Supplemental Digital Content is available in the text
Keywords: atrial fibrillation, diagnostic overuse, high-value care, implantable cardiac monitors, syncope
Abstract
Implantable cardiac monitors (ICMs) provide long-term electrocardiographic monitoring for a number of indications. However, frequencies of use by indication and temporal changes have not been characterized on a national scale. We sought to characterize overall use and changes between 2011 and 2018. We used generalized linear models to characterize the incidence rate per 1,000,000 patient-quarters at risk and an autoregressive integrated moving average model to account for autocorrelation in this time series data. We studied commercially-insured patients and their insured dependents in the IBM MarketScan Commercial Database who had an ICM placed. We described the characteristics of individuals who received ICMs and the frequency of placements into 3 guideline concordance groups. We estimated the mean change per quarter in ICM placements (mean quarterly change in incidence rate per 1,000,000 patient-quarters at risk) for quarter (Q)1 2011 through Q1 2014, Q1 2014 to Q2 2014, and Q2 2014 through Q4 2018 for each guideline concordance group. The most common indications for categorizable ICM placement were syncope (24%), atrial fibrillation (11%), and stroke (11%). For each of the 3 guideline concordance groups except guideline unaddressed inpatient ICM placements, there was a significant increase in use either during the Q1 2014 to Q2 2014 or the Q2 2014 through Q4 2018 periods. A significant portion of ICM placements were for indications that lack strong evidence, such as established atrial fibrillation. The incidence of ICM placement for most of the indications and settings increased after miniaturization and technical improvements.
1. Introduction
Implantable cardiac monitors (ICMs) are being increasingly used in the US and globally. A 2017 analysis estimated the global market at $410.4 million and projected it to grow to $678.3 million by 2023.[1] Evidence- and guideline-based indications have been established, yet ICMs are being placed in scenarios for which they may have lower clinical utility.[2]
Clinical evidence supports the use of ICMs to determine the etiology of recurrent unexplained syncope and cryptogenic stroke. Both the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS), and the European Society of Cardiology (ESC) have recommendations on use of ICMs for syncope evaluation. The 2017 ACC/AHA/HRS syncope guidelines grade the use of ICMs for certain cases of unexplained syncope as a class IIa recommendation with level B-R evidence.[3] The 2018 ESC syncope guidelines recommend ICMs (level I-A) for recurrent, unexplained syncope or for the initial management of syncope in patients with high-risk criteria without an indication for pacemaker or implantable cardioverter defibrillator.[4] It recommends consideration of ICMs (level IIa-B) with “suspected or certain reflex syncope presenting with frequent or severe syncopal episodes” and for patients with epilepsy refractory to treatment or with unexplained falls (level IIb-B). Finally, a scientific statement on indications for ICMs by the ESC in 2009 suggested ICMs are appropriate in patients with infrequent but severe palpitations when external loop recorders or other electrocardiogram monitoring has not determined a cause (Class IIA-B).[5]
A 2016 systematic review and meta-analysis showed that ICMs have a higher diagnostic yield than conventional monitoring for diagnosing atrial fibrillation (AF) in cryptogenic stroke and underlying arrhythmias in unexplained recurrent syncope.[6] The 2020 ESC AF Guidelines recommend consideration of ICM placement to evaluate for silent AF as the cause of a cryptogenic stroke as a class IIa recommendation with level B evidence.[7] The 2019 update to the 2014 ACC/AHA/HRS AF guidelines contains a similar recommendation for use of ICMs in this clinical setting when external ambulatory monitoring is inconclusive (class IIa-B-R).[8] Neither the European nor the US AF guidelines contain any specific recommendation for use of ICMs in patients with established AF, either to assess AF burden or to monitor efficacy of rate control.
We sought to characterize trends in placement of ICMs in commercially-insured patients in the US between 2011 and 2018, including around the time of device miniaturization in early 2014. Specifically, we aimed to quantify the use of ICMs for different indications, hypothesizing that the overall rate of placement increased when these devices were miniaturized and thus became easier and faster to implant. Moreover, we hypothesized that placement for indications less supported by clinical evidence or guidelines might have contributed disproportionately to this increase. Placement of this device when its use is not well-supported by guidelines and unlikely to change clinical management may represent low-value care and poor use of healthcare dollars.
2. Methods
2.1. Data
Institutional Review Board approval was not required for this study because our dataset is considered exempt by the Johns Hopkins Institutional Review Board. We used the IBM MarketScan Commercial Database which contains inpatient and outpatient claims for commercially-insured patients and their insured dependents. It includes claims from over 47 million US residents, mostly under the age of 65, including patients in all states and the District of Columbia. We analyzed claims from 2011 through 2018 and used 2010 claims where necessary for lookback data. Placement of an ICM was identified from a claim for Current Procedural Terminology code 33282 in the inpatient or outpatient files. We excluded ICM placements for patients under the age of 18 and those for whom age or sex was missing; we included only the first observed ICM placement for a given individual. We required that all individuals included in the analysis have at least 1 year of continuous health plan enrollment prior to ICM placement.
We extracted patient-level information including age, sex, procedure setting (inpatient or outpatient), commercial insurance plan type, region of the country, and comorbid diagnoses known from International Classification of Disease (ICD)-9 or ICD-10 diagnosis codes in the 12 months preceding ICM placement. With these data, we generated a Charlson Comorbidity Index score for each individual.[9]
2.2. Guideline concordance of indications for placement of ICMs
We classified ICM placements to 1 of 3 groups based on concordance with guideline recommendations. The placements were considered to be definitely guideline concordant, possibly guideline concordant, and guideline unaddressed based on the primary ICD-9 or ICD-10 diagnosis code associated with the procedure (Table S1, Supplemental Digital Content, http://links.lww.com/MD/G554). The guideline unaddressed group includes ICD codes associated with ICM placement that the guidelines do not explicitly support. ICM placements for recurrent syncope and cryptogenic stroke comprised the definitely guideline concordant group. Placements for recurrent falls, epilepsy, non-recurrent syncope, palpitations, dizziness, and stroke/transient ischemic attack comprised the possibly guideline concordant group. Placements for AF, atrial flutter, and paroxysmal supraventricular tachycardia were categorized as guideline unaddressed. ICM placements that did not fall into any of these groups were considered non-classifiable and were not further considered in our analyses.
Classification into these guideline concordance groups relied on identifying consistent diagnosis codes at 2 different times – on the day of ICM placement and at least once within the preceding 3 months. For the 2 indications that required the recurrence of an event (recurrent syncope and recurrent falls), 2 diagnoses within the preceding 12 months were required, one of which needed to be within the 3 months preceding device placement. If there was no classifiable diagnosis on the day of placement, despite a classifiable diagnosis in the preceding 3 months, this ICM placement was considered non-classifiable. Additionally, if the diagnosis on the day of ICM placement could not be confirmed in the 3-month lookback window, the placement was considered non-classifiable.
2.3. Descriptive statistics
We tabulated the number of ICM placements that fell into each of the concordance groups for the entire study period (2011–2018) as well as before and after the start of quarter (Q)2 in 2014. We generated means and standard deviations for continuous descriptive variables and percentages for categorical variables. These data are reported for the entire cohort and for the cohort stratified by inpatient or outpatient placement, stratified by date (January 2011–March 2014 and April 2014–December 2018), and stratified by guideline concordance group.
2.4. Statistical analyses
We generated an incidence rate for ICM placements in 3-month intervals from January 1, 2011 through December 31, 2018 as follows. We created risk sets of individuals “eligible” or “at risk” to receive an ICM for each concordance category and each setting (representing the denominator of the incidence rate). Each individual with a relevant diagnosis code contributed person-time to this risk set beginning on the date of his/her qualifying diagnosis (noted from an inpatient or outpatient visit) and for the next 3 months; the person-time was apportioned to the appropriate calendar quarter beginning with January 2011. For example, a person with an outpatient encounter on March 15, 2013 with a primary diagnosis code of AF was at risk of ICM placement for 91 days after that encounter, and thus contributed 0.18 person-quarters (16/91 = 0.18) to Q1 and 0.82 person-quarters ([91–16]/91 = 0.82) to Q2 of 2013 in the guideline unaddressed outpatient group. ICM placements within each calendar quarter were counted as events to the appropriate risk set. The “eligible” or “at risk” population for each guideline concordance group was limited by the specific diagnosis codes included in that group.
We used generalized linear models to characterize the incidence rate per 1,000,000 patient-quarters at risk. We used an autoregressive integrated moving average model to account for autocorrelation in this time series data. We used the smallest canonical correlation method to guide selection of the model parameters and used Akaike Information Criterion to compare models for each guideline concordance group, by setting. All but one of the models contained a linear term to describe the trend in placement rates from Q1 2011 through Q1 2014, an intercept to represent the immediate change in rates at Q2 2014, and a term for the linear trend after Q2 2014. We plotted the incidence rates, and after visual inspection, added a second data-driven intercept and third line segment, where needed. We visually inspected the plots of incidence rates to look for seasonality, and where necessary, adjusted the autoregressive integrated moving average model for seasonality with an indicator variable for season (Table S2, Supplemental Digital Content, http://links.lww.com/MD/G554).
We tested our hypothesis by calculating the mean change per quarter in ICM placements (quarterly change in incidence rate per 1,000,000 patient quarters at risk) for the Q1 2011 through Q1 2014, Q1 2014 to Q2 2014, and Q2 2014 through Q4 2018 with 95% confidence intervals from the model. For the Q1 2011 through Q1 2014 period, if the 95% confidence interval did not include 0, this suggests that the slope of this segment is different than 0. For the Q1 2014 to Q2 2014 period, if the 95% confidence interval did not include 0, this suggests there is a statistically significant change between these 2 data points. For the Q2 2014 to Q4 2018 period, if the 95% confidence interval does not include zero, this suggests that the slope of this segment is significantly different than the Q1 2011 to Q1 2014 segment.
2.5. Sensitivity analysis
We performed a sensitivity analysis using a 12-month (rather than 3-month) lookback period to define qualifying diagnoses for placement. We measured how this alternative criterion changed the proportion of ICM placements in the 3 guideline concordance groups (and their sizes relative to the non-classifiable group) and how it impacted the mean quarterly change of incidence rates for the time periods of interest. The person-time (denominator) was adjusted accordingly such that each individual contributed 4 person-quarters to the risk set (rather than 1 as in the main analysis) in the appropriate concordance group and setting.
3. Results
3.1. Patient characteristics
After applying exclusion criteria, we identified 23,396 adults with ICM placements between 2011 and 2018 (Figure S1, Supplemental Digital Content, http://links.lww.com/MD/G554). Most (87%) ICMs were placed in the outpatient setting (Table 1). The plurality of patients in both the inpatient and outpatient settings was between 55 and 64 years of age, had a preferred provider organization-like health plan, and resided in the South. (Tables S3 and S4, Supplemental Digital Content, http://links.lww.com/MD/G554 show the entire cohort and cohort stratified by time of placement, respectively).
Table 1.
Description of a set of commercially-insured US recipients of ICMs from 2011 to 2018, stratified by procedure setting.
| Inpatient n = 3113 (13%) | Outpatient n = 20,283 (87%) | |
| Age, yrs; mean (standard deviation) | 52.8 (10.1) | 50.6 (11.7) |
| 18–34 | 213 (7%) | 2461 (12%) |
| 35–44 | 350 (11%) | 2686 (13%) |
| 45–54 | 821 (26%) | 5400 (27%) |
| 55–64 | 1713 (55%) | 9681 (48%) |
| 65 | 16 (1%) | 55 (< 1%) |
| Sex | ||
| Male | 1806 (58%) | 9867 (49%) |
| Plan type | ||
| Basic major medical or comprehensive | 127 (4%) | 651 (3%) |
| PPO-like | 2279 (73%) | 15,590 (77%) |
| HMO-like | 411 (13%) | 2046 (10%) |
| HDHP | 153 (5%) | 1190 (6%) |
| Missing | 143 (5%) | 806 (4%) |
| Region | ||
| South | 1318 (42%) | 8762 (43%) |
| North Central | 713 (23%) | 4589 (23%) |
| Northeast | 812 (26%) | 4312 (21%) |
| West | 246 (8%) | 2448 (12%) |
| Unknown | 24 (1%) | 172 (1%) |
| Charlson Comorbidity Index > 1 | 1216 (39%) | 4832 (24%) |
HDHP = high deductible health plan, HMO = health maintenance organization, ICM = implantable cardiac monitor, PPO = preferred provider organization.
3.2. Recipients by guideline concordance groups
Among the classifiable placements, the most common indications were recurrent syncope (12%), non-recurrent syncope (12%), and AF (11%). Of note, 67 of 332 (20%) of the ICMs in the inpatient setting for AF or atrial flutter were placed after an ablation procedure. Placements for stroke (other than cryptogenic stroke)/transient ischemic attack and cryptogenic stroke, combined, were approximately 11% of all placements. The definitely guideline concordant ICM placements comprised 19% of placements, possibly guideline concordant comprised 22%, guideline unaddressed comprised 14%, and non-classifiable placements comprised 45% (Table 2). For the majority of non-classifiable placements, the day of procedure coding could not be confirmed during the 3-month lookback period. The rest had day of procedure coding that did not fall into any of the guideline categories (Table S5, Supplemental Digital Content, http://links.lww.com/MD/G554). Among the concordance groups, the definitely guideline concordant placements were the most likely to occur in the inpatient as opposed to outpatient setting (Table 3).
Table 2.
Guideline concordance of the primary diagnosis codes on the date of placement of the ICM.
| % of total (n = 23,396) | |
| Definitely guideline concordant | 4459 (19%) |
| Recurrent syncope | 2868 (12%) |
| Cryptogenic stroke | 1591 (7%) |
| Possibly guideline concordant | 5070 (22%) |
| Recurrent falls | 0 |
| (Uncontrolled) epilepsy | 0 |
| Non-recurrent syncope | 2798 (12%) |
| Palpitations | 1208 (5%) |
| Dizziness | 71 (<1%) |
| Stroke/transient ischemic attack | 993 (4%) |
| Guideline unaddressed | 3274 (14%) |
| Atrial fibrillation | 2634 (11%) |
| Atrial flutter | 184 (1%) |
| Paroxysmal supraventricular tachycardia | 456 (2%) |
| Non-classifiable placements | 10,593 (45%) |
| Primary diagnosis on day of procedure not confirmed in 3-month lookback | 7267 (31%) |
| Syncope | 3124 (13%) |
| Cryptogenic stroke | 400 (2%) |
| Epilepsy | 1 (< 1%) |
| Palpitations | 1026 (4%) |
| Dizziness | 92 (< 1%) |
| Stroke/transient ischemic attack | 273 (1%) |
| Atrial fibrillation | 1758 (8%) |
| Atrial flutter | 119 (1%) |
| Paroxysmal supraventricular tachycardia | 474 (2%) |
| All other primary diagnoses on day of procedure | 3326 (14%) |
ICM = implantable cardiac monitor.
Table 3.
Description of a set of commercially-insured US recipients of ICMs from 2011 to 2018, stratified by guideline concordance.
| Definitely guideline concordant n = 4459 (19%) | Possibly guideline concordant n = 5070 (22%) | Guideline unaddressed n = 3274 (14%) | Non-classifiable n = 10,593 (45%) | |
| Age, yrs; mean (standard deviation) | 51.0 (11.4) | 49.4 (12.1) | 54.4 (9.3) | 50.5 (11.7) |
| 18–34 | 496 (11%) | 724 (14%) | 164 (5%) | 1290 (12%) |
| 35–44 | 569 (13%) | 747 (15%) | 267 (8%) | 1453 (14%) |
| 45–54 | 1209 (27%) | 1399 (28%) | 797 (24%) | 2816 (27%) |
| 55–64 | 2167 (49%) | 2192 (43%) | 2030 (62%) | 5005 (47%) |
| 65 | 18 (< 1%) | 8 (< 1%) | 16 (1%) | 29 (< 1%) |
| Sex | ||||
| Male | 2245 (50%) | 2317 (46%) | 2008 (61%) | 5103 (48%) |
| Procedure setting | ||||
| Outpatient | 3270 (73%) | 4336 (86%) | 3040 (93%) | 9637 (91%) |
| Plan type | ||||
| Basic major medical or comprehensive | 172 (4%) | 145 (3%) | 111 (3%) | 350 (3%) |
| PPO-like | 3329 (75%) | 3881 (76%) | 2547 (78%) | 8112 (77%) |
| HMO-like | 509 (11%) | 542 (11%) | 311 (10%) | 1095 (10%) |
| HDHP | 280 (6%) | 287 (6%) | 207 (6%) | 569 (5%) |
| Missing | 169 (4%) | 215 (4%) | 98 (3%) | 467 (5%) |
| Region | ||||
| South | 1986 (45%) | 2254 (45%) | 1308 (40%) | 4532 (43%) |
| North Central | 1078 (24%) | 1081 (21%) | 776 (24%) | 2367 (22%) |
| Northeast | 930 (21%) | 1037 (20%) | 823 (25%) | 2334 (22%) |
| West | 442 (10%) | 641 (13%) | 350 (11%) | 1,261 (12%) |
| Unknown | 23 (<1%) | 57 (1%) | 17 (<1%) | 99 (1%) |
| Charlson Comorbidity Index > 1 | 1441 (32%) | 1183 (23%) | 744 (23%) | 2680 (25%) |
HDHP = high deductible health plan, HMO = health maintenance organization, ICM = implantable cardiac monitor, PPO = preferred provider organization.
3.3. Change in use over the study period
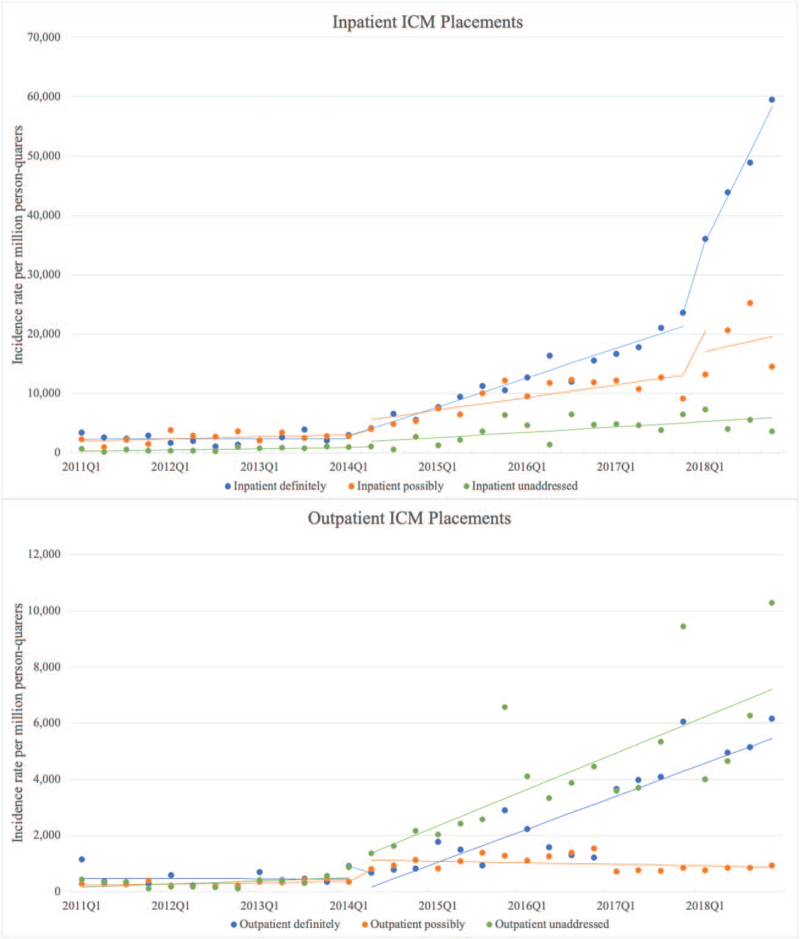
The incidence rate of ICM placements per 1,000,000 person-quarters increased for each of the 6 groups over the course of the study period (Fig. 1). The change over time in ICM placement in the period before Q1 2014 was non-significant for all groups except the outpatient guideline unaddressed group for which there was a trend toward an increase. For the guideline unaddressed outpatient group, the mean change per quarter in incidence rate for Q1 2014 to Q2 2014 was not modelled separately from the period after Q2 2014, as visual inspection suggested no abrupt change in slope. For each guideline concordance group in both settings except guideline unaddressed inpatient placements, there was either a statistically significant immediate increase in mean ICM placement from Q1 2014 to Q 2014 or a sustained increasing trend after Q2 2014 (Table 4). The largest mean increase in magnitude after Q2 2014 were in the definitely guideline concordant inpatient group (1215 ICMs per 1,000,000 patient-quarters at risk) and the possibly guideline concordant inpatient group (497 ICMs per 1,000,000 patient-quarters at risk). There was a mean increase in the possibly guideline concordant outpatient group from Q1 2014 to Q2 2014 of 648 ICMs per 1,000,000 patient-quarters).
Figure 1.
Inpatient and outpatient ICM placements between 2011 and 2018. Mean incidence rates of ICM placement per million person-quarters “at risk”, with lines of best fit for each interval modeled using ARIMA. ARIMA = autoregressive integrated moving average, ICM = implantable cardiac monitor.
Table 4.
Mean change per quarter per 1,000,000 patient-quarters at risk for ICM placement by guideline concordance categories, stratified by setting.
| Mean change (95% CI∗) per quarter, Q1 2011 through Q1 2014 | Immediate change (95% CI†) from Q1 2014 to Q2 2014 | Mean change (95% CI‡) per quarter, after Q2 2014 | |
| Inpatient | |||
| Definitely guideline concordant | 26 (–174, 226) P = .80 | –122 (-2223, 1979) P = .91 | 1215 (945, 1485) P < .0001 |
| Possibly guideline concordant | 54 (–230, 338) P = .71 | 2169 (–777, 5115) P = .15 | 497 (148, 846) P = .005 |
| Guideline unaddressed | 53 (–127, 233) P = .57 | 763 (–970, 2496) P = .39 | 174 (–34, 382) P = .10 |
| Outpatient | |||
| Definitely guideline concordant | –8 (–130, 114) P = .89 | –561 (–1715, 593) P = .34 | 304 (161, 447) P < .0001 |
| Possibly guideline concordant | 17 (–24, 58) P = .40 | 648 (297, 999) P = .0003 | –26 (–77, 25) P = .31 |
| Guideline unaddressed | 78 (0, 156) P = .05 | --- Not modeled | 256 (140, 372) P < .0001 |
CI = confidence interval, ICM = implantable cardiac monitor, Q = quarter.
If the 95% confidence interval did not include 0, this suggests that the slope of this segment is different than 0.
If the 95% confidence interval did not include 0, this suggests there is a statistically significant change between Q1 2014 and Q2 2014.
If the 95% confidence interval does not include 0, this suggests that the slope of this segment is different than the Q1 2011 to Q1 2014 segment.
3.4. Sensitivity analysis
In the sensitivity analysis using a 12-month lookback, the percentage of ICM placements that were non-classifiable dropped from 45% to 31% (Table S6, Supplemental Digital Content, http://links.lww.com/MD/G554). In this analysis, the most common indications for ICM placement were non-recurrent syncope (16%), AF (16%), and recurrent syncope (14%). The trends of significant increases in ICM placement were similar to that of the main analysis, with the only exception being the guideline unaddressed outpatient group which no longer had a significant increase before Q1 2014 or after Q2 2014 (Table S7, Supplemental Digital Content, http://links.lww.com/MD/G554).
4. Discussion
We characterized placement of ICMs using a large national commercial claims database by describing the indications for placement and their temporal changes. We hypothesized, based on clinical observation, that the frequency of device placement was increasing and that this increase coincided with miniaturization and technical improvements. Moreover, we hypothesized that placement of ICMs for indications with a less well-established clinical evidence base had increased disproportionately. ICMs were most commonly placed for syncope, AF, and stroke. This was a consistent finding for both the main analysis and the sensitivity analysis. As hypothesized, there was an increase in ICM placements that coincided temporally with miniaturization of the device. However, this increase was not immediate in any group, except the possibly guideline concordant group receiving ICMs as outpatients.
Our hypothesis that the increase in ICM placement occurred disproportionately for indications with a weaker evidence base was not supported by the data. The largest increase was for the definitely guideline concordant inpatient group and the smallest but still statistically significant increase was for the guideline unaddressed outpatient group. Of course, the magnitude of these trends is also impacted by the patient-quarters at risk (i.e., the denominator). We suspect this is the reason that there is a significant mean increase in placement of ICMs for the guideline unaddressed outpatient group in the main analysis but not the sensitivity analysis – extending the lookback period to 12 months markedly increased the patient-quarters at risk for the diagnoses in this group.
Our analysis confirms that ICMs are being placed at an increasing rate and that a significant proportion are placed for indications with a less well-established evidence base, particularly for established AF. A previous study of change in ICM placement at 2 hospitals between 2012 and 2016 showed a marked increase over this period, with an increase in placement for AF, consistent with our findings.[2] The utility of ICMs in the management of patients with a previously confirmed diagnosis of AF is uncertain as it is unclear how findings from these devices would change management of AF in most cases. While some research suggests that patients with a low burden of AF after an ablation can have their anticoagulation safely discontinued,[10] this is not yet a guideline-supported practice for patients at high thromboembolic risk. Surveillance for recurrent AF for other reasons can usually be accomplished with less invasive and lower-cost methods, such as Holter or non-invasive event monitors. Therefore, evaluation of AF burden with an invasive device is not indicated for most patients. As noted previously, only 20% of ICMs placed in the inpatient setting for AF or atrial flutter were placed after an ablation.
We recognize certain limitations in our approach. We used administrative claims data to describe changing utilization of ICMs, and thus the limitations of this study are those associated with claims data, including changes in coding practices over time, “upcoding,” and inaccurate coding. Claims data also do not include clinical measures that may have informed the appropriateness of ICM placement. The requirement that the diagnosis be coded twice (on the procedure day and in the preceding 3 months) likely improved the specificity of concordance group placement. However, individuals in the risk set did not need to have a second qualifying diagnosis, thus our methodology to define the at-risk population is less precise. Our categorization process relied on arbitrary cut points regarding the intervals in which diagnosis codes were sought. However, our similar results in the sensitivity analysis support our findings. Additionally, while MarketScan© is a large national commercial claims database, it is not representative of all commercially- insured patients and also may not be applicable to an older population or to patients without commercial insurance. Finally, we did not control for patient-level or physician-level covariates that may have changed over time, and we are not able to say whether an electrophysiologist or general cardiologist placed the ICM though we suspect the vast majority were placed by electrophysiologists. We recognize that our finding of an increase in ICM placement after the release of a smaller and technically improved device does not prove that this change caused the rapid uptick in use.
The ease of implantation of current generation ICMs may contribute to overuse, and their cost is substantial. They can be placed with a minor procedure. Medicare payment in an outpatient surgical center and hospital outpatient setting in 2019 were $6375 and $7404, respectively.[11] These figures do not include recurring costs for long-term remote monitoring and interpretation. ICMs have a favorable safety profile, with rates of unsuccessful insertion or placement-related complications under 1% and total adverse events under 5%.[12] The combination of a favorable reimbursement rate, ease of implantation, and minimal risk of complications could lead to overuse in clinical scenarios for which the benefit is marginal.
Recent evidence suggests a lack of a relationship between short duration AF detected in patients with pacemaker/implantable cardioverter defibrillators who do not have an established diagnosis of AF and incidence of stroke.[13] These findings have not changed guidelines for anticoagulation in AF.[8] Presumably, more evidence is needed before AF duration (as measured by pacemaker, defibrillator, or ICM) is incorporated into anticoagulation decisions. If there is a relationship between AF burden and management of AF, particularly with respect to anticoagulation, then placement of ICMs in patients with established AF could have clinical utility. However, at this time, AF burden does not impact treatment, and thus placement of ICMs in this setting is likely to represent low-value care.
Additionally, given the high cost of ICMs, cost-benefit analyses for the indications that are less evidence-based – non-recurrent syncope, palpitations, and dizziness – should be undertaken. These analyses could be used to inform specific appropriate use criteria. Studies to evaluate the downstream impact (both in terms of clinical and utilization endpoints) of ICM placement on patients with indications in the possibly guideline concordant and guideline unaddressed groups should be conducted. Low-value diagnostic testing can lead to wasteful cascades of additional testing.[14] Finally, future studies should evaluate whether increased placement of ICMs in the Medicare population is similar to that in the commercially-insured population.
We found increasing utilization of ICMs in US patients with commercial insurance between 2011 and 2018, coinciding with device miniaturization and technical improvements. A significant proportion of these devices were placed for established AF. The clinical value of ICM monitoring in patients with established AF is not yet well-supported by clinical evidence or guidelines. Our findings suggest a need for appropriate use and/or guideline criteria for ICMs for the diagnoses for which they are being frequently placed.
Acknowledgments
Access to MarketScan at Johns Hopkins University is made possible through the following data license sponsors: Center for Drug Safety and Effectiveness, Department of Health Policy and Management, Center for Surgical Trials and Outcomes Research, Institute for Clinical and Translational Research, as well as Jodi Segal MD MPH, Morgan Grams MD PhD, and Gerard Anderson PhD.
Author contributions
Conceptualization: Michael I. Ellenbogen, Kathleen M. Andersen, Joseph E. Marine, Nae-Yuh Wang, Jodi B. Segal.
Data curation: Kathleen M. Andersen, Nae-Yuh Wang.
Formal analysis: Kathleen M. Andersen, Nae-Yuh Wang.
Funding acquisition: Jodi B. Segal.
Investigation: Michael I. Ellenbogen, Kathleen M. Andersen, Joseph E. Marine, Jodi B. Segal.
Methodology: Michael I. Ellenbogen, Kathleen M. Andersen, Joseph E. Marine, Nae-Yuh Wang, Jodi B. Segal.
Supervision: Michael I. Ellenbogen, Nae-Yuh Wang, Jodi B. Segal.
Validation: Kathleen M. Andersen, Nae-Yuh Wang, Jodi B. Segal.
Writing – original draft: Michael I. Ellenbogen, Kathleen M. Andersen, Joseph E. Marine, Nae-Yuh Wang, Jodi B. Segal.
Writing – review & editing: Michael I. Ellenbogen, Kathleen M. Andersen, Joseph E. Marine, Nae-Yuh Wang, Jodi B. Segal.
Footnotes
Abbreviations: ACC = American College of Cardiology, AF = atrial fibrillation, AHA = American Heart Association, ESC = European Society of Cardiology, HRS = Heart Rhythm Society, ICD = International Classification of Disease, ICM = implantable cardiac monitor, Q = quarter.
How to cite this article: Ellenbogen MI, Andersen KM, Marine JE, Wang NY, Segal JB. Changing patterns of use of implantable cardiac monitors from 2011 to 2018 for a large commercially-insured U.S. population. Medicine. 2021;100:51(e28356).
Dr Ellenbogen is supported by the Johns Hopkins Hospitalist Scholars Fund.
Ms Andersen receives doctoral training support from the National Heart, Lung and Blood Institute Pharmacoepidemiology T32 Training Program (T32HL139426-03).
Dr Wang is supported by UL1TR003098 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health, and NIH Roadmap for Medical Research.
Dr Segal is supported by K24AG049036 from National Institute on Aging.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1]. Implantable Cardiac Monitors Market Expected to Reach $678.3 Million, Globally, by 2023. Available at: https://www.alliedmarketresearch.com/press-release/implantable-cardiac-monitors-market.html. Accessed October 8, 2018. [Google Scholar]
- [2].Pillarisetti J, Vinas A, Roy D, Panday M, Lakkireddy D. The Era of LINQ Implantable Loop Recorders: Expanding Indications or Ease of Implantation? Circulation. 138(Suppl_1):A11573, November 6, 2018. Available at: https://insights.ovid.com/crossref/00003017-201811061-00588?isFromRelatedArticle=Y. Accessed August 26, 2019. [Google Scholar]
- [3].Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS Guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol 2017;70:e39–110. [DOI] [PubMed] [Google Scholar]
- [4].Brignole M, Moya A, de Lange FJ, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–948. [DOI] [PubMed] [Google Scholar]
- [5].Brignole M, Vardas P, et al. Task Force members. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009;11:671–87. [DOI] [PubMed] [Google Scholar]
- [6].Burkowitz J, Merzenich C, Grassme K, Brüggenjürgen B. Insertable cardiac monitors in the diagnosis of syncope and the detection of atrial fibrillation: a systematic review and meta-analysis. Eur J Prev Cardiol 2016;23:1261–72. [DOI] [PubMed] [Google Scholar]
- [7].Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- [8].January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 2019;140:02. [DOI] [PubMed] [Google Scholar]
- [9].Bannay A, Chaignot C, Blotière P-O, et al. The best use of the Charlson Comorbidity index with electronic health care database to predict mortality: medical care 2016;54:188–94. [DOI] [PubMed] [Google Scholar]
- [10].Zuern CS, Kilias A, Berlitz P, et al. Anticoagulation after catheter ablation of atrial fibrillation guided by implantable cardiac monitors. Pacing Clin Electrophysiol 2015;38:688–93. [DOI] [PubMed] [Google Scholar]
- [11].Shepard, K. 2019 Reimbursement Changes for Subcutaneous Cardiac Rhythm Monitors. December 6, 2018. Available at: http://www.medtronic.me/content/dam/medtronic-com/products/cardiac-rhythm/coding-coverage-reimbursement/documents/reimbursement-subcutaneous-cardiac-rhythm-monitors-2019-slides.pdf. Accessed August 26, 2019. [Google Scholar]
- [12].Rogers JD, Sanders P, Piorkowski C, et al. In-office insertion of a miniaturized insertable cardiac monitor: results from the Reveal LINQ In-Office 2 randomized study. Heart Rhythm 2017;14:218–24. [DOI] [PubMed] [Google Scholar]
- [13].Swiryn S, Orlov MV, Benditt DG, et al. Clinical implications of brief device-detected atrial tachyarrhythmias in a cardiac rhythm management device population: results from the registry of atrial tachycardia and atrial fibrillation episodes. Circulation 2016;134:1130–40. [DOI] [PubMed] [Google Scholar]
- [14].Ganguli I, Lupo C, Mainor AJ, et al. Prevalence and cost of care cascades after low-value preoperative electrocardiogram for cataract surgery in fee-for-service medicare beneficiaries. JAMA Intern Med 2019;179:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]