Abstract
Herein, the genetic diversity of the local Přeštice Black-Pied pig breed was assessed by the simultaneous analysis of the pedigree and single nucleotide polymorphism (SNP) data. The information about sire line, dam, date of birth, sex, breeding line, and herd for 1971 individuals was considered in the pedigree analysis. The SNP analysis (n = 181) was performed using the Illumina PorcineSNP60 BeadChip kit. The quality of pedigree and SNPs and the inbreeding coefficients (F) and effective population size (Ne) were evaluated. The correlations between inbreeding based on the runs of homozygosity (FROH) and pedigree (FPED) were also calculated. The average FPED for all animals was 3.44%, while the FROH varied from 10.81% for a minimum size of 1 Mbp to 3.98% for a minimum size of 16 Mbp. The average minor allele frequency was 0.28 ± 0.11. The observed and expected within breed heterozygosities were 0.38 ± 0.13 and 0.37 ± 0.12, respectively. The Ne, obtained using both the data sources, reached values around 50 animals. Moderate correlation coefficients (0.49–0.54) were observed between FPED and FROH. It is necessary to make decisions that stabilize the inbreeding rate in the long-term using optimal contribution selection based on the available SNP data.
Keywords: Přeštice Black-Pied pig, pedigree, SNP, inbreeding, ROH
1. Introduction
The Přeštice Black-Pied (PBP) is a Czech local pig breed (Figure 1). At the end of the 19th century, the local pig breeds (mostly, Yorkshire, Sussex, Berkshire, and Essex British breeds) were crossed with boars imported into the Bohemia territory [1,2]. Subsequently, pig breeds including Wessex Saddleback (in 1952), Pietrain (in 1966), Welsh (in 1985), and Landrace (in 1986) were also used in crossing of the PBP breed [3]. However, during the WWII, the first bottleneck effect on genetic variation of pigs was observed after the ban on breeding black-spotted breeds of pigs was tightened even when their breeding was secretly maintained. Although the use of unlicensed boars and inbreeding contributed to the stability of the breed, there was an apparent impact on the genetic diversity of PBP pigs [1]. Since 1992, the PBP breed has been categorized as an Animal Genetic Resource (AnGR) and listed by the Food and Agriculture Organization of the United Nations (FAO) as threatened with extinction [4]. The PBP pig is recognized as a breed with a small body frame originally bred for good reproduction and fitness. It was reported that the litter size of the PBP pigs was 11.0 piglets. Among them, 10.3 piglets were born alive and 9.6 piglets were weaned. At 21 days of age, the litter weight was 54.4 kg [5]. In terms of meat production, the PBP pigs have showed, on average, 561 g of daily weight gain, 11.4 mm of backfat thickness, and 59.1% of lean meat content [6].
Figure 1.
Přeštice Black-Pied sows.
The biological diversity of the local pig breeds plays an important role in food security and nutrition. Nevertheless, it is seriously endangered by the continuous loss of genetic diversity [7]. Plentiful information is available regarding the genetic diversity of pigs, often provided by international cooperation [8]. It is important to determine the historical trend for small, closed, or isolated populations along with parameters that define genetic diversity parameters. This can be done only by detailed analysis of all available data about population under consideration. Only then, it is possible to make adequate management decisions to prevent the genetic diversity loss.
At present, two basic sources of data about population can be obtained. The pedigree data still represents the base for analyses of genetic diversity. In general, they have been collected over ten generations. However, the results obtained from pedigree data alone could misrepresent the extent of genetic diversity. This disadvantage could be addressed by the inclusion of population data from the SNP analyses. Single nucleotide polymorphism (SNP) analysis (preceded by the analysis of microsatellites) has contributed to a considerable refinement of the assessment of genetic diversity, especially in endangered animal populations. In PBP breed, assessment of genetic diversity based on microsatellites analysis [3] and pedigree data [9] has been studied. However, simultaneous analysis of the SNP and pedigree data of the PBP pig breed has not been performed till date.
The objective of the present study was to assess the genetic diversity of PBP pigs using the pedigree and SNP data.
2. Materials and Methods
2.1. Animals
The PBP breed belongs to the primitive indigenous breeds reared in semi-intensive or extensive production systems. This breed has been kept in situ in closed population for more than 25 years. It is characterized by a smaller body frame and mottled black and white coloration. The average weight of mature live boars and sows is 260–280 kg and 215–235 kg, respectively. Despite the fact that individuals are bred in herds with other commercial breeds, they do not have a strong selection pressure. In animals selected for breeding, the basic meat production and reproductive traits (listed in the Supplementary Table S1) are routinely determined and assessed. The database of Czech National program for Pig breeding (called CzePig) gathered and guided by the Czech Pig Breeders Association was used for determining the traits used for animal selection.
2.2. Pedigree Analysis
An active population of 324 dams and sires (animals used in breeding) in 2020 was considered the reference population for pedigree analysis of the PBP breed. Basic characteristics of the population are summarized in Table 1. The information about sire, dam, date of birth, sex, breeding line, and herd for 1971 individuals were considered for pedigree analysis. The pedigree of each individual in the reference population was tracked back to its earliest ancestor. The analyzed pedigree contains animals born between 1982 and 2020. Average generation interval (GI) was calculated as the average age of parents when their selected offspring was born (in years). The GI was computed for the four selection pathways (SS: sires to sons, SD: sires to daughters, DS: dams to sons, and DD: dams to daughters).
Table 1.
Basic characteristics of the population in pedigree analysis.
| Parameter (Unit) | Sire | Dam | Total |
|---|---|---|---|
| Total number of animals in pedigree | 507 | 1466 | 1971 |
| Reference population in pedigree | 47 | 277 | 324 |
| Number of evaluated herds | 12 | 11 | 12 |
| Average number of: dams in a herd | 15.3 | ||
| Sires in a herd | 2.71 | ||
| Number of offspring selected per dam | 1.71 | ||
| Number of offspring selected per sire | 4.50 | ||
| Number of sires per dam | 1.19 | ||
| Number of dams per sire | 3.02 | ||
Inbreeding coefficient (F) of an individual was calculated using Wright’s coefficients and Meuwissen and Luo’s algorithm [10,11]. The differences of the inbreeding effect which were achieved during recent generations compared with inbreeding achieved during the more distant past were evaluated. Therefore, the total inbreeding coefficient of a given animal (FAnT) has been split into the “new” inbreeding coefficient (FAnN) and the “old” inbreeding coefficient (FAnO). The FAnT was calculated as inbreeding coefficient of ancestors up to five generations back. In addition to the calculation of the inbreeding coefficient of an individual, the influence of inbreeding was also evaluated by the sires (FSrN, FSrO, and FSrT as new, old, and total inbreeding, respectively), and dams (FDmN, FDmO, and FDmT as new, old, and total inbreeding, respectively). Expected inbreeding coefficient was computed as the co-ancestry of the breeding animals assuming random mating [12]. The co-ancestry coefficient was obtained by applying Colleau’s algorithm [13].
Pedigree completeness index (PCI) was calculated to express the quality of the pedigree using the algorithm devised by MacCluer et al., which summarized the proportion of known ancestors in each ascending generation [14]. It quantifies the change in observed inbreeding in the pedigree [15]. The following equations were used:
And
where k is the dam or sire line of an animal, ai is the proportion of known ancestors in generation i, and d is number of generations considered in the calculation of the pedigree completeness. For description of methods used to calculate maximum generations traced back, average complete equivalent generations, number of founders and number of ancestors explaining appropriate proportion of gene pool [16].
Different methods were used to compute the effective population size (Ne). The Ne was calculated based on standard equation:
where ΔF is rate of inbreeding coefficient. Here, ΔFp is the ratio of the average inbreeding in an offspring and their direct parents and ΔFg was the ratio of the average inbreeding in an offspring to the average inbreeding in average parent’s generation. Both methods were described previously [12]. An algorithm devised by Pérez-Ensico was used for the calculation of Ne-Ln and used the slope from logarithmic regression performed on year of birth of the PBP pigs in the pedigree analysis [17]. The approach defined by Gutierrez et al. [18], which uses the sum of the inbreeding coefficients of all individuals and their known ancestors, was used to compute the Ne-Ecg. Ne-Coan was computed using the additive genetic relationship against the inbreeding coefficient as described by Falconer and Mackay [12].
The basic pedigree data manipulations were performed using the R-project package [19]. The POPREP package v. 2.0, the CFC software v. 1.0, the PEDIG package, and the Endog software v. 4.5 were used for the pedigree analysis [20,21,22,23].
2.3. SNP Analysis
The genome-wide data were analyzed for 181 individuals (65 sows and 116 boars) born between 2005 and 2020. Bristles, frozen blood samples, and used insemination doses were the sources of DNA. All DNA analyses were performed at the laboratory of genetics of the Czech-Moravian Breeders’ Corporation. DNA was isolated using the magnetic bead method and the silicate column method for bristles and insemination doses, respectively. DNA from the blood samples was isolated using the GeneAll Exgene DNA micro isolation kit (GeneAll Biotechnology, Seoul, Korea). Selected DNA isolates were applied to PorcineSNP60 v2 BeadChips (Illumina, San Diego, CA, USA) using the Illumina HD Infinium technology protocol. A total of 61 565 SNPs were identified in the selected DNA isolates. The average call rate was 0.99, 0.97, and 0.94 for the bristles, blood samples, and insemination doses, respectively. The animals with call rate > 0.90 were used in the subsequent analyses.
Only the SNPs mapped on autosomes were considered in this study. The PLINK v. 1.9 software was used for quality control and subsequent analyses [24]. The SNPs with call rate > 0.90, p-value of Hardy–Weinberg equilibrium > 0.0001, and minor allele frequency (MAF) > 0.05 were included in the SNP analyses. After the quality control analysis of all SNPs, the data from 174 animals containing 39 779 SNPs were used for further analysis. Runs of homozygosity (ROH) segments were expressed by a specific minimum number of continuous homozygous SNP markers with a maximum spacing of 1 Mb and a minimum density of one SNP marker per 100 kb. The minimum number of SNPs in ROH (n = 39) was determined based on the approach proposed in the study by Lencz et al. to avoid the detection of false-positive ROH segments (α = 0.05) [25]. As shown in previous studies, the length of ROH segments is related to the proportion of genomic information coming from different ancestral generations [26,27,28,29]. Therefore, based on the terminology proposed previously, the ROH segments were divided into five classes on the basis of their length (>1 Mb, >2 Mb, >4 Mb, >8 Mb, and >16 Mb) [27,29]. These trace back approximately 50, 25, 12, 6, and 3 ancestral generations.
The inbreeding coefficients, calculated based on the SNP data, were calculated by four different methods. The genomic inbreeding coefficient was calculated for each ROH (FROH) class by dividing the sum of the ROH segments length (kb) in a particular class by the total length of the autosomal genome (kb) covered by the SNPs and expressed in percentage. The inbreeding coefficient, Fhat1 is the variance-standardized relationship minus one. The inbreeding coefficient, Fhat2 is approximately equal to the estimates (observed and expected autosomal homozygous genotype counts for each sample and reported method of moments F coefficient estimates). The inbreeding coefficient Fhat3 is based on the correlation between uniting gametes. All the inbreeding coefficients were calculated using PLINK 1.9 software [24]. The correlations between evaluated inbreeding coefficients were calculated. The effective population size based on the genomic data was calculated by GONE software [30]. The method is based on the linkage disequilibrium (LD) between SNP markers and implements a genetic algorithm published previously [31]. The parameters were set to 2000 generations for which the linkage data is obtained in bins. Only pairs of SNPs with recombination fraction, c > 1/4000 were considered. Numbers of bins were set to 400, making a five-year generations gap, obtained by dividing the number of generations with the number of bins. However, the first 10 generations were analyzed using 2-generation gaps and the rest using 5-generation gaps. The Ne was calculated separately for K1, K2, K3, K4, and K8, which represent 1, 2, 4, and 8 centiMorgans per mega base, respectively. The overall degree of population stratification was assessed by calculating Nei’s genetic distances (Da) between animals and the pair-wise Wright’s index at the group level using the StAMPP R package [32].
3. Results
3.1. Quality of the Pedigree
Results from the pedigree data evaluation are presented in Figure 2, Figure 3 and Figure 4 and Table 2, Table 3 and Table 4. The pedigree of the animals in the reference population could be traced back 28 generations at maximum and 12.99 generations on average. The mean value of complete generations was 4.58. For all animals involved in pedigree analysis, the mean equivalent generations were 7.53 and the total number of founders was 269. The number of founders explaining 50% of the diversity was 15 and 7 in animals of pedigree analysis and reference population, respectively.
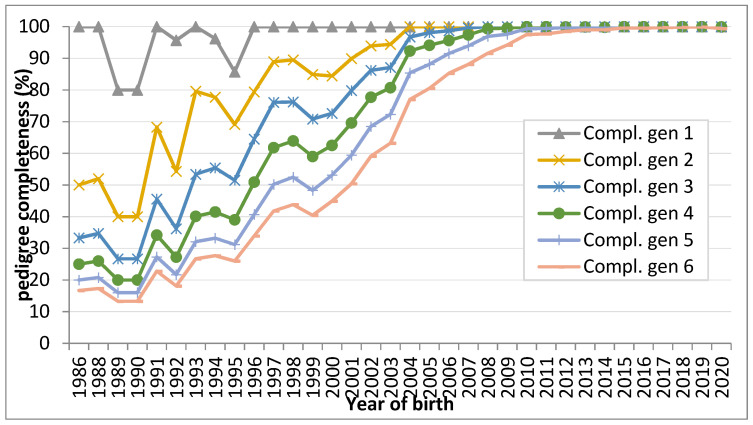
Figure 2.
Average pedigree completeness for generation one to six.
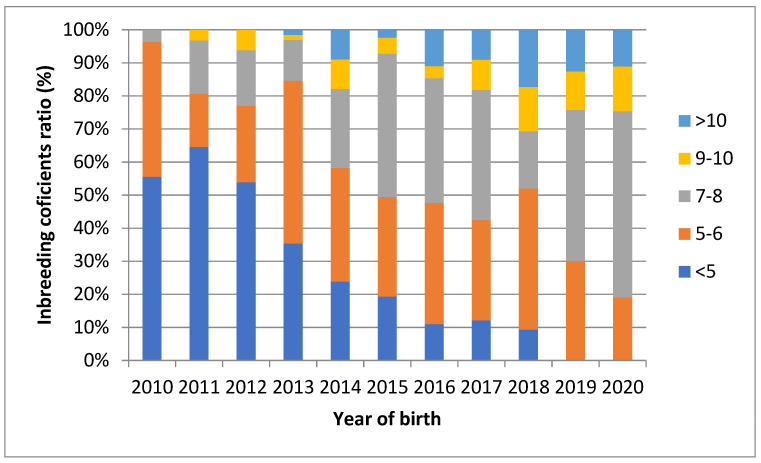
Figure 3.
Inbreeding coefficients ratio of animals born between 2010 and 2020. Note: Animals are divided into five groups based on the FPED value. Groups are specified in the figure legend.
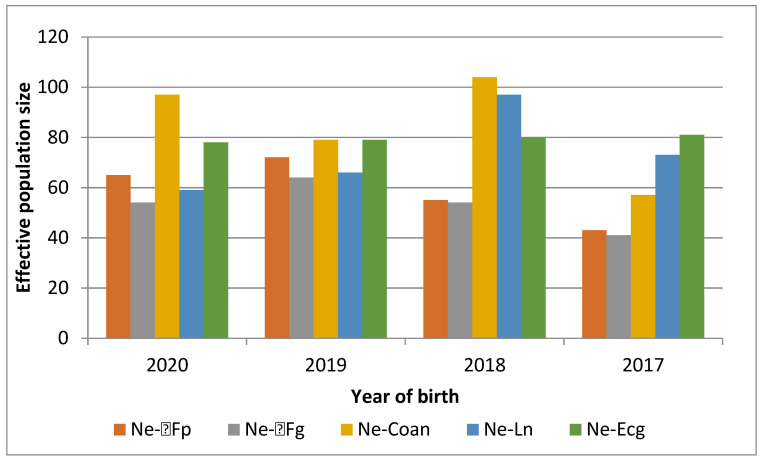
Figure 4.
Effective population size (Ne) based on pedigree data calculated by five methods (described in detail in the Materials and Methods section).
Table 2.
Generation interval of animals 1 in the pedigree analysis during the study period.
| Year of Evaluation | Generation Interval (in Years) in Different Paths 1 | ||||
|---|---|---|---|---|---|
| SS | SD | DS | DD | Population | |
| 2005 | 3.6 | 2.4 | 2.3 | 2.4 | 2.6 |
| 2010 | 1.8 | 2.0 | 2.3 | 2.4 | 2.1 |
| 2015 | 2.3 | 2.3 | 1.8 | 2.1 | 2.2 |
| 2020 | 3.3 | 2.8 | 2.2 | 2.2 | 2.6 |
| Mean | 3.0 | 2.5 | 2.6 | 2.2 | 2.5 |
1 Generation interval expresses the average age of animals when their offspring were born in the year of study. Animals are assigned to the four selection paths: SS: sire to son, SD: sires to daughter, DS: dam to son, and DD: dam to daughter.
Table 3.
Number and average inbreeding coefficients of animals born in last three years and their sires and dams.
| Year | Animals | Sires | Dams | |||
|---|---|---|---|---|---|---|
| n 1 in Total | F 2 | n in Total | F | n in Total | F | |
| 2018 | 75 | 7.49% | 26 | 7.03% | 54 | 6.82% |
| 2019 | 103 | 8.11% | 24 | 7.37% | 73 | 7.21% |
| 2020 | 126 | 8.10% | 32 | 6.67% | 82 | 7.43% |
1 number of observations.2 average inbreeding coefficient.
Table 4.
Average new, old, and total inbreeding coefficients of animals born in three recent years (2018–2020) and their sires and dams (%).
| FAnN | FAnO | FAnT | FSrN | FSrO | FSrT | FDmN | FDmO | FDmT | |
|---|---|---|---|---|---|---|---|---|---|
| Avg. | 2.31 | 5.64 | 7.95 | 2.15 | 5.49 | 7.64 | 2.32 | 5.65 | 7.97 |
| s.d. | 2.51 | 0.71 | 2.61 | 1.54 | 0.71 | 1.79 | 1.6 | 2.61 | 1.83 |
| Min. | 0.00 | 2.87 | 4.31 | 0.00 | 2.87 | 5.05 | 0.00 | 3.14 | 4.31 |
| Max | 9.38 | 7.86 | 14.46 | 5.47 | 7.26 | 12.11 | 9.38 | 7.86 | 14.46 |
FAnN: new inbreeding of animals; FAnO: old inbreeding of animals; FAnT: total inbreeding of animals; FSrN: new inbreeding of sires; FSrO: old inbreeding of sires; FSrT: total inbreeding of sires; FDmN: new inbreeding of dams; FDmO: old inbreeding of dams; FDmT: total inbreeding of dams; Avg.: arithmetic mean; s.d.: standard deviation.
The PCI calculated for one to six generations back is shown in Figure 2. A PCI value of 100% was observed for all the analyzed generations from 2012. Moreover, a continuous increase in the PCI can be seen from the year 1999 for all generations.
An overview of the GI of animals in the pedigree analysis is presented in Table 2. The average GI for the selection path from sire to son was 3.0 during the study period. This value is the average sire age of 255 selected sons born in the last 30 years. Additionally, the average GI for other selection paths were 2.2 (for both dam to son and dam to daughter) and 2.8 years (for sire to daughter). The average age of parent sires, dams, and overall population was slightly increased by 0.6, 0.2, and 0.4 years in the last 6 years, respectively. This indicates the increased age of parents, especially of sire animals.
3.2. Analysis Based on the Pedigree Data
The total inbreeding (FPED) observed in the animals from the completely evaluated pedigree was 3.44% on average. Although this value is high, more in-depth analysis is required for a closed population of a genetic resource. Average inbreeding coefficient calculated from the pedigree data for animals born in 2020 was 8.10 ± 1.50%, ranking from 5.50% to 13.00% (see Table 3). The data presented in Table 3 also indicates a gradual increase in the F value in animals born within last three years and also in their parents. There was a slight decrease in the FPED of sires born in 2020. This could be due to the use of boars with less inbreeding. This intervention had only a slight effect on the average F value of their offspring due to the high F value of their mothers used in that particular year.
The overall negative trend of FPED is also observed in Figure 3. The figure shows the proportion of animals with inbreeding divided into groups over the last 10 years. The share of animals with the lowest FPED has been continuously decreasing since 2013. A similar trend was observed for FPED in the 5–6% group. In contrast, there is a continuous increase in FPED in 7–8% group, which in the following generations will cascade leading to an increase in F with a higher proportion. From the context of population development in the future, the rate of the inbreeding (∆F) is an important parameter and is expressed as the difference of the measured inbreeding between two consecutive generations. This value increased at a rate of +1.58% per generation in average. As expected, the inbreeding rate, as calculated by balancing the founder’s contribution, was positive, and was 0.43% per generation.
Table 4 shows the total, new, and old inbreeding calculated for animals born over three recent years (2018–2020) and for their sires and dams. A relatively higher share of the total inbreeding of an individual is coming from the older generations. The ratio FAnN vs. FAnO in animals born in the last three years was 1:2.44. The total inbreeding observed in mothers had a slightly higher share in an individual’s inbreeding than fathers (the ratio of total F between dams and sires was 49:51). However, in a more detailed investigation, we observed differences during the acquisition of inbreeding.
The Ne was derived based on multiple parameters depending on various conditions of population under consideration. Different methods also take into account different time windows of pedigree data in calculations and therefore respond differently to rapid changes in population size with different sensitivity. The calculated Ne by five methods is compared in Figure 4.
3.3. SNP Data Analysis
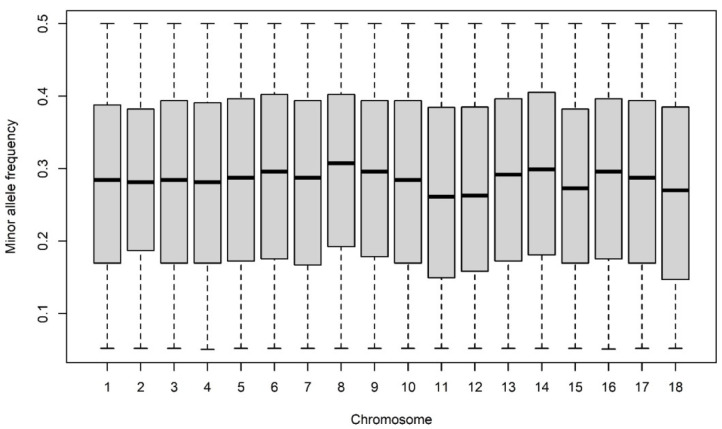
The MAF distributed over each autosome are summarized in Figure 5. The average MAF was 0.28 ± 0.11 and ranked from 0.05 to 0.50. There were more than 28% of SNPs with MAF higher than 0.40, whereas less than 10% of SNPs have an MAF of lower than 0.10 in all chromosomes. In total, 2188 SNPs on chromosome eight showed highest average MAF (0.30). In contrast, 1441 SNPs on chromosome six showed the lowest MAF (0.27). The observed (Ho) and expected (He) within breed heterozygosity was 0.38 ± 0.13 and 0.37 ± 0.12, respectively.
Figure 5.
Minor allele frequency (MAF) for each autosome.
The inbreeding coefficients (F), calculated based on the SNP data, were analyzed by different methods. Table 5 summarizes the results for ROH, namely the number of ROH and FROH for all considered ROH classes. The F value based on ROH decreased with the increase in the minimum length of ROH. The highest average value for FROH was observed in class 1 (10.81, varied from 3.23 to 23.66), while the lowest average value for FROH was in class 16 (3.98, varied from 0.00 to 14.80).
Table 5.
Inbreeding coefficient derived from runs of homozygosity (ROH).
| ROH Class | No. of ROH | FROH (%) | ||||
|---|---|---|---|---|---|---|
| ± SD | Min | Max | ± SD | Min | Max | |
| 1 Mb | 41.931 ± 8.974 | 21 | 71 | 10.810 ± 3.634 | 3.225 | 23.663 |
| 2 Mb | 38.477 ± 8.725 | 19 | 68 | 10.571 ± 3.637 | 3.097 | 23.289 |
| 4 Mb | 21.190 ± 5.834 | 8 | 36 | 9.308 ± 3.578 | 2.438 | 23.486 |
| 8 Mb | 9.500 ± 3.941 | 1 | 21 | 6.722 ± 3.320 | 0.333 | 20.430 |
| 16 Mb | 3.517 ± 2.190 | 0 | 10 | 3.984 ± 2.758 | 0 | 14.801 |
The average value of Fhat1 was −0.026 ± 0.06 and varied from −0.131 to 0.109. The inbreeding coefficient expressed as Fhat2 showed a lower average value compared to that of Fhat1 (−0.032 ± 0.05 and ranged from −0.116 to 0.104). The correlation coefficient between uniting gametes, Fhat3 showed an average value of −0.028 ± 0.046 and varied between −0.086 and 0.107). Sows showed lower Fhat1 values compared to boars (on average, −0.051 and −0.017, respectively). In contrast, an opposite trend was observed for Fhat2 (−0.012 and −0.038 for sows and boars, respectively).
The correlation coefficients between inbreeding coefficients, as determined based on the pedigree and SNP data, are shown in Table 6. The presented coefficients values were highly statistically significant (p < 0.001) except for the correlation between FPED and Fhat1. FPED had a moderate correlation with inbreeding coefficients based on ROH and varied from 0.491 (with FROH in ROH class 16) to 0.542 (with FROH in ROH class 8). The correlation observed between FPED and Fhat2 was also moderate (0.508). However, no relationship was observed between FPED Fhat1.
Table 6.
Correlations between inbreeding coefficients 1.
| FROH1 | FROH2 | FROH4 | FROH8 | FROH16 | Fhat1 | Fhat2 | Fhat3 | |
|---|---|---|---|---|---|---|---|---|
| FPED | 0.519 | 0.513 | 0.510 | 0.542 | 0.491 | −0.026 | 0.508 | 0.307 |
| FROH1 | 0.998 | 0.967 | 0.895 | 0.795 | 0.222 | 0.844 | 0.659 | |
| FROH2 | 0.964 | 0.889 | 0.797 | 0.220 | 0.838 | 0.651 | ||
| FROH4 | 0.942 | 0.834 | 0.262 | 0.830 | 0.689 | |||
| FROH8 | 0.893 | 0.288 | 0.785 | 0.686 | ||||
| FROH16 | 0.237 | 0.716 | 0.585 | |||||
| Fhat1 | 0.032 | 0.771 | ||||||
| Fhat2 | 0.606 |
1 Significant at p < 0.001 except for the correlation between FPED and Fhat1. Detailed description of individual inbreeding coefficients is given in Materials and Methods section.
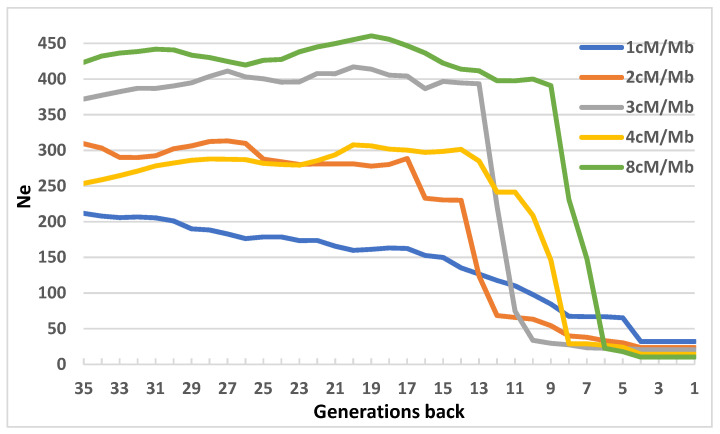
The genetic structure all of the individuals in which the SNP data was derived from the Nei’s genetic distance matrix is presented in Supplementary Figure S1. The temporal effective population size (Ne) calculated using different constant recombination rates is displayed in Figure 6. The presented results show Ne up to 35 past generations. In particular, for 1 cM per Mb, the Ne value has decreased slowly and could be observed from 8 to 35 past generations. Then, a short period of three generations without any change in Ne was observed, followed by a relatively rapid decrease of Ne between the fifth to fourth generation. Similar trends were observed for other constant recombination rates with relatively stable historical period from 9 or 13 to 35 past generations, followed by a rapid decrease in Ne.
Figure 6.
Effective population size derived from the single nucleotide polymorphism data.
4. Discussion
4.1. Analysis Based on Pedigree Data
Based on the established performance testing and recording of the local PBP breed, the results from the quality of the pedigree are similar to our previous study on the PBP breed [9]. Nevertheless, continued increase of PCI can be seen from 1999 in all generations of pigs under study. For most of the commercial breeds (such as the Czech large white dam and sire line, Czech landrace, and Pietrain) used in the national breeding program called CzePig [16], the completeness of pedigree for more than 10 years was determined only for the first generation of animals. In contrast, slow reduction of PCI recorded in the commercial population at the beginning of the early 1990s also can be seen in the PBP breed. It indicates that, irrespective of the genotype, a breed could be slightly influenced by the import of animals that were unrelated to the PBP breed and/or with the unknown pedigree. Generally, pedigree data, analyzed in present study forms a very good basis for accurate and reliable calculations of basic and derived parameters of genetic diversity of the PBP population.
The GI of the PBP breed (2.5 years on average, see Table 2) is relatively long compared to that in commercial breeds (ranking from 1.40 to 1.95 years), as observed previously [16,33,34]. In contrast, comparable values of average parent age (2.24 to 2.69 years) were measured in various local pig breeds [35,36]. The differences of GI found in our study within the individual selection paths indicate the application of divergent selection pressure. In the context of basic production parameters of the breed (given in the Supplement Table S1), the average number of litters per year is relatively low (1.54 on average), even though the farrowing interval was short (157 days). This corresponds to a longer suckling period of piglets confirmed by the PBP breed farmers (personal communication). In brief, the levels of all meat production and reproduction parameters ratifies the almost extensive production system of this breed.
The average FPED values and average rate of inbreeding (∆F) presented in our study are higher than these values published in the literature. The slightly higher value of F was observed in a previous study for Guadyerbas pig breed maintained in small isolated herds [37]. They reported a value of 3.90%, which is moderately higher than that described in a previous study by Toro et al. on the same breed [38]. We obtained a similar value for FPED in our previous study [9]. However, ∆F observed in the present study is higher. Kock et al. studied the effect of total and partial inbreeding of pigs in Austria; they reported a low correlation (under 0.30) between old and new inbreeding [39]. A study by Silio et al., which analyzed inbreeding coefficients for Iberian pig, reported slightly higher values for inbreeding from the last five generations (57% and 43% contribution of new and old inbreeding, respectively) [40]. In the present study, we reported 71% old inbreeding (Table 4).
It is generally recommended to perform a detailed analysis of the observed Ne before making conclusions regarding the genetic diversity of populations [20]. In our study, the Ne value of the PBP breed calculated by various methods (described in Materials and Methods section) ranged from 40 to 80 individuals during the years of study (see Figure 3). Similar values were reported in our previous study [9]. In an ideal situation, when population is mating randomly, the values of Ne-Fg and Ne-Coan should be the same or very similar to each other. The population is exposed to a certain selection pressure, when Ne-Coan reaches a higher value than Ne-Fg. The most appropriate method could be marked Ne-ecg (as a most stable method with negligible variations over years) or Ne-ln, which uses shortest time window. Some authors made suggestions for minimal value of Ne. Welsh et al. stated 50 individuals as the minimum value of Ne [41]. Meuwissen and Woolliams recommended that the Ne value be maintained between 31 and 250 animals for good population fitness [42]. Tang et al. reported an effective population size of 50 as a threshold necessary to avoid the negative impact of inbreeding in population; these authors recommend that at least 500 animals are needed for maintenance of genetic diversity in population for several generations [34].
4.2. SNP Data Analysis
Over the last two decades, enormous progress has been made in using of the SNP data for assessment of genetic diversity. At present, high-density SNPs can be used to analyze the past and current population demography [43]. The MAF observed in the study is relatively high compared to other studies. Muñoz et al. analyzed twenty-one different pig breeds across Europe [8]. They reported relatively moderate differences in MAF (average of 0.228, approximately). Similarly, the results reported in our study suggested that the explanatory power of the SNPs is slightly lower; this could be justified by the fact that local indigenous breeds have not been considered for design of commercial porcine chip. This could be indicated by the results of Hlongwane et al., who reported the genetic variation and population distinctiveness of three commercial, four village, two indigenous, three wild, and one Asian pig breeds [44].
In general, observed and expected heterozygosity of the PBP population are comparable to other studies performed on wide range of pig breeds and they are on the lower range of values published in previous literature [43,45,46]. The observed inbreeding coefficients from the pedigree data (FPED) and SNP data were different. It should also be mentioned that the animals in which SNP analysis was preformed represent a wide range of boars and sows born during 2005 to 2020. Even though, all of them are parents of animals used in next breeding, they were not equal to the number of animals in reference populations (only 58% were born in last four years). Schiavo et al. reported a similar trend; they analyzed three commercial and four autochthonous pig breeds raised in Italy for calculation of inbreeding coefficient values and correlation coefficients among the evaluated types of inbreeding [29]. In accordance with their results, in our study the correlations between FPED and FROH did not vary significantly depending on the changes in ROH length. In contrast, few authors reported a negligible correlation between FROH and FPED [37]. Zanella et al. reported that this correlation was low in Landrace (0.24) and negligible in Large White (0.015) breed raised in Brazil. The differences in inbreeding coefficients obtained from the pedigree and SNP data could be caused by unknown level of inbreeding in the founder animals in a pedigree [47]. It is assumed, that these founder animals are unrelated with no inbreeding. Additionally, Daetwyler et al. suggested the fact that genomic information estimates the Mendelian sampling effects more accurately, which consequently improves the estimates of the inbreeding rate [48].
From the perspective of current and past effective population size, the population analyzed in our study required an additional attention. In our study, the overall trend in Ne is slightly negative, especially over the last three years. Reduction of the number of effective animals recorded in the analyzed breed population has been confirmed both by pedigree and LD analysis.
5. Conclusions
The present study reports a detailed analysis of the local PBP pig breed based on all available data. From the results, it is evident that the PBP pig population showed a high degree of inbreeding. Consequently, this results in low value of effective population size. It is necessary to make decisions that stabilize the rate of inbreeding in the long-term by using optimal contribution selection (OCS). The specific mating management, which considers OCS, whereby animals with higher genetic diversity will be preferred for breeding, will be applied in the PBP population in cooperation with breeders’ association. Despite a high volume of pedigree data being recorded, the results of the SNP data analysis should be considered for decision-making in animal conservation programs. However, the final decision-making depends on the understanding of the breeders. For some local populations with very limited pedigree information, the evaluation of genomic information is one of the ways to identify inbred individuals and to quantify the degree of increase in homozygosity due to mating of related animals, respectively.
Acknowledgments
Thanks are due to Czech Pig Breeders Association for making pedigree data available.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes12121972/s1, Table S1: Basic meat production and reproduction characteristics of the Přeštice Black-Pied pig population in 2020. Figure S1: Genetic structure all of the genotyped individuals in which the SNP data was derived from the Nei’s genetic distance matrix7.
Author Contributions
Conceptualization and methodology, E.K. and N.M.; validation, Z.K. and E.Ž.; formal analysis, E.K., N.M. and Z.K.; investigation, E.K. and N.M.; resources, E.Ž. and E.K.; data curation, E.Ž.; writing—original draft preparation, E.K., Z.K. and N.M.; writing—review and editing, E.K. and N.M.; visualization, E.K. and N.M.; supervision and project administration, E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture of the Czech Republic (grant No. QK1910217 and by the project No. MZE-RO0718-V001) and by Slovak Research and Development Agency (grant numbers APVV-20-0161 and APVV-17-0060).
Institutional Review Board Statement
Ethical review and approval were waived for this study, because the study was oriented on analysis of DATA.
Informed Consent Statement
Not applicable.
Data Availability Statement
The derived data supporting this study is available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fiedler J., Fiedlerová M., Smital J. Přeštické Černostrakaté Plemeno Prasat. 1st ed. VÚŽV; Praha, Czech Republic: 2004. p. 166. [Google Scholar]
- 2.Hodan J. Historie vzniku a průběh zvelebování chovu přeštického prasete. Pod Zelenou Horou Vlastivědný Sborník Jižního Plzeňska. 1998;1:13. [Google Scholar]
- 3.Vrtková I. Genetic Admixture Analysis in Prestice Black-Pied Pigs. Arch. Anim. Breed. 2015;58:115–121. doi: 10.5194/aab-58-115-2015. [DOI] [Google Scholar]
- 4.Vaclavková E., Rozkot M., Dostálová A. Přeštické Černostrakaté Prase. Živé Dědictví po Předcích (Přestice Black-Pied Pig—Living Heritage from Our Ancestors (Czech Language) 1st ed. VÚŽV; Praha, Czech Republic: 2012. p. 70. [Google Scholar]
- 5.Wolf J., Horáčková Š., Wolfová M. Genetic Parameters for the Black Pied Přeštice Breed: Comparison of Different Multi-Trait Animal Models. Czech J. Anim. Sci. 2001;46:165–171. [Google Scholar]
- 6.Matoušek V., Kernerová N., Hyšplerová K., Komosný M. Performance Traits of Prestice Black-Pied Pig Breed at the Effect of Genealogical Line. Res. Pig Breed. 2016;10:10–15. [Google Scholar]
- 7.FAO . The State of the World’s Biodiversity for Food and Agriculture. Food and Agriculture Organization; Rome, Italy: 2019. [Google Scholar]
- 8.Muñoz M., Bozzi R., García-Casco J., Núñez Y., Ribani A., Franci O., García F., Škrlep M., Schiavo G., Bovo S., et al. Genomic Diversity, Linkage Disequilibrium and Selection Signatures in European Local Pig Breeds Assessed with a High Density SNP Chip. Sci. Rep. 2019;9:13546. doi: 10.1038/s41598-019-49830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krupa E., Krupová Z., Žáková E., Kasarda R., Svitáková A. Population Analysis of the Local Endangered Přeštice Black-Pied Pig Breed. Poljoprivreda. 2015;21:155–158. doi: 10.18047/poljo.21.1.sup.36. [DOI] [Google Scholar]
- 10.Wright S. Coefficients of Inbreeding and Relationship. Am. Nat. 1922;56:330–338. doi: 10.1086/279872. [DOI] [Google Scholar]
- 11.Meuwissen T.H.E., Luo Z. Computing in Breeding Coefficients in Large Populations. Genet. Sel. Evol. 1992;24:305–313. doi: 10.1186/1297-9686-24-4-305. [DOI] [Google Scholar]
- 12.Falconer D.S., Mackay T.F.C. Introduction to Quantitative Genetics. 4th ed. Longman Scientific and Technical; Harlow, UK: 1996. p. 448. [Google Scholar]
- 13.Colleau J.J. An Indirect Approach to the Extensive Calculation of Relationship Coefficients. Genet. Sel. Evol. 2002;34:409–421. doi: 10.1186/1297-9686-34-4-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maccluer J.W., Boyce A.J., Dyke B., Weitkamp L.R., Pfenning D.W., Parsons C.J. Inbreeding and Pedigree Structure in Standardbred Horses. J. Hered. 1983;74:394–399. doi: 10.1093/oxfordjournals.jhered.a109824. [DOI] [Google Scholar]
- 15.Sørensen A.C., Sørensen M.K., Berg P. Inbreeding in Danish Dairy Cattle Breeds. J. Dairy Sci. 2005;88:1865–1872. doi: 10.3168/jds.S0022-0302(05)72861-7. [DOI] [PubMed] [Google Scholar]
- 16.Krupa E., Žáková E., Krupová Z. Evaluation of Inbreeding and Genetic Variability of Five Pig Breeds in Czech Republic. Asian-Australas. J. Anim. Sci. 2015;28:25–36. doi: 10.5713/ajas.14.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-Enciso M. Use of the Uncertain Relationship Matrix to Compute Effective Population Size. J. Anim. Breed. Genet. 1995;112:327–332. doi: 10.1111/j.1439-0388.1995.tb00574.x. [DOI] [Google Scholar]
- 18.Gutiérrez J.P., Cervantes I., Goyache F. Improving the Estimation of Realized Effective Population Sizes in Farm Animals. J. Anim. Breed. Genet. 2009;126:327–332. doi: 10.1111/j.1439-0388.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 19.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [(accessed on 5 October 2021)]. Available online: https://www.R-project.org. [Google Scholar]
- 20.Groeneveld E., Van der Westhuizen B., Maiwashe A., Voordewind F., Ferraz J.B.S. POPREP: A Generic Report for Population Management. Genet. Mol. Res. 2009;8:1158–1178. doi: 10.4238/vol8-3gmr648. [DOI] [PubMed] [Google Scholar]
- 21.Sargolzaei M., Iwaisaki H., Colleau J.J. CFC: A Tool for Monitoring Genetic Diversity; Proceedings of the 8th World Congress on Genetics Applied to Livestock Production (WCGALP); Belo Horizonte, Brazil. 13–18 August 2006; pp. 27–28. [Google Scholar]
- 22.Boichard D. PEDIG: A Fortran Package for Pedigree Analysis Suited for Large Populations; Proceedings of the 7th World Congress on Genetics Applied to Livestock Production (WCGALP); Montpellier, France. 19–23 August 2002; Castanet-Tolosan, France: INRA; 2002. pp. 19–23. [Google Scholar]
- 23.Gutiérrez J.P., Goyache F. A Note on ENDOG: A Computer Program for Analysing Pedigree Information. J. Anim. Breed. Genet. 2005;122:172–176. doi: 10.1111/j.1439-0388.2005.00512.x. [DOI] [PubMed] [Google Scholar]
- 24.Chang C.C., Chow C.C., Tellier L.C.A.M., Vattikuti S., Purcell S.M., Lee J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lencz T., Lambert C., DeRosse P., Burdick K.E., Morgan T.V., Kane J.M., Kucherlapati R., Malhotra A.K. Runs of Homozygosity Reveal Highly Penetrant Recessive Loci in Schizophrenia. Proc. Natl. Acad. Sci. USA. 2007;104:19942–19947. doi: 10.1073/pnas.0710021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marras G., Gaspa G., Sorbolini S., Dimauro C., Ajmone-Marsan P., Valentini A., Williams J.L., Macciotta N.P. Analysis of Runs of Homozygosity and Their Relationship with Inbreeding in Five Cattle Breeds Farmed in Italy. Anim. Genet. 2015;46:110–121. doi: 10.1111/age.12259. [DOI] [PubMed] [Google Scholar]
- 27.Ferenčakovič M., Hamzíc E., Gredler B., Solberg T.R., Klemetsdal G., Curik I., Sölkner J. Estimates of Autozygosity Derived from Runs of Homozygosity: Empirical Evidence from Selected Cattle Populations. J. Anim. Breed. Genet. 2013;130:286–293. doi: 10.1111/jbg.12012. [DOI] [PubMed] [Google Scholar]
- 28.Ferenčakovič M., Sölkner J., Curik I. Estimating Autozygosity from High-Throughput Information: Effects of SNP Density and Genotyping Errors. Genet. Sel. Evol. 2013;45:42. doi: 10.1186/1297-9686-45-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiavo G., Bovo S., Bertolini F., Tinarelli S., Dall’Olio S., Nanni Costa L., Gallo M., Fontanesi L. Comparative Evaluation of Genomic Inbreeding Parameters in Seven Commercial and Autochthonous Pig Breeds. Animal. 2020;14:910–920. doi: 10.1017/S175173111900332X. [DOI] [PubMed] [Google Scholar]
- 30.Santiago E., Novo I., Pardiñas A.F., Saura M., Wang J., Caballero A. Recent Demographic History Inferred by High-Resolution Analysis of Linkage Disequilibrium. Mol. Biol. Evol. 2020;37:3642–3653. doi: 10.1093/molbev/msaa169. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell M. An Introduction to Genetic Algorithms. MIT Press; Cambridge, UK: 1998. [Google Scholar]
- 32.Pembleton L.W., Cogan N.O.I., Forster J.W. StAMPP: An R Package for Calculation of Genetic Differentiation and Structure of Mixed-Ploidy Level Populations. Mol. Ecol. Resour. 2013;13:946–952. doi: 10.1111/1755-0998.12129. [DOI] [PubMed] [Google Scholar]
- 33.Melka M.G., Schenkel F. Analysis of Genetic Diversity in Four Canadian Swine Breeds Using Pedigree Data. Can. J. Anim. Sci. 2010;90:331–340. doi: 10.4141/CJAS10002. [DOI] [Google Scholar]
- 34.Tang G.Q., Xue J., Lian M.J., Yang R.F., Liu T.F., Zeng Z.Y., Jiang A.A., Jiang Y.Z., Zhu L., Bai L., et al. Inbreeding and Genetic Diversity in Three Imported Swine Breeds in china Using Pedigree Data. Asian-Australas. J. Anim. Sci. 2013;26:755–765. doi: 10.5713/ajas.2012.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veroneze R., Lopes P.S., Guimarães S.E.F., Guimarães J.D., Costa E.V., Faria V.R., Costa K.A. Using Pedigree Analysis to Monitor the Local Piau Pig Breed Conservation Program. Arch. Zootec. 2014;63:45–54. doi: 10.4321/S0004-05922014000100005. [DOI] [Google Scholar]
- 36.Mariani E., Summer A., Ablondi M., Sabbioni A. Genetic Variability and Management in Nero di Parma Swine Breed to Preserve Local Diversity. Animals. 2020;10:538. doi: 10.3390/ani10030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saura M., Fernández A., Varona L., Fernández A.I., de Cara M.Á., Barragán C., Villanueva B. Detecting Inbreeding Depression for Reproductive Traits in Iberian Pigs Using Genome-Wide Data. Genet. Sel. Evol. 2015;47:1. doi: 10.1186/s12711-014-0081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toro M.A., Rodrigañez J., Silio L., Rodriguez C. Genealogical Analysis of a Closed Herd of Black Hairless Iberian Pigs. Conserv. Biol. 2008;14:1843–1851. doi: 10.1111/j.1523-1739.2000.99322.x. [DOI] [PubMed] [Google Scholar]
- 39.Köck A., Fürst-Waltl B., Baumung R. Effects of Inbreeding on Number of Piglets Born Total, Born Alive and Weaned in Austrian Large White and Landrace Pigs. Arch. Anim. Breed. 2009;52:51–64. doi: 10.5194/aab-52-51-2009. [DOI] [Google Scholar]
- 40.Silió L., Rodríguez M.C., Fernández A., Barragán C., Benítez R., Óvilo C., Fernández A.I. Measuring Inbreeding and Inbreeding Depression on Pig Growth from Pedigree or SNP-Derived Metrics. J. Anim. Breed. Genet. 2013;130:349–360. doi: 10.1111/jbg.12031. [DOI] [PubMed] [Google Scholar]
- 41.Welsh C.S., Stewart T.S., Schwab C., Blackburn H.D. Pedigree Analysis of 5 Swine Breeds in the United States and the Implications for Genetic Conservation. J. Anim. Sci. 2010;88:1610–1618. doi: 10.2527/jas.2009-2537. [DOI] [PubMed] [Google Scholar]
- 42.Meuwissen T.H.E., Woolliams J.A. Effective Sizes of Livestock Populations to Prevent a Decline in Fitness. Theor. Appl. Genet. 1994;89:1019–1026. doi: 10.1007/BF00224533. [DOI] [PubMed] [Google Scholar]
- 43.Herrero-Medrano J.M., Megens H.J., Groenen M.A., Ramis G., Bosse M., Pérez-Enciso M., Crooijmans R.P.M.A. Conservation Genomic Analysis of Domestic and Wild Pig Populations from the Iberian Peninsula. BMC Genet. 2013;14:106. doi: 10.1186/1471-2156-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hlongwane N.L., Hadebe K., Soma P., Dzomba E.F., Muchadeyi F.C. Genome Wide Assessment of Genetic Variation and Population Distinctiveness of the Pig Family in South Africa. Front. Genet. 2020;11:344. doi: 10.3389/fgene.2020.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laval G., Iannuccelli N., Legault C., Milan D., Groenen M.A., Giuffra E., Andersson L., Nissen P.H., Jørgensen C.B., Beeckmann P., et al. Genetic Diversity of Eleven European Pig Breeds. Genet. Sel. Evol. 2000;32:187–203. doi: 10.1186/1297-9686-32-2-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blackburn H., Faria D.A., Wilson C., Paiva S.R. P4066 Genetic Diversity of Pig Populations from the US Mainland, Pacific Islands and China: Autosomal SNP Evaluation. J. Anim. Sci. 2016;94:112. doi: 10.2527/jas2016.94supplement4112x. [DOI] [Google Scholar]
- 47.Zanella R., Peixoto J.O., Cardoso F.F., Cardoso L.L., Biegelmeyer P., Cantão M.E., Otaviano A., Freitas M.S., Caetano A.R., Ledur M.C. Genetic Diversity Analysis of Two Commercial Breeds of Pigs Using Genomic and Pedigree Data. Genet. Sel. Evol. 2016;48:24. doi: 10.1186/s12711-016-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daetwyler H.D., Villanueva B., Bijma P., Woolliams J.A. Inbreeding in Genome-wide Selection. J. Anim. Breed. Genet. 2007;124:369–376. doi: 10.1111/j.1439-0388.2007.00693.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The derived data supporting this study is available from the corresponding author on reasonable request.