Abstract
Studies of infective endocarditis (IE) have relied on International Classification of Disease (ICD) codes to identify cases, a method vulnerable to misclassification. Clinical narrative data could offer greater accuracy and richness to cohort identification. We evaluated two algorithms:
-
1.
a standard query of ICD-9/10 billing codes, with or without procedure codes for echocardiogram and
-
2.
a text query of discharge summaries (DS) that selected on the term “endocarditis” in fields headed by “Discharge Diagnosis” or “Admission Diagnosis” or similar.
Further coding extracted valve involved and organism responsible if present. All cases were chart reviewed using pre-specified criteria. Positive predictive value (PPV), sensitivity and specificity were calculated. The ICD-based query identified 612 individuals from July 2015 to July 2019 who had a hospital billing code for infective endocarditis; of these, 534 had an echocardiogram. The DS query identified 387 cases. PPV for the DS query was 84.5% (95% CI 80.6%, 87.8%) compared with 72.4% (95% CI 68.7%, 75.8%) for ICD only (P < .001) and 75.8% (95% CI 72.0%, 79.3%) for ICD + echo queries (P = .002). Sensitivity was 75.9% for DS query and 86.8% to 93.4% for ICD queries (P < .02 for these comparisons). Specificity was high for all queries >94%. The DS query also yielded valve data (prosthetic, tricuspid, aortic, etc) in 60% and microbiologic agent in 73% of identified cases with an accuracy of 94% and 90%, respectively when assessed by chart review. Compared with ICD-based queries, text-based queries of discharge summaries have the potential to improve precision of IE case ascertainment and extract key clinical variables.
Keywords: discharge summary, endocarditis, ICD code
1. Introduction
Even in the modern antibiotic era, infective endocarditis (IE) is associated with a 1-year mortality in the range of 20% to 30%.[1,2] The incidence of IE is increasing in the United States, particularly among younger individuals and those who inject drugs.[3–5] Gaining a contemporary understanding of this severe infection is a priority and requires accurate cohort identification. Observational studies to date have relied heavily on International Classification of Disease (ICD) diagnosis codes to extract IE cases.[3–6] This method, while highly time-efficient, may be prone to misclassification due to coding inaccuracies.[7–9] ICD coding for hospital care is often not performed by clinicians but rather professional coders[10] and can be inconsistent across clinical institutions.[11] Use of ICD codes without verification of event accuracy is a common practice, and few studies have validated ICD codes for IE.[12–15]
Discrimination between historical and current clinical events is critical for patient identification for clinical registries and outcomes research but may be suboptimal with ICD codes.[16] Additionally, key clinical characteristics of IE, such as valvular features or microbiologic data, are often not appropriately captured in ICD codes, or captured at all. Examination of clinical narrative data, specifically discharge summaries, could offer greater accuracy and richness to efforts to define a clinical cohort.[17,18] The aim of this study was to evaluate the comparative performance characteristics—positive predictive value, sensitivity, and specificity—of two algorithms for IE case identification:
-
1.
ICD codes with or without a procedure code for echocardiogram and
-
2.
a rule-based keyword search of discharge summaries to identify patients hospitalized and newly diagnosed with infective endocarditis.
2. Methods
We conducted a retrospective methodological research study of diagnostic accuracy of two methods of IE cohort identification. We identified patients who were discharged from one of two academic teaching hospitals in Seattle, Washington any time from July 1, 2015 to July 31, 2019:
-
1.
University of Washington Medical Center (UWMC), a 570-bed tertiary/quaternary care facility and
-
2.
Harborview Medical Center (HMC), a 413-bed acute care hospital that serves as a public safety-net hospital and level 1 trauma center.
This activity was approved by the UW human subjects division.
2.1. Algorithm 1 (ICD-based query)
Inpatient hospital billing data were used to identify and extract patients discharged with a primary or secondary diagnostic code for infective endocarditis within our clinical data warehouse using Microsoft SQL Server Management Studio (SQL). Selected codes were Ninth Revision (ICD-9) 424.9, 424.91, 424.99, 421.0, 421.1, 421.9, 112.81, 036.42, and Tenth Revision (ICD-10) I38, I39, I33, I33.9, B37.6, A39.51. This query was performed with and without a Current Procedural Terminology (CPT) code for echocardiogram (93303–93356).
2.2. Algorithm 2 (discharge summary-based query)
After conducting a review of thirty randomly selected discharge summaries (DS) over the period of interest, we identified four commonly employed patterns for discharge (or death) summary. From these, a key-word, pattern-based text query of discharge summaries (DS) was generated that selected on the term “endocarditis” in the fields headed by “Discharge Diagnosis” or “Admission Diagnosis” or “Other disease affecting hospitalization.” Patients containing these DS features were extracted from the data warehouse using SQL. Further coding involved the removal of possible “history of endocarditis” word combinations (e.g., “Hx of,” “Hx,” “H/O,” “history of”). Additional coding was performed to extract the nature and type of valve (prosthetic, tricuspid, pulmonic, aortic, or mitral), and the microbiologic agent responsible for the IE, if present in the diagnosis fields.
2.3. Case adjudication
DS query cases were chart reviewed by clinicians using a standardized collection form using REDCap electronic data capture hosted at the University of Washington.[19] Four medical students conducted this first-pass review. The pre-specified criterion for a confirmed IE case was evidence of endocarditis mentioned but also verified within an infectious diseases (ID) consultation note. When patients did not have this ID consultation or verification, or had a clinical diagnosis of endocarditis but no evidence of valvular vegetation on transthoracic or transesophageal echocardiogram, charts were subsequently reviewed by two ID specialists, and cases were included in the cohort if they met Duke's criteria for definite IE (2 major or 1 major + 3 minor criteria).[20] Interrater reliability was also assessed for a random 10% of all DS-identified student-reviewed cases with a secondary review by an ID specialist blinded to the original ascertainment; agreement with the first-pass review and kappa were calculated.[21] Endocarditis cases that presented first from an outside facility were included as long as they continued to be treated for IE on transfer. Only incident cases of IE were included—such that if the patient was readmitted for the same endocarditis infection that was managed in an earlier hospitalization, only the earlier one was counted. Cardiovascular implantable electronic device infections were excluded unless they involved a valvular vegetation. Right-sided endocarditis was considered present in the absence of a tricuspid valve vegetation if there was septic pulmonary embolism in the absence of another embolic source.
Because we did not have a gold standard for case ascertainment of all possible cases of IE in our system that could be utilized without our screening methods, a separate set of IE cases was derived from two independent sources of data:
-
1.
the HMC Infectious Diseases clinic's list of outpatient parenteral antibiotic therapy (OPAT) cases from 2016 to 2018 (n = 90) and
-
2.
the Cardiology division's UWMC IE cases from their 2017 to 2018 echocardiogram lists (n = 100).
These were also adjudicated by an ID specialist to fulfill the same criteria as noted above for a final list of 166 confirmed IE cases. This cleaned list was used as a benchmark to evaluate the sensitivity of either algorithm. A list of 119 cases that were verified as non-IE cases from the HMC OPAT list was used to benchmark specificity.
ICD-identified cases (with or without CPT code) were assessed against the DS-identified and clinician-confirmed IE cases as well as the manually identified true IE cases for verified matches. All remaining ICD-screened cases that did not match these two confirmed lists of cases were then reviewed by an ID specialist using the adjudication criteria outlined above.
Baseline characteristics including age, sex, race, and ethnicity, as well as clinical comorbidities, were provided for descriptive purposes. Comorbidities were extracted using ICD-9/10 codes previously validated for Charlson comorbidities.[22]
2.4. Statistical analysis
The positive predictive value was calculated for each algorithm as the total number of verified/confirmed cases over the algorithm-selected cases. Sensitivity was calculated as the total number of algorithm-matched cases over the final list of 166 manually extracted true IE cases. Specificity was defined as the total number 119 non-cases minus the number of algorithm-matched cases over the 119 non-cases. Comparisons of these performance characteristics by algorithm were done with Chi-square testing. We calculated 95% Wilson score confidence intervals (CI)[23] using R version 4.0.3.
3. Results
The ICD-based query identified 612 individuals with hospitalizations from July 1, 2015 to July 31, 2019 that included a billing code for infective endocarditis. Of these, 534 individuals also had an echocardiogram as part of this hospitalization. In contrast, for the same timeframe, the discharge summary-based query yielded 387 individuals. Baseline characteristics including age, sex, race, ethnicity, and comorbidities were similar between these groups (Table 1). Comparable to other endocarditis cohorts,[1] the majority of patients were male.
Table 1.
Baseline characteristics of two endocarditis cohorts, identified by diagnostic codes plus echocardiogram or by discharge summary.
| Characteristic | ICD + ECHO n = 534 | Discharge summary n = 387 | P-valuea | ||
|---|---|---|---|---|---|
| Age, median years (IQR) | 47 (33, 63) | 45 (32, 61) | .41 | ||
| Female | 185 | 35% | 148 | 38% | .29 |
| Race | .96 | ||||
| White | 412 | 77% | 299 | 77% | |
| Black or African American | 53 | 10% | 34 | 9% | |
| American Indian or Alaska Native | 27 | 5% | 21 | 5% | |
| Asian/Pacific Islander | 31 | 6% | 23 | 6% | |
| Unavailable or Unknown | 11 | 2% | 10 | 3% | |
| Ethnicity | .57 | ||||
| Hispanic or Latino | 32 | 6% | 17 | 4% | |
| Not Hispanic or Latino | 487 | 91% | 359 | 93% | |
| Unavailable or Unknown | 15 | 3% | 11 | 3% | |
| Comorbidities | |||||
| Any malignancy | 91 | 17% | 56 | 14% | .34 |
| Cerebrovascular disease | 222 | 42% | 158 | 41% | .87 |
| Chronic pulmonary disease | 107 | 20% | 66 | 17% | .29 |
| Congestive heart failure | 304 | 57% | 236 | 61% | .24 |
| Liver Disease | 24 | 4% | 19 | 5% | .89 |
| Metastatic solid tumor | 46 | 9% | 17 | 4% | .02 |
| Myocardial infarction | 18 | 3% | 13 | 3% | 1.00 |
| Peripheral vascular disease | 126 | 24% | 94 | 24% | .87 |
| Renal disease | 164 | 31% | 120 | 31% | .98 |
ECHO = echocardiogram, ICD = International Classification of Diseases diagnostic code, IQR = interquartile range.
P-values for comorbidities conducted per category instead of overall distribution since individual patients can have multiple comorbidities.
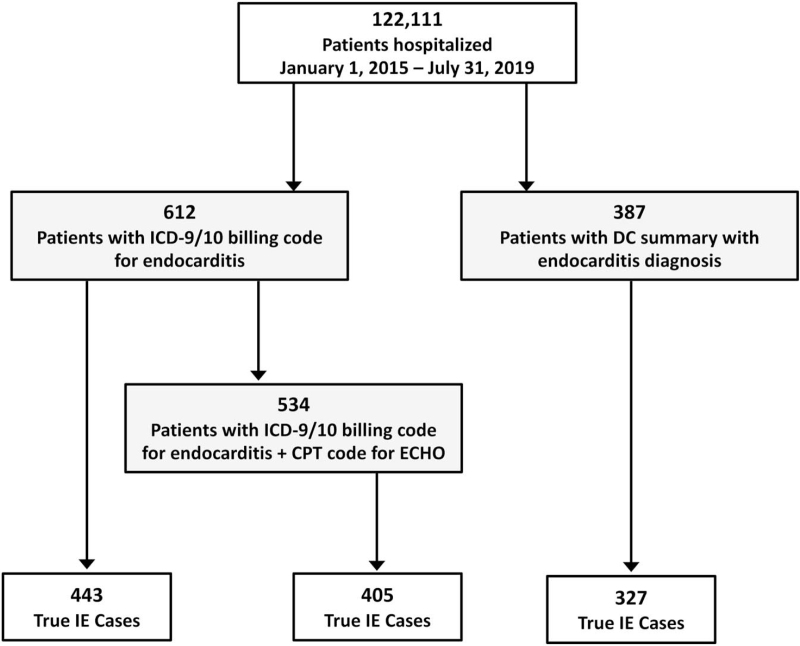
Chart review of the 387 individuals identified through the DS query resulted in 327 patients with confirmed endocarditis. In contrast, the ICD code query resulted in only 443 true cases out of the 612 total identified; ICD code + ECHO resulted in 405 true cases out of 534 (Fig. 1).
Figure 1.
Flowchart of individuals identified through diagnostic codes (with or without Echocardiogram Procedure Codes) versus discharge summary.
The DS query demonstrated a higher positive predictive value (PPV) at 84.5% (95% CI 80.6%, 87.8%) compared with 72.4% (95% CI 68.7%, 75.8%) for ICD alone (P < .001) or 75.8% (95% CI 72.0%, 79.3%) with ICD + echocardiogram (P = .002, Table 2). The sensitivity of the DS algorithm was lower at 75.9% compared with 93.4% for ICD alone (P < .001) or 86.8% with ICD + echocardiogram (P = .017). Specificity was high for all algorithms at 94% to 98%.
Table 2.
Test Characteristics of ICD-based versus discharge summary-based algorithms for endocarditis cohort identification.
| Positive predictive value 95% confidence interval | Sensitivity 95% confidence interval | Specificity 95% confidence interval | |
| ICD code only | 72.4% (443/612) 68.7%, 75.8% | 93.4% (155/166) 88.5%, 96.3% | 94.1% (112/119) 88.4%, 97.1% |
| ICD code + ECHO | 75.8% (405/534) 72.0%, 79.3% | 86.8% (144/166) 80.8%, 91.1% | 94.1% (112/119) 88.4%, 97.1% |
| Discharge Summary | 84.5% (327/387) 80.6%, 87.8% | 75.9% (126/166) 68.9%, 81.8% | 98.3% (117/119) 94.1%, 99.5% |
| P-value comparing ICD only vs Discharge summary | <0.001 | <0.001 | 0.174 |
| P-value comparing ICD + ECHO vs Discharge summary | .002 | .017 | .174 |
ECHO = echocardiogram, ICD = International Classification of Diseases.
A secondary review of student-reviewed cases by an ID specialist demonstrated excellent concordance with 97% agreement in assessments and a kappa of 0.78.
The DS query yielded information on valve involvement (prosthetic, tricuspid, pulmonic, aortic, or mitral) in 60% and/or the responsible organism in 73% of identified cases with an accuracy of 94% and 90%, respectively when assessed by chart review.
There were 78 true cases of IE missed by the DS query but captured by the ICD + ECHO query. Examination of a sample of 36 (46% of the 78) of these cases revealed three main reasons for lack of capture within the DS query:
-
1.
the missed DS had a different pattern or formatting that resulted in the query not detecting the case,
-
2.
“endocarditis” was not mentioned in the discharge diagnosis list (e.g., “bacteremia” was used instead) or
-
3.
an incorrect spelling of “endocarditis” occurred.
An atypical pattern/format, typo or lack of mention of IE within the DS were the primary reasons encountered for misses in all of the cases reviewed.
4. Discussion
We evaluated the diagnostic performance of two different case identification methods for infective endocarditis (IE): a traditional query using ICD codes with and without CPT codes for echocardiogram compared with a key word, pattern-based search of discharge summaries. We found that the DS query had a higher PPV than the ICD query with the ability to identify the type and nature of valve and organism involved in a majority of cases, albeit with some loss of sensitivity. The ICD query had suboptimal PPV, even with the addition of echocardiogram codes. Both methods were reasonably specific.
Our ICD-based query was not as predictive for IE as previously reported,[12] a reminder that the performance characteristics of ICD codes may not be generalizable across different healthcare systems. In our health system, coding was not able to distinguish between true cases and situations where endocarditis was considered but ultimately ruled out. Historical cases and hospitalizations that did not address the endocarditis as a current problem were often not appropriately coded. As with any complex clinical diagnosis, cases that were treated as probable endocarditis, that did not officially meet Dukes’ criteria for definite IE, may have been excluded in our adjudication but included in other settings. Our ascertainment criteria were not only stringent regarding the fidelity of diagnosis but the timeliness since our hope was to use this cohort for longitudinal assessment of clinical outcomes such as mortality, which requires an accurate anchoring to time of presentation. Using ICD in conjunction with a diagnostic procedure (or other defining data element) may be helpful in improving PPV and anchoring to the initial presentation.[17,24] However, the inclusion of echocardiogram did not significantly increase the PPV of our ICD-based algorithm, nor differentiate the rule-out cases.
The higher PPV with the DC summary algorithm was not surprising. The query was designed to target the region of a clinician-generated document that outlined the key diagnoses of the hospitalization and was therefore intrinsically less likely to contain false positives. The logic of this coding was straightforward and replicable, and could be adapted for other conditions and scaled in a way that natural language processing may not. Narrative data also has the ability to move beyond the absence or presence of a condition to other more nuanced features such as timing, severity, and relationship to other factors.[25]
The loss in sensitivity for the DS query was notable and suggests that this mode of case identification may not be optimal when used for the purpose of comprehensive case capture (for example, when the intent is to determine incidence or prevalence). One option for streamlining case identification might be to start with ICD code query (to optimize sensitivity) and follow this with DS query to minimize chart review; we were unable to evaluate this combination method. The reduced sensitivity of the DS query was mainly attributable to atypical patterns or formats in the DC summary as well as unusual choice or spelling of words. These aberrations were sporadic in nature and unlikely to be associated with significant selection bias. Accurate and comprehensive retrieval of data from clinical documentation for secondary use could benefit from structured and consistent formats in notes, as well as features such as auto-filling of clinician-selected diagnoses or prepopulated options for data elements rather than free-form typing or transcription.[26,27]
Our study had some limitations. This was a single academic health system with two hospitals, and our findings may not be applicable to other settings. While multiple chart reviewers with different levels of training could have introduced variability in ascertainment, a second-pass review for accuracy suggested good concordance. Lastly, the manually extracted list of true cases that we used for benchmarking sensitivity was a not randomly selected sample of known cases but a convenience sample drawn from two specialty practices over a narrower period of time, so our estimate of sensitivity may have been influenced by the selected nature of these lists. A strength of our study was that we evaluated all identified candidates for case ascertainment with a sufficiently large sample to obtain greater precision around the PPV estimate. We also utilized a standardized verification criteria and explored missed cases to gain a better understanding of potential deficiencies in these strategies.
When compared to traditional ICD code-based queries, text-based queries have the potential to improve accuracy of IE case ascertainment and extract key clinical variables from the electronic medical record. Although such methods may come at a slight cost to sensitivity, they have the potential to quickly and accurately define a cohort of patients with complex clinical diagnoses such as IE and reduce the burden of clinical chart review.
Acknowledgments
Authors would like to acknowledge Tanner N. Muggli, Jody Sharninghausen, Jordan M. Takasugi, and Ty J Tietjen for their assistance with the chart review for this study.
Author contributions
Conceptualization: Hyang Nina Kim.
Data curation: Ayushi Gupta, Kristine Lan.
Formal analysis: Ayushi Gupta, Kristine Lan.
Investigation: Hyang Nina Kim, Ayushi Gupta, Kristine Lan, Jenell Stewart, Shireesha Dhanireddy, Maria A. Corcorran.
Methodology: Hyang Nina Kim, Ayushi Gupta, Kristine Lan.
Resources: Shireesha Dhanireddy.
Supervision: Hyang Nina Kim.
Validation: Hyang Nina Kim, Jenell Stewart, Maria A. Corcorran.
Writing – original draft: Hyang Nina Kim.
Writing – review & editing: Hyang Nina Kim, Ayushi Gupta, Kristine Lan, Jenell Stewart, Shireesha Dhanireddy, Maria A. Corcorran.
Footnotes
Abbreviations: CPT = Current Procedural Terminology, DS = discharge summary, ECHO = echocardiogram, ICD = International Classification of Diseases, ID = infectious diseases, IE = infective endocarditis, OPAT = outpatient parenteral antibiotic therapy, PPV = positive predictive value.
How to cite this article: Kim HN, Gupta A, Lan K, Stewart J, Dhanireddy S, Corcorran MA. Diagnostic accuracy of ICD code versus discharge summary based query for endocarditis cohort identification. Medicine 2021;100:51(e28354).
This work was supported by the University of Washington Division of Allergy & Infectious Diseases. The authors received no other outside funds for this work.
The authors have no conflicts of interest to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
The datasets generated during and/or analyzed during the present study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016;387:882–93. [DOI] [PubMed] [Google Scholar]
- [3].Meisner JA, Anesi J, Chen X, Grande D. Changes in infective endocarditis admissions in Pennsylvania during the opioid epidemic. Clin Infect Dis 2020;71:1664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wong CY, Zhu W, Aurigemma GP, et al. Infective endocarditis among persons aged 18–64 years with HIV, hepatitis C infection, or opioid use disorder—United States, 2007–2017. Clin Infect Dis 2020;72:1767–81. [DOI] [PubMed] [Google Scholar]
- [5].Schranz AJ, Fleischauer A, Chu VH, Wu L-T, Rosen DL. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med 2019;170:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015;65:2070–6. [DOI] [PubMed] [Google Scholar]
- [7].Burles K, Innes G, Senior K, Lang E, McRae A. Limitations of pulmonary embolism ICD-10 codes in emergency department administrative data: let the buyer beware. BMC Med Res Methodol 2017;17:01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lawrence K, Joos C, Jones AE, Johnson SA, Witt DM. Assessing the accuracy of ICD-10 codes for identifying acute thromboembolic events among patients receiving anticoagulation therapy. J Thromb Thrombolysis 2019;48:181–6. [DOI] [PubMed] [Google Scholar]
- [9].Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care 2005;43:480–5. [DOI] [PubMed] [Google Scholar]
- [10].O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res 2005;40:1620–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chang TE, Lichtman JH, Goldstein LB, George MG. Accuracy of ICD-9-CM codes by hospital characteristics and stroke severity: Paul Coverdell National Acute Stroke Program. J Am Heart Assoc 2016;5: doi: 10.1161/jaha.115.003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH, Egorova NN. Trends in infective endocarditis in California and New York State, 1998–2013. JAMA 2017;317:1652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kobayashi T, Beck B, Miller A, Polgreen P, O'Shea AMJ, Ohl ME. Positive predictive values of 2 algorithms for identifying patients with intravenous drug use-associated endocarditis using administrative data. Open Forum Infect Dis 2020;7:ofaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ball LJ, Sherazi A, Laczko D, et al. Validation of an algorithm to identify infective endocarditis in people who inject drugs. Med Care 2018;56:e70–5. [DOI] [PubMed] [Google Scholar]
- [15].Marks LR, Nolan NS, Jiang L, Muthulingam D, Liang SY, Durkin MJ. Use of ICD-10 codes for identification of injection drug use-associated infective endocarditis is nonspecific and obscures critical findings on impact of medications for opioid use disorder. Open Forum Infect Dis 2020;7:ofaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Miller E. To code or not to code. J AHIMA. 2017. Available at: https://journal.ahima.org/to-code-or-not-to-code/. Accessed April 26, 2021. [Google Scholar]
- [17].Wei W-Q, Teixeira PL, Mo H, Cronin RM, Warner JL, Denny JC. Combining billing codes, clinical notes, and medications from electronic health records provides superior phenotyping performance. J Am Med Inform Assoc 2016;23:e20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shivade C, Raghavan P, Fosler-Lussier E, et al. A review of approaches to identifying patient phenotype cohorts using electronic health records. J Am Med Informatics Assoc 2014;21:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Informatics 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- [21].McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–82. [PMC free article] [PubMed] [Google Scholar]
- [22].Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- [23].Brown LD, Cai TT, Das Gupta A. Interval estimation for a binomial proportion. Stat Sci 2001;16:101–17. [Google Scholar]
- [24].Singla M, Hutfless S, Al Kazzi E, Rodriguez B, Betteridge J, Brant S. Clinical codes combined with procedure codes increase diagnostic accuracy of Crohn's disease in a US Military health record. BMJ Open Gastroenterol 2020;7:e000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hripcsak G, Albers DJ. High-fidelity phenotyping: richness and freedom from bias. J Am Med Inform Assoc 2018;25:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Johnson SB, Bakken S, Dine D, et al. An electronic health record based on structured narrative. J Am Med Informatics Assoc 2008;15:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rosenbloom ST, Denny JC, Xu H, Lorenzi N, Stead WW, Johnson KB. Data from clinical notes: a perspective on the tension between structure and flexible documentation. J Am Med Informatics Assoc 2011;18:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]