Abstract
This study aimed to evaluate the effect and safety of anlotinib combined with S-1 in the treatment of recurrent or metastatic esophageal cancer patients who refused or were intolerant to intravenous chemotherapy.
This study retrospectively reviewed 22 recurrent or metastatic esophageal cancer patients who refused or were intolerant to intravenous chemotherapy between June 1, 2018 and February 28, 2019. All patients did not previously receive anlotinib or S-1.
Of 22 patients, 20 patients had squamous cell cancer. Seventeen patients received at least 2 cycles of anlotinib plus S-1. The objective response rate (ORR) was 35.3%, and the disease control rate (DCR) was 82.4%. The median progression-free survival (PFS) was 3.5 months, and median overall survival (OS) was 5.2 months. In the first-line treatment subgroup, the ORR was 50%, the DCR was 80%, the median PFS was 4.5 months, and the median OS was 5.8 months. In the second-line and above treatment subgroup, the ORR was 14.3%, the DCR was 85.7%, the median PFS was 3.0 months, and the median OS was 3.7 months. The main adverse events (AEs) of anlotinib combined with S-1 were fatigue (58.8%), hypertension (47.1%), hemoptysis (29.4%), anemia (29.4%), nausea (23.5%), liver function damage (23.5%), albuminuria (17.6%), abdominal pain (17.6%), leukopenia (17.6%), neutropenia (11.8%), fever (11.8%), and hand-foot syndrome (11.8%). Grade 3 AEs included nausea (5.9%) and hypertension (5.9%), and no grade 4 or more AEs were reported.
Anlotinib combined with S-1 achieved promising disease control and satisfactory survival with tolerable safety in recurrent metastatic esophageal cancer who refused or were intolerant to intravenous chemotherapy.
Keywords: anlotinib, antiangiogenesis drug, chemotherapy, esophageal cancer, S-1
1. Introduction
Esophageal cancer has a high incidence in China, and more than 90% is squamous cell carcinoma. These patients are mainly middle-aged and elderly, of which 30% patients are over 70 years old.[1,2] Surgical resection and simultaneous chemoradiotherapy are the first choices for the treatment of patients in the early and locally advanced stages. However, even with standard treatment, the local control rate and overall survival (OS) of patients with esophageal cancer are still poor, and about 27% to 50% of patients will relapse and metastasize.[3,4] The median OS of patients with recurrence and metastasis is only 6.0 to 8.2 months.[5,6] For relapsed and metastatic advanced esophageal cancer, intravenous chemotherapy is preferred, with an objective response rate (ORR) of approximately 20% to 60%.[7,8] But for older patients, the performance status is poor and patients who cannot tolerate intravenous chemotherapy have limited treatment option and a worse prognosis. Therefore, there is an urgent need to explore safer and more effective treatment options for patients with recurrent metastatic esophageal cancer to improve the quality of life and prolong survival.
S-1 is a fluorouracil antitumor drug, which is mostly used in the treatment of multiple cancers. It is metabolized to fluorouracil in the body to block 5-fluorouracil phosphorylation, thereby inhibiting disease progression. Domestic studies have reported that first-line treatment of S-1 in patients with advanced esophageal cancer has an ORR of 24% to 60%.[9,10] The development and metastasis of tumors are closely related to the formation of new blood vessels, so effective inhibition of the formation of new blood vessels is the key to enhancing the antitumor effect. Anlotinib, a new antiangiogenesis drug developed in China, is a multi-target receptor tyrosine kinase inhibitor, which can highly select vascular endothelial growth factor receptor 2, inhibit vascular endothelial growth factor receptor 2 kinase activity, and block angiogenesis. At the same time, it can also inhibit platelet-derived growth factor receptor, fibroblast growth factor receptor, and c-Kit and other kinases to exert antiangiogenic effects.[11] Anlotinib has been approved by the China Food and Drug Administration for advanced non-small cell lung cancer as a third-line or further treatment with ORR 9.18%, refractory metastatic soft-tissue sarcoma as a two-line or further treatment with ORR 19.75%, advanced small cell lung cancer as two or a third-line or further treatment with ORR 4.94%, and locally advanced or metastatic medullary thyroid carcinoma as first-line therapy with ORR 48.39%. This study retrospectively analyzes the effect and safety of anlotinib combined with S-1 in the treatment of patients with advanced relapsed or metastatic esophageal cancer patients who refused or were intolerant to intravenous chemotherapy.
2. Methods
2.1. Patients
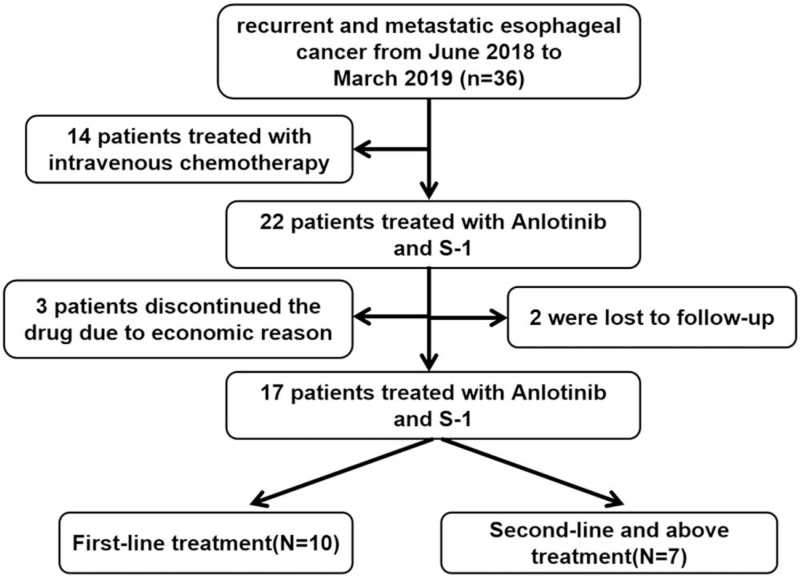
This retrospective study enrolled patients with recurrent or metastatic esophageal cancer who underwent anlotinib combined with S-1 in the oncology department of the Second Affiliated Hospital of Nanchang University between June 1, 2018 and February 28, 2019 (Fig. 1). Eligible patients were aged 18 to 85 years with histologically confirmed esophageal cancer, who underwent surgical treatment or chemoradiotherapy and refused or were intolerant to intravenous chemotherapy. Patients also were diagnosed as distant metastasis by computed tomography (CT), magnetic resonance imaging, positron emission tomography/computed tomography, and other imaging examinations, with at least one measurable lesion. Other key inclusion criteria included normal liver and kidney function, no contraindications to chemotherapy, Eastern Cooperative Oncology Group Performance Status of 1 or 2 and no indication for local treatment. Patients were excluded if they had treated with antitumor angiogenesis drugs; other malignant tumors; refractory hypertension; gastrointestinal bleeding and other bleeding tendencies. This study was conducted in accordance with the Declaration of Helsinki and approved by the Second Affiliated Hospital of Nanchang University ethics committee. The requirement for informed consent was waived due to the retrospective study design.
Figure 1.
Study profile.
2.2. Treatment regimen
Anlotinib 12 mg orally, once a day, days 1–14. For S-1, the dose is calculated based on body surface area (see Table 1), orally, twice a day, days 1–14. Twenty one days per cycle. All patients received at least 2 cycles of chemotherapy.
Table 1.
S-1 dose calculation.
| Body surface area (m2) | Dose (mg) |
| <1.25 | 40 |
| 1.25–1.50 | 50 |
| >1.50 | 60 |
2.3. Outcomes
Tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors system (version 1.1). At baseline, all patients underwent enhanced CT examination of the abdomen, chest, and neck before treatment, and CT should be reviewed every 2 cycles during treatment. The effective evaluation is divided into complete response (CR), partial response (PR), stable disease (SD), and disease progression (PD). ORR (%) = (CR + PR) / total number of cases × 100%. Disease control rate (DCR, %) = (CR + PR + SD) / total number of cases × 100%. Progress free survival (PFS) defined as time from the beginning of clinical research to radiographic progression or death from any cause. Overall survival (OS) defined as the time from the beginning of clinical research to death from any cause. Toxicities were assessed according to the Common Terminology Criteria for Adverse Events (AEs), version 4.0.
2.4. Statistics
The data obtained are expressed in numerical values and percentages (%). Statistical analysis was performed by SPSS 25.0. The continuous variables were expressed by median. The survival curve was constructed using the Kaplan–Meier method and Log Rank test.
3. Results
3.1. Patient characteristics
This retrospective study enrolled 22 patients. Among them, 3 patients discontinued the drug due to economic reason, 2 were lost to follow-up, and finally 17 patients were included in the analysis. The average age of 17 patients was 69.7 years (range 61–82 years), four patients of them had a history of comorbid conditions, including gastric bleeding, laryngeal carcinoma resection, hepatitis, and cholecystolithiasis, respectively. The median time from diagnosis to inclusion was 8.4 months (range 5.9–25.9 months). Prior to receiving anlotinib combined with S-1, six patients had undergone radical resection of esophageal carcinoma, five patients had received radical chemoradiation, one patient had received docetaxel plus nedaplatin and radiotherapy for supraclavicular lymph node, one patient had received docetaxel plus nedaplatin, one patient had received radiotherapy for the primary lesion, one patient had received etoposide and lobaplatin, one patient had received sequential chemoradiotherapy, and one patient without any treatment. The characteristics of the 17 patients are shown in Table 2.
Table 2.
Baseline characteristics of the 17 esophageal cancer patients.
| Characteristics | n | Percentage (%) |
| Sex | ||
| Male | 14 | 82.4 |
| Female | 3 | 17.6 |
| Age | ||
| ≤70 yrs | 11 | 64.7 |
| >70 yrs | 6 | 35.3 |
| ECOG PS | ||
| 1 | 5 | 29.4 |
| 2 | 12 | 70.6 |
| Histology | ||
| Squamous carcinoma | 15 | 88.2 |
| Adenocarcinoma | 1 | 5.9 |
| Small cell carcinoma | 1 | 5.9 |
| Treatment | ||
| First-line treatment | 10 | 58.8 |
| Second-line and above treatment | 7 | 41.2 |
| Metastatic location | ||
| Distant lymph node | 6 | 35.3 |
| Lung | 8 | 47.1 |
| Liver | 5 | 29.4 |
| Bone | 2 | 11.8 |
| Brain | 2 | 11.8 |
| Metastases | ||
| Single organ | 10 | 58.8 |
| Multiple organs | 7 | 41.2 |
ECOG PS = Eastern Cooperative Oncology Group Performance Status.
3.2. Evaluation of tumor response
Of the 17 patients, 0 patient achieved CR, 6 patients achieved PR (35.3%), 8 patients achieved SD (47.1%), and 3 patients had PD (17.6%); the ORR was 35.3%, and the DCR was 82.4%. Ten patients received first-line treatment. Among them, no patient achieved CR, 5 patients achieved PR (50%), 3 patients achieved SD (30%), and 2 patients had PD (20%); the ORR was 50%, and the DCR was 80%. Seven patients received second-line or further-line treatment. Among them, no patient achieved CR, 1 patient achieved PR (14.3%), 5 patients achieved SD (71.4%), and 1 patient had PD (14.3%); the ORR was 14.3% and the DCR was 85.7% (Table 3).
Table 3.
Response of anlotinib combined with S-1 in the treatment of recurrent and metastatic advanced esophageal cancer.
| n | CR | PR | SD | PD | ORR (%) | DCR (%) | |
| First-line treatment | 10 | 0 | 5 | 3 | 2 | 50 | 80 |
| Second-line and above treatment | 7 | 0 | 1 | 5 | 1 | 14.3 | 85.7 |
| Total | 17 | 0 | 6 | 8 | 3 | 35.3 | 82.4 |
CR = complete response, DCR = disease control rate, ORR = objective response rate, PD = disease progression, PR = partial response, SD = stable disease.
3.3. Progression-free survival (PFS) and OS
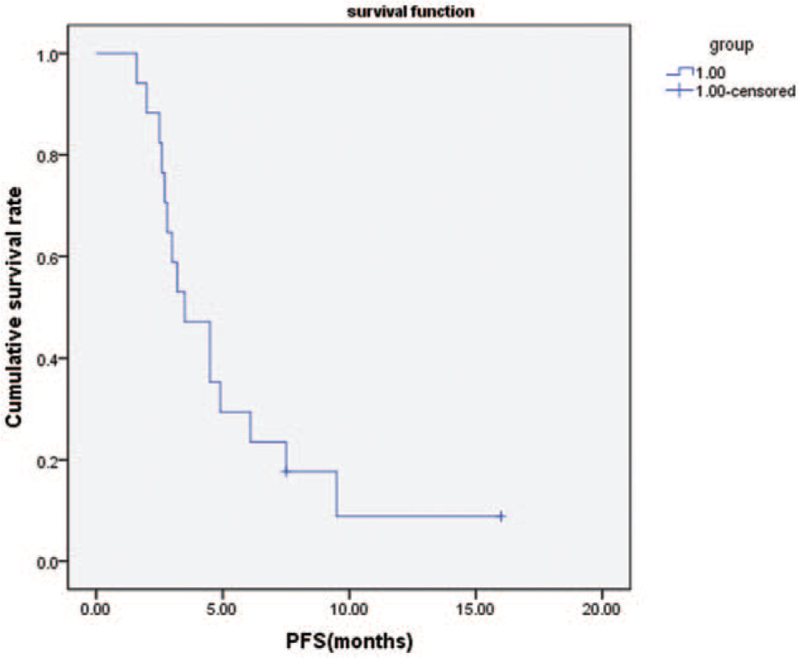
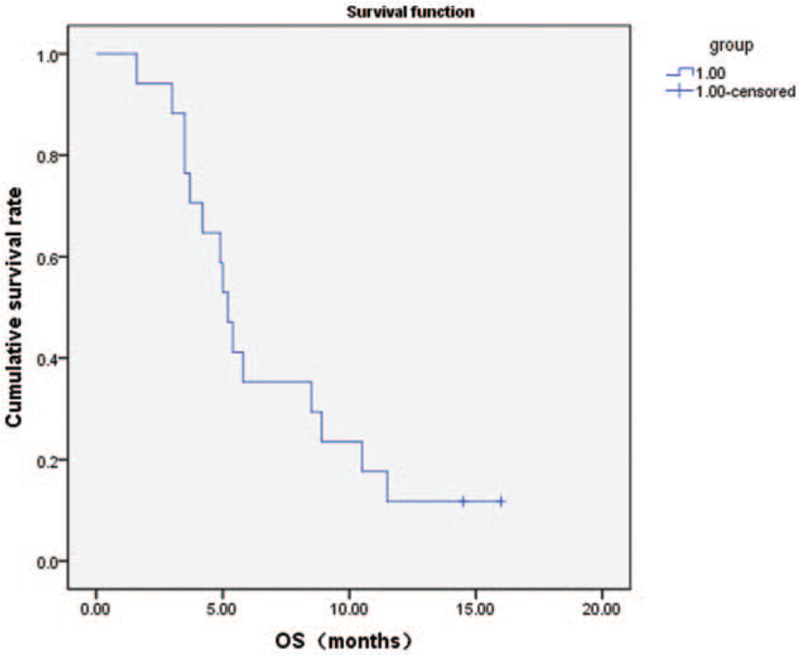
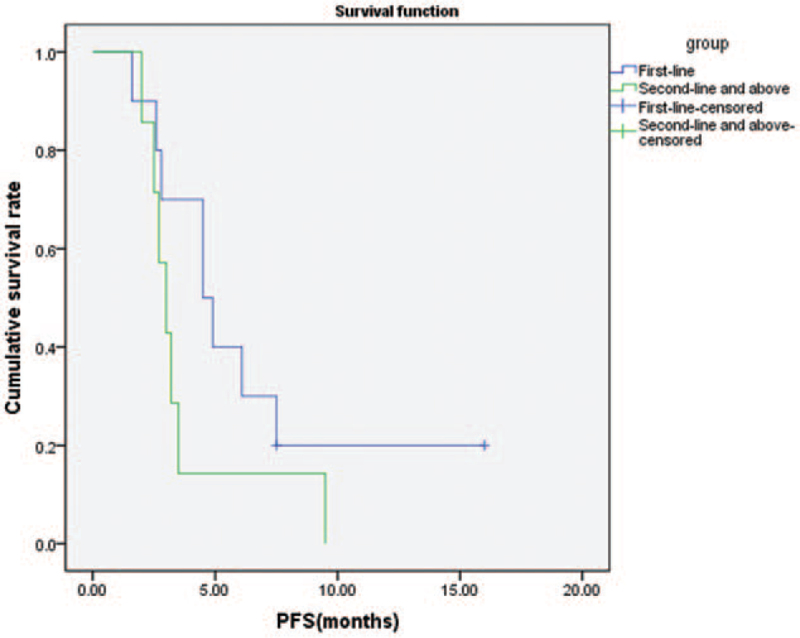
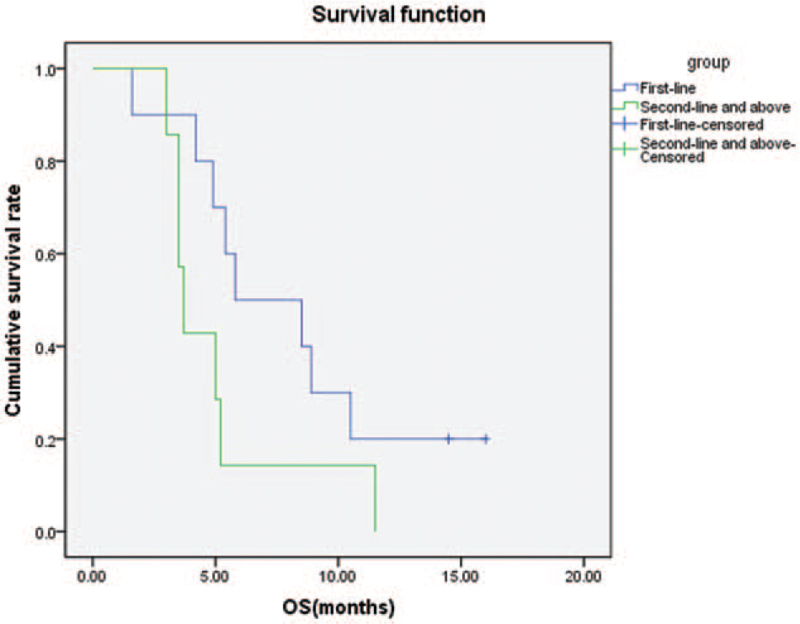
In all patients, the median PFS was 3.5 months (Fig. 2) and the median OS was 5.2 months (Fig. 3). The median PFS of first-line treatment and second-line or further-line treatment was 4.5 months and 3.0 months, respectively (Fig. 4). The median OS of first-line treatment and second-line or further-line treatment was 5.8 months and 3.7 months, respectively (Fig. 5). Among them, one patient had esophageal small cell carcinoma with extensive metastasis who previously had received etoposide and lobaplatin regimen as first line treatment. The patient was effectively controlled by anlotinib and S-1 with stable disease 3 months until died of diffuse brain metastases.
Figure 2.
Kaplan–Meier analysis of progression-free survival.
Figure 3.
Kaplan–Meier analysis of overall survival.
Figure 4.
Kaplan–Meier analysis of progression-free survival in patients with first-line treatment and second-line or further-line treatment.
Figure 5.
Kaplan–Meier analysis of overall survival in patients with first-line treatment and second-line or further-line treatment.
3.4. Safety
All patients were evaluated for toxicity according to Common Terminology Criteria for Adverse Events 4.0. The common AEs in 17 patients included fatigue, hypertension, hemoptysis, nausea, anemia, hand-foot syndrome, abdominal pain, liver function impairment, leukopenia, neutropenia, fever, and proteinuria. Level 3 AEs include hypertension and nausea. No grade 4 or above AEs were observed. One patient in the anlotinib group discontinued treatment because of grade 3 hypertension which could not be relieved with antihypertensive therapy. One case reduced the dosage to 10 mg and symptomatic treatment was given to relieve symptoms because of grade 3 nausea and poor general nutritional status (Table 4).
Table 4.
17 patients with toxicities.
| Toxicities | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||||
| N | % | N | % | N | % | N | % | |
| Hypertension | 7 | 41.2 | 0.8 | 0 | 1 | 5.9 | 0 | 0 |
| Leukopenia | 2 | 11.8 | 1 | 5.9 | 0 | 0 | 0 | 0 |
| Neutropenia | 2 | 11.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 5 | 29.4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liver damage | 4 | 23.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 9 | 52.9 | 1 | 5.9 | 0 | 0 | 0 | 0 |
| Hemoptysis | 5 | 29.4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hand-foot syndrome | 2 | 11.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 3 | 17.6 | 1 | 5.9 | 1 | 5.9 | 0 | 0 |
| Stomach ache | 3 | 17.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 2 | 11.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Proteinuria | 3 | 17.6 | 0 | 0 | 0 | 0 | 0 | 0 |
4. Discussion
This study retrospectively analyzed 17 advanced esophageal cancer patients who refused or not intolerate to intravenous chemotherapy and received anlotinib combined with S-1 chemotherapy. In 17 patients, the ORR was 35.3%, the DCR was 82.4%, the PFS was 3.5 months, and the median OS was 5.2months.
Chemotherapy therapy is the cornerstone of advanced esophageal cancer, such as cisplatin, oxaliplatin, 5-flourouracil, but mainly given by intravenous injection.[12] Therefore, the patients who refused or were intolerant to intravenous chemotherapy have limited treatment options. Moreover, patients with older and poor performance status had a poor response to chemotherapy. S-1 is an orally antitumor chemotherapy agent, and anlotinib an orally multi-targeted tyrosine kinase inhibitor showing a promising antitumor activity and manageable toxicity in advanced esophageal squamous-cell carcinoma. Anlotinib and S-1 combination is a convenient and effective regimen. In 10 patients receiving first-line treatment, the ORR was 50%, the DCR was 80%, the median PFS was 4.5 months, and the median OS was 5.8 months. Compared with previous studies, the ORR of 5-FU and platinum as the first-line treatment for patients with relapsed advanced esophageal cancer is reported to be 35% to 40%.[7] Our outcomes suggest that anlotinib combined with S-1 could be an effective treatment option for advanced esophageal cancer patient who are older, poor performance score, and unwilling or intolerant to intravenous chemotherapy. In 7 patients with second-line or further-line treatment, the ORR was 14.3%, DCR was 85.7%, the median PFS was 3.0 months, and the median OS was 3.7 months. At present, the palliative single-agent chemotherapy or programmed cell death-1 (PD-1) inhibitor is commonly used for second-line treatment, but the efficiency of single-agent chemotherapy is maintained at about 10%, such as paclitaxel, docetaxel, irinotecan and are given by intravenous injection. The patients with a good ECOG PS (PS 0–1) produced a response rate of about 20% after receiving single-agent PD-1 inhibitor. In the study, patients enrolled were older, poor performance status and unwilling or intolerant to intravenous chemotherapy, with 2 PS scores accounted for 70.6%. Our outcomes suggest that anlotinib combined with S-1 could be an effective treatment option for those patients. The effectiveness of new drug treatments such as gefitinib, icotinib, and nivolumab is not ideal.[13,14] Apatinib, an orally tyrosine kinase inhibitor, can specifically inhibit vascular endothelial growth factor receptor 2. Wu Yayun and others reported that the use of apatinib combined with S-1 in the treatment of advanced esophageal cancer patients can significantly improve the clinical efficacy and quality of life. Zhao et al reported that the median PFS of apatinib combined with S-1 in the second-line treatment of advanced esophageal cancer was 6.23 months, and the median OS was 8.83 months.[15] The DCR and ORR were 91.67% and 16.67%, respectively, and the adverse reactions were tolerable. It also proved that the use of antiangiogenic agents combined with S-1 had better survival benefits.
In safety evaluation, the most common AEs of anlotinib combined with S-1 include fatigue, hypertension, bone marrow suppression, hemoptysis, nausea, proteinuria, fever, and liver function impairment. Grade 3 AEs included hypertension, nausea, and no treatment-related deaths. Many studies reported that compared with single-agent intravenous chemotherapy, multi-agent intravenous chemotherapy increased the toxicity and side effects significantly, making serious adverse reactions up to 17.1% to 48.8% and treatment-related deaths up to 16%.[13,14] The 17 patients with relapse and metastasis were unwilling or intolerant to intravenous chemotherapy, but they were able to tolerate anlotinib combined with S-1. These results suggested that the anlotinib combined with S-1 could be well tolerated and safe. Anlotinib as an anti-vascular drug, the main side effects include bleeding, hypertension, and proteinuria. VEGF plays an important role in the function of normal physiological blood vessels to form blood vessels. Anlotinib might affect the growth and proliferation of normal endothelial cells, causing endothelial cell dysfunction.[16] Five patients in the anlotinib combined with S-1 developed grade 1–2 hemoptysis, and all symptoms were significantly relieved after symptomatic treatment. Anlotinib can also inhibit vasodilation through VEGF and produce vasodilator molecule nitric oxide and other mechanisms leading to increased blood pressure.[17,18] One patient achieved grade 3 hypertension, and the blood pressure was still high after multi-drug combined with antihypertensive therapy, and discontinued anlotinib. The past history should be asked in detail before drug treatment, and blood pressure changes should be monitored daily before and during treatment. VEGF is widely expressed in renal tissue cells and maintains the normal structure and function of glomerular endothelial cells. Anlotinib inhibits the production of glomerular endothelial cells and podocytes by inhibiting the production of VEGF, affecting the formation of glomerular capillaries, and ultimately destroys glomerular filtration function resulting in proteinuria.[19] In this study, 3 patients developed grade 1–2 proteinuria and continued to be treated with anlotinib. If the urine protein quantitative ≥2 g/24 h, anlotinib need to be suspended and given symptomatic treatment. The anlotinib can be used after the urine protein quantitative is less than 2 g/24 h.
With the rapid development of targeted drugs, the application of precision and individualized treatment of tumors has become more and more widespread. For patients with recurrent and metastatic advanced esophageal cancer, especially patients with poor efficacy of intravenous chemotherapy, older age, and intolerant to intravenous chemotherapy, we hope to use antiangiogenic agents combined with chemotherapy to improve patients’ symptoms, improve their quality of life, and prolong their survival time.
This study is a retrospective study with some limitations, such as no control group. More randomized clinical studies with large samples are needed to further clarify the efficacy and adverse reactions of anlotinib combined with S-1 in patients with recurrent and metastatic advanced esophageal cancer.
In this study, anlotinib combined with S-1 achieved promising disease control and satisfactory survival with tolerable safety in recurrent metastatic esophageal cancer who were unwilling or intolerant to intravenous chemotherapy. In the future, large-sample, prospective, randomized clinical trials will further study the role of anlotinib in patients with metastatic advanced esophageal cancer.
Acknowledgments
The authors thank the participants, the coordinators, and administrators for their supports during the study.
Author contributions
Data curation: Jing Cai, Anwen Liu.
Formal analysis: Jing Cai, Shan Zhou.
Investigation: Jing Cai, Anwen Liu.
Methodology: Jing Cai, Anwen Liu.
Writing – original draft: Jing Cai, Shan Zhou.
Writing – review & editing: Yuxi Luo, Anwen Liu.
Footnotes
Abbreviations: AEs = adverse events, CR = complete response, DCR = disease control rate, ORR = objective response rate, OS = overall survival, PFS = progress free survival, PD = disease progression, PD-1 = programmed cell death-1, PR = partial response, SD, stable disease, VEGFR 2, vascular endothelial growth factor receptor 2.
How to cite this article: Cai J, Zhou S, Luo Y, Liu A. Effect and safety of anlotinib combined with S-1 for recurrent or metastatic esophageal cancer patients who refused or were intolerant to intravenous chemotherapy. Medicine. 2021;100:51(e28126).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med 2017;14:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yousheng M, Shugeng G, Qun W. Big data analysis of clinical epidemiological characteristics and surgical treatment profiles of esophageal cancer in China. Chin J Oncol 2020;3:228–33. [Google Scholar]
- [3].Ni W, Yang J, Deng W, et al. Patterns of recurrence after surgery and efficacy of salvage therapy after recurrence in patients with thoracic esophageal squamous cell carcinoma. BMC Cancer 2020;20:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zeng Y, Yu W, Liu Q, et al. Difference in failure patterns of pT3-4N0-3M0 esophageal cancer treated by surgery vs surgery plus radiotherapy. World J Gastrointest Oncol 2019;11:1172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mikhail S, Wei L, Salem ME, Bekaii-Saab T. Outcomes of definitive chemoradiation in patients with esophageal cancer. Dis Esophagus 2017;30:01–7. [DOI] [PubMed] [Google Scholar]
- [6].Taniyama Y, Sakurai T, Heishi T, et al. Different strategy of salvage esophagectomy between residual and recurrent esophageal cancer after definitive chemoradiotherapy. J Thorac Dis 2018;10:1554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hiramoto S, Kato K, Shoji H, et al. A retrospective analysis of 5-fluorouracil plus cisplatin as first-line chemotherapy in the recent treatment strategy for patients with metastatic or recurrent esophageal squamous cell carcinoma. Int J Clin Oncol 2018;23:466–72. [DOI] [PubMed] [Google Scholar]
- [8].Hamai Y, Hihara J, Emi M, et al. Treatment outcomes and prognostic factors after recurrence of esophageal squamous cell carcinoma. World J Surg 2018;42:2190–8. [DOI] [PubMed] [Google Scholar]
- [9].Haiying W, Hong T, Mengqiang Z. Clinical effect of tegio in the treatment of advanced esophageal squamous cell carcinoma in the elderly. Chin J Gerontol 2018;38:1103–5. [Google Scholar]
- [10].Jianyi Y, Yongting Y, Zhihua Z. Therapeutic effect analysis of 15 cases of elderly esophageal cancer treated with tegio. Mod Pract Med 2015;27:1359–60. [Google Scholar]
- [11].Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci 2018;109:1207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tanaka Y, Yoshida K, Yamada A, et al. Phase II trial of biweekly docetaxel, cisplatin, and 5-fluorouracil chemotherapy for advanced esophageal squamous cell carcinoma. Cancer Chemother Pharmacol 2016;77:1143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ku GY. Systemic therapy for esophageal cancer: chemotherapy. Chin Clin Oncol 2017;6:49. [DOI] [PubMed] [Google Scholar]
- [14].Forde PM, Kelly RJ. Chemotherapeutic and targeted strategies for locally advanced and metastatic esophageal cancer. J Thorac Oncol 2013;8:673–84. [DOI] [PubMed] [Google Scholar]
- [15].Zhao J, Lei J, Yu J, et al. Clinical efficacy and safety of apatinib combined with S-1 in advanced esophageal squamous cell carcinoma. Invest New Drugs 2020;38:500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018;11:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shao L, Wang W, Song Z, Zhang Y. The efficacy and safety of anlotinib treatment for advanced lung cancer. Onco Targets Ther 2019;12:6549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Si X, Zhang L, Wang H, et al. Management of anlotinib-related adverse events in patients with advanced non-small cell lung cancer: experiences in ALTER-0303. Thorac Cancer 2019;10:551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]