Abstract
The mortality rate of patients with anti-neutrophil cytoplasm antibody -associated vasculitis (AAV) is higher than the general population. To date, no studies have evaluated the factors associated with unfavorable outcomes in Brazilian patients, who represent a miscegenated population. Our objective was to identify clinical and laboratory features associated with mortality in Brazilian patients with AAV.
One hundred twenty eight patients fulfilling the American College of Rheumatology and Chapel Hill Classification Criteria followed between 2000 and 2018 in our Rheumatology Outpatient Clinics were included. Data were obtained from an ongoing electronic database. Patients were divided into 2 groups (dead or alive in 2018), and disease activity (Birmingham vasculitis activity score [BVAS]), vasculitis-related damage (VDI), and laboratory parameters were compared at the most recent attendance and at the last attendance before death.
Of the 128 patients followed, 78.9% had granulomatosis with polyangiitis, 16.4% had eosinophilic granulomatosis with polyangiitis, and 4.6% had microscopic polyangiitis. In 2018, 78 patients were alive, 25 had died, and 25 had lost contact. The main cause of death was infection. According to the univariate analysis, the Birmingham vasculitis activity score, VDI, and glucocorticoid dose were higher in the group of patients who died. Laboratorial features related to mortality were creatinine, hemoglobin, erythrocyte sedimentation ratio, and C-reactive protein (CRP). Logistic regression analysis showed that high VDI, creatinine levels, and CRP levels were independent factors associated with mortality. Survival was significantly decreased in patients with renal impairment.
This is the first study to use this approach performed in a Brazilian population and it showed that damage index, renal impairment, and CRP levels were associated with mortality in a miscegenated population with AAV.
Keywords: anti-neutrophil cytoplasm antibody, anti-neutrophil cytoplasm antibody-associated vasculitis, mortality, vasculitis
1. Introduction
Anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) represents a group of diseases that cause small-vessel inflammation, with 3 recognized conditions: granulomatosis with polyangiitis (GPA, formerly called Wegener granulomatosis), eosinophilic granulomatosis with polyangiitis (EGPA, formerly called Churg-Strauss syndrome), and microscopic polyangiitis (MPA).[1,2] Although the 3 diseases each have a unique clinical presentation, they share many manifestations due to the systemic nature of the underlying small-vessel vasculitis and, in GPA and EGPA, granulomatous inflammation.[3] GPA is highly associated with anti-proteinase 3 (PR3) antibodies, while MPA and, less frequently, EGPA are associated with anti-myeloperoxidase (MPO) antibodies.[3–5]
Mortality is increased among patients with AAV.[6–8] A few papers have tried to understand what disease features or patterns are associated with these unfavorable outcomes. Lai et al[9] found that age, secondary infection, pulmonary involvement, and initial kidney function were independent predictors of mortality in a Chinese population with AAV (26.1% with GPA and 67.6% with MPA). On the other hand, Pu et al[10] also analyzed 123 Chinese patients in a Nephrology center and showed that a high Birmingham vasculitis activity score (BVAS), pulmonary hemorrhage, digestive system involvement, and a high creatinine level were features associated with worse prognosis. A Swedish cohort of 195 patients with AAV (48% GPA, 46% MPA, and 6% EGPA) divided by cluster affiliation demonstrated that increasing age, renal impairment, and cardiovascular and gastrointestinal clusters were predictors of mortality.[11]
A few studies[12,13] have evaluated the factors associated with mortality among patients with AAV from Latin America, who represent a miscegenated population, and none with Brazilian patients. The objective of this study was to improve the understanding of mortality and survival in these patients and to identify clinical or laboratory features associated with worse prognosis.
2. Methods
All the new-diagnosed patients fulfilling the American College of Rheumatology (1990)[14,15] and Chapel Hill (2012)[16] classification criteria for ANCA-associated vasculitis who were followed between 2000 and 2018 in our Rheumatology Vasculitis Outpatient Clinics at the Clinics Hospital of University of Sao Paulo were included.
Data were obtained during the visits to an ongoing electronic database protocol, established in January 2000, that was carried out for all patients at 1- to 6-month intervals; the protocol consisted of an extensive clinical and laboratorial evaluation. This is a retrospective analysis from the electronic database of an ongoing prospective cohort. Data regarding disease activity and damage were calculated by the attending clinician at the last follow-up visit for all the patients. Information regarding death was taken from medical records as well as from death certificates, which were available in the database of the Program of Information on Mortality in Sao Paulo. Family members were contacted by telephone when data were not available in the medical records. Patients who have lost follow-up and could not be found by telephone were excluded from the analysis.
Patients were divided into 2 groups—dead or alive in 2018—and their clinical and laboratorial features were compared. The variables analyzed included demographic features (sex, age at the onset of vasculitis, ethnicity), ANCA frequency (at any time during follow-up), disease activity using the BVAS[17,18] and vasculitis-related damage using the vasculitis damage index (VDI)[19] (both completed during patient's evaluation at the last available outpatient follow up), and laboratory parameters (blood cell count, creatinine, C-reactive protein [CRP], and erythrocyte sedimentation rate [ESR]) at the most recent attendance (for the living patients) or at the last attendance before death. For those patients who had no dosed anti-PR3 and anti-MPO, dosing was done in 2019 with stockpiled serum. The immunosuppressive treatments administered, including the current dose of prednisone, were also compared.
Qualitative characteristics were described using absolute frequencies and the association between mortality with the use of chi-squared test or Fisher exact test. Quantitative characteristics were described according to mortality using means and standard deviations or medians and interquartile intervals according to the probability distribution of the data assessed using Kolmogorov–Smirnov test[20] compared between groups using test analysis Student t or Mann–Whitney U tests.[20] Odds ratios (OR) were estimated with the respective 95% confidence intervals for each characteristic for mortality using unadjusted logistic regression.[21]
The joint model was adjusted to explain the mortality of patients according to the characteristics of the last outpatient assessment using multiple logistic regression.[21] The variables that showed statistical significance in the bivariate analyzes and that present clinical relevance with the disease and its worst prognosis and all variables included in the final model (full model) were maintained.
Each item of the VDI was tested separately with the mortality of patients to explore which ones most influenced their final outcome using chi-squared test or Fisher exact test.
The analyzes were performed using the IBM-SPSS for Windows version 20.0 software and tabulated using the Microsoft-Excel 2003 software. The tests were performed with a 5% significance level.
The protocol was approved by the Ethics Committee of the Clinics Hospital of University of Sao Paulo, School of Medicine. According to the Ethics Committee, the present study waives consent declaration from the patients, once there is no intervention related to them or data disclosure (Ethics Committee opinion no. 2,903,237, September 19, 2020).
3. Results
One hundred and twenty-eight patients were initially included. One hundred and one of these patients had GPA (78.9%), 21 had EGPA (16.4%), and 6 had MPA (4.6%). In 2018, 78 (60.9%) of the patients were alive, 25 (19.5%) had died, and 25 (19.5%) had lost contact and could not be assessed via telephone, and therefore were excluded from the analysis. The mean time of follow up was 10.77 (±6.13) years for the living patients until 2018 and 8.44 (±5.96) years for the group of patients who died (P = .10).
The patients were asked about their self-referred ethnicity: 75.7% of them were White, 1.9% were Black, 20.4% were Mulatto, and 1.9% were Yellow (oriental).
In the group of living patients, 34 out of 78 (43%) tested positive for anti-PR3 antibodies, while 10 out of 78 (12.8%) tested positive for anti-MPO antibodies. In the group of patients who died, 11 out 25 tested positive for anti-PR3 antibodies (44%) and 3 out of 25 tested positive for anti-MPO antibodies (12%). Seventeen out of 25 (68%) patients who died and 55 out of 78 (70.5%) living patients had histological confirmation of ANCA-associated vasculitis.
Both activity levels and damage index scores (BVAS and VDI) were higher in the group of patients who died (P = .001 and P < .001, respectively). The laboratorial features related to higher mortality were creatinine (P < .001), hemoglobin (P < .001), ESR (P = .037), and CRP (P < .001). The current glucocorticoid dose taken at the last attendance was higher in the patients who died (P = .014). No significant difference was observed regarding the presence of ANCA, anti-PR3 antibodies, and anti-MPO antibodies or the immunosuppressive treatment. No significant difference was observed regarding pulmonary involvement by the vasculitis (52% in patients who died vs 72% in living patients, P = .11). The results are shown in Table 1.
Table 1.
Demographic characteristics and clinical/laboratory features analyzed.
| 95% CI | ||||||
| Variable | Live (n = 78) | Dead (n = 25) | OR | Lower | Upper | P |
| Gender (female), n (%) | 51 (65.4) | 15 (60) | 0.79 | 0.32 | 2.01 | .625∗ |
| Age at diagnosis, yrs, mean ± SD | 41 ± 16.5 | 51.3 ± 16.1 | 1.04 | 1.01 | 1.07 | .008∗∗ |
| Disease duration, yrs, mean ± SD | 10.8 ± 6.1 | 8.4 ± 6 | 0.94 | 0.86 | 1.01 | .009∗∗ |
| c-ANCA, n (%) | 46 (59) | 14 (56) | 0.89 | 0.36 | 2.20 | .793∗ |
| p-ANCA, n (%) | 19 (24.4) | 4 (16) | 0.59 | 0.18 | 1.94 | .382∗ |
| BVAS, median (IQR) | 2 (0;4) | 8 (2;12) | 1.21 | 1.09 | 1.34 | .001 |
| VDI, median (IQR) | (3 (2;5) | 6 (3;8.5) | 1.43 | 1.18 | 1.73 | <.001 |
| Hemoglobin, g/dL, median (IQR) | 13.4 (12.5;14.3) | 11.3 (8.5;13.1) | 0.49 | 0.35 | 0.69 | <.001 |
| Leukocytes (103/mm3), median (IQR) | 6.38 (5.32;9.08) | 9.47 (6.02;11.79) | 1.14 | 0.99 | 1.32 | .037 |
| Neutrophils median (IQR) | 4.20 (3.11;6.40) | 6.50 (4.83;9.10) | 1.29 | 1.08 | 1.54 | .002 |
| Lymphocytes, median (IQR) | 1.55 (1.05;2.15) | 1.71 (0.680;2.62) | 0.95 | 0.53 | 1.70 | .818 |
| Platelets, 103/μL, median (IQR) | 232 (210;304) | 217 (131.5;295;3) | 0.99 | 0.99 | 1.00 | .125 |
| Creatinine, mg/dL, median (IQR) | 0.9 (0.7;1.1) | 1.6 (0.9;3.9) | 1.39 | 1.09 | 1.76 | <.001 |
| CRP, mg/L, median (IQR) | 2.9 (1.3;6.1) | 33.1 (6.1;142.1) | 1.07 | 1.02 | 1.13 | <.001 |
| ESR (mm/1st h), median (IQR) | 14 (5;25) | 31 (7.3;64) | 1.03 | 1.01 | 1.05 | .037 |
| Prednisone dose, mg/d, median (IQR) | 2.5 (0;10) | 15 (1.3;30) | 1.03 | 1.00 | 1.05 | .014 |
Mann–Whitney's U test.
BVAS = Birmingham vasculitis activity score, CRP = C-reactive protein, ESR = erythrocyte sedimentation ratio, VDI = vasculitis damage index.
Chi-square's test.
Student's t test.
Logistic regression analysis showed that high VDI scores (OR 1.43, CI 95% 1.08–1.89, P = .013), creatinine levels (OR 1.27, CI 95% 1.03–1.56, P = .024), and CRP levels (OR 1.05, CI 95% 1.00–1.09, P = .031) were independent factors associated with mortality (Table 2).
Table 2.
Logistic regression analysis of the mortality predictors.
| 95% CI | ||||
| Variable | OR | Lower | Upper | P |
| BVAS | 0.98 | 0.81 | 1.19 | .851 |
| VDI | 1.43 | 1.08s | 1.89 | .013 |
| Creatinine, mg/dL | 1.27 | 1.03 | 1.56 | .024 |
| CRP, mg/L | 1.05 | 1.00 | 1.09 | .031 |
| Prednisone dose, mg/d | 1.02 | 0.99 | 1.06 | .226 |
Multivariate logistic regression (full model).
BVAS = Birmingham vasculitis activity score, CRP = C-reactive protein, VDI = vasculitis damage index.
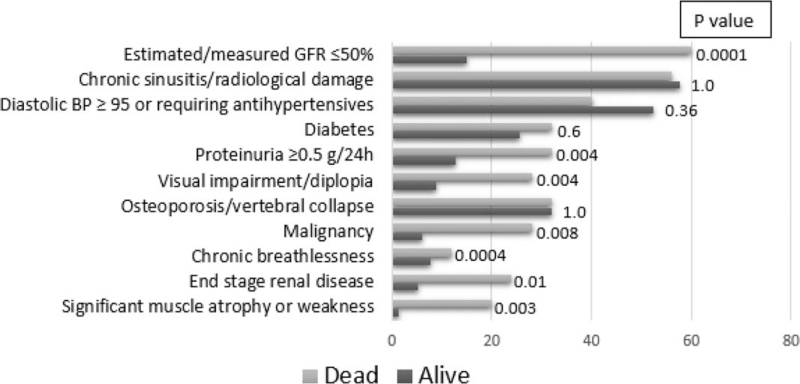
Of the 64 items in the VDI, 8 were significantly different between the 2 groups: significant muscle atrophy or weakness, estimated/measured glomerular filtration rate (GFR) ≤50%, proteinuria ≥0.5 g/24 h, end-stage renal disease, chronic breathlessness, malignancy, visual impairment/diplopia, and oral ulcers (Fig. 1). A logistic regression was made with the 8 items of the VDI that were considered statistically different in the first analysis. In the logistic regression, 4 of the 8 items remained different between the groups: visual impairment/diplopia (OR 6.22, CI 95% 1.27–30.41, P = .024), chronic breathlessness (OR 6.03, CI 95% 1.10–32.84, P = .038), estimated/measured GFR (by CKD-EPI equation), <50% (OR 6.08, CI 95% 1.08–33.94, P = .04), and malignancy (OR 9.27, CI 95% 1.66–51.66, P = .01).
Figure 1.
Comparison of the vasculitis damage index (VDI) most scored items between the groups. BP = blood pressure, GFR = glomerular filtration ratio.
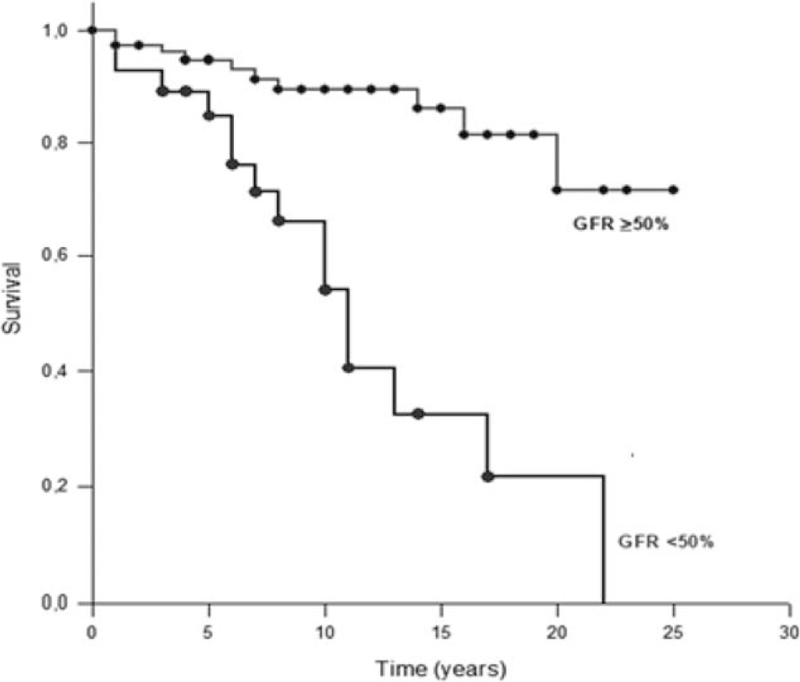
A survival analysis was performed comparing the patients who had kidney dysfunction, which was defined as a GFR ≤50% (same definition used in the VDI), with those who did not. The duration of the disease was calculated from the time of the diagnosis until 2018, the year of the study, for the living patients or until the time of the death for the patients who died. This analysis showed that survival was significantly decreased among patients with AAV and renal impairment (11.98 ± 1.58 years vs 21.53 ± 1.05 years; P < .001) (Fig. 2).
Figure 2.
Survival analysis of ANCA-associated vasculitis patients with or without kidney dysfunction. ANCA = anti-neutrophil cytoplasm antibody, GFR = glomerular filtration ratio.
The main cause of death was infection (64%), according to the immediate cause of death registered in the death certificate. Of all the infection-related deaths, 62% were due to pneumonia, 12.5% were due to urinary tract infections, 6% were due to bloodstream infections, and 6% were due to skin infections. In Brazilian death certificates, the physician must specify the immediate cause of death in the first line and, below it, other conditions that the patient had that were not immediately the cause of death but have contributed to it in some degree. Diseases not related to death are specified in a different section. Death was attributed to the vasculitis in only 2 of the 25 patients (8%), but the disease was part of the chain of events that led to death in 10 (40%) of the patients, according to the death certificates (Table 3). There was no difference in the number of deaths attributable to infections between the groups with normal and impaired renal function (70% vs 60%, respectively, P = .69).
Table 3.
Causes of death within and after the first year of follow-up, respectively.
| <1 year | >1 year | ||||
| Cause of death | Primary cause | Contributing factor | Primary cause | Contributing factor | Total (%) |
| Vasculitis | 1 | 1 | 1 | 7 | 10 (40) |
| Infection | 1 | 0 | 15 | 0 | 16 (64) |
| Cardiovascular | |||||
| Cerebrovascular accident | 0 | 0 | 1 | 1 | 2 (8) |
| Pulmonary embolism | 1 | 0 | 0 | 0 | 1 (4) |
| Cardiomyopathy | 0 | 0 | 1 | 1 | 2 (8) |
| Malignancy | 1 | 1 | 0 | 3 | 5 (20) |
| Kidney disease | 0 | 0 | 1 | 1 | 2 (8) |
| Unknown | 2 | 2 (8) | |||
4. Discussion
This inception cohort study was conducted to identify the factors associated with mortality in Brazilian patients with AAV. A better understanding of the factors affecting prognosis will help with the choice of appropriate interventions in individual patients.
The multivariable analysis showed that VDI scores, creatinine levels, and CRP levels were independent factors related to mortality at the most recent/last attendance of AAV patients, predominantly those with GPA. The VDI is an extensively validated scoring system of 64 items that represent organic damage/dysfunction related to vasculitis itself or to the treatment administered.[19,22] The items with the highest scores in the group of patients who died were estimated/measured GFR ≤50%, chronic sinusitis/radiological damage, and diastolic blood pressure ≥95 or requiring antihypertensive drugs. However, comparing the 2 groups, we found that 8 of the 63 items were significantly different (Fig. 1), and 3 of them were related to kidney involvement (estimated/measured GFR ≤50%, proteinuria ≥0.5 g/24 h, and end-stage renal disease, defined by GFR < 15 mL/min/1.73 m2 for at least 3 months). This finding, in addition to the fact that serum creatinine was found to be an independent predictor of mortality, is evidence that kidney involvement in vasculitis is the most important factor related to an unfavorable prognosis in these AAV patients. In a large retrospective descriptive study conducted in Brazilian patients with GPA, the prevalence of end-stage renal disease was 20%,[23] while in this study, the prevalence was 10%.
C-reactive protein, as an independent predictor of mortality, must be carefully interpreted, considering that the main cause of death in this study was infection. However, the laboratory tests analyzed were from the last outpatient clinic attendance, up to 6 months before death, when patients were stable. The median time between the laboratory analysis and the death was 3 months. Laboratory results collected when patients were hospitalized or at a known infectious process were not considered. The BVAS was higher in the group of patients who died, showing that the acute phase reactants (CRP and ESR) may reflect disease activity in some cases. Even so, infection screening is mandatory before assigning active vasculitis as the cause of these abnormal laboratory results.
In this study, pulmonary involvement by the vasculitis was not found to be associated to higher mortality, as it has been in similar studies.[9,10] However, there was a significant difference between the groups concerning the item “chronic breathlessness” of the VDI. This may suggest that the patients who died could have respiratory impairment due to other causes, such as chronic kidney failure. Dyspnoea by other causes was not evaluated in this study.
Our overall mortality rate, 24.27%, is similar to that of other studies on AAV.[24,25] Only 3 patients died in the first year of follow-up (1 due to infection, 1 due to pulmonary embolism, and 1 due to lymphoma). Patients who died were older at the onset of the disease (40 vs 51 years old), and most of them died due to causes not related to vasculitis. The leading cause of death was infection, and vasculitis itself was considered a contributing factor in 40% of the deaths. Other studies have also identified infection and active vasculitis as major causes of mortality in AAV patients.[9,10,25,26]
Compared to populations in other studies that analyzed mortality among AAV patients,[9–11] our population had a higher proportion of patients with GPA and a smaller proportion of patients with MPA. A recent study on the epidemiology features of systemic vasculitides in Brazilian patients was published and it showed that in our population the frequency of EGPA is higher than MPA, indeed, as it was in our work.[27] GPA can present as a localized disease that does not affect the kidneys, and renal involvement is usually more frequent in MPA than in GPA.[9,28] There is a predominance of MPA in Chinese patients with AAV, and mortality in 2 studies that analyzed a Chinese AAV population was indeed higher than that in our study.[9,10]Table 4 shows a comparison among the types of vasculitis, the mortality rates and the conclusions in four recent studies and in our own study. Nevertheless, our results show that renal impairment is also an important predictor of outcome in this population with GPA vasculitis predominance.
Table 4.
Comparison of studies which analyzed mortality of patients with AAV.
| Author, yr | Lai (2014) | Pu (2017) | Heijl (2017) | Flossman (2010) | This study |
| Population | Chinese | Chinese | Swedish | European | Brazilian |
| Number of patients | 398 | 123 | 195 | 535 | 128 |
| GPA/MPA/EGPA (%) | 26.1/67.6/0 | Not described | 48/46/6 | 52.5/47.5/0 | 78.9/4.6/16.4 |
| Mortality (%) | 33.9 | 37.4 | 50.2 | 24.9 | 24.3 |
| Mortality predictors | Age, secondary infection, pulmonary involvement, initial kidney function | BVAS, pulmonary hemorrhage, digestive system involvement, higher creatinine | Age, renal impairment, cardiovascular and gastrointestinal clusters | GFR <15 mL/min, age, BVAS, lower hemoglobin, higher white cell count | VDI, creatinine, CRP |
AAV = ANCA-associated vasculitis, BVAS = Birmingham vasculitis activity score, CRP = C-reactive protein, EGPA = eosinophilic granulomatosis with polyangiitis, GFR = glomerular filtration ratio, GPA = granulomatosis with polyangiitis, MPA = microscopic polyangiitis, VDI = vasculitis damage index.
Our study has some limitations. First, a considering number of patients were lost during the follow-up and excluded from the analysis, which could possibly change some of the results finded. Second, the course of the disease during follow up and the effect of the immunosuppressant treatment were not assessed. We understand that data collected in a longitudinal way or earlier in the course of the disease would probably be better predictors of long-term mortality risk in these patients. However, our rationale was to find clinical and laboratory parameters, during the follow up, that should be “red flags” and alert the physician that the patient is evolving to a greater severity of the disease and higher mortality risk.
Our conclusion is that creatinine, C-reactive protein, and the VDI are independent factors related to mortality in Brazilian patients with ANCA-associated vasculitis. Furthermore, survival is significantly decreased among patients with renal impairment compared with those with normal kidney function, and the main cause of death in AAV patients with normal or impaired kidney function is infection.
There is still a lack of disease activity biomarker in vasculitis, specially concerning renal activity.[29] Serum and urine calprotectin[30–32] and Activin A[33] are promising options currently under investigation. Physicians frequently have doubt about interpreting a rise in creatinine as a sign of disease activity or progression of a chronic kidney disease, which implicates in under treatment and renal failure. Hematuria is a sensitive but not specific finding in active renal vasculitis, and can be persistent after remission is achieved in some cases.[34,35] Therefore, another important conclusion of this study is that renal vasculitis should always be screened and promptly treated in all AAV patients. Research agenda should include the search for a better diagnostic tool or activity biomarker for renal vasculitis.
Author contributions
Conceptualization: Marília Ambiel Dagostin, Sergio Luiz Oliveira Nunes, Samuel Katsuyuki Shinjo, Rosa Maria Rodrigues Pereira.
Data curation: Marília Ambiel Dagostin, Sergio Luiz Oliveira Nunes, Samuel Katsuyuki Shinjo, Rosa Maria Rodrigues Pereira.
Formal analysis: Marília Ambiel Dagostin, Sergio Luiz Oliveira Nunes, Rosa Maria Rodrigues Pereira.
Funding acquisition: Rosa Maria Rodrigues Pereira.
Investigation: Marília Ambiel Dagostin, Sergio Luiz Oliveira Nunes, Samuel Katsuyuki Shinjo, Rosa Maria Rodrigues Pereira.
Methodology: Marília Ambiel Dagostin, Sergio Luiz Oliveira Nunes, Rosa Maria Rodrigues Pereira.
Project administration: Marília Ambiel Dagostin, Rosa Maria Rodrigues Pereira.
Resources: Marília Ambiel Dagostin, Rosa Maria Rodrigues Pereira.
Software: Marília Ambiel Dagostin, Rosa Maria Rodrigues Pereira.
Supervision: Samuel Katsuyuki Shinjo, Rosa Maria Rodrigues Pereira.
Validation: Marília Ambiel Dagostin, Rosa Maria Rodrigues Pereira.
Visualization: Marília Ambiel Dagostin, Rosa Maria Rodrigues Pereira.
Writing – original draft: Marília Ambiel Dagostin, Rosa Maria Rodrigues Pereira.
Writing – review & editing: Marília Ambiel Dagostin, Rosa Maria Rodrigues Pereira.
Footnotes
Abbreviations: AAV = ANCA-associated vasculitis, ACR = American College of Rheumatology, ANCA = anti-neutrophil cytoplasm antibody, BVAS = Birmingham vasculitis activity score, CI = confidence interval, CRP = C-reactive protein, EGPA = eosinophilic granulomatosis with polyangiitis, ESR = erythrocyte sedimentation ratio, GFR = glomerular filtration rate, GPA = granulomatosis with polyangiitis, MPA = microscopic polyangiitis, MPO = myeloperoxidase, OR = odds ratios, PR3 = Proteinase 3, VDI = vasculitis damage index.
How to cite this article: Dagostin MA, Nunes SL, Shinjo SK, Pereira RM. Mortality predictors in ANCA-associated vasculitis: experience of a Brazilian monocentric cohort of a rheumatology center. Medicine. 2021;100:51(e28305).
This work was supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPQ) # 305556/2017–7 (RMRP).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Pagnoux C. Updates in ANCA-associated vasculitis. Eur J Rheumatol 2016;3:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yates M, Watts R. ANCA-associated vasculitis. Clin Med (Lond) 2017;17:60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lamprecht P, Kerstein A, Klapa S, et al. Pathogenetic and clinical aspects of anti-neutrophil cytoplasmic autoantibody-associated vasculitides. Front Immunol 2018;9: article 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hatemi G, Esatoglu SN, Yazici Y. Biomarkers in vasculitis. Curr Opin Rheumatol 2018;30:30–5. [DOI] [PubMed] [Google Scholar]
- [5].Kang EH, Ha YJ, Lee YJ. Autoantibody biomarkers in rheumatic diseases. Int J Mol Sci 2020;21: article 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tan JA, Dehgan N, Chen W, Xie H, Esdaile JM, Avina-Zubieta JA. Mortality in ANCA-associated vasculitis: ameta-analysis of observational studies. Ann Rheum Dis 2017;76:1566–74. [DOI] [PubMed] [Google Scholar]
- [7].Garen T, Lerang K, Hoffman-Vold AM, et al. Mortality and causes of death across the systemic connective tissue diseases and the primary systemic vasculitides. Rheumatology 2019;58:313–20. [DOI] [PubMed] [Google Scholar]
- [8].Jardel S, Puéchal X, Quellec AL, et al. Mortality in systemic necrotizing vasculitides: a retrospective analysis of the French Vasculitis Study Group Registry. Autoimmun Rev 2018;17:653–9. [DOI] [PubMed] [Google Scholar]
- [9].Lai Q, Ma T, Li Z, Chang D, Zhao M, Chen M. Predictors for mortality in patients with antineutrophil cytoplasmic. J Rheumatology 2014;41:1849–55. [DOI] [PubMed] [Google Scholar]
- [10].Pu L, Li GS, Zou YR, Zhang P, Wang L. Clinical predictors of outcome in patients with anti-neutrophil cytoplasmic autoantibody-related renal vasculitis: experiences from a single-center. Chin Med J 2017;130:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heijl C, Mohammad AJ, Westman K, Hoglund P. Long-term patient survival in a Swedish population-based cohort of patients with ANCA-associated vasculitis. RMD Open 2017;3: article 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marcela CM, Lilian SS, Sergio JG, et al. Clinical features of Wegener granulomatosis and microscopic polyangiitis in Chilean patients, 1990-2001. Rev méd Chile 2005;133:273–8. [DOI] [PubMed] [Google Scholar]
- [13].Gamron S, Eugenia Muscellini M, Onetti L, et al. Wegener's granulomatosis: its prevalence in a ten-year period in the rheumatology service of the Clinic Hospital, Cordoba, Argentina. Rev Fac Cien Med Univ Nac Cordoba 2006;63:53–6. [PubMed] [Google Scholar]
- [14].Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum 1990;33:1101–7. [DOI] [PubMed] [Google Scholar]
- [15].Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990;33:1094–100. [DOI] [PubMed] [Google Scholar]
- [16].Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum 2013;65:01–11. [DOI] [PubMed] [Google Scholar]
- [17].Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham vasculitis activity score (BVAS) in systemic necrotizing vasculitis. QJM 1994;87:671–8. [PubMed] [Google Scholar]
- [18].Bai YH, Li ZY, Chanag DY, Chen M, Kallenberg CG, Zhao MH. The BVAS is an independent predictor of cardiovascular events and cardiovascular related mortality in patients with ANCA-associated vasculitis: a study of 504 cases in a single Chinese Center. Semin Arthritis Rheum 2018;47:524–9. [DOI] [PubMed] [Google Scholar]
- [19].Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the vasculitis damage index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum 1997;40:371–80. [DOI] [PubMed] [Google Scholar]
- [20].Kirkwood BR, Sterne JAC. Essential Medical Statistics. 2nd ed.2006;MA, USA: Blackwell Science, p. 502. [Google Scholar]
- [21].Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied Linear Statistical Models. 4th ed.1996;IL, USA: Richard D. Irwing, 1408p. [Google Scholar]
- [22].Robson J, Doll H, Suppiah R, et al. Damage in the anca-associated vasculitides: long-term data from the European Vasculitis Study Group (EUVAS) therapeutic trials. Ann Rheum Dis 2015;74:177–84. [DOI] [PubMed] [Google Scholar]
- [23].De Souza FHC, Halpern ASR, Barbas CSV, Shinjo SK. Wegener's granulomatosis: experience from a Brazilian tertiary center. Clin Rheumatol 2010;29:855–60. [DOI] [PubMed] [Google Scholar]
- [24].Flossman O, Berden A, Groot K, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011;70:488–94. [DOI] [PubMed] [Google Scholar]
- [25].Wallace ZS, Fu X, Harkness T, Stone JH, Zhang Y, Choi H. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA Type. Rheumatology 2019;0:01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Little MA, Nightingale P, Verburgh CA, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis 2010;69:1036–43. [DOI] [PubMed] [Google Scholar]
- [27].Belem JMFM, Pereira RMR, Perez MO, et al. Epidemiologic features of systemic vasculitides in the Southeast Region of Brazil: hospital-based survey. J Clin Rheumatol 2020;26: (7s suppl 2): S106–10. [DOI] [PubMed] [Google Scholar]
- [28].Jennette JC, Nachman PH. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol 2017;12:1680–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].O’reilly VP, Wong L, Kennedy C, et al. Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol 2018;29:2906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Valenzuela LM, Draibe J, Ramos MQ, et al. Calprotectin as a smoldering activity detection tool and renal prognosis biomarker in ANCA associated vasculitis. PLoS One 2018;13: article e0205982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Romand X, Bernardy C, Nguyen MCY, et al. Systemic calprotectin and chronic inflammatory rheumatic diseases. Joint Bone Spine 2019;86:691–8. [DOI] [PubMed] [Google Scholar]
- [32].Pepper RJ, Hamour S, Chavele KM, et al. Leukocyte and serum S100A8/S100A9 expression reflects disease activity in ANCA-associated vasculitis and glomerulonephritis. Kidney Int 2013;83:1150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Takei Y, Takahashi S, Nakasatomi M, et al. Urinary Activin A is a novel biomarker reflecting renal inflammation and tubular damage in ANCA-associated vasculitis. PLoS One 2019;14: article e0223703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rhee RL, Davis JC, Ding L, et al. The utility of urinalysis in determining the risk of renal relapse in ANCA-associated vasculitis. Clin J Am Soc Nephrol 2018;13:251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vandernbussche C, Bitton L, Bataille P, et al. Prognostic value of microscopic hematuria after induction of remission in antineutrophil cytoplasmic antibodies-associated vasculitis. Am J Nephrol 2019;49:479–86. [DOI] [PubMed] [Google Scholar]