Supplemental Digital Content is available in the text
Keywords: acquired immunodeficiency syndrome, bronchoalveolar lavage, CD8+ T cells, human herpesvirus 8, pulmonary Kaposi sarcoma
Abstract
Pulmonary Kaposi sarcoma (pKS) caused by Human herpesvirus 8 (HHV-8) is a devastating form of KS in patients with advanced acquired immunodeficiency syndrome (AIDS) and is associated with increased morbidity and mortality. Blood T cells play a central role in the response of HIV-1 and HHV-8. However, little information is available on T cells in the alveolar space of HIV-1-associated pKS patients.
Therefore, we examined CD8+ and CD4+ T cells in the alveolar space in comparison with the blood of patients with pKS. We recruited 26 HIV-1 positive patients with KS, including 15 patients with pKS. Bronchoalveolar lavage (BAL) cells and blood mononuclear cells were analyzed for T cell memory phenotypes, surface markers associated with exhaustion, and intracellular cytokine staining (ICS) using flow cytometry. HIV-1 and HHV-8 viral loads were measured in plasma by quantitative PCR.
BAL T cells showed reduced inflammatory capacities and significantly diminished polyfunctionality compared to blood T cells from patients with pKS. This was not accompanied by increased expression of exhaustion markers, such as TIM-3 and PD-1.
More importantly, we found a negative correlation between the production of MIP1-β and TNF-α in T cells in BAL and blood, indicating compartmentalised immune responses to pKS and accentuated chronic HIV-1/HHV-8 pathogenesis via T cells in the lungs of people with pKS.
1. Introduction
Acquired immunodeficiency syndrome (AIDS)-associated lung complications such as pulmonary Kaposi sarcoma (pKS) cause irreversible tissue damage with unfavourable outcomes after initiation of antiretroviral therapy (ART). Blood samples are frequently collected to improve our knowledge of cellular immune responses accompanying such severe disease stages. However, the concordance of the immune profiles of circulating and mucosal immune cells in pulmonary diseases is not well understood.
Human herpesvirus 8 (HHV-8) is the causative agent of Kaposi sarcoma (KS), the most common cancer among HIV-1 with AIDS and one of the most common cancers in countries with high HIV-1 prevalence, such as Zimbabwe.[1–3] It has contributed to the increase in morbidity and mortality of AIDS patients worldwide.[4] Persistent chronic inflammation and immunosuppression might drive tumor formation on the skin and in the mouth, and advanced KS disease involves visceral organs such as the gastrointestinal tract and lungs.[5] pKS is a devastating illness that may involve the lung parenchyma, endobronchial tree, and visceral pleura, and is associated with high mortality.[6] Diagnosis of pKS is made by a combination of clinical presentation, the presence of KS skin lesions, radiological abnormalities, and bronchoscopy. The latter allows visualisation of lesions characteristic of pKS in the endobronchial mucosa and collection of bronchoalveolar lavage fluid (BAL), to exclude other infectious conditions that can cause similar radiological findings.[7–10] BAL has also been used to characterise the immunopathology and cellular immune responses against HIV-1 to better understand the mechanisms of infectious and non-infectious AIDS-associated lung complications.[11–13] However, in the context of HIV-1-associated pKS, BAL immune cells have not been investigated to date.
HIV-1 infection induces an early strong T cell response[14] which is associated with reduced viral loads and protection in people naturally controlling HIV-1 replication,[15–17] whereas chronic HIV-1 infection with high viral burden is associated with viral escape, reduced Nef-mediated HIV-1 antigen presentation, and malfunction of T cells by upregulation of surface markers associated with exhaustion, such as PD-1, TIM-3, or CTLA4.[18–23] Like HIV-1, HHV-8 mounts a strong T cell response in individuals without clinical symptoms, while patients with KS show a strong decline in HHV-8 specific T cell responses.[24–28] The vast majority of the literature investigating the role of T cells in HIV-1 and HHV-8 infection has been generated by investigations using blood samples. However, HIV-1/AIDS-associated co-morbidities primarily affect lymphoid organs and mucosal tissues.[29] Mucosal sites in particular, and thus mucosal resident immune cells, play a crucial role in HIV-1 dissemination and disease progression.[30,31] Lung mucosa integrity and immunity are highly affected by HIV-1, causing a significant number of HIV-1-associated infectious and non-infectious lung complications such as tuberculosis (TB), chronic obstructive pulmonary disease (COPD), and pKS.[11,32] Investigations of this mucosal site in HIV-1 infected individuals by bronchoscopy revealed that chronic HIV-1 infection causes inflammatory alveolitis by infiltration of T cells from the circulation into the alveolar space.[33] BAL T cells show an exhausted phenotype, which is associated with high viral replication, chronic inflammation, and severe depletion of tissue-resident CD4+ T cells, driving some of the HIV-1 associated lung complications.[34,35] Interestingly, during chronic HIV-1 infection, T cell profiles and dynamics differ significantly between BAL and blood, suggesting concordant and discordant immune responses in different body sites.[36–39] Tumor growth during HIV-1-associated pKS in the lung mucosa most likely affects the local immune environment. However, the impact of pKS on lung T cells compared to that on blood has not been investigated.
Based on the observations from other cohorts of HIV-1-associated lung complications and the gap in knowledge on BAL cells in HIV-1-associated pKS, we aimed to characterize BAL versus blood T cells in patients with chronic HIV-1 infection and KS disease with or without pKS. Therefore, we collected blood and BAL samples by bronchoscopy and analyzed the phenotype of T cells and expression of markers associated with exhaustion and cytokine/chemokine production by flow cytometry. We show that BAL and blood T cells differ significantly in their phenotype, and that pKS is associated with decreased cytokine/chemokine production of T cells in BAL. Importantly, we found a negative correlation between the T cell profile in the BAL and blood.
2. Materials and methods
2.1. Cohort and ethical statement
The study was approved by the Joint Parirenyatwa Hospital and College of Health Sciences Research Ethics Committee (JREC), Medical Research Council of Zimbabwe IRB, and Colorado Multiple Institutional Review Board (COMIRB). Written consent was obtained from all the patients prior to sample collection. The detailed inclusion and exclusion criteria and study protocol are provided in the Supplementary Methods section (see Supplemental Digital Content, Methods, http://links.lww.com/MD/G533). We received de-identified anonymized samples from 11 HIV-1 positive patients with KS confined to the skin (pKS-negative) and 15 samples from HIV-1 positive patients with endobronchial (pulmonary) KS (pKS positive) according to the KS Staging ACTG criteria. pKS was diagnosed by visual examination of the airways using bronchoscopy. After the initial assessment, all participants initiated ART, if not already started, and the standard of care KS treatment. T cell analysis was performed on all subjects with a sufficient number of cells collected (pKS negative = 10 subjects and pKS positive = 11 subjects). CD4 counts and viral loads for HIV-1 and HHV-8 in the blood were not significantly different between patients with and without pKS (Table 1 and see Supplemental Digital Content, Table S1, http://links.lww.com/MD/G531).
Table 1.
Summary of Kaposi sarcoma patient cohort and clinical information.
| Pulmonary KS negative | Pulmonary KS positive | Significance (P value) | |
| #Of patients | 11 | 15 | N/A |
| gender | 1 female: 10 male | 2 female: 13 male | 1.00 ∗ |
| Median age (median, IQR range) | 35 (30–44) years | 33 (28–40) years | .699 |
| Duration of HIV infection (median, IQR range) | 19 (3–32) months | 42 (2–79) months | .391 |
| Duration of ART (median, IQR range) | 7 (0–24) months | 6 (1–70) months | .530 |
| HIV-1 viral load (median, IQR range) | 20 (TND–592) copies/mL | 2329 (39–210 032) copies/ml | .393 |
| HHV-8 viral load (median, IQR range) | 186 (25–363) copies/ml | 540 (183–631) copies/ml | .189 |
| CD4+ Count (median, IQR range) | 196 (49–479) cells/ml | 245 (94–305) cells/ml | 1.00 |
| BAL Cell Recovery (median, IQR range) | 9.5 (6.75–18.5) million cells | 14 (8–27.25) million cells | .421 |
| Occurrence of mouth/oral lesions | 36% (4) | 53% (8) | .267 ∗ |
| CXR – normal | 64% (7) | 33% (5) | .431 ∗ |
| Use of Cotrimoxazole - yes | 82% (9) | 67% (10) | .658∗ |
| Previous respiratory infection | 18% (2) | 36% (5) | .391 ∗ |
| Sputum culture - negative | 78% (7) | 92% (12) | .544∗ |
| Sputum GeneXpert - negative | 100% | 100% | N/A∗ |
| BAL culture - negative | 64% (7) | 73% (11) | .683∗ |
| BAL GeneXpert - negative | 100% | 100% | N/A∗ |
| BAL PCP - negative | 100% | 100% | N/A∗ |
Mann–Whitney U and ∗Fisher exact tests were used to determine statistical differences between the 2 groups.
ART = antiretroviral treatment, CXR = chest X-ray, HIV-1 = human immunodeficiency virus 1, IQR = interquartile range, KSHV = Kaposi sarcoma-associated herpesvirus, n/a = not available, TND = target not detected.
2.2. Sample collection and processing
Peripheral blood was collected 7 days before bronchoscopy, and peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation. PBMCs were stored in freezing medium (90% fetal bovine serum (FBS) and 10% DMSO) for several weeks at −80°C and analyzed in batches. Bronchoscopy was performed as described in the Supplementary Material (see Supplemental Digital Content, Methods, http://links.lww.com/MD/G533). Bronchoalveolar lavage (BAL) fluid was collected from all 26 patients and processed immediately. BAL cells were obtained by centrifugation and used for downstream analyses.
2.3. Flow cytometry
BAL cells and PBMCs were stained with antibodies to investigate T cells for cytokine/chemokine production, memory phenotypes, and exhaustion markers. Details of all antibody clones and manufacturers are provided in Supplementary Data (see Supplemental Digital Content, Table S2, http://links.lww.com/MD/G533). For intracellular cytokine and chemokine production, cells were stimulated for a short period with phorbol myristate acetate (PMA) and ionomycin (PMA/I, 1:500; BioLegend, Cat. 423301) In the presence of 2.5 μL of the Golgi inhibitor Brefeldin A (BioLegend) and 2.5 μL anti-CD49d/CD28 (BD) for 4 hours in a CO2 incubator at 37°C and 5% CO2. To exclude dead cells, samples were stained with LIVE/DEAD Fixable dyes (ThermoFisher) for 10 minutes at room temperature (RT). Cells were washed with washing buffer (PBS + 1% FCS) and centrifuged for 5 minutes at 400xg. The following antibodies were used to phenotype T cells in the ICS panel: CD3 (BD Bioscience, UCHT1), CD4 (BioLegend, OKT4), CD8a (BioLegend, HIT8a), and CCR7 (BD Bioscience, 150503). For the memory and exhaustion panel, CD3 (BioLegend, OKT3), CD4 (BD Bioscience, SK3), CD8 (BioLegend, RPA-T8), CD14 (BioLegend, M5E2), CD19 (BioLegend, HIB19), CD56 (BioLegend, HCD56) and CD45RA (BioLegend, HI100) were used. CD19, CD56, and CD14 were used as exclusion markers for non-T cells. These antibodies were incubated for 10 minutes at RT, followed by intracellular staining of cytokines and chemokines with the antibodies IFN-γ (BioLegend, 4S.B3), IL17A (BioLegend, BL168), MIP1-β (BD Biosciences, D21–1351), TNF-α (BioLegend, Mab11), CD28 (BioLegend, CD28.2), CTLA4 (BD Bioscience, BNI3), PD-1 (BioLegend, EH12.2H7), and TIM-3 (BioLegend, F38–2E2) using the perm/wash buffer (BD Bioscience) for intracellular staining, according to the manufacturer's instructions. The stained cells were fixed with 4% paraformaldehyde (PFA, Sigma) and analyzed by flow cytometry using a BD LSRFortessa. Cell profiles and frequencies were analyzed using FlowJo v10.6 to v10.6.2 (BD). SPICE v6.1 algorithm[40] was used for the analysis of polyfunctional T cells.
2.4. Viral loads
Plasma was collected and stored at −80°C to determine viral loads of HIV-1 and HHV-8. For HIV-1, the COBAS AmpliPrep/COBAS Taqman HIV-1 Test v2.0 kit was used to detect HIV-1 RNA following the manufacturer's instructions. After extraction of DNA from 200 μL of plasma (Qiagen blood kit), plasma HHV-8 DNA was quantified by real-time PCR amplification of a conserved region of the ORF 26 minor capsid gene, as previously described.[41]
2.5. Statistical analysis
All statistical analyses were performed using the Prism software (GraphPad v5.02). All data are expressed as medians and interquartile ranges. For comparison of 2 groups (Figs. 1 and 3), we used a nonparametric Mann–Whitney U test and for multiple group comparisons a nonparametric one-way ANOVA with Dunn's test for multiple comparisons (Fig. 2). For correlations, we used a nonparametric Spearman's correlation test (Fig. 4). For the comparison of specific clinical data, Fisher exact test was used, as the variables were nominal (Table 1). For all tests, differences were considered significant at P < .05.
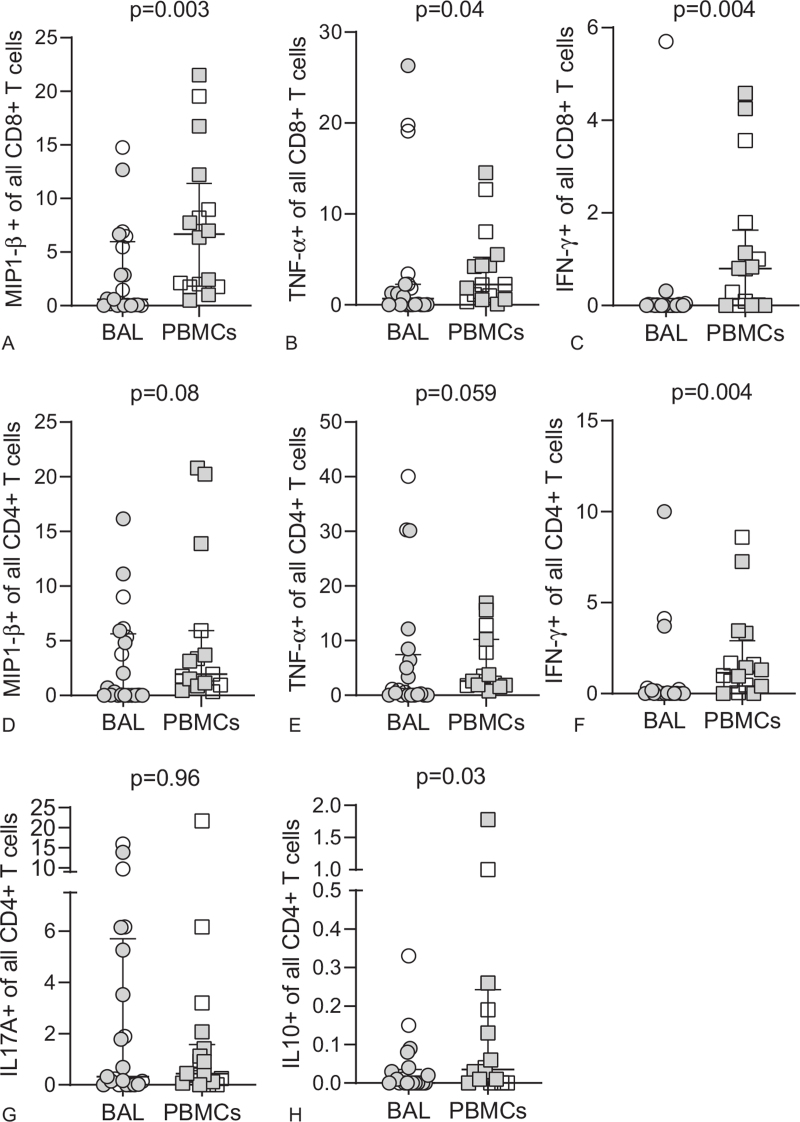
Figure 1.
Decreased Cytokine expression of BAL T cells compared to PBMCs. BAL or PBMCs CD8+ (A-C) and CD4+ (D-H) T cells from KS patients (open circles and squares) and pKS patients (closed circles and squares) were stimulated with PMA/I and their pro-inflammatory capacity measured by intracellular detection of MIP1-β, TNF-α, IFN-γ, IL17A or IL10. P values were determined by non-parametric Mann Whitney U test. Scatter plots are labelled with median and interquartile range.
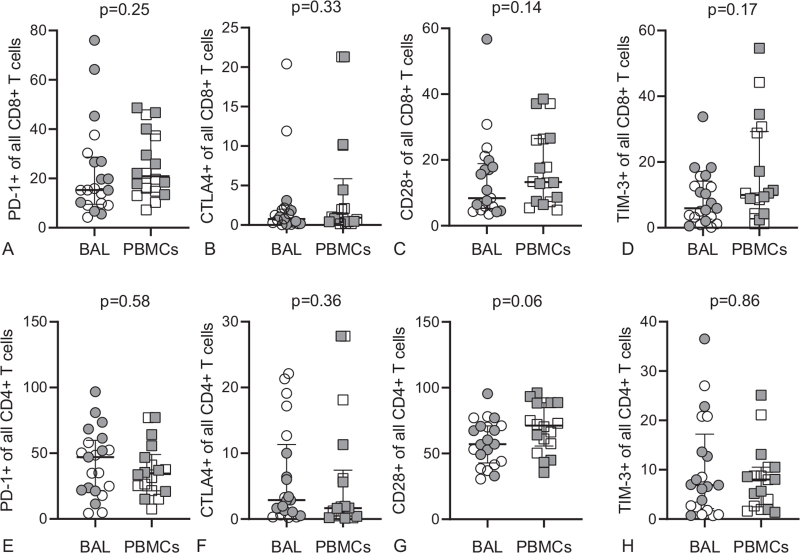
Figure 3.
Comparable expression of exhaustion marker in BAL T cells compared to PBMCs. BAL or PBMCs CD8+ (A-D) and CD4+ (E-H) T cells from KS patients (open circles and squares) and pKS patients (closed circles and squares) were analysed for the expression of PD-1, CTLA4, CD28 and TIM-3. P values were determined by non-parametric Mann Whitney U test. Scatter plots are labelled with median and interquartile range.
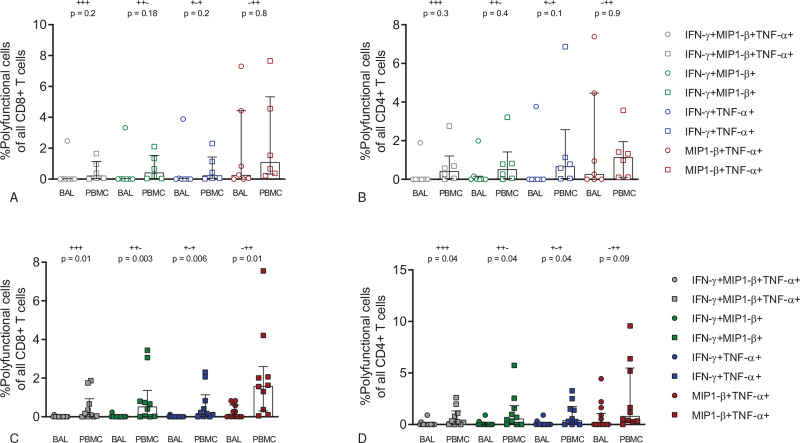
Figure 2.
Decrease of polyfunctional CD8+ and CD4+ T cells in BAL of patients with pKS. CD8+ and CD4+ T cells in patients with KS (A and B) or pKS (C and D) were analysed for the expression of multiple pro-inflammatory cytokines/chemokines using the software package pestle and spice. Scatter plots are labelled with median and interquartile range. P values were determined by a non-parametric Kruskal-Wallis test followed by Dunn's multiple comparison test.
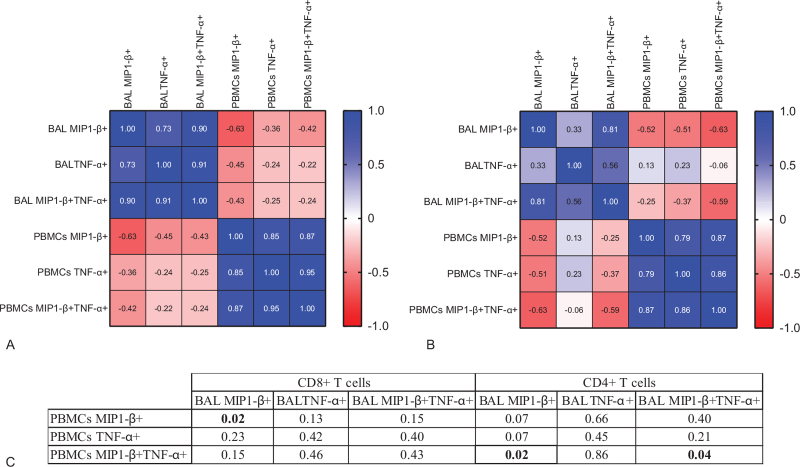
Figure 4.
Negative correlation of TNF-α and MIP1-β expressing T cells in BAL versus PBMCs. TNF-α+, MIP1-β+ or MIP1-β+ TNF-α+ CD8+ T cells (A) or CD4+ (B) T cells from BAL and PBMCs were analysed in a correlation matrix. Each square (A and B) contains the r value of a nonparametric Spearman's correlation test and (C) significant p values are highlighted in bold in the corresponding table.
3. Results
3.1. Reduced inflammatory responses of BAL CD8+ T cells from patients with pKS
The profile of T cells from mucosal compartments, such as in BAL, differs significantly from T cells in the circulation.[42] Mucosal T cells in BAL primarily comprise TEM cells compared to naïve and TCM cells in the periphery.[43] In our cohort, we also found a higher frequency of effector memory CD8+ and CD4+ T cells in BAL compared to PBMCs, while T cell phenotypes in blood were enriched for CD8+ TEMRA and CD4+ TCM cells (see Supplemental Digital Content, Figure S1 and S2, http://links.lww.com/MD/G533). The phenotypes of T cells in each compartment did not change when analyzed by pKS status (see Supplemental Digital Content, Figure S3, http://links.lww.com/MD/G533). Chronic HIV-1 infection leads to infiltration of pro-inflammatory CD8+ TEM cells in the alveolar space, but also to increased expression of exhaustion markers, which might result in a reduced cytokine response in the BAL of patients with pKS compared to KS.[44] Therefore, we set up an ex vivo stimulation assay to investigate the inflammatory potential of BAL and blood T cells from patients with KS and pKS after stimulation with PMA/I by ICS flow cytometry (see Supplemental Digital Content, Figure S4, http://links.lww.com/MD/G533). MIP1-β, TNF-α, and IFN-γ expression was significantly higher in CD8+ T cells from PBMCs than in BAL (Fig. 1 A–C), with similar trends for CD4+ T cells (Fig. 1 D–H). Overall, we did not observe any difference between BAL and blood T cells in samples from pKS-negative patients (see Supplemental Digital Content, Figure S5, http://links.lww.com/MD/G533). In contrast, pKS patients were significantly different between BAL and blood T cells (see Supplemental Digital Content, Figure S6, http://links.lww.com/MD/G533). Therefore, our data suggest that the pro-inflammatory capacity of BAL CD8+ and, to some extent, BAL CD4+ T cells are significantly reduced in patients with pKS compared to blood T cells. To further understand the impact of pKS on BAL T cells, we performed an analysis of polyfunctional T cells after PMA/I stimulation. TNF-α+ MIP1-β+ T cells constituted the highest proportion of all polyfunctional CD8+ or CD4+ T cells (Fig. 2). Interestingly, the frequency of all polyfunctional subpopulations was not altered in patients without pKS (Fig. 2A and B), while polyfunctional (IFN-γ+ MIP1-β+ TNF-α+, IFN-γ+ MIP1-β+, IFN-γ+TNF-α+, and MIP1-β+ TNF-α+) CD8+ and CD4+ T cells were significantly reduced in BAL compared to blood T cells from patients with pKS (Fig. 2C and D). In summary, we observed reduced single- and polyfunctional capacities of BAL, but not blood CD8+ and CD4+ T cells from patients with pKS to produce pro-inflammatory cytokines such as TNF-α and MIP1-β.
3.2. BAL T cell surface marker of exhaustion are not increased in patients with pKS
T cell exhaustion during chronic HIV-1 infection is well reported and is associated with a loss of the inflammatory and cytotoxic activities of these cells.[20] Thus, we investigated the impact of pKS on T cell exhaustion by comparing the frequency of the surface receptors TIM-3, PD-1, CD28, and CTLA4 between BAL and blood T cells (Supplemental Digital Content, Figure S7, http://links.lww.com/MD/G533). We did not find a significant difference in the expression of exhaustion markers between BAL and blood (Fig. 3), independent of pKS status (see Supplemental Digital Content, Figure S8 and S9, http://links.lww.com/MD/G533). A poly exhaustion marker analysis for cells expressing more than 1 exhaustion marker revealed a high proportion of TIM-3 and PD-1 double-positive cells in the BAL and blood. However, this was not upregulated in BAL T cells from patients with pKS (see Supplemental Digital Content, Figure S10, http://links.lww.com/MD/G533). In conclusion, the analysis of cell surface markers associated with T cell exhaustion in T cells from BAL and blood did not reveal a significant upregulation of single or multiple exhaustion markers in BAL T cells from patients with pKS, suggesting that the reduced capacity of BAL T cells to produce MIP1-β, TNF-α, and IFN-γ compared to blood is not associated with the upregulation of exhaustion markers.
3.3. Negative correlation of BAL CD8+ T cell cytokine expression compared to blood in patients with pKS
pKS significantly impacted the production of cytokines and chemokines by BAL T cells compared to blood. Instead, we observed a trend of increased TNF-α and MIP1-β production in blood CD8+ T cells from patients with pKS (see Supplemental Digital Content, Figure S11, http://links.lww.com/MD/G533). This suggests that pKS might have a different impact on circulating CD8+ T cells compared to BAL CD8+ T cells. To further investigate this observation, we ran a correlation matrix between donor-matched BAL and blood T cells expressing either TNF-α, MIP1-β, or both (TNF-α+MIP1-β+). We found a significant positive correlation between TNF-α and MIP1-β expression in CD8+ and CD4+ T cells in the same compartment, suggesting that increased expression of either cytokine or chemokine was associated with a higher expression of other pro-inflammatory analytes (Fig. 4). However, the same comparisons were negatively correlated between the compartments in a cross-sectional analysis, indicating that an increased expression of TNF-α or MIP1-β in blood is associated with a decreased expression in the BAL of the same patients (Fig. 4).
In conclusion, in this cohort of AIDS-associated KS patients, BAL CD8+ T cells were almost exclusively of a TEM phenotype, independent of pKS diagnosis, but significantly different from blood CD8+ T cells. pKS diagnosis was associated with decreased production of MIP1-β, TNF-α, IFN-γ, and polyfunctionality of BAL T cells. Interestingly, blood CD8+ T cells from patients with pKS did not follow this trend. Instead, we found a negative association between the inflammatory cytokine production of MIP1-β and TNF-α between BAL and blood CD8+ T cells. Therefore, the state of the local immune response in the mucosa of patients with HIV-1-associated pKS was not predictable by sampling the blood of these patients.
4. Discussion
Chronic HIV-1 infection has a significant impact on lung function and is associated with a vast number of infectious and non-infectious lung complications, such as pKS.[45,46] T cell responses are an essential component of the immune system to control acute and chronic viral infections[47]; however, immune responses to HIV-1-associated lung diseases are primarily studied by sampling the blood. Thus, it is not well understood how these findings reflect the immune response in mucosal tissues. Our analysis of BAL and blood T cells in patients with and without pKS confirmed the dominant presence of CD8+ and CD4+ TEM cells in BAL compared to blood. In patients with pKS, we found a decrease in the poly-functionality of BAL T cells compared to that in blood. Importantly, we found a negative association between BAL and blood T cells with the ability to produce MIP1-β and TNF-α after stimulation. Our data suggest that pulmonary KS might induce compartmentalised changes that are not reflected in the periphery of the same patient.
It is generally well described that the majority of T cells in the alveolar space are of a TEM phenotype, based on the absence of CD45RA and CD62L on their cell surface.[48] In our cohort, this phenotype was not altered by pKS. CD8+ T cells infiltrating the alveolar space during HIV-1 and other infections have been described as primarily of a TEM cell phenotype, suggesting that TEM cells, due to their expression of the lung-homing receptors CXCR3 and CCR5 are the primary recruited cell type in this compartment in health and disease.[33,49] The frequency of TEM cells was significantly lower in blood CD8+ T cells, which might result in a compartmentalised response to HIV-1 and HHV-8, such as differences in cytokine and chemokine expression. The high frequency of CD8+ TEMRA cells in the blood might have contributed to the observed differences. However, we were not able to investigate the impact of KS on the BAL CD8+ T cell phenotype or CD8+ T cell infiltration, since we did not recruit a KS negative control group. The focus of this study was to investigate the T cell profile at the mucosal site compared to the periphery in the context of pKS. The HIV-1 or HHV-8 viral load was not significantly different in plasma from pKS-positive versus-negative patients. However, pKS patients had relatively high and persistent HIV-1 viral loads in the peripheral blood, despite ART for at least 5 months before the bronchoscopy, which might indicate a role for viral replication in driving the decreased pro-inflammatory response of BAL CD8+ T cells. We did not measure the viral load in the BAL fluid; therefore, it remains to be determined whether our findings in this compartment are associated with increased viral replication. In this context, the lung and the alveolar space have been suggested to serve as a cellular reservoir for HIV-1, whereas cell-free virus might correlate between plasma and BAL fluid.[38,50,51] However, the heterogeneity of our cohort might have resulted in other confounders which could have contributed to our findings. Chronic HIV-1 infection has been reported to facilitate the recruitment of CD8+ T cells to the lungs[52] and ultimately leads to the dysfunction of CD8+ T cells and a reduced presence of CD4+ T helper cells.[30,53] Therefore, a reduction in total CD4+ T cells or HHV-8 specific BAL CD4+ T cells might significantly contribute to the development of pKS.[54] Likewise, an increased frequency of regulatory CD4+ T cells could impact the CD8+ T cell response against HHV-8 and enhance the development of KS.[24] Thus, HHV-8 specific T cell responses should be further investigated in this compartment.
In our data set, cell surface markers associated with T cell exhaustion were not increased in BAL versus blood T cells from pKS patients. However, the frequency of PD-1+ and TIM3+PD-1+ CD8+ T cells was high in this cohort, independent of the pKS diagnosis. TIM-3+PD-1+ CD8+ T cells have been shown to have reduced function and increased exhaustion.[55] TIM-3 expression also leads to exhaustion of T cells in the context of chronic stimulation and HIV-1 infection, which results in reduced cytotoxic activity, inflammatory capacities, or polyfunctionality.[55–57] However, we did not directly measure the cytokine production or cytotoxicity of TIM-3+PD-1+ CD8+ T cells. Other escape mechanisms of HHV-8 have been described and might also play a role in reducing T cell responses in BAL, but were not investigated in our study.[27,28]
In our experimental setup, we investigated the capacity of CD8+ T cells to produce pro-inflammatory cytokines/chemokines by strongly stimulating the cells with the protein kinase C activating compound PMA/I. This enabled us to measure the general inflammatory capacity of T cells independent of T cell receptor (TCR) specificity. Thus, for TCR-dependent stimulation, it needs to be further explored in a larger cohort to determine whether pKS diagnosis is associated with a decreased response in BAL T cells compared to blood. In addition to the possible presence of ongoing HIV-1 and HHV-8 replication, pKS patients often present clinically with other co-infections or co-morbidities such as TB or chronic obstructive pulmonary disease. Although not statistically significant in our cohort, there was a trend for increased mouth/oral lesions, abnormal chest X-rays, and previous diagnosis of respiratory illnesses in the pKS group. It cannot be excluded that additional lung complications accelerated the decreased cytokine and chemokine production of BAL T cells specific for additional pathogens such as M. tuberculosis in patients with HIV-1-associated pKS. However, all participants were tested for active tuberculosis and pneumocystis pneumonia (PCP), and therefore might not account for these differences. Our study is limited by its small sample size, which may not allow generalised conclusions. A larger cohort would be required to investigate the specificity of BAL T cells, association with viral loads, and the pro-inflammatory and cytotoxic capacity of BAL CD8+ T cells in a TCR-dependent manner. Furthermore, our study was biased in the recruitment of primarily male patients, which likely reflects the higher burden of AIDS-KS in men compared to women in African countries.[58–60] Nevertheless, these limitations cannot fully explain the discrepancy between BAL and PBMCs in pKS, with decreased expression of inflammatory cytokines and reduced polyfunctionality in BAL CD8+ T cells compared to blood T cells. Thus, our data indicate a negative correlation between the production of MIP1-β and TNF-α in CD8+ cells in BAL and blood. In the periphery, pKS was associated with a trending increase in the expression of inflammatory markers, while in the alveolar space with a diminished inflammatory T cell response. Our study underscores the relevance of investigations of the alveolar space in patients with HIV-1-associated lung complications in order to improve our knowledge about the mechanisms driving the pathology in these patients. Bronchoscopy is more invasive than collection of blood samples and requires specialized clinical training. However, we would like to argue that BAL sampling significantly improves our understanding of HIV-1-associated lung complications such as pKS and may contribute to the development of new treatment strategies against pKS.
Acknowledgments
We would also like to thank the respiratory medicine specialists’ team in the Department of Medicine at the Parirenyatwa Group of Hospitals in Harare, Zimbabwe, working under the supervision of Dr. Rebecca Lyall: Dr. F Manyeruke, Dr. T Nyagura, and Dr. T Makoni.
Author contributions
Conceptualization: Björn Corleis, Rebecca A Lyall, Douglas S Kwon, Alan M McGregor, Suzanne Fiorillo, Thomas B Campbell.
Data curation: Björn Corleis, Tarisiro Matiza, Kathryn F Boyd, Rebecca A Lyall, Suzanne Fiorillo, Thomas B Campbell.
Formal analysis: Björn Corleis, Tarisiro Matiza.
Funding acquisition: Thomas B Campbell, Margaret Borok.
Investigation: Björn Corleis, Tarisiro Matiza, Kathryn F Boyd, Rebecca A Lyall.
Methodology: Björn Corleis, Tarisiro Matiza, Kathryn F Boyd.
Project administration: Kathryn F Boyd, Alan M McGregor, Thomas B Campbell, Margaret Borok.
Supervision: Björn Corleis, Douglas S Kwon, Alan M McGregor, Thomas B Campbell, Margaret Borok.
Validation: Suzanne Fiorillo.
Visualization: Björn Corleis, Tarisiro Matiza.
Writing – original draft: Björn Corleis, Tarisiro Matiza.
Writing – review & editing: Björn Corleis, Rebecca A Lyall, Douglas S Kwon, Alan M McGregor, Suzanne Fiorillo, Thomas B Campbell, Margaret Borok.
Footnotes
Abbreviations: ART = antiretroviral therapy, AIDS = acquired immunodeficiency syndrome, BAL = bronchoalveolar lavage, HHV-8 = human herpesvirus 8, KS = Kaposi sarcoma, pKS = pulmonary Kaposi sarcoma.
How to cite this article: Matiza T, Boyd KF, Lyall RA, Kwon DS, McGregor AM, Fiorillo S, Campbell TB, Borok M, Corleis B. Compartmentalized T cell profile in the lungs of patients with HIV-1-associated pulmonary Kaposi sarcoma. Medicine. 2021;100:51(e28328).
This work was supported by funds from the National Institutes of Health (NIH) awarded to TBC and MB (Supplement to NIH P30 CA046934).
The authors have no conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].Borok M, Fiorillo S, Gudza I, et al. Evaluation of plasma human herpesvirus 8 DNA as a marker of clinical outcomes during antiretroviral therapy for AIDS-related Kaposi sarcoma in Zimbabwe. Clin Infect Dis 2010;51:342–9. [DOI] [PubMed] [Google Scholar]
- [2].Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS 2008;22:1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nelson BC, Borok MZ, Mhlanga TO, Makadzange AT, Campbell TB. AIDS-associated Kaposi sarcoma: outcomes after initiation of antiretroviral therapy at a university-affiliated hospital in urban Zimbabwe. Int J Infect Dis 2013;17:e902–6. [DOI] [PubMed] [Google Scholar]
- [4].Chokunonga E, Levy LM, Bassett MT, et al. Aids and cancer in Africa: the evolving epidemic in Zimbabwe. AIDS 1999;13:2583–8. [DOI] [PubMed] [Google Scholar]
- [5].Pinzone MR, Berretta M, Caopardo B, Nunnari G. Epstein-barr virus- and Kaposi sarcoma-associated herpesvirus-related malignancies in the setting of human immunodeficiency virus infection. Semin Oncol 2015;42:258–71. [DOI] [PubMed] [Google Scholar]
- [6].Aboulafia DM. The epidemiologic, pathologic, and clinical features of AIDS-associated pulmonary Kaposi's sarcoma. Chest 2000;117:1128–45. [DOI] [PubMed] [Google Scholar]
- [7].Alwassia A, Alshathri Z, Khosla R, Spagnolo SV. Pulmonary Kaposi sarcoma presenting as complete lung consolidation. BMJ Case Rep 2017;2017:bcr2016219048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Epelbaum O, Go R, Patel G, Braman S. Pulmonary Kaposi's sarcoma and its complications in the HAART era: a contemporary case-based review. Lung 2016;194:163–9. [DOI] [PubMed] [Google Scholar]
- [9].Gasparetto TD, Marchiori E, Lourenco S, et al. Pulmonary involvement in Kaposi sarcoma: correlation between imaging and pathology. Orphanet J Rare Dis 2009;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yoo DJ, Lee KHL, Munderi P, Shin KC, Lee JK. Clinical and bronchoscopic findings in Ugandans with pulmonary Kaposi's sarcoma. Korean J Intern Med 2005;20:290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Crothers K, Humang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 2011;183:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Petrache I, Diab K, Knox KS, et al. HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax 2008;63:463–9. [DOI] [PubMed] [Google Scholar]
- [13].Mwale A, Hummel A, Mvaya L, et al. B cell, CD8 (+) T cell and gamma delta T cell infiltration alters alveolar immune cell homeostasis in HIV-infected Malawian adults. Wellcome Open Res 2017;2:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ndhlovu ZM, Kamya P, Mewalal N, et al. Magnitude and kinetics of CD8+ T cell activation during hyperacute HIV infection impact viral set point. Immunity 2015;43:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gaiha GD, Rossin EJ, Urbach J, et al. Structural topology defines protective CD8 (+) T cell epitopes in the HIV proteome. Science 2019;364:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cartwright EK, Spicer L, Smith SA, et al. CD8(+) lymphocytes are required for maintaining viral suppression in SIV-infected macaques treated with short-term antiretroviral therapy. Immunity 2016;45:656–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jin X, Bauer DE, Tuttleton SE, et al. Dramatic rise in plasma viremia after CD8 (+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med 1999;189:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McGary CS, Deleage C, Harper J, et al. CTLA-4(+)PD-1(-) memory CD4(+) T cells critically contribute to viral persistence in antiretroviral therapy-suppressed, SIV-infected rhesus macaques. Immunity 2017;47:776–88. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fujita T, Burwitz BJ, Chew GM, et al. Expansion of dysfunctional Tim-3-expressing effector memory CD8+ T cells during simian immunodeficiency virus infection in rhesus macaques. J Immunol 2014;193:5576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fenwick C, Joo V, Jacquier P, et al. T-cell exhaustion in HIV infection. Immunol Rev 2019;292:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006;443:350–4. [DOI] [PubMed] [Google Scholar]
- [22].Boutwell CL, Rolland MM, Herbeck JT, et al. Viral evolution and escape during acute HIV-1 infection. J Infect Dis 2010;202:S309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wonderlich ER, Leonard JA, Collins KL. HIV immune evasion disruption of antigen presentation by the HIV Nef protein. Adv Virus Res 2011;80:103–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lepone LM, Rappocciolo G, Piazza PA, et al. Regulatory T cell effect on CD8 (+) T cell responses to human herpesvirus 8 infection and development of Kaposi's Sarcoma. AIDS Res Hum Retroviruses 2017;33:668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lepone L, Rappocciolo G, Knowlton E, et al. Monofunctional and polyfunctional CD8+ T cell responses to human herpesvirus 8 lytic and latency proteins. Clin Vaccine Immunol 2010;17:1507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Roshan R, Labo N, Trivett M, et al. T-cell responses to KSHV infection: a systematic approach. Oncotarget 2017;8:109402–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Misstear K, Chanas SA, Rezaee SA, et al. Suppression of antigen-specific T cell responses by the Kaposi's sarcoma-associated herpesvirus viral OX2 protein and its cellular orthologue, CD200. J Virol 2012;86:6246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Coscoy L. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Nat Rev Immunol 2007;7:391–401. [DOI] [PubMed] [Google Scholar]
- [29].Maciel RA, Klück HM, Durand M, Sprinz E. Comorbidity is more common and occurs earlier in persons living with HIV than in HIV-uninfected matched controls, aged 50 years and older: a cross-sectional study. Int J Infect Dis 2018;70:30–5. [DOI] [PubMed] [Google Scholar]
- [30].Corleis B, Bucsan AN, Deruaz M, et al. HIV-1 and SIV infection are associated with early loss of lung interstitial CD4+ T cells and dissemination of pulmonary tuberculosis. Cell Rep 2019;26:1409–18. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004;200:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fitzpatrick ME, Kunisaki KM, Morris A. Pulmonary disease in HIV-infected adults in the era of antiretroviral therapy. AIDS 2018;32:277–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Agostini C, Facco M, Siviero M, et al. CXC chemokines IP-10 and mig expression and direct migration of pulmonary CD8+/CXCR3+ T cells in the lungs of patients with HIV infection and T-cell alveolitis. Am J Respir Crit Care Med 2000;162:1466–73. [DOI] [PubMed] [Google Scholar]
- [34].Neff CP, Chain JL, MaWhinney S, et al. Lymphocytic alveolitis is associated with the accumulation of functionally impaired HIV-specific T cells in the lung of antiretroviral therapy-naive subjects. Am J Respir Crit Care Med 2015;191:464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Enyindah-Asonye G, Nwankwo A, Rahman MA, et al. Overexpression of CD6 and PD-1 identifies dysfunctional CD8(+) T-cells during chronic SIV infection of rhesus macaques. Front Immunol 2019;10:3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brenchley JM, Knox KS, Asher AI, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol 2008;1:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bunjun R, Riou C, Soares AP, et al. Effect of HIV on the frequency and number of mycobacterium tuberculosis-specific CD4+ T cells in blood and airways during latent M. tuberculosis Infection. J Infect Dis 2017;216:1550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Costiniuk CT, Salahuddin S, Farnos O, et al. HIV persistence in mucosal CD4+ T cells within the lungs of adults receiving long-term suppressive antiretroviral therapy. AIDS 2018;32:2279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Knox KS, Vinton C, Hage CA, et al. Reconstitution of CD4 T cells in bronchoalveolar lavage fluid after initiation of highly active antiretroviral therapy. J Virol 2010;84:9010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011;79:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].White IE, Campbell TB. Quantitation of cell-free and cell-associated Kaposi's sarcoma-associated herpesvirus DNA by real-time PCR. J Clin Microbiol 2000;38:1992–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Snyder ME, Farber DL. Human lung tissue resident memory T cells in health and disease. Curr Opin Immunol 2019;59:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Connors TJ, Baird JS, Yopes MC, et al. Developmental regulation of effector and resident memory T cell generation during pediatric viral respiratory tract infection. J Immunol 2018;201:432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Popescu I, Drummond MB, Gama L, et al. Activation-induced cell death drives profound lung CD4(+) T-cell depletion in HIV-associated chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014;190:744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cribbs SK, Crothers K, Morris A. Pathogenesis of HIV-related lung disease: immunity, infection, and inflammation. Physiol Rev 2020;100:603–32. [DOI] [PubMed] [Google Scholar]
- [46].Fitzpatrick M, Brooks JT, Kaplan JE. Epidemiology of HIV-associated lung disease in the United States. Semin Respir Crit Care Med 2016;37:181–98. [DOI] [PubMed] [Google Scholar]
- [47].Collins DR, Gaiha GD, Walker BD. CD8(+) T cells in HIV control, cure and prevention. Nat Rev Immunol 2020;20:471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Szabo PA, Levitin HM, Miron M, et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat Commun 2019;10:4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-gamma Production. J Immunol 2015;195:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Costiniuk CT, Jenabian MA. The lungs as anatomical reservoirs of HIV infection. Rev Med Virol 2014;24:35–54. [DOI] [PubMed] [Google Scholar]
- [51].Wood KL, Chaiyarit P, Day RB, et al. Measurements of HIV viral loads from different levels of the respiratory tract. Chest 2003;124:536–42. [DOI] [PubMed] [Google Scholar]
- [52].Twigg HL, Soliman DM, Day RB, et al. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med 1999;159:1439–44. [DOI] [PubMed] [Google Scholar]
- [53].Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006;12:1198–202. [DOI] [PubMed] [Google Scholar]
- [54].Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15:486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010;207:2187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kassu A, Marcus RA, D'Souza MB, et al. Suppression of HIV replication by antiretroviral therapy reduces TIM-3 expression on HIV-specific CD8(+) T cells. AIDS Res Hum Retroviruses 2011;27:01–3. [DOI] [PubMed] [Google Scholar]
- [57].Jones RB, Ndhlovu LC, Barbour JD, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 2008;205:2763–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Parkin DM. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int J Cancer 2013;133:721–9. [DOI] [PubMed] [Google Scholar]
- [59].Iregbu KC, Elegba OY. Prevalence of Kaposi's sarcoma among adult HIV-seropositive patients seen in a designated HIV treatment and care center in Abuja, Nigeria. J Int Assoc Physicians AIDS Care (Chic) 2006;5:115–8. [DOI] [PubMed] [Google Scholar]
- [60].Meditz AL, Borok M, MaWhinney, et al. Gender differences in AIDS-associated Kaposi sarcoma in Harare, Zimbabwe. J Acquir Immune Defic Syndr 2007;44:306–8. [DOI] [PubMed] [Google Scholar]