mediterr. 2021;37:1203–1208. [Google Scholar]
- 6.Casula E., Manca M.L., Manconi M. An integrative review on the uses of plant-derived bioactives formulated in conventional and innovative dosage forms for the local treatment of damaged nasal cavity. Int. J. Pharm. 2021;610:121229. doi: 10.1016/j.ijpharm.2021.121229. [DOI] [PubMed] [Google Scholar]
- 7.Ciprandi G., La Mantia I., Damiani V., Passali D. Local Bacteriotherapy—A promising preventive tool in recurrent respiratory infections. Expert Rev. Clin. Immunol. 2020;16:1047–1052. doi: 10.1080/1744666X.2021.1833720. [DOI] [PubMed] [Google Scholar]
- 8.Sanders M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008;46((Suppl. 2)) doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- 9.Ren J., Zhao Y., Huang S., Lv D., Yang F., Lou L., Zheng Y., Zhang J., Liu S., Zhang N., et al. Immunomodulatory effect of Bifidobacterium breve on experimental allergic rhinitis in BALB/c mice. Exp. Med. 2018;16:3996–4004. doi: 10.3892/etm.2018.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi S.P., Oh H.N., Choi C.Y., Ahn H., Yun H.S., Chung Y.M., Chun T. Oral administration of Lactobacillus plantarum CJLP133 and CJLP243 alleviates birch pollen-induced allergic rhinitis in mice. J. Appl. Microbiol. 2018;124:821–828. doi: 10.1111/jam.13635. [DOI] [PubMed] [Google Scholar]
- 11.Kim W.G., Kang G.D., Kim H.I., Han M.J., Kim D.H. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 alleviate allergic rhinitis in mice by restoringTh2/Treg imbalance and gut microbiota disturbance. Benef Microbes. 2019;10:55–67. doi: 10.3920/BM2017.0146. [DOI] [PubMed] [Google Scholar]
- 12.La Mantia I., Varricchio A., Ciprandi G. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a nasal spray for preventing recurrent acute otitis media in children: A real-life clinical experience. Int. J. Gen. Med. 2017;10:171–175. doi: 10.2147/IJGM.S137614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andaloro C., Santagati M., Stefani S., La Mantia I. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a oral spray for children with recurrent streptococcal pharyngotonsillitis: A randomized placebo-controlled clinical study. Eur. Arch. Otorhinolaryngol. 2019;276:879–887. doi: 10.1007/s00405-019-05346-3. [DOI] [PubMed] [Google Scholar]
- 14.Dekaboruah E., Suryavanshi M.V., Chettri D., Verma A.K. Human microbiome: An academic update on human body site specific surveillance and its possible role. Arch. Microbiol. 2020;202:2147–2167. doi: 10.1007/s00203-020-01931-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu D.M., Ma J., Prince A.L., Antony K.M., Seferovic M.D., Aagaard K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017;23:314–326. doi: 10.1038/nm.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominguez-Bello M.G., Godoy-Vitorino F., Knight R., Blaser M.J. Role of the microbiome in human development. Gut. 2019;68:1108–1114. doi: 10.1136/gutjnl-2018-317503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatt A.P., Redinbo M.R., Bultman S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017;67:326–344. doi: 10.3322/caac.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeGruttola A.K., Low D., Mizoguchi A., Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016;22:1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esposito S., Principi N. Impact of nasopharyngeal microbiota on the development of respiratory tract diseases. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:1–7. doi: 10.1007/s10096-017-3076-7. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y.J. Nasopharyngeal microbiota: Gatekeepers or fortune tellers of susceptibility to respiratory tract infections? Am. J. Respir Crit. Care Med. 2017;196:1504–1505. doi: 10.1164/rccm.201707-1470ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan M., Pamp S.J., Fukuyama J., Hwang P.H., Cho D.Y., Holmes S., Relman D.A. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe. 2013;14:631–640. doi: 10.1016/j.chom.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy B.L., Merrell D.S. Friend or Foe: Interbacterial Competition in the Nasal Cavity. J. Bacteriol. 2021;203:e00480-20. doi: 10.1128/JB.00480-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kluytmans J.A., Wertheim H.F. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection. 2005;33:3–8. doi: 10.1007/s15010-005-4012-9. [DOI] [PubMed] [Google Scholar]
- 24.Tong S.Y., Davis J.S., Eichenberger E., Holland T.L., Fowler V.G., Jr Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi E.B., Hong S.W., Kim D.K., Jeon S.G., Kim K.R., Cho S.H., Kim Y.K. Decreased diversity of nasal microbiota and their secreted extracellular vesicles in patients with chronic rhinosinusitis based on a metagenomic analysis. Allergy. 2014;69:517–526. doi: 10.1111/all.12374. [DOI] [PubMed] [Google Scholar]
- 26.Lal D., Keim P., Delisle J., Barker B., Rank M.A., Chia N., Cope E.K. Mapping and comparing bacterial microbiota in the sinonasal cavity of healthy, allergic rhinitis, and chronic rhinosinusitis subjects. Int. Forum Allergy Rhinol. 2017;7:561–569. doi: 10.1002/alr.21934. [DOI] [PubMed] [Google Scholar]
- 27.Dimitri-Pinheiro S., Soares R., Barata P. The Microbiome of the Nose—Friend or Foe? Allergy Rhinol. 2020;11:2152656720911605. doi: 10.1177/2152656720911605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Boeck I., Wittouck S., Martens K., Claes J., Jorissen M., Steelant B., Van den Broek M.F.L., Seys S.F., Hellings P.W., Vanderveken O.M., et al. Anterior nares diversity and pathobionts represent sinus microbiome in chronic rhinosinusitis. MSphere. 2019;4:e00532-19. doi: 10.1128/mSphere.00532-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivasubramaniam R., Douglas R. The microbiome and chronic rhinosinusitis. World J. Otorhinolaryngol. Head Neck Surg. 2018;4:216–221. doi: 10.1016/j.wjorl.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner Mackenzie B., Waite D.W., Hoggard M., Douglas R.G., Taylor M.W., Biswas K. Bacterial community collapse: A meta-analysis of the sinonasal microbiota in chronic rhinosinusitis. Environ. Microbiol. 2017;19:381–392. doi: 10.1111/1462-2920.13632. [DOI] [PubMed] [Google Scholar]
- 31.Yamanishi S., Pawankar R. Current advances on the microbiome and role of probiotics in upper airways disease. Curr. Opin. Allergy Clin. Immunol. 2020;20:30–35. doi: 10.1097/ACI.0000000000000604. [DOI] [PubMed] [Google Scholar]
- 32.Wood A.J., Antoszewska H., Fraser J., Douglas R.G. Is chronic rhinosinusitis caused by persistent respiratory virus infection? Int. Forum Allergy Rhinol. 2011;1:95–100. doi: 10.1002/alr.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y.C., Bassiouni A., Tanjararak K., Vreugde S., Wormald P.J., Psaltis A.J. Role of fungi in chronic rhinosinusitis through ITS sequencing. Laryngoscope. 2018;128:16–22. doi: 10.1002/lary.26702. [DOI] [PubMed] [Google Scholar]
- 34.Zhang I., Pletcher S.D., Goldberg A.N., Barker B.M., Cope E.K. Fungal microbiota in chronic airway inflammatory disease and emerging relationships with the host immune response. Front Microbiol. 2017;8:2477. doi: 10.3389/fmicb.2017.02477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gevers D., Knight R., Petrosino J.F., Huang K., McGuire A.L., Birren B.W., Nelson K.E., White O., Methé B.A., Huttenhower C. The Human Microbiome Project: A communityresource for the healthy human microbiome. PLoS Biol. 2012;10:e1001377. doi: 10.1371/journal.pbio.1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fastenberg J.H., Hsueh W.D., Mustafa A., Akbar N.A., Abuzeid W.M. Biofilms in chronic rhinosinusitis: Pathophysiology and therapeutic strategies. World J. Otorhinolaryngol. Head Neck Surg. 2016;2:219–229. doi: 10.1016/j.wjorl.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suh J.D., Cohen N.A., Palmer J.N. Biofilms in chronic rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck. Surg. 2010;18:27–31. doi: 10.1097/MOO.0b013e328334f670. [DOI] [PubMed] [Google Scholar]
- 38.Galli J., Calò L., Ardito F., Imperiali M., Bassotti E., Passali G.C., La Torre G., Paludetti G., Fadda G. Damage to ciliated epithelium in chronic rhinosinusitis: What is the role of bacterial biofilms. Ann. Otol. Rhinol. Laryngol. 2008;117:902–908. doi: 10.1177/000348940811701207. [DOI] [PubMed] [Google Scholar]
- 39.Ramakrishnan V.R., Feazel L.M., Gitomer S.A., Ir D., Robertson C.E., Frank D.N. The microbiome of the middle meatus in healthy adults. PLoS ONE. 2013;8:e85507. doi: 10.1371/journal.pone.0085507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Copeland E., Leonard K., Carney R., Kong J., Forer M., Naidoo Y., Oliver B.G.G., Seymour J.R., Woodcock S., Burke C.M., et al. Chronic rhinosinusitis: Potential role of microbial dysbiosis and recommendations for sampling sites. Front. Cell Infect. Microbiol. 2018;8:57. doi: 10.3389/fcimb.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gómez-López A. Microbioma, salud y enfermedad: Probióticos, prebióticos y simbióticos. Biomedica. 2019;39:617–621. [PMC free article] [PubMed] [Google Scholar]
- 42.Williams N.T. Probiotici. Am. J. Health Syst. Pharm. 2010;67:449–458. doi: 10.2146/ajhp090168. [DOI] [PubMed] [Google Scholar]
- 43.Martens K., Pugin B., De Boeck I., Spacova I., Steelant B., Seys S.F., Lebeer S., Hellings P.W. Probiotics for the airways: Potential to improve epithelial and immune homeostasis. Allergy. 2018;73:1954–1963. doi: 10.1111/all.13495. [DOI] [PubMed] [Google Scholar]
- 44.De Boeck I., Spacova I., Vanderveken O.M., Lebeer S. Lactic acid bacteria as probiotics for the nose? Microb. Biotechnol. 2021;14:859–869. doi: 10.1111/1751-7915.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Habermann W., Zimmermann K., Skarabis H., Kunze R., Rusch V. Reduction of acute recurrence in patients with chronic recurrent hypertrophic sinusitis by treatment with a bacterial immunostimulant (Enterococcus faecalis Bacteriae of human origin) Arzneim. Forsch. 2002;52:622–627. doi: 10.1055/s-0031-1299941. [DOI] [PubMed] [Google Scholar]
- 46.Mukerji S.S., Pynnonen M.A., Kim H.M., Singer A., Tabor M., Terrell J.E. Probiotics as adjunctive treatment for chronic rhinosinusitis: A randomized controlled trial. Otolaryngol. Head Neck Surg. 2009;140:202–208. doi: 10.1016/j.otohns.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 47.Mårtensson A., Abolhalaj M., Lindstedt M., Mårtensson A., Olofsson T.C., Vásquez A., Greiff L., Cervin A. Clinical efficacy of a topical lactic acid bacterial microbiome in chronic rhinosinusitis: A randomized controlled trial. Laryngoscope Investig. Otolaryngol. 2017;2:410–416. doi: 10.1002/lio2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Endam L.M., Alromaih S., Gonzalez E., Madrenas J., Cousineau B., Renteria A.E., Desrosiers M. Intranasal Application of Lactococcus lactis W136 is safe in chronic rhinosinusitis patients with previous sinus surgery. Front. Cell Infect. Microbiol. 2020;10:440. doi: 10.3389/fcimb.2020.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz J.S., Peres A.G., Mfuna Endam L., Cousineau B., Madrenas J., Desrosiers M. Topical probiotics as a therapeutic alternative for chronic rhinosinusitis: A preclinical proof of concept. Am. J. Rhinol. Allergy. 2016;30:202–205. doi: 10.2500/ajra.2016.30.4372. [DOI] [PubMed] [Google Scholar]
- 50.Cho D.Y., Skinner D., Lim D.J., Mclemore J.G., Koch C.G., Zhang S., Swords W.E., Hunter R., Crossman D.K., Crowley M.R., et al. The impact of Lactococcus lactis (probiotic nasal rinse) co-culture on growth of patient-derived strains of Pseudomonas aeruginosa. Int. Forum. Allergy Rhinol. 2020;10:444–449. doi: 10.1002/alr.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abreu N.A., Nagalingam N.A., Song Y., Roediger F.C., Pletcher S.D., Goldberg A.N., Lynch S.V. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci. Transl. Med. 2012;4:151ra124. doi: 10.1126/scitranslmed.3003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cleland E.J., Drilling A., Bassiouni A., James C., Vreugde S., Wormald P.J. Probiotic manipulation of the chronic rhinosinusitis microbiome. Int. Forum. Allergy Rhinol. 2014;4:309–314. doi: 10.1002/alr.21279. [DOI] [PubMed] [Google Scholar]