Abstract
The aim of this study was to investigate the modulation of gut microbiota by fermented raspberry juice (FRJ) both in vitro and in vivo. Results showed that total phenolic content and antioxidant activities of FRJ reached the highest after fermentation for 42 h. Seventeen phenolic compounds were contained in FRJ, mainly including ellagic acid (496.64 ± 2.91 μg/g) and anthocyanins (total concentration: 387.93 μg/g). FRJ modulated the gut microbiota into a healthy in vitro status, with increase of valeric and isovaleric acids production. In healthy mice, all FRJ treatments improved the production of acetic, butyric and isovaleric acids as well as the gene expression of ZO-1, Claudin-1, Claudin-4, Ocdudin, E-cadherin and Muc-2. Moreover, variable gut microbial compositions were found among the groups fed diet-supplemented the different doses of FRJ, within low and median doses of FRJ may regulate the microbiota to a healthier state compared to the high dose supplementation. This study indicated that fermentation is a potential way to produce plant-based juices, which could reshape the gut microbiota and improve the host health.
Keywords: non-dairy probiotic juice, Lactobacillus fermentation, polyphenols, antioxidant activity, microbiome
1. Introduction
Raspberry is one of the most popular berries around the world, especially in Europe, America, Australia and China, due to its unique flavour and various health benefits [1]. Raspberries are a rich source of bioactive compounds, such as vitamin A, C, and polyphenols [2]. It was reported that the health benefits of raspberries are primarily mediated by phenolic compounds, mainly ellagitannins, ellagic acid and anthocyanins [3,4]. As a perishable soft fruit, raspberries are often processed and consumed as wines, juices, and jams [5]. However, processing techniques (e.g., thermal processing) may alter the bioactive compounds in raspberries, leading to a decrease in the health-promoting properties of the end products [6]. Thus, it is a challenge for the food industry to find alternative ways to preserve the nutritional and nutraceutical properties of fruit products.
As fruit juices have been revealed to be potential carriers for probiotics [7], fermentation by probiotic lactic acid bacteria (LAB) is a promising way to improve the nutritional characteristics and health-related aspects of plant-based juices [8]. So far, several studies regarding the fermentation of raspberry juice by LAB have been carried out, mainly focused on the changes in chemical compounds, including acid, glucose, and polyphenol contents, as well as in vitro antioxidant capacity [9,10]. However, to our knowledge, no in vivo investigation on the health benefits of LAB-fermented raspberry juice (FRJ) has been published.
Recently, the gut microbiota has attracted intense attention due to its important role in host health [11]. Accumulating evidence supported the effect of berry polyphenols on modulating gut microbiota and decreasing risks of microbiota-associated diseases [12]. Our previous research showed that Lactobacillus casei fermentation increased the phenolic contents in blueberry pomace and the fermented pomace improved the fecal microbial community structure [13], which indicates that L. casei fermentation may enhance the gut-improving functions of berry products. Whereas, information is lacking regarding the influence of FRJ on gut microbial community structure and functionality. Therefore, the objective of this study is to investigate the gut microbial modulation by L. casei FRJ both in vitro and in vivo. As a complementary analysis, antioxidant capacity and effects of FRJ on the gene expressions of colon mechanical barrier were also measured.
2. Materials and Methods
2.1. Materials
The raspberries (Rubus idaeus L.) were kindly provided by Gold berry Co., Ltd. (Tianmen, Hubei, China) and stored at −20 °C for further use. Lactobacillus casei (CICC 20280) was used for fermentation.
2.2. Preparation and Fermentation of Raspberry Juice
Frozen raspberries were thawed at room temperature, then mixed with distilled water (1:1, m/v) in a juice blender (300 W) for 1 min. The mixture was homogenized for 5 min, then the whole raspberry juice was collected and stored at 4 °C until further processing.
After pasteurization at 80 °C for 15 min, the sterilized juice was fermented with 5% (v/v) of L. casei in exponential phase at 37 °C for 0, 18, 42 and 72 h without agitation, comprising the fermented raspberry juice (FRJ) group. The non-fermented raspberry juice (NFRJ) group was inoculated with 5% (v/v) of MRS broth. After fermentation, the bacteria counts and pH values were determined. After centrifuge, FRJ and NFRJ supernatants were collected for antioxidant activity determination.
2.3. Determination of Antioxidant Activity
2.3.1. The 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) Radical Cation Scavenging Activity Assay (ABTS)
The ABTS was carried out following the previous study [14] with slight modification. In brief, the supernatant of FRJ/NFRJ (200 μL) was mixed with 0.8 mL ABTS solution, then incubated in dark at 25 °C for 6 min. The absorbance of the mixture was recorded at 734 nm.
2.3.2. The 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Activity (DPPH)
DPPH was measured according to the previous method [15] with some modification. Briefly, supernatant of FRJ/NFRJ (200 μL) was mixed with DPPH solution (1.8 mL, 0.08 mg/mL), incubating in dark at 25 °C for 20 min. Absorbance was recorded at 517 nm.
2.4. The Total Phenolic Content (TPC) Analysis
The TPC of FRJ/NFRJ was determined by the Folin–Ciocalteu method. A half milliliter of ciocalteu reagent (50%) was mixed with 1.0 mL of sample. Then Na2CO3 (5%, 1.0 mL) was added and mixed for 1 min. After incubation (25 °C, 60 min), the absorbance of the mixture was measured at 760 nm.
2.5. In Vitro Digestion and Colonic Fermentation
In vitro digestion of 25 mL sample was carried out as described previously [16]. Afterward, the in vitro colonic fermentation of the FRJ/NFRJ digestion(45 mL, equivalent to 10% of diet) was carried out as descried previously [17]. After centrifuging at 7000× g, 4 °C for 20 min, precipitates were used for DNA extraction while supernatants were used for short chain fatty acids (SCFAs) analysis, storing at −20 °C until use.
2.6. Determination of Phenolic Compounds in FRJ
Phenolic compounds of the FRJ sample were detected using UPLC-MS (Thermo Scientific Q Exactive, Newton Drive, Carlsbad, USA)and analyzed using Xcalibur (Thermo Scientific, Newton Drive, Carlsbad, CA, USA) as described previously [18]. All standards were dissolved to 1 mmol/L by dimethyl sulfoxide respectively, then 50 μL standard solutions were mixed and added the diluent (acetonitrile:water = 3:7, both with 0.1% formic acid-water) to 500 μL.
2.7. Animal Study
All the animal procedures and experiments were strictly carried out according to the legislation for care and use of laboratory animals of China and the U.K. Animals (Scientific Procedures) Act (1986). The study was approved by the Animal Ethics Committee of Huazhong Agricultural University (permission no. SYXK (Hubei) 2015-0084). Male Kun Ming mice (17–22 g) were purchased from Huazhong Agricultural University, China, and randomly allocated to 4 groups of 8 animals each. All the mice were provided with a 12 h light/dark cycle under a specific pathogen-free condition with relative humidity (40–60%) and steady temperature (22–24 °C). Diets and sterile water were provided ad libitum. For 30 days, each group was fed an experimental diet with different portions of freeze-dried FRJ powders as follows: (1) Standard diet without FRJ supplementation (group C), (2) Standard diet with 3% (wt:wt) FRJ supplementation (group L), (3) Standard diet with 6% (wt:wt) FRJ supplementation (group M), (4) Standard diet with 9% (wt:wt) FRJ supplementation (group H). The doses of freeze-dried FRJ powders, as well as the preparation of experiment diets, were set according to previous studies [19]. Details about the composition of the standard diet AIN93 are shown in Supplementary Information Table S1. The weight and feed utilization rate of the mice were recorded every 2 days.
After 30 days of feeding, the mice were deprived of food for 14 h and then euthanized by diethyl ether inhalation and killed by cervical dislocation. The heart, liver, kidneys and spleen were collected to calculate organ index. All samples were immediately frozen and stored at −80 °C for further analysis.
2.8. In Vivo Redox Status Analysis
The hepatic and colonic tissues were homogenized in pre-cooled PBS (m/v = 1:9) and centrifuged at 10,000× g, 4 °C for 10 min. The total antioxidant capacity (T-AOC, A015-1-2) and superoxide dismutase (SOD, A001-1-2) in homogenates were measured using kits for mice (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions.
2.9. DNA/RNA Extraction and qPCR
The fecal microbial DNA extraction of precipitates from Section 2.5 and the total RNA extraction of colon tissues from Section 2.7 was performed according to the previous research [18].
The quantity of gut microbiota was amplified by specific primers (Supplementary Information Table S2) of Akkermansia, Bacteroides, Bifidobacterium, butyric acid-producing bacteria, Escherichia coli, Enterococcus, Lactobacillus and Ruminococcus. Primers (Supplementary Information Table S3) of ZO-1(zonula occluden-1), Claudin-1, Claudin-4, Occludin, E-cadherin and Muc-2(mucin-2) were designed using BLAST (Basic local alignment search tool) of NCBI (USA) and synthesized by Tsingke Biotech Co., Ltd. (Wuhan, China). The program used for amplification was detailed described in the study of Tang et al. [18]. The relative expression of the intestinal barrier related genes was normalized by internal reference gene GADPH.
2.10. Analysis of 16S rRNA Illumina Sequencing
The total bacterial DNA was extracted using Omega Stool DNA Kit (Omega Bio-Tek, USA) according to the manufacturer’s instructions. The V3-V4 regions of bacterial 16S rDNA were amplified by PCR using forward primer (5′-ACTCCTACGGGAGGCAGCAG-3′) and reverse primer (5′-GGACTACHVGGGTWTCTAAT-3′). Samples were sequenced on Illumina HiSeq platforms according to the manufacturer’s manual at Biomarker Technologies Co, Ltd. (Beijing, China).
2.11. SCFAs Analysis
Concentrations of 5 SCFAs in supernatants from Section 2.5 and feces from Section 2.7 were determined by gas chromatography according to the previous research [13]. The internal standard (2-ethylbutyric acid) and external standards (acetic, propionic and butyric acids) were purchased from Yuanye Biotech Co., Ltd. (Shanghai, China).
2.12. Statistical Analysis
Data were presented as the mean ± standard deviation. ANOVA and Pearson’s correlation analysis were carried out using SPSS 16.0. Statistically significant differences at p < 0.05 were calculated using Ducan test.
3. Results
3.1. Growth of L. casei and pH of FRJ
The number of L. casei and the pH value of FRJ with different fermentation time are shown in Supplementary Information Table S4. The density of L. casei in FRJ was first decreased from 0 to 18 h, then rebounded to the initial level at 42 h. After 72 h, no colony was observed. The pH value of FRJ was stable around 3.15 during the first 18 h, then significantly dropped to 3.05 at 42 h, and slightly increased to 3.10 at 72 h. For NFRJ, no significant change of the pH was observed during fermentation.
3.2. Antioxidant Activities of FRJ and NFRJ
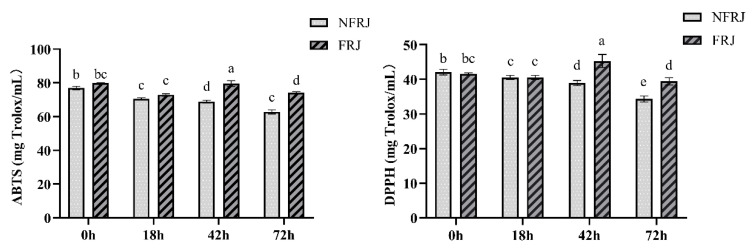
The antioxidant activities (ABTS and DPPH) of FRJ/NFRJ are listed in Figure 1. Both assays showed the same trend of NFRJ antioxidant activity, which gradually decreased over the time, whereas the antioxidant activity of FRJ reached the highest value at 42 h. In addition, the antioxidant activity of FRJ was significantly higher than that of NFRJ at 42 and 72 h.
Figure 1.
Antioxidant capacities (ABTS and DPPH) of NFRJ and FRJ. Different letters indicated significantly different at p < 0.05. ABTS:The 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical cation scavenging activity assay; DPPH:The 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity; NFRJ:Non-fermented raspberry juice; FRJ: Fermented raspberry juice.
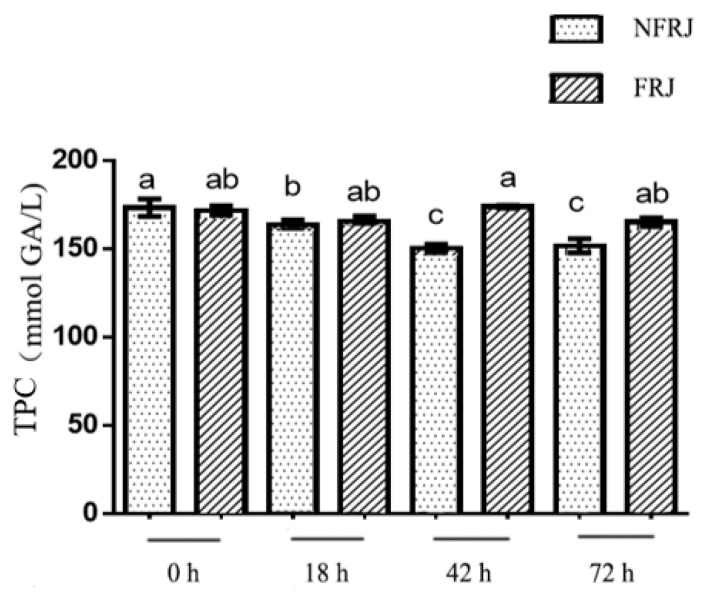
3.3. The Total Phenolic Content (TPC) of FRJ and NFRJ
The TPC of FRJ and NFRJ are displayed in Figure 2. With increasing fermentation time, the TPC of NFRJ decreased while no significant difference in the FRJ TPC was observed. The TPC of FRJ was significantly higher than the TPC of NFRJ at 42 h (173.67 ± 2.16 versus (vs) 150.19 ± 6.04 mmol gallic acid/L) and 72 h (165.00 ± 6.35 vs. 151.64 ± 10.29 mmol gallic acid/L).
Figure 2.
Total phenolic content(TPC) of NFRJ and FRJ. Different letters indicated significantly different at p < 0.05.
3.4. Determination of Phenolic Compounds in FRJ
As shown in Table 1, a total of 17 phenolic compounds were quantified in the FRJ, including 5 anthocyanins, 2 flavanols, 4 flavonols, and 6 phenolic acids. The dominant compounds were ellagic acid (496.64 μg/g) and anthocyanins (total concentration: 387.93 μg/g).
Table 1.
Phenolic composition in FRJ.
| No. | Rt (min) |
[M−H]+ | Error (ppm) |
MS2 | [M−H]− | Error (ppm) |
MS2 | Formula | Identification | Concentration (μg/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Anthocyanins | ||||||||||
| 1 | 5.49 | 449.10703 | −3.020 | 287 | C21H20O11 | cyanidin-3-O-glucoside | 298.34 ± 2.58 | |||
| 2 | 5.69 | 595.16431 | −3.335 | 287 | C27H30O15 | cyanidin-3-O-rutinoside | 10.06 ± 0.05 | |||
| 3 | 6.30 | 463.12244 | −3.447 | 301 | C22H22O11 | peonidin-3-O-glucoside/ peonidin-3-O-galactcoside |
2.20 ± 0.01 | |||
| 4 | 7.38 | 287.05429 | −4.434 | 287 | C15H10O6 | cyanidin | 58.22 ± 1.44 | |||
| 5 | 9.31 | 465.10172 | −3.399 | 303 | C21H20O12 | delphinidin-3-O-glucoside/ delphinidin-3-O-galactcoside |
19.11 ± 0.25 | |||
| Total Anthocyanins | 387.93 | |||||||||
| Flavanols | ||||||||||
| 6 | 6.07 | 289.07187 | 2.273 | C15H14O6 | catechin | 0.04 ± 0.00 | ||||
| 7 | 6.47 | 577.13519 | 1.021 | C30H26O12 | procyanidin B | 32.17 ± 1.03 | ||||
| Total Flavanols | 32.21 | |||||||||
| Flavonols | ||||||||||
| 8 | 5.50 | 447.09314 | 0.903 | 300 | C21H20O11 | quercetin-3-O-rhamnoside | 10.03 ± 0.15 | |||
| 9 | 9.30 | 463.08835 | 1.510 | 300 | C21H20O12 | quercetin-3-O-glucoside/ quercetin-3-O-galactcoside |
5.07 ± 0.04 | |||
| 10 | 13.06 | 301.03534 | 1.703 | C15H10O7 | quercetin | 16.42 ± 0.20 | ||||
| 11 | 14.97 | 285.04041 | 1.744 | C15H10O6 | kaempferol | 0.06 ± 0.00 | ||||
| Total Flavonols | 31.58 | |||||||||
| Phenolic acids | ||||||||||
| 12 | 2.55 | 169.01335 | −2.060 | C7H6O5 | gallic acid | 0.12 ± 0.00 | ||||
| 13 | 5.94 | 137.02328 | −4.298 | C7H6O3 | p -hydroxybenzoic acid | 0.36 ± 0.02 | ||||
| 14 | 8.53 | 153.01830 | −3.160 | C7H6O4 | protocatechuic acid | 0.27 ± 0.00 | ||||
| 15 | 6.85 | 179.03415 | −1.584 | C9H8O4 | caffeic acid | 0.46 ± 0.01 | ||||
| 16 | 8.81 | 300.99899 | 1.821 | C14H6O8 | ellagic acid | 496.64 ± 2.91 | ||||
| 17 | 13.38 | 147.04410 | −3.430 | C9H8O2 | cinnamic acid | 0.29 ± 0.05 | ||||
| Total phenolic acids | 498.14 | |||||||||
Rt, retention time; [M−H]+, precursor ion obtained from positive mode; [M−H]−, precursor ion obtained from negative mode; MS2, fragment ions of precursor ion obtained from tandem mass spectrum.
3.5. Effects of FRJ on Fecal Microbiota In Vitro
After 42 h fermentation and in vitro digestion, the effect of FRJ on 8 fecal bacteria was shown in Table 2. Compared to NFRJ, FRJ increased the abundance of Escherichia coli, butyric acid-producing bacteria, Lactobacillus and Akkermansia in varying degrees, among which the increase of Lactobacillus was the highest (5.56 fold). On the other hand, FRJ treatment decreased the abundance of Bacteroides and Ruminococcus. No significant change in the abundance of Bifidobacterium and Enterococcus was observed.
Table 2.
The relative abundance of fecal microbiota with FRJ and NFRJ treatment.
| Bacteroides | Bifidobacterium | Ruminococcus | Escherichia coli | Butyrate- Producing Bacteria |
Lactobacillus | Enterococcus | Akkermansia | |
|---|---|---|---|---|---|---|---|---|
| NFRJ | 1.00 ± 0.00 a | 1.00 ± 0.00 b | 1.00 ± 0.00 a | 1.00 ± 0.00 c | 1.00 ± 0.00 b | 1.00 ± 0.00 c | 1.00 ± 0.00 a | 1.00 ± 0.00 b |
| FRJ | 0.16 ± 0.02 b | 1.14 ± 0.12 b | 0.82 ± 0.14 b | 1.28 ± 0.42 b | 1.37 ± 0.22 a | 5.56 ± 0.19 b | 1.06 ± 0.14 a | 1.74 ± 0.14 a |
Different letters indicated significantly different at p < 0.05. The NFRJ sample was used as the control, of which the relative abundance of the microbiota was set as 1.00.
3.6. Effects of FRJ and NFRJ on In Vitro Production of Short Chain Fatty Acids (SCFAs)
The SCFAs production of in vitro colonic fermentation is presented in Table 3. Compared to NFRJ, treatment with FRJ significantly increased the concentration of valeric (1.63 mmol/L vs. 1.23 mmol/L) and isovaleric acids (8.03 mmol/L vs. 3.94 mmol/L). Whereas, no significant difference in acetic, propionic, and butyric acids production was observed between NFRJ and FRJ treatments.
Table 3.
SCFAs production of in vitro colonic fermentation with FRJ and NFRJ treatments.
| Group | Acetic Acid (mmol/L) | Propionic Acid (mmol/L) |
Butyric Acid (mmol/L) |
Valeric Acid (mmol/L) |
Isovaleric Acid (mmol/L) |
|---|---|---|---|---|---|
| NFRJ | 275.07 ± 24.38 a | 5.37 ± 1.86 a | 16.69 ± 2.06 a | 1.23 ± 0.23 b | 3.94 ± 0.24 b |
| FRJ | 266.83 ± 12.92 a | 4.54 ± 0.23 ab | 17.75 ± 0.62 a | 1.63 ± 0.05 a | 8.03 ± 0.29 a |
Different letters indicated significantly different at p < 0.05.
3.7. Effects of FRJ on Growth Performance of Mice
As shown in Supplementary Information Figure S1 and Table S5, there was no significant difference in body weight, feed utilization rate nor organ index among the different groups.
3.8. Effects of FRJ on Redox Status in the Liver and Colon of Mice
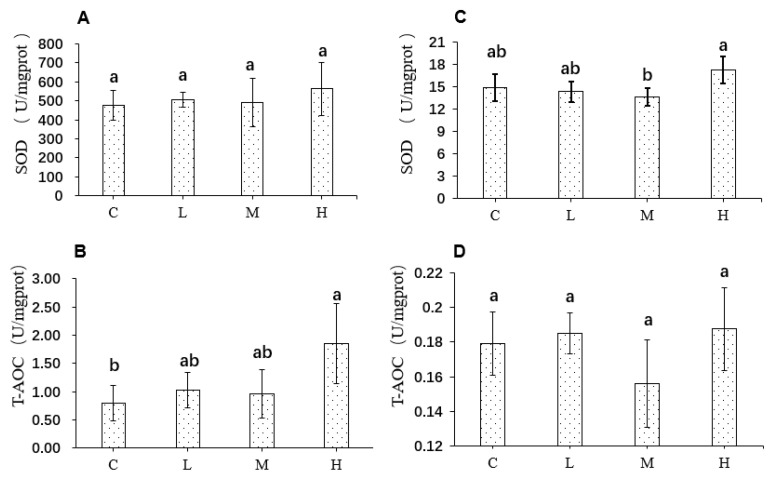
The SOD and T-AOC levels in the liver and colon of mice are shown in Figure 3. In the liver, there was no significant difference in SOD level among the four groups, while T-AOC significantly increased in the group treated with high dose FRJ compared to the control group (1.85 ± 0.71 and 0.79 ± 0.31 U/mgprot, respectively). In the colon, no significant difference in T-AOC was observed among the four groups. Also, there was no significant difference in SOD level between the FRJ groups and the control group. However, the SOD of group H was higher than that of group M (17.3 ± 1.8 and 13.7 ± 1.2 U/mgprot, respectively, p < 0.05).
Figure 3.
Redox status in the liver (A,B) and colon (C,D) of mice after treatments of FRJ. C: mice fed with standard diet; L: mice fed with 3% (wt:wt) FRJ supplementation; M: mice fed with 6% (wt:wt) FRJ supplementation; H: mice fed with 9% (wt:wt) FRJ supplementation. Different letters indicated significantly different at p < 0.05. SOD:Superoxide dismutase; T-AOC:Total antioxidant capacity.
3.9. Effects of FRJ on Fecal Microbiota In Vivo
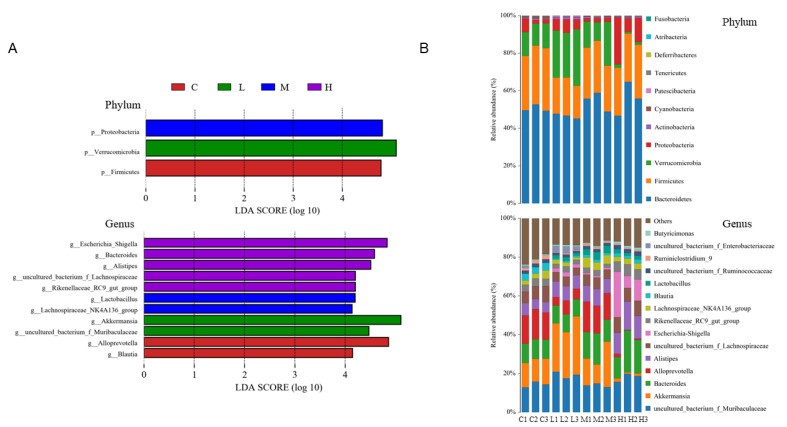
Effects of FRJ on the gut microbiota of mice was evaluated by 16S rRNA Illumina sequence. Although there was no difference in the alpha diversity among all groups (data not shown), the principal component analysis (PCA) showed that FRJ treatments altered the beta diversity of mice, especially in groups L and H, which presented a very distinct cluster from the control group (Supplementary Information Figure S2). Moreover, linear discriminant analysis (LDA) effect size (LEfSe) analysis was performed to identify the statistically significant biomarkers at phylum and genus level of each group (Figure 4A).
Figure 4.
The dominant composition of gut microbiota after FRJ treatments. (A) Linear discriminant analysis (LDA) effect size (LEfSe) analyses at phylum and genus level (LDA score > 4.0). (B) Relative abundance of microbiota at phylum and genus level. C: mice fed with standard diet; L: mice fed with 3% (wt:wt) FRJ supplementation; M: mice fed with 6% (wt:wt) FRJ supplementation; H: mice fed with 9% (wt:wt) FRJ supplementation. Each number of the samples represents one replicate.
The main composition of group M and the control was quite similar, as their dominant phyla were Bacteroidetes (50.50% and 54.48%), Firmicutes (31.27% and 26.51%), Verrucomicrobia (12.55% and 15.68%) and Proteobacteria (3.64% and 1.99%), respectively, in descending order. Treatment with FRJ significantly decreased the Firmicutes to Bacteroidetes ratio in groups L, M, and H (40.99%, 48.76%, 48.80%, respectively) compared to the control group (61.98%). In addition, Verrucomicrobia was significantly increased to 26.29% in group L, while considerably dropped to 1.36% in group H. Among all groups, the group H showed the most different gut microbiota with a very high relative abundance of Proteobacteria (14.96%).
At the genus level, compared to the control group, all the groups treated with FRJ exhibited a significant decrease in the relative abundance of Blautia, Ruminiclostridium_9, and an increase in the relative abundance of Lactobacillus (especially group M). Alistipes, Escherichia-Shigella, Rikenellaceae_RC9_gut_group and Butyricimonas were significantly increased only in group H. On the other hand, a significant increase of Muribaculaceae and Enterobacteriaceae were only found in group L. Furthermore, the low and high dose FRJ treatments even had some reverse effects. Besides, the abundance of Akkermansia markedly increased to 26.29% in the group L (p < 0.05 vs. control) while considerably decreased to 1.36% in the group H (p < 0.05 vs. control).
3.10. Effects of FRJ on Production of SCFAs in Mice
As shown in Table 4, four SCFAs were determined and the administration of FRJ increased the production of acetic, butyric and isovaleric acids (p < 0.05) while no significant difference in propionic acid production was observed among the four groups. Among the three groups treated with FRJ, only the group M presented a higher content of acetic and butyric acids (378.63 and 395.48 mmol/L, respectively), indicating that the median dose of FRJ improved acetic and butyric acids production in mice.
Table 4.
SCFAs production of mice after treatments of FRJ.
| Group | Acetic Acid (mmol/L) | Propionic Acid (mmol/L) |
Butyric Acid (mmol/L) |
Isovaleric Acid (mmol/L) |
|---|---|---|---|---|
| C | 281.42 ± 8.29 c | 331.57 ± 60.75 a | 308.10 ± 42.75 b | 162.46 ± 12.59 b |
| L | 358.65 ± 14.44 b | 366.74 ± 57.29 a | 350.27 ± 17.38 ab | 169.08 ± 31.04 b |
| M | 378.63 ± 40.78 a | 377.61 ± 19.52 a | 395.48 ± 20.66 a | 207.00 ± 11.03 a |
| H | 342.17± 8.40 b | 362.43 ± 30.35 a | 374.40 ± 23.03 a | 202.75 ± 11.96 a |
C: mice fed with standard diet; L: mice fed with 3% (wt:wt) FRJ supplementation; M: mice fed with 6% (wt: wt) FRJ supplementation; H: mice fed with 9% (wt:wt) FRJ supplementation. Different letters indicated significantly different at p < 0.05.
3.11. Effects of FRJ on the Gene Expression of Colon Mechanical Barrier
Table 5 demonstrates that the intake of the 3 different doses of FRJ significantly improved the gene expression of ZO-1, Claudin-1, Claudin-4, Occludin, E-cadherin, and Muc-2 compared to the control. In addition, groups M and H had a better increase of those 6 genes than the group L.
Table 5.
Effects of FRJ on gene expression of mechanical barrier of colon.
| Group | ZO-1 | Claudin-1 | Claudin-4 | Occludin | E-cadherin | Muc-2 |
|---|---|---|---|---|---|---|
| C | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a |
| L | 1.41 ± 0.13 b | 1.31 ± 0.13 b | 1.30 ± 0.06 b | 1.13 ± 0.08 b | 1.19 ± 0.09 b | 1.49 ± 0.21 b |
| M | 1.82 ± 0.06 c | 1.48 ± 0.13 c | 1.43 ± 0.08 c | 1.33 ± 0.11 c | 1.35 ± 0.24 b | 1.82 ± 0.33 c |
| H | 1.58 ± 0.10 bc | 1.61 ± 0.04 d | 1.42 ± 0.12 c | 1.32 ± 0.05 c | 1.30 ± 0.07 b | 1.67 ± 0.18 bc |
C: mice fed with standard diet; L: mice fed with 3% (wt:wt) FRJ supplementation; M: mice fed with 6% (wt:wt) FRJ supplementation; H: mice fed with 9% (wt:wt) FRJ supplementation. Different letters indicated significantly different at p < 0.05.
4. Discussion
The present study, for the first time, investigated the influence of FRJ on gut microbiota both in vitro and in vivo. It has demonstrated that (i) after L. casei fermentation for 42 h, the total phenolic content and antioxidant capacity of raspberry juice was improved in vitro, whereas, only high dose of FRJ intake slightly improved antioxidant activity in vivo, (ii) FRJ reshaped the microbial composition associated with increase in SCFAs production in vitro and in vivo, (iii) the different doses of FRJ had different regulating effects on microbial community structure in vivo. Furthermore, low and median doses of FRJ may regulate the microbiota to a healthier state in the groups L and M compared to the high dose group.
Upon the L. casei fermentation, the growth of L. casei decreased during the first 18 h. There was also a slight decrease of LAB in Punica granatum juice at the early fermentation stage, which was explained by the stress-induced due to the difference of pre-culture and fermentation medium [20]. From 18 h to 42 h, the drop of pH was accompanied by increased microbial growth, probably due to the production of lactic acid by LAB fermentation. It was reported that L. casei was able to grow about 2 Log cycles in elderberry juice after 48 h fermentation [21]. However, in the present study, L. casei only rebounded to its initial inoculum at 42 h and could no longer survive after 72 h. As it was reported that soluble solids in raspberry juice were the least compared to blackcurrant and red kiwifruit juices [22], raspberry juice may not be a perfect matrix without nutritional supplementation for LAB fermentation.
The antioxidant capacities of the NFRJ determined by ABTS and DPPH decreased over the time in the same trend as the TPC, whereas the L. casei fermentation relieved these declines. At 42 h, the antioxidant activity and the TPC of FRJ were both significantly higher than those of NFRJ. Similar results were reported by Ryu et al., showing that TPC, flavonoid content, and DPPH radical scavenging activity of the black raspberry juice increased significantly after LAB fermentation [23]. This positive correlation between antioxidant activity and TPC was also demonstrated in our previous study on blueberry pomace fermented by L. casei [13]. The increase of antioxidant capacity may due to the LAB fermentation, as bound polyphenols might be released and degraded into smaller phenolic compounds, exerting higher bioactivity [24]. It is worth noting that, from 42 h to 72 h, the antioxidant activity of FRJ decreased (p < 0.05), while TPC was relatively stable. Combined with the fact that no L. casei was detected at 72 h, the improvement of antioxidant activity was partly due to L. casei. Likewise, previous studies have demonstrated the potential effect of LAB strains on the decrease of oxidative stress and the accumulation of reactive oxygen species [25,26].
The detailed phenolic composition of FRJ was further analyzed by UPLC-MS. Overall, a total of 17 phenolic compounds were detected in the FRJ, among which ellagic acid and anthocyanins were the predominant compounds. Ellagic acid and anthocyanins have been well documented for their antioxidant activity [27]. In this study, they might be the main cause of the increase of FRJ antioxidant activity.
Numerous findings on the gut microbial community suggest the link with the brain, respiratory and urogenital tracts, heart, and skin. Thus understanding the gut microbial structure and function could help to find new approaches to health maintenance [28]. As the TPC and antioxidant capacity of FRJ reached the highest level at 42 h, raspberry juice fermented for 42 h was selected to further investigate its gut microbiota related effects. Firstly, FRJ (equivalent to 10% of diet) was applied to the in vitro digestion and human fecal fermentation. It was found that FRJ had the ability to modulate specific gut microbiota, increasing the abundance of Lactobacillus (5.56 fold), Akkermansia (1.74 fold), butyric acid-producing bacteria (1.37 fold) and Escherichia coli (1.28 fold), while decreasing Bacteroides (0.16 fold) and Ruminococcus (0.82 fold). As is well known, Lactobacillus is an important probiotic, improving gut function, stimulating immune system as well as regulating metabolism [29]. Akkermansia, a mucin-degrading bacterium, has been reviewed to have various health benefits, combating diabetes mellitus, obesity, atherosclerosis, cancer, and inflammatory bowel disease [30]. It was reported that Bacteroides can weaken gut permeability [31], eventually promoting the migration of enteric pathogens through the intestinal barrier [32]. Consistent with our results, Lactobacillus and Akkermansia were increased, while Bacteroidetes was decreased in cyclophosphamide-induced mice after treatment with litchi juice fermented by L. casei [33]. SCFAs are one of the most important microbial metabolites, which have demonstrated positive effects, including inhibition of pathogenic bacteria, maintenance of gut barrier integrity and protection against diet-induced obesity [34,35]. In our in vitro mode, valeric and isovaleric acids were significantly increased by FRJ, whereas acetic, propionic, and butyric acids were not affected. This indicated that FRJ influenced differently the producers of SCFAs. Butyric acid-producing bacteria was promoted by FRJ while Ruminococcus, which was also a butyric acid producer [36] was decreased. Overall, FRJ could modulate the gut microbiota community into a healthier status in vitro, with an improvement of SCFAs production to some extent.
We further examined whether FRJ could exert similar effects in vivo, growth performance, redox status in liver and colon, and alterations of the microorganisms in mice fed diet-supplemented with the 3 doses (3%, 6%, and 9% of FRJ). All FRJ treatments had no adverse effects on the growth performance of mice. Moreover, although no significant antioxidant activity of FRJ was found in the colon, all the 3 doses improved the total antioxidant activity of liver tissue with the high dose exerting the strongest effect. These results indicated that FRJ could, to a certain extent, alleviate oxidative stress in vivo. Upon the gut microbial change in vivo, all FRJ treatments reshaped the gut microbiota with different doses gave rise to distinct microbial communities. At phylum level, the relative abundance of Firmicutes with respect to Bacteroidetes (F/B ratio) was decreased among all the FRJ groups. Since an increment of F/B ratio was related to an imbalance in the taxonomic composition of gut microbiota [37], eventually resulting in metabolic disorders [38], it indicated that the FRJ might promote a healthier state of microbial composition. This hypothesis was supported by facts that all the FRJ doses improved the SCFAs production and the gene expression of ZO-1, Claudin-1, Claudin-4, Ocdudin, E-cadherin, and Muc-2 in mice, which were positively correlated to the intestinal barrier function [19].
At the genus level, the dominant microbiota were Akkermansia and unclassified_Muribaculaceae for the group L, and Lactobacillus and Lachnospiraceae_NK4A136_group for the group M, which either are positively correlated with intestinal immune factors and antimicrobial peptides [19] or their implication in the production of acetic and butyric acids [20]. Compared with the low and median doses, high dose of FRJ exerted dramatic different modulation of the initial microbial community. The dominant microbiota in the group H were Escherichia-Shigella, Bacteroidetes, Alistipes, unclassified_Lachnospiraceae and Rikenellaceae_RC9_gut_group, most of which were reported to be obesity-related bacteria [39]. Escherichia-Shigella, Bacteroidetes and Alistipes were reported as conditional pathogenic bacteria [40] or potentially harmful bacteria with proinflammatory effects [20]. In addition, the high dose FRJ significantly inhibited Akkermansia, whereas the low dose had the opposite effects. In our previous in vitro study on Lactobacillus plantarum-fermented mulberry pomace, it was also found that the abundance of Akkermansia decreased by high dose of fermented mulberry pomace while increased by low dose treatment [18]. These consistent in vitro and in vivo results indicated that high dose of Lactobacillus-fermented berry material inhibits the abundance of Akkermansia, by contrast, the low dose promotes the growth. Henning et al. [41] reported that ellagic acid of 10 μM did not inhibit Akkermansia while 0.28 mg/mL pomegranate extract (equivalent to 50 μM ellagic acid) significantly inhibited Akkermansia growth. As FRJ contains ellagic acids, the observed inhibition effect of the high dose FRJ on Akkermansia might due to its high amount of ellagic acid. Since Akkermansia can withstand highly oxidative environments [42], another possible explanation for the decrease of Akkermansia in the group H could be due to the strongest antioxidant ability observed in the group H compared to the other groups. In summary, treatment with high dose FJR promoted obesity-related bacteria and some potentially harmful bacteria, and inhibited probiotic Akkermansia in the group H, whereas treatment with low and median doses FRJ mainly boost some beneficial bacteria in groups L and M. Therefore, low and median doses FRJ could lead to a healthier state of the microbiota. Another study also reported that the gut microbial modulation by 3 different concentrations of cherries juices were significantly different from each other and from the baseline, indicating that dietary modulation of gut microbiota may follow a multi-modal response to nutrient/food doses [43]. Therefore, amounts of juice products or other foods for microbial regulation research need to be well considered and validated.
5. Conclusions
The present study demonstrated that FRJ could modulate the profile of gut microbiota both in vitro and in vivo with improvement of antioxidant activity and SCFAs production. Moreover, variable gut microbial compositions were found among the groups fed diet-supplemented the different doses of FRJ, within low and median doses of FRJ may regulate the microbiota to a healthier state compared to the high dose supplementation. Our study indicated that fermentation by L. casei is a potential way to produce plant-based juices which could reshape the gut microbiota and improve the host health. However, to realize precise manipulation of gut microbial profile, the amounts of fermented juices need to be considered and validated. Futures studies are needed to understand the underlying mechanisms of the gut microbial change, dynamics of changes in the composition of phenolic compounds in raspberry juice as well as the interplay between gut bacteria and phenolic compounds.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods10123055/s1, Figure S1: Body weight change (left) and feed utilization rate (right) of mice in each group, Figure S2: PCA analysis of fecal microbiota after FRJ treatments with different doses., Table S1: Compositions of the standard diet AIN93, Table S2: Primers of gut microbiota used in this study, Table S3: Primers of related genes used in this study, Table S4: Changes of total number of L.casei colonies and pH of NFRJ/FRJ, Table S5: Organ index of mice in each group
Author Contributions
Conceptualization, T.W. and X.X.; methodology, X.C. and Y.C.; software, T.W. and X.C.; validation, S.T.; formal analysis, T.W., X.C. and S.T.; investigation, Y.C.; resources, T.W.; data curation, X.C.; writing—original draft preparation, T.W.; writing—review and editing, D.Z. and S.P.; visualization, T.W. and X.C.; supervision, X.X.; project administration, X.X.; funding acquisition, T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grant No. 32001605) and Project (No. 2662019QD037) supported by the Fundamental Research Funds for the Central Universities.
Institutional Review Board Statement
The study was conducted according to the guidelines of the legislation for care and use of laboratory animals of China and the U.K. Animals (Scientific Procedures) Act (1986) and was approved by the Animal Ethics Committee of Huazhong Agricultural University (permission no. SYXK (Hubei) 2015-0084).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang J., Cui J., Chen J., Yao J., Hao Y., Fan Y., Liu Y. Evaluation of physicochemical properties in three raspberries (Rubus idaeus) at five ripening stages in northern China. Sci. Hortic. 2020;263:109146. doi: 10.1016/j.scienta.2019.109146. [DOI] [Google Scholar]
- 2.Borges G., Degeneve A., Mullen W., Crozier A. Identification of Flavonoid and Phenolic Antioxidants in Black Currants, Blueberries, Raspberries, Red Currants, and Cranberries. J. Agric. Food Chem. 2010;58:3901–3909. doi: 10.1021/jf902263n. [DOI] [PubMed] [Google Scholar]
- 3.García-Niño W.R., Zazueta C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol. Res. 2015;97:84–103. doi: 10.1016/j.phrs.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Gomes-Neto J.C., Mantz S., Held K., Sinha R., Munoz R.R.S., Schmaltz R., Benson A.K., Walter J., Ramer-Tait A.E. A real-time PCR assay for accurate quantification of the individual members of the Altered Schaedler Flora microbiota in gnotobiotic mice. J. Microbiol. Methods. 2017;135:52–62. doi: 10.1016/j.mimet.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siracusa L., Giuseppe R. Not Only What Is Food Is Good—Polyphenols From Edible and Nonedible Vegetable Waste- ScienceDirect. Polyphen. Plants. 2019:3–21. doi: 10.1016/B978-0-12-813768-0.00001-3. [DOI] [Google Scholar]
- 6.Piccolo E.L., Garcìa L.M., Landi M., Guidi L., Remorini D. Influences of Postharvest Storage and Processing Techniques on Antioxidant and Nutraceutical Properties of Rubus idaeus L.: A Mini-Review. Horticulturae. 2020;6:105. doi: 10.3390/horticulturae6040105. [DOI] [Google Scholar]
- 7.Panghal A., Janghu S., Virkar K., Gat Y., Kumar V., Chhikara N. Potential non-dairy probiotic products–A healthy approach. Food Biosci. 2018;21:80–89. doi: 10.1016/j.fbio.2017.12.003. [DOI] [Google Scholar]
- 8.Bancalari E., Castellone V., Bottari B., Gatti M. Wild Lactobacillus casei Group Strains: Potentiality to Ferment Plant Derived Juices. Foods. 2020;9:314. doi: 10.3390/foods9030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J.Y., Lee M.Y., Ji G.E., Lee Y.S., Hwang K.T. Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int. J. Food Microbiol. 2009;130:12–16. doi: 10.1016/j.ijfoodmicro.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Zhang M., Wang W., Lv H., Ta N.Z. The in vitro Effects of the Probiotic Strain, Lactobacillus casei ZX633 on Gut Microbiota Composition in Infants With Diarrhea. Front. Cell. Infect. Microbiol. 2020;10:576185. doi: 10.3389/fcimb.2020.576185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flint H.J., Duncan S.H., Scott K.P., Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015;74:13–22. doi: 10.1017/S0029665114001463. [DOI] [PubMed] [Google Scholar]
- 12.Lavefve L., Howard L.R., Carbonero F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020;11:45–65. doi: 10.1039/C9FO01634A. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y., Wu T., Chu X., Tang S., Xu X. Fermented blueberry pomace with antioxidant properties improves fecal microbiota community structure and short chain fatty acids production in an in vitro mode. LWT. 2020;125:109260. doi: 10.1016/j.lwt.2020.109260. [DOI] [Google Scholar]
- 14.Kwaw E., Ma Y., Tchabo W., Apaliya M.T., Wu M., Sackey A.S., Xiao L., Tahir H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018;250:148–154. doi: 10.1016/j.foodchem.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez J.E., Zambrano R., Sepulveda B., Kennelly E.J., Simirgiotis M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC–HR-ESI-ToF-MS. Food Chem. 2015;176:106–114. doi: 10.1016/j.foodchem.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Koehnlein E.A., Koehnlein É.M., Corrêa R., Nishida V.S., Correa V.G., Bracht A., Peralta R.M. Analysis of a whole diet in terms of phenolic content and antioxidant capacity: Effects of a simulated gastrointestinal digestion. Int. J. Food Sci. Nutr. 2016;67:614–623. doi: 10.1080/09637486.2016.1186156. [DOI] [PubMed] [Google Scholar]
- 17.Corrêa R., Haminiuk C., Barros L., Dias M.I., Calhelha R.C., Kato C.G., Correa V.G., Peralta R.M., Ferreira I. Stability and biological activity of Merlot (Vitis vinifera) grape pomace phytochemicals after simulated in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods. 2017;36:410–417. doi: 10.1016/j.jff.2017.07.030. [DOI] [Google Scholar]
- 18.Tang S., Cheng Y., Wu T., Hu F., Pan S., Xu X. Effect of Lactobacillus plantarum-fermented mulberry pomace on antioxidant properties and fecal microbial community. LWT. 2021;147:111651. doi: 10.1016/j.lwt.2021.111651. [DOI] [Google Scholar]
- 19.Cheng Y., Wu T., Tang S., Liang F., Fang Y., Cao W., Pan S., Xu X. Fermented blueberry pomace ameliorates intestinal barrier function through the NF-κB-MLCK signaling pathway in high-fat diet mice. Food Funct. 2020;11:3167–3179. doi: 10.1039/C9FO02517K. [DOI] [PubMed] [Google Scholar]
- 20.Kong C., Gao R., Yan X., Huang L., Qin H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition. 2019;60:175–184. doi: 10.1016/j.nut.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Mousavi Z.E., Mousavi S.M., Razavi S.H., Emam-Djomeh Z., Kiani H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J. Microbiol. Biotechnol. 2011;27:123–128. doi: 10.1007/s11274-010-0436-1. [DOI] [Google Scholar]
- 22.Cirlini M., Ricci A., Galaverna G., Lazzi C. Application of lactic acid fermentation to elderberry juice: Changes in acidic and glucidic fractions. LWT. 2019;118:108779. doi: 10.1016/j.lwt.2019.108779. [DOI] [Google Scholar]
- 23.Clark C.J., Cooney J.M., Hopkins W.A., Currie A. Global Mid-Infrared Prediction Models Facilitate Simultaneous Analysis of Juice Composition from Berries of Actinidia, Ribes, Rubus and Vaccinium Species. Food Anal. Methods. 2018;11:3147–3160. doi: 10.1007/s12161-018-1296-9. [DOI] [Google Scholar]
- 24.Ryu J., Kang H.R., Cho S.K. Changes Over the Fermentation Period in Phenolic Compounds and Antioxidant and Anticancer Activities of Blueberries Fermented by Lactobacillus plantarum. J. Food Sci. 2019;84:2347–2356. doi: 10.1111/1750-3841.14731. [DOI] [PubMed] [Google Scholar]
- 25.Filannino P., Bai Y., Cagno R.D., Gobbetti M., Gaenzle M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015;46:272–279. doi: 10.1016/j.fm.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Chait Y.A., Gunenc A., Hosseinian F., Bendali F. Antipathogenic and probiotic potential of Lactobacillus brevis strains newly isolated from Algerian artisanal cheeses. Folia Microbiol. 2021;66:429–440. doi: 10.1007/s12223-021-00857-1. [DOI] [PubMed] [Google Scholar]
- 27.Yang S.-J., Lee J.-E., Lim S.-M., Kim Y.-J., Lee N.-K., Paik H.-D. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Sci. Biotechnol. 2019;28:491–499. doi: 10.1007/s10068-018-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yxa B., Rong F.C., Jing S., Amt C., Scc D., Rta B. Polyphenolic fractions isolated from red raspberry whole fruit, pulp, and seed differentially alter the gut microbiota of mice with diet-induced obesity -ScienceDirect. J. Funct. Foods. 2020;76:104288. [Google Scholar]
- 29.Reid G., Abrahamsson T., Bailey M., Bindels L.B., Bubnov R., Ganguli K., Martoni C., O’Neill C., Savignac H.M., Stanton C., et al. How do probiotics and prebiotics function at distant sites? Benef. Microbes. 2017;8:521–533. doi: 10.3920/BM2016.0222. [DOI] [PubMed] [Google Scholar]
- 30.Linares D.M., Carolina G., Erica R., Fresno J.M., Tornadijo M.E., Ross R.P., Catherine S. Lactic Acid Bacteria and Bifidobacteria with Potential to Design Natural Biofunctional Health-Promoting Dairy Foods. Front. Microbiol. 2017;8:846. doi: 10.3389/fmicb.2017.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayachandran M., Chung S., Xu B. A critical review of the relationship between dietary components, the gut microbe Akkermansia muciniphila, and human health. Crit. Rev. Food Sci. Nutr. 2019;60:1–12. doi: 10.1080/10408398.2019.1632789. [DOI] [PubMed] [Google Scholar]
- 32.Xing H., Goedert J.J., Pu A., Yu G., Shi J. Allergy associations with the adult fecal microbiota: Analysis of the American Gut Project. EBioMedicine. 2016;3:172–179. doi: 10.1016/j.ebiom.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren X., Zhu Y., Gamallat Y., Ma S., Chiwala G., Meyiah A., Xin Y.E. Coli O124 K72 alters the intestinal barrier and the tight junctions proteins of guinea pig intestine. Biomed. Pharmacother. 2017;94:468–473. doi: 10.1016/j.biopha.2017.07.123. [DOI] [PubMed] [Google Scholar]
- 34.Wen J., Ma L., Xu Y., Wu J., Li L. Effects of probiotic litchi juice on immunomodulatory function and gut microbiota in mice. Food Res. Int. 2020;137:109433. doi: 10.1016/j.foodres.2020.109433. [DOI] [PubMed] [Google Scholar]
- 35.Lin H.V., Frassetto A., Kowalik E.J., Jr., Nawrocki A.R., Lu M.M., Kosinski J.R., Hubert J.A., Szeto D., Yao X., Forrest G., et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ríos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., Reyes-Gavilán C., Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie M.G., Fei Y.Q., Wang Y., Wang W.Y., Wang Z. Chlorogenic Acid Alleviates Colon Mucosal Damage Induced by a High-Fat Diet via Gut Microflora Adjustment to Increase Short-Chain Fatty Acid Accumulation in Rats. Oxidative Med. Cell. Longev. 2021;2021:1–18. doi: 10.1155/2021/3456542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ley R.E., Backhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin N.R., Whon T.W., Bae J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Tx A., Wd A., Zz A., Sl A., Yz A., Bg A., Yu Z.A., Jyb C., Min W.A. Polyphenol-rich vinegar extract regulates intestinal microbiota and immunity and prevents alcohol-induced inflammation in mice. Food Res. Int. 2021;140:110064. doi: 10.1016/j.foodres.2020.110064. [DOI] [PubMed] [Google Scholar]
- 41.Henning S.M., Summanen P.H., Lee R.-P., Yang J., Finegold S.M., Heber D., Li Z. Pomegranate ellagitannins stimulate the growth of Akkermansia muciniphila in vivo. Anaerobe. 2017;43:56–60. doi: 10.1016/j.anaerobe.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Reunanen J., Kainulainen V., Huuskonen L., Ottman N., Belzer C., Huhtinen H., De Vos W.M., Satokari R., Goodrich-Blair H. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015;81:3655–3662. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Othaim A.A., Marasini D., Carbonero F. Impact of increasing concentration of tart and sweet cherries juices concentrates on healthy mice gut microbiota. Food Front. 2020;1:224–233. doi: 10.1002/fft2.46. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.