Abstract
Rationale:
Pulmonary toxoplasmosis (PT) is an infectious disease that can be fatal if reactivation occurs in the recipients of hematopoietic stem cell transplantation (HSCT) who were previously infected with Toxoplasma gondii. However, whether the toxoplasmosis reactivation is an actual risk factor for patients receiving immunosuppressive therapies without HSCT remains unclear. Therefore, reactivated PT is not typically considered as a differential diagnosis for pneumonia other than in patients with HSCT or human immunodeficiency virus (HIV).
Patient concerns:
A 77-year-old man presented with fever and nonproductive cough for several days. He was hospitalized due to atypical pneumonia that worsened immediately despite antibiotic therapy. Before 4 months, he was diagnosed with immune thrombocytopenia (ITP) and received corticosteroid therapy. Trimethoprim–sulfamethoxazole (ST) was administered to prevent pneumocystis pneumonia resulting from corticosteroid therapy.
Diagnosis:
The serological and culture test results were negative for all pathogens except T. gondii immunoglobulin G antibody. Polymerase chain reaction, which can detect T. gondii from frozen bronchoalveolar lavage fluid, showed positive results. Therefore, he was diagnosed with PT.
Intervention:
ST, clindamycin, and azithromycin were administered. Pyrimethamine and sulfadiazine could not be administered because his general condition significantly worsened at the time of polymerase chain reaction (PCR) examination.
Outcomes:
The patient died of acute respiratory distress syndrome despite anti-T. gondii treatment. An autopsy revealed a severe organizing pneumonia and a small area of bronchopneumonia.
Lessons:
PT should be considered as a differential diagnosis in patients with pneumonia, particularly in seropositive patients who receive immunosuppressive therapies even for other than HSCT or HIV.
Keywords: acute respiratory distress syndrome, immune thrombocytopenia, pulmonary toxoplasmosis, trimethoprim–sulfamethoxazole prophylaxis
1. Introduction
Toxoplasmosis is caused by the protozoan parasite Toxoplasma gondii. Further, it is an opportunistic infection that can be fatal among immunocompromised patients such as those with human immunodeficiency virus (HIV) and those who received hematopoietic stem cell transplantation (HSCT).[1–3] Pulmonary toxoplasmosis (PT) is not rare among HSCT recipients previously infected with T. gondii. The serological assessment of anti-Toxoplasma antibodies is recommended before starting immunosuppressive therapies.[4] However, in patients treated with immunosuppressive drugs, PT is not commonly considered as a differential diagnosis for pneumonia, even though the condition is a common complication of T. gondii infection. There are few reports about reactivated PT without HIV infection or HSCT. However, whether the reactivation of toxoplasmosis is an actual risk among patients receiving immunosuppressive therapies remains unclear.
Herein, we present a patient who died of acute respiratory distress syndrome (ARDS) that was likely attributed to PT during corticosteroid therapy for immune thrombocytopenia (ITP). Moreover, a literature review of case reports about PT in patients without HIV infection or organ transplantation was performed.
2. Case report
A 77-year-old man presented to the hematologic department due to fever and nonproductive cough for several days. He visited the general internal medicine department to seek consultation for fever and colds approximately 4 months back. His blood test results showed thrombocytopenia (platelet count: 1000/μL). Thus, he was referred to the hematology department. He was then diagnosed with virus-associated or drug-induced ITP as bone marrow examination revealed the typical signs of ITP. Moreover, other differential diagnoses for thrombocytopenia such as collagen and aplastic diseases and hematological malignancies were ruled out. The patient was initially treated with prednisolone (PSL) 1 mg/kg (60 mg/day), and he had good response. During the current admission, the PSL dose was tapered to 10 mg/day. At the time of PSL therapy, trimethoprim–sulfamethoxazole (ST) 80/400 mg/day was administered as a prophylactic treatment for pneumocystis pneumonia. The patient had no comorbidities other than ITP. Further, he had no history of contact with animals (particularly cats), eating raw meat, or recent visits at certain areas considered at high risk for T. gondii. His medical or family history was unremarkable.
The vital signs (including oxygen saturation [96%] on room air and axial temperature) upon admission were normal. Coarse crackles were auscultated in the right lower lung field. Blood examination showed leukocytosis (white blood cell count: 16,390/μL, neutrophil ratio: 93%, and lymphocyte ratio: 2%). Blood chemistry revealed elevated C-reactive protein (27.51 mg/dL), lactate dehydrogenase (LDH) (277 U/L), and β-D glucan (20.0 pg/mL) levels. Computed tomography scan and plain chest radiography revealed a mixed appearance of ground glass opacity, consolidation, and hypertrophy of the interlobular septa in the whole right lobe and the left upper lobe (Figures 1A, 1B, and 1C). The patient was diagnosed with pneumonia and admitted to the hematology department.
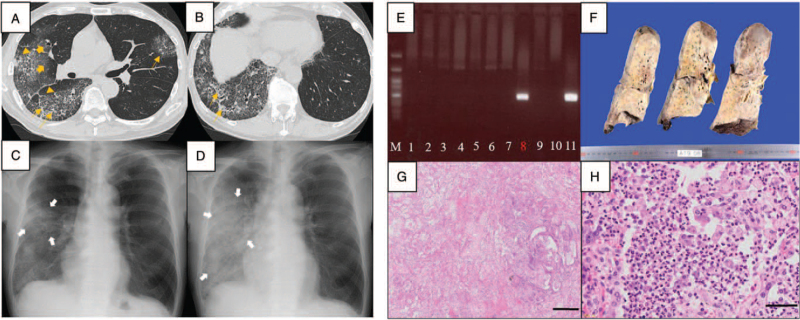
Figure 1.
(A, B) Computed tomography findings during hospitalization. The right lung lobe mainly presented with areas with ground glass opacity (broad orange arrows), consolidation (thin orange arrow), and hypertrophy of the interlobular septa (orange triangles). (C, D) Plain chest radiography (C) during hospitalization and (D) at day 4. An infiltrative shadow (white arrows) in the right lobe spread immediately within 4 days. (E) Nested polymerase chain reaction results. Genomic DNA was purified from peripheral blood leukocytes (PBL) and bronchoalveolar lavage fluid (BALF). PCR was performed using primers specific for the T. gondii B1 gene. Lane M: molecular size marker; lanes 1–6: PBL; lanes 7–9: BALF; lane 10: negative control; lane 11: positive control. T. gondii DNA was identified in lane 8. (F–H) Autopsy findings. (F) Macroscopic finding of the right lung. The lung had diffuse consolidation. (G) Microscopic finding revealed extensive organizing pneumonia with bronchopneumonia focus. Scale bar: 400 μm. (H) Infiltration of neutrophils and macrophages are observed in bronchopneumonia. Scale bar: 50 μm.
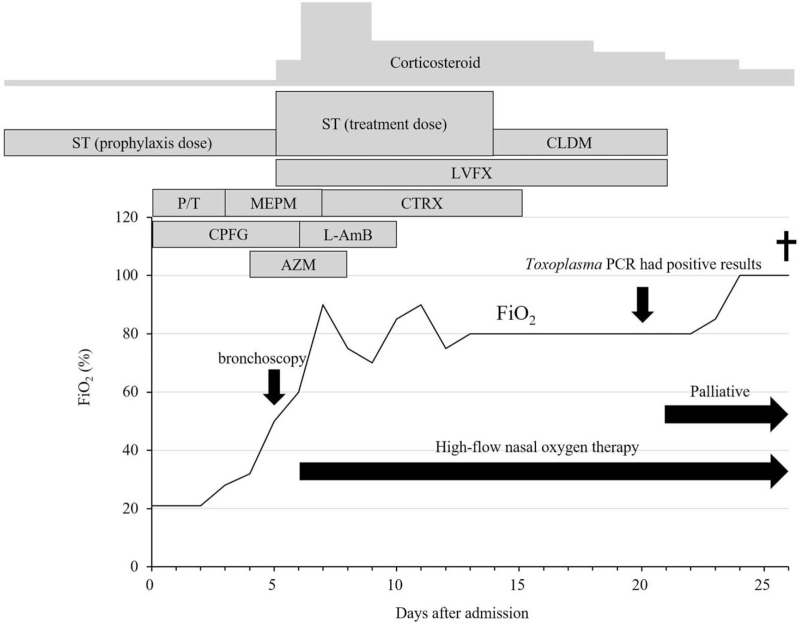
Piperacillin/tazobactam and caspofungin (CPFG) were administered as an empirical therapy. However, pneumonia worsened immediately (Fig. 1D). Hence, the treatment was changed to meropenem, azithromycin, and CPFG. After performing bronchoscopy on day 5 after admission, treatment with ST 960/4800 mg/day, PSL 40 mg/day, and levofloxacin were started for the management of pneumocystis pneumonia and other types of bacterial pneumonia. On day 6, the patient's respiratory status deteriorated, and it did not stabilize with oxygen therapy at 15 L/min via a reservoir mask. Blood chemistry revealed elevated LDH (368 U/L), Krebs von den Lungen-6 (1218 U/mL), pulmonary surfactant protein-D (1026 ng/mL), pulmonary surfactant protein-A (97.7 ng/mL), fibrin degradation product (251 μg/mL), and ferritin (1611 ng/mL) levels. Thus, the patient was diagnosed with ARDS and disseminated intravascular coagulation (DIC). Next, high-flow oxygen therapy via a nasal cannula was started in addition to corticosteroid pulse and DIC therapy. All examinations, which were performed to determine the etiology during the study period, had negative results. These included multiple sputum and blood cultures; bronchoalveolar lavage fluid (BALF) culture; Grocott staining of BALF; polymerase chain reaction (PCR), which can detect Pneumocystis jirovecii and Legionella from BALF and Mycobacteria from BALF and sputum; and testing for aspergillus antigen, HIV antibody, cytomegalovirus antigenemia, and Chlamydia antibody. The patient's β-D glucan level normalized on day 4. On day 7, as Pneumocystis was ruled out, the ST dose was reduced to 640/3200 mg/day. On day 10, the patient tested positive for anti-Toxoplasma immunoglobulin G antibody (38 IU/mL) and negative for anti-Toxoplasma IgM antibody. Moreover, his anti-Toxoplasma IgG avidity index was 0.54, thereby indicating chronic infection caused by T. gondii. To obtain a definitive diagnosis of T. gondii infection, we collected blood samples on day 13 and frozen BALF on day 5 for nested PCR.[5] However, as there was a risk of CPFG refractory fungal infection, CPFG was changed to liposomal amphotericin B. In addition, as PT or anaerobic pneumonia was suspected, clindamycin was added to the therapy. His general status might be temporarily reasonable, and his FiO2 ratio did not decrease below 60%. On day 14, as the patient had difficulties in taking ST and his platelet count decreased, ST was discontinued. On day 20, nested PCR revealed the presence of Toxoplasma in the BALF, but not in the blood (Fig. 1E). As his general condition further deteriorated and both lungs had severe infiltration, treatment with pyrimethamine and sulfadiazine, which are the mainstay therapy for toxoplasmosis, could not be started. Hence, the patient and his family were consulted regarding palliative treatment. The patient died from respiratory failure on day 26. Figure 2 shows the clinical course.
Figure 2.
Clinical course of the patient. The FiO2 conversion before high-flow nasal oxygen was as follows: room air: 21%, oxygen flow rate of 2 L/min via nasal cannula: 28%, oxygen flow rate of 3 L/min via nasal cannula: 32%, oxygen flow rate of 6 L/min via a face mask: 50%. AZSM = azithromycin, CLDM = clindamycin, CPFG = caspofungin, CTRX = ceftriaxone, L-AmB = liposomal amphotericin B, LVFX = levofloxacin, MEPM = meropenem, P/T = piperacillin/tazobactam, ST = trimethoprim–sulfamethoxazole.
On autopsy, the lungs were heavy (left: 950 g and right: 850 g). The cut surface showed congestion and extensive tan-yellow consolidation (Fig. 1F). Histologically, there was organizing fibrosis in the alveolar spaces without remodeling of the lung architecture, thereby showing a pattern of organizing pneumonia and a small area of bronchopneumonia (Fig. 1G). Neutrophils and swollen macrophages infiltrated the bronchiole and alveolar spaces in the bronchopneumonia region (Fig. 1H). Toxoplasma was not apparent in these specimens, and PCR testing for T. gondii in the lung slides obtained during autopsy yielded negative results (data not shown). Stenotrophomonas maltophilia (1+) was identified via culture of the lung autopsy specimen. However, it was not apparent under microscopic view during autopsy.
3. Discussion
Our patient who was treated with glucocorticoid therapy for ITP died of ARDS, and the only causative pathogens even based on the pathological autopsy were S. maltophilia and T. gondii. S. maltophilia can be detected in routine culture tests. However, in the current case, pneumonia could not be attributed to S. maltophilia as it was not detected in any tests other than the lung culture by autopsy. Multiple sputum, blood, and BALF cultures tested negative for S. maltophilia. In addition, its presence was not validated via pathological investigation. S. maltophilia might have colonized in the lungs, which was caused by microbial substitution due to the continuous use of broad-spectrum antimicrobials for approximately 1 month while the patient's general condition was poor. In the current case, PT was the most likely diagnosis due to the following reasons: First, PCR of BALF showed positivity to T. gondii, which strongly indicated that the lungs were infected with T. gondii. Second, the tests for the other causative of microorganisms of pneumonia, except for S. maltophilia, yielded negative results. Third, the clinical and pathological findings were consistent with those described in previous reports of PT in HSCT recipients.[4] In addition, T. gondii could have easily reactivated in our patient as his immune status deteriorated due to treatment with glucocorticoids. T. gondii and its DNA were not found in the autopsy slides, probably because the parasites had been successfully eradicated via treatment with ST, clindamycin, and azithromycin at therapeutic doses for more than 1 week. However, the patient died of concomitant ARDS and DIC that could not be controlled.
Commonly, PT is only mildly symptomatic in immunocompetent hosts. However, it can be life-threatening in significantly immunocompromised hosts.[6] The symptoms of PT are nonspecific, and these include fever, nonproductive cough, myalgia, arthralgia, lymphadenopathy, and dyspnea. Moreover, the laboratory findings are nonspecific, which include lymphopenia, thrombocytopenia, rhabdomyolysis, and LDH elevation.[7] Further, an elevated C-reactive protein level is nonspecific and may be influenced by differences in the underlying disease, treatment, and/or causative T. gondii strain.[4,8,9] The diagnosis of PT can become more challenging due to computed tomography findings such as ground glass opacity and peribronchovascular thickening, which indicate other pulmonary conditions of atypical pneumonia.[4,6] This disease might be misdiagnosed, or proper treatment could be delayed because of its nonspecific clinical features and imaging findings and insufficient knowledge among clinicians. Thus, it has a high mortality rate.[4,10,11]
There are no systematic reports about toxoplasmosis in immunocompromised patients, except HSCT recipients and patients with HIV. There are several case reports of toxoplasmosis. However, most discussed about cerebral toxoplasmosis.[12,13] A PubMed search was performed on August 8, 2021 using the phrase PT, and 932 articles were identified. Nevertheless, only 8 reports were considered after the exclusion of studies with transplant recipients, patients with HIV, disseminated cases with main symptoms other than respiratory, nonhuman cases, articles published in languages other than English, and unknown cases (Table 1).[8,9,11,14–18] As shown in Table 1, in most cases, the patient's condition improved with T. gondii-specific approaches such as T. gondii serologic testing and pyrimethamine–sulfadiazine treatment despite the presence of various and nonspecific underlying diseases and symptoms. Thus, suspected T. gondii and availability of pyrimethamine–sulfadiazine are important for the timely initiation of appropriate treatment.
Table 1.
Case reports of pulmonary toxoplasmosis in patients without HIV infection or organ transplantation.
| Authors∗ | Sex | Age | Underlying disease | Treatment at toxoplasmosis onset | Symptoms | Affected organs | Diagnostic method | Primary infection or reactivation∗∗ | Toxoplasmosis treatment | Outcome |
| Brown et al[14] | F | 41 | Acute myelomonocytic leukemia | Consolidation chemotherapy | Cough, dyspnea | Lung, spleen, bone marrow, lymph node, pancreas | Autopsy | Reactivation | None | Death |
| De Salvador-Guillouët et al[8] | M | 19 | None | None | Fever, fatigue, dyspnea | Lung | Histology (BALF), serology (IgG, IgM, and IgG avidity) | Primary | Pyrimethamine and sulfadiazine | Improved |
| Leal et al[15] | M | 41 | None | None | Fever, myalgia, headache, nausea, vomiting | Lung, cerebrospinal fluid | Serology (IgG and IgM), PCR (cerebrospinal fluid) | Primary | Pyrimethamine and sulfadiazine | Improved |
| de Souza Giassi et al[9] | M | 36 | Type 2 diabetes | None | Fever, cough, dyspnea | Lung | Serology (IgG, IgM, and IgG avidity) | Primary | Pyrimethamine and sulfadiazine | Improved |
| F | 56 | Type 2 diabetes | ||||||||
| F | 38 | None | ||||||||
| Lu et al[16] | F | 64 | Lung cancer | None | Nonproductive cough, chest pain | Lung | Histology (BALF), serology (IgG and IgM) | Primary | Unknown | Unknown |
| Abdulkareem et al[11] | F | 55 | Inflammatory arthritis | Methotrexate and corticosteroids | Nonproductive cough, shortness of breath | Lung | Histology (lung biopsy) | Reactivation | Trimethoprim-sulfamethoxazole | Improved |
| Matsuzawa et al[17] | M | 74 | Myelodysplastic syndrome | None | Fever, dyspnea | Lung | Serology (IgG and IgM) | Primary | Pyrimethamine and sulfadiazine | Improved |
| Steinhauser Motta et al[18] | M | 30 | None | None | Dry cough, dyspnea, cervical lymphadenopathy | Lung, lymph node, liver, spleen | Serology (IgG and IgM), PCR (BALF) | Primary | Pyrimethamine and sulfadiazine | Improved |
| Omori et al, (current report) | M | 77 | Immune throbocytopenia | Corticosteroids and trimethoprim-sulfamethoxazole | Fever, nonproductive cough | Lung | PCR (BALF) | Reactivation | Trimethoprim-sulfamethoxazole and clindamycin∗∗∗ | Death |
Reference number.
Toxoplasma gondii IgM-positive cases were defined as primary.
These medicines were used as empiric therapy targeting not only Toxoplasma pneumonia but also Pneumocystis and anaerobic pneumonia.
BALF = broncheoalveolar lavage fluid, PCR = polymerase chain reaction.
The measurement of anti-Toxoplasma IgG antibody is recommended before HSCT to reduce the incidence of severe infection.[2] However, this is not generally performed on patients receiving immunosuppressive therapies, as the reactivation risk associated with other immunosuppressive therapies such as corticosteroid administration and chemotherapy is not elucidated. In addition, the diagnosis of PT requires considerable effort and time in Japan where Toxoplasma PCR cannot be routinely performed despite the large number of target patients. Thus, it is challenging to differentiate PT in Toxoplasma-seropositive patients compared with HSCT recipients in Japan. Furthermore, patients receiving immunosuppressive therapies (such as corticosteroids and chemotherapy) have a milder level of immunosuppression than HSCT recipients. Therefore, the positivity rate of Toxoplasma PCR can be significantly lower in patients receiving immunosuppressive therapies than in HSCT recipients. To overcome these issues, Toxoplasma-seropositive patients with an unknown cause of pneumonia should directly undergo transbronchial lung biopsy via bronchoscopy and BALF cytology and pathologists should cautiously investigate for the presence of Toxoplasma. PT might be overlooked because of its rarity. Hence, clinicians must be aware of which patients should be suspected with PT and how to investigate for toxoplasmosis.
Toxoplasmosis can be effectively prevented with ST. That is, ST at a dosage of 80/400 mg/day can be administered daily. However, ST at a dose of 160/800 mg/day is generally administered either twice or 3 times a week. However, previous reports have documented the onset of toxoplasmosis during prophylactic ST treatment (80/400 mg/day),[19] as in the current case. Failed prophylactic treatment can be explained by inefficient drug absorption, intolerance, and discontinuation.[20] ST prophylactic regimens comprising ST administration less than 3 times a week have a lower efficacy than regimens requiring more frequent administrations.[19] In addition, anti-T. gondii medications (both chemoprophylaxis and treatment for T. gondii) are not effective against latent bradyzoite forms, such as cysts, involved in chronic infection, and they do not prevent encysted-bradyzoite reactivation.[20] Thus, clinicians should be aware that toxoplasmosis can still occur in patients who have received prophylactic ST therapy.
The current case showed that pneumonia caused by T. gondii reactivation can occur during corticosteroid and prophylactic ST therapies. PT is challenging to diagnose because of its nonspecific clinical features and laboratory and imaging findings. However, it should be considered as a differential diagnosis for pneumonia, particularly in seropositive patients receiving immunosuppressive treatments.
Acknowledgments
We want to thank all the staffs involved in medical treatment. We also would like to thank Enago (www.enago.jp) for English language review.
Author contributions
Conceptualization: Naoto Imoto.
Data curation: Koji Omori, Naoto Imoto.
Formal analysis: Naoto Imoto.
Investigation: Kazumi Norose, Matsuyoshi Maeda, Kenji Hikosaka.
Methodology: Naoto Imoto.
Project administration: Naoto Imoto.
Supervision: Shingo Kurahashi.
Validation: Kazumi Norose, Matsuyoshi Maeda, Kenji Hikosaka, Shingo Kurahashi.
Visualization: Naoto Imoto, Kazumi Norose.
Writing – original draft: Koji Omori, Naoto Imoto.
Writing – review & editing: Kazumi Norose.
Footnotes
Abbreviations: ARDS = acute respiratory distress syndrome, BALF = bronchoalveolar lavage fluid, CPFG = caspofungin, HIV = human immunodeficiency virus, HSCT = hematopoietic stem cell transplantation, ITP = immune thrombocytopenia, LDH = lactate dehydrogenase, PCR = polymerase chain reaction, PSL = prednisolone, PT = pulmonary toxoplasmosis, ST = trimethoprim–sulfamethoxazole.
How to cite this article: Omori K, Imoto N, Norose K, Maeda M, Hikosaka K, Kurahashi S. Acute exacerbation of pulmonary toxoplasmosis during corticosteroid therapy for immune thrombocytopenia: a case report and literature review. Medicine. 2021;100:51(e28430).
As the patient is deceased, permission was obtained from the family to publish this manuscript.
The authors have no conflicts of interests to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Martino R, Bretagne S, Einsele H, et al. Early detection of Toxoplasma infection by molecular monitoring of Toxoplasma gondii in peripheral blood samples after allogeneic stem cell transplantation. Clin Infect Dis 2005;40:67–78. [DOI] [PubMed] [Google Scholar]
- [2].Mulanovich VE, Ahmed SI, Öztürk T, Khokhar FA, Kontoyiannis DP, de Lima M. Toxoplasmosis in allo-SCT patients: risk factors and outcomes at a transplantation center with a low incidence. Bone Marrow Transplant 2011;46:273–7. [DOI] [PubMed] [Google Scholar]
- [3].Jarque I, Salavert M, Pemán J. Parasitic infections in hematopoietic stem cell transplantation. Mediterr J Hematol Infect Dis 2016;8:e2016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sumi M, Norose K, Hikosaka K, et al. Clinical characteristics and computed tomography findings of pulmonary toxoplasmosis after hematopoietic stem cell transplantation. Int J Hematol 2016;104:729–40. [DOI] [PubMed] [Google Scholar]
- [5].Grigg ME, Boothroyd JC. Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondii by PCR-restriction fragment length polymorphism analysis at the B1 gene. J Clin Microbiol 2001;39:398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pomeroy C, Filice GA. Pulmonary toxoplasmosis: a review. Clin Infect Dis 1992;14:863–70. [DOI] [PubMed] [Google Scholar]
- [7].Azoulay E, Russell L, Van de Louw A, et al. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med 2020;46:298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].De Salvador-Guillouët F, Ajzenberg D, Chaillou-Opitz S, et al. Severe pneumonia during primary infection with an atypical strain of Toxoplasma gondii in an immunocompetent young man. J Infect 2006;53:e47–50. [DOI] [PubMed] [Google Scholar]
- [9].de Souza Giassi K, Costa AN, Apanavicius A, et al. Tomographic findings of acute pulmonary toxoplasmosis in immunocompetent patients. BMC Pulm Med 2014;14:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sumi M, Hata S, Sato K, et al. Severe pulmonary toxoplasmosis mimicking viral pneumonitis after a third allogeneic stem cell transplantation in a man with acute lymphoblastic leukemia. Intern Med 2012;51:2943–7. [DOI] [PubMed] [Google Scholar]
- [11].Abdulkareem A, D'Souza RS, Patel N, Donato AA. A rare case of pulmonary toxoplasmosis in a patient with undifferentiated inflammatory arthritis on chronic methotrexate and corticosteroid therapy. BMJ Case Rep 2017;2017:bcr2017221252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Herold MA, Kühne R, Vosberg M, Ostheeren-Michaelis S, Vogt P, Karrer U. Disseminated toxoplasmosis in a patient with non-Hodgkin lymphoma. Infection 2009;37:551–4. [DOI] [PubMed] [Google Scholar]
- [13].Furuya H, Ikeda K, Iida K, et al. Disseminated toxoplasmosis with atypical symptoms which developed with exacerbation of systemic lupus erythematosus. Lupus 2019;28:133–6. [DOI] [PubMed] [Google Scholar]
- [14].Brown NJ, McKenzie S, Decker MD. Case report: fatal pulmonary toxoplasmosis following chemotherapy. Am J Med Sci 1991;302:152–4. [DOI] [PubMed] [Google Scholar]
- [15].Leal FE, Cavazzana CL, de Andrade HF, Galisteo AJ, de Mendonça JS, Kallas EG. Toxoplasma gondii pneumonia in immunocompetent subjects: case report and review. Clin Infect Dis 2007;44:e62–6. [DOI] [PubMed] [Google Scholar]
- [16].Lu N, Liu C, Wang J, Ding Y, Ai Q. Toxoplasmosis complicating lung cancer: a case report. Int Med Case Rep J 2015;8:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Matsuzawa Y, Adachi E, Takahashi A, et al. Cytokine profile in Sweet's syndrome under the treatment of pulmonary toxoplasmosis complicated with myelodysplastic syndrome. Intern Med 2019;58:2079–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Steinhauser Motta JP, Barbosa Cleinman I, Palermo Bruno L. An immunocompetent young man with diffuse pulmonary infiltrates. Breathe (Sheff) 2020;16:200165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gajurel K, Dhakal R, Montoya JG. Toxoplasma prophylaxis in haematopoietic cell transplant recipients: a review of the literature and recommendations. Curr Opin Infect Dis 2015;28:283–92. [DOI] [PubMed] [Google Scholar]
- [20].Dard C, Marty P, Brenier-Pinchart MP, et al. Management of toxoplasmosis in transplant recipients: an update. Expert Rev Anti-Infect Ther 2018;16:447–60. [DOI] [PubMed] [Google Scholar]