Supplemental Digital Content is available in the text
Keywords: adiposis dolorosa, dercum disease, frequency rhythmic electrical modulation system therapy, genetic analysis, lipomatosis, pain management, quality of life
Abstract
Dercum's disease (DD), or adiposis dolorosa, is a rare condition of unknown etiology characterized by growth of painful subcutaneous adipose tissue. No specific treatment exists. Pain is often invalidating and resistant to analgesic drugs. We tested the efficacy of Frequency Rhythmic Electrical Modulation System (FREMS) therapy on pain relief. Subcutaneous biopsies were performed for genetic analysis.
Nine DD patients were enrolled. Five cycles of FREMS at 3-month intervals during 1 year were administered. Visual analogue scale (VAS), Bartel Index Questionnaire and Short Form 36 questionnaire were used to measure pain and general health status at baseline, 6 and 12 months. Dual-energy X-ray absorptiometry (DEXA) quantified fat mass. Next-Generation Sequencing (NGS) was performed on adipose tissue biopsies and peripheral blood sample to search for somatic variants and specific protein pathway mutation.
Seven patients were included in the final analysis. FREMS induced a reduction in VAS score (from 92 to 52.5, P = .0597) and a significant improvement in SF-36 domains (Physical functioning, Role limitation due to physical health, Body pain, Vitality, Social functioning, P < .05). No modification in anthropometrics and DEXA values was observed. The analysis of the mitochondrial Displacement loop (D-loop) region confirmed the clonality of all lipomatous lesions. The presence of the mitochondrially encoded tRNA-Lysine (MT-TK) m.8344A>G variant, occasionally identified in patients with multiple symmetric lipomatosis, was excluded in all subjects. On the other hand, we observed variants in genes belonging to signaling pathways involved in cell cycle and proliferation (Phosphoinositide 3-kinase/AKT/mTOR, MAPK/ERK, and Hippo).
FREMS can be a useful tool to alleviate pain and improve overall quality of life in patients with DD. Genetic analysis highlighted the molecular heterogeneity of lipomas.
1. Introduction
Dercum's disease (DD) is a rare and clinically variable condition characterized by growth of painful, subcutaneous adipose tissue.[1] The majority of cases occurs sporadically. However, a few familiar cases have been reported,[1–3] suggesting autosomal dominant inheritance. The genetic cause is unknown, with the only exception of the c.8344A-G mutation in the mitochondrially encoded tRNA-Lysine (MT-TK) mitochondrial gene, which has occasionally been found in patients with multiple symmetric lipomatosis.[4,5]
The disease, recognized by the National Organization for Rare Disorders, occurs more frequently in middle-age women, although the exact prevalence of DD is not known.[1] It is classified in 4 types according to the distribution of the affected adipose tissue and the association with lipomas. The diagnosis is clinical and is based on the presence of overweight or obesity and chronic pain in the subcutaneous tissue, after the exclusion of other possible causal conditions.[1] Many approaches have been explored to relief pain: surgical removal of lipomas by liposuction,[6,7] pregabalin and manual lymphatic drainage,[8] systemic analgesics,[1] steroids and non-steroidal anti-inflammatory drugs,[1] methotrexate,[9] infliximab, interferon alpha,[10] lidocaine as local injections[11] or intravenously,[12,13] cyclic hypobaric pressure,[14] subcutaneous adipose tissue therapy.[15] These treatments are associated with variable and temporary results, mostly regarding one or few patients, while larger clinical trials are missing.
We previously described successful treatment of DD-associated pain in a single patient treated with repeated cycles of Frequency Rhythmic Electrical Modulation System (FREMS)[16] which prompted us to explore this approach through a specific trial in patients with DD.
2. Methods
The study was a pilot, single center, open label, not controlled and not randomized trial. The aim of the study was to investigate the efficacy of FREMS therapy on DD-associated pain. Adult patients with a diagnosis of DD[1] were recruited from March 2015 to March 2017 from the Rare Disease Outpatient Clinic at our institution. Key exclusion criteria were wearing a pacemaker, pregnancy and a previous diagnosis of cancer. The research protocol included an ancillary study for genetic analysis performed on peripheral blood and on subcutaneous fat tissue (obtained through an ultrasound guided biopsy from 2 different painful sites). The study was approved by the Ethic Committee at San Raffaele Hospital in Milan (protocol number 7/int/2015) and was conducted in accordance with the Helsinki Declaration.
At the first visit, patients signed an informed consent, underwent complete physical examination, evaluation of total body composition by dual-energy X-ray absorptiometry (DEXA) and ultrasound of painful sites. We also graded pain using the visual analogue scale (VAS from 0 “no pain” to 100 “worst pain”), physical disability using the Barthel Index of Activities of Daily Living questionnaire (from 0 “worst disability” to 20 “no disability”) and health status using the Short Form 36 questionnaire (SF-36, from 0 to 100). DEXA, ultrasound, visual analogue scale, Barthel Index of Activities of Daily Living questionnaire and SF-36 questionnaire were also repeated at 6 and 12 months.
Primary outcome was changes of analogue visual scale scores at 1 year. Secondary outcomes were changes of analogue visual scale scores at 6 months, results of Barthel Index of Activities of Daily Living questionnaire and SF-36 questionnaire and values of total body composition by DEXA at 6 and 12 months.
2.1. FREMS treatment
Five cycles of FREMS therapy were performed over the one-year study period: at baseline, at 3, 6, 9 and 12 months. FREMS is a transcutaneous electrical stimulation administered as a sequence of software-driven modulated electrical stimuli that vary automatically as of pulse frequency, duration and voltage amplitude through a specific device (Aptiva, Lorenz Lifetech, Ozzano dell’Emilia, Italy). The electric stimulation is delivered in session of 30 minutes and is characterized by sequences of biphasic, asymmetric pulses consisting of an active phase of high negative voltage spike of extra short duration, and a recharging phase of low voltage activity of longer duration. Pulse frequency is variable, mainly in the range of 1 to 50 Hz. Patients were asked to set their own threshold of maximal electrical stimulation in a voltage range up to 300 V, by progressively increasing the voltage through a hand-held remote control device increasing the voltage by 1 V per step to the maximum allowed, which corresponds to a local burning sensation. Electrodes were positioned over the most painful areas of the body; positioning was tailored on each patient characteristics. FREMS therapy has established documented efficacy in treating pain related to diabetic neuropathy,[17,18] painful leg ulcers[19,20] and other painful conditions.[21,22]
2.2. Genetic analysis
2.2.1. Detection of the m.8344A>G variant
Genomic DNA (gDNA) was extracted from peripheral blood and lipomatous tissue by using the DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions.
A preliminary screening of the m.8344A>G variant affecting the mitochondrial MT-TK gene was performed on gDNA extracted from peripheral blood of each patient by Sanger sequencing using specific primers for the region of interest (sequence available upon request).
2.2.2. Analysis of the D310 sequence
To characterize the clonal origin of the lipomatous lesions obtained through the biopsy, the mitochondrial D-loop (Displacement loop) sequence of the bioptic material was compared with the corresponding germline sample (peripheral blood) of each patient. Notably, we amplified and sequenced the hypervariable D310 sequence within the D-loop region, consisting of a poly-cytosine tract, which contains polymorphisms associated with nucleotide expansion/deletion.[23,24]
2.2.3. Germline and somatic whole-exome sequencing
The gDNA extracted from lipomatous tissue and peripheral blood samples (1.5 μg) was used to generate libraries by using a commercial target enrichment kit (SureSelectXT Clinical Research Exome, Agilent Technologies, Santa Clara, CA) and sequenced through a paired-end 150 bp protocol on a HiSeq 2500 (Illumina, San Diego, CA, USA), producing on average 68 million read pairs corresponding to about 90X coverage, after mapping and removal of duplicated reads. Briefly, reads were quality-filtered and aligned to the reference human genome sequence (GRCh37/hg19) with Burrow-Wheeler Aligner. The Sequence Alignment Map output file was converted into a sorted binary alignment map (BAM) file using Sequence Alignment Map tools. BAM files underwent local realignment around insertion-deletion sites, duplicate marking, and recalibration steps with Genome Analysis Toolkit v3.7. Variant calling was performed with Unified Genotyper, and output Variant Call Format files were recalibrated with Variant Recalibrator from Genome Analysis Toolkit v3. Genomic variant annotation was carried out with VarSeq v1.4.5 (Golden Helix, Inc., Bozeman, MT) and only variants with a minimum quality score of 30 and a minimum read depth of 10X were included in the downstream analysis. Finally, the manual inspection of the Bam files, by using Integrative Genomics Viewer, allowed us to evaluate the coverage and quality of the aligned reads and, together with the EXCAVATOR tool (https://sourceforge.net/projects/excavatortool/), to identify potential insertions or deletions. Thereafter, we excluded variants with a population frequency above 5% in 1000 Genomes Project, Exome Sequencing Project (National Heart, Lung and Blood Institute Exome Sequencing Project), Exome Aggregation Consortium, Genome Aggregation Database, and The Cancer Genome Atlas. We considered only variants predicted to alter the protein structure or function by at least one of the in-silico prediction tools we used (namely Mutation Taster, Sorting Intolerant From Tolerant and Polyphen-2, Mutation Assessor, Functional Analysis through Hidden Markov Models and Functional Analysis through Hidden Markov Models-MKL) and affecting non-synonymous exonic, or splice site (beyond 30 bp of exon/intron boundaries) regions.
2.3. Statistical analysis
Due to a lack of data in literature about epidemiology of DD, we decided to use a convenience sample, based on the number of patients followed at the Rare Disease Outpatient Clinic of our Institution. Continuous variables are presented as median with interquartile range in parenthesis and categorical variables as frequency and percent.
Comparisons between baseline, 6 and 12 months were conducted using a mixed random-effect model, in order to accommodate for dependencies and missing values (Stata [Stata Corp. College Station, TX], version 10.1). No adjustments were made for potential confounders. To document goodness of fit, we generated standardized residuals and checked that they followed a standard normal distribution.
3. Results
3.1. Clinical evaluation
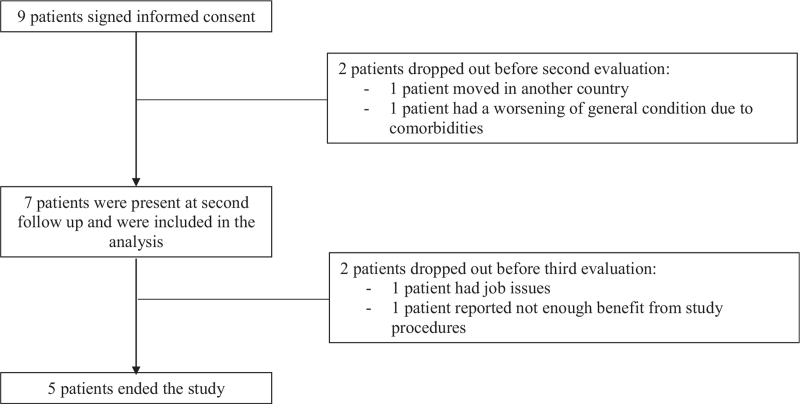
Nine patients with the type II generalised nodular form of DD were enrolled in this study. Two patients dropped out after the second cycle of FREMS therapy, 1 case because of worsening of known comorbidities and in the other case because of personal issues (patient moved to another country). Therefore, we included in the final analysis 7 patients, of which 2 completed 3 cycles of FREMS therapy (missing cycles were due to job issue, lack of pain improvement), 1 patient completed 4 cycles (personal issue) and 4 patients completed all 5 cycles. The flow chart of study participants is shown in Figure 1. Baseline general characteristics of study participants are shown in Table 1.
Figure 1.
Flow chart of study participants.
Table 1.
Baseline general characteristics of study participants.
| N | 7 |
| Age (yr) | 49 (40–64) |
| Gender (% female) | 7 (100%) |
| Duration of Dercum disease (yr) | 5 (2–12) |
| Menopause | 4 (57.1%) |
| Smoking habits | 3 (42.9%) |
| Physically active | 3 (42.9%) |
Continuous variables are presented as median with interquartile range in parenthesis; categorical variables as frequency and percent in parenthesis. ∗Comorbidities: hypertension (3 patients), type 2 diabetes (2 patients), clinical depression (2 patients), hypothyroidism (2 patients), dyslipidemia (2 patients), migraine, anemia, polycystic ovary syndrome (PCOS), gastric reflux, atrial fibrillation (1 patient).
Characteristics of patients and efficacy measures during study period are shown in Table 2. Questionnaires scores revealed an amelioration of clinical conditions and a reduction in pain perception. VAS scores decreased during the study period from 92 to 52.5 (P = .0597) and SF-36 scores increased in physical health domains (Physical functioning, Role limitation because of impaired physical health, Body pain, P < .05) and mental health domains (Vitality, Social functioning, P < .05). We did not observe statistically significant differences in any anthropometrics or body fat composition variables.
Table 2.
Patients’ anthropometrics, pain and functional status assessment and body composition by DEXA over the study.
| Baseline | 6 Months | 12 Months | ||
| Anthropometrics | ||||
| BMI | 30.8 (27–30.8) | 30.8 (27.6–32.6) | 32.5 (30.1–33.1) | 0.6424 |
| Abdominal circumference (cm) | 102 (91–103) | 101 (92–107) | 103 (98–103) | 0.2515 |
| Right leg circumference (cm) | 56 (51–63) | 60 (51–62) | 58 (51–60) | 0.8974 |
| Left leg circumference (cm) | 60 (50–64) | 59 (50–62) | 58.5 (48–61) | 0.6764 |
| Right arm circumference (cm) | 31 (28–33) | 32.5 (26–34) | 33 (30–34) | 0.8913 |
| Left arm circumference (cm) | 30 (27–33) | 31 (25–34) | 34 (29.5–35) | 0.5972 |
| Pain and functional status assessment | ||||
| VAS score | 92 (70–100) | 84 (68–89) | 52.5 (35–69.5) | 0.0597 |
| Barthel Index | 20 (18–20) | 19.5 (18–20) | 19.5 (19–20) | 0.5578 |
| SF-36 score: | ||||
| Physical functioning | 40 (25–55) | 47.5 (35–70) | 70 (62.5–70) | 0.0470 |
| Role limitation because of impaired physical health | 0 (0–0) | 0 (0–25) | 50 (0–100) | 0.0128 |
| Body pain | 22.25 (0–22.5) | 28.75 (20–45) | 55 (43.75 -67.5) | 0.0015 |
| General health | 20 (5–50) | 42.5 (35–65) | 37.5 (25–70) | 0.1940 |
| Vitality | 15 (5–20) | 35 (20–50) | 30 (22.5–50) | 0.0439 |
| Social functioning | 25 (0–37.5) | 43.75 (37.5–50) | 56.25 (43.75–81.25) | 0.0210 |
| Role limitation because of emotional problems | 0 (0–33.3) | 0 (0–0) | 33.3 (0–83.3) | 0.1255 |
| Mental health | 40 (24–56) | 52 (44–52) | 54 (36–82) | 0.2042 |
| DEXA body composition (fat mass, g) | ||||
| Left arm | 1703 (1176–2108) | 1647 (1385–1839) | 1933 (1673–2395) | 0.6060 |
| Right arm | 1719 (1320–2071) | 1761.5 (1415–2108) | 2023 (1845–2113) | 0.4938 |
| Trunk | 13688 (9560–17469) | 13758.5 (10987–16310) | 14792 (14250–15590) | 0.1755 |
| Left thigh | 5039 (4623–6792) | 5296.5 (5052–6700) | 5266 (4724–6597) | 0.5926 |
| Right thigh | 5444 (4900–7285) | 5360 (5130–6369) | 5488 (4821–6335) | 0.7765 |
| Total body | 32249 (22519–32952) | 29991 (25771–33445) | 30722 (29821–32608) | 0.3788 |
BMI = body mass index, DEXA = dual X-ray absorptiometry, VAS = visual analogue scale, SF = short form 36, VAS = visual analogue scale.
Data are presented as median with interquartile range in parenthesis.
Almost all patients assumed analgesic drugs to relief pain and to control comorbidities: drugs taken during study period are summarized in Table 3.
Table 3.
Analgesic therapy and daily dose during study period.
| Baseline | 6 Months | 12 Months | |
| Patient 2 | Oxycodon/naloxon 20/10 mg twice daily | Transdermal fentanyl 75 mcg/h | Transdermal fentanyl 75 mcg/h |
| Patient 3 | Analgesic on demand | Analgesic on demand | Analgesic on demand |
| Patient 4 | Ketoprofen 80 mg on demand | Transdermal fentanyl 75 mcg/h | Transdermal fentanyl 75 mcg/h |
| Patient 6 | Ibuprofen 1200 mg | Ibuprofen 600 mg | Ibuprofen 600 mg |
| Patient 7 | Analgesic on demand | None | None |
| Patient 8 | Oxycodon/paracetamol 20/750 mg | Oxycodon/paracetamol 30/975 | Oxycodon/paracetamol 30/975 |
| Patient 9 | Paracetamol/codein 500/30 on demand | Oxycodon/paracetamol 10/325 mg | Oxycodon/paracetamol 10/325 mg |
No major adverse event occurred; minor events were an episode of thoracic pain (cardiac event was excluded) and an episode of urinary tract infection, none of them were related to FREMS treatment. One patient underwent orthopedic surgery for shoulder ligament injury during the study period (patient 9).
3.2. Genetic analysis results
The analysis of the mitochondrial D-loop region confirmed the clonality of lipomatous lesions obtained from different anatomical regions in all patients. Furthermore, we excluded in all study participants the presence of the MT-TK m.8344A>G variant, the only genetic cause that has been sporadically associated with lipomatosis until now.[1]
Whole-exome sequencing data were initially filtered for single nucleotide variants and copy number variations affecting genes involved in the so-called overgrowth syndromes. Indeed, lipomatous lesions are frequently observed in patients with PIK3CA Related Overgrowth Syndromes, such as Bannayan-Riley-Ruvalcaba syndrome (BRRS; Online Mendelian Inheritance in Man [OMIM] #158350), Proteus syndrome (OMIM #176920), and Congenital Lipomatous Overgrowth, Vascular Malformations, Epidermal Nevi and Spinal/Skeletal Anomalies (OMIM# 612918).[25–28] These conditions are caused by mosaic gain-of-function variations in different genes of the Phosphoinositide 3-kinase/AKT/mTOR pathway, which is also involved in many different cancers. We compared all patients’ samples to identify a shared pathogenic variant and/or gene. However, this analysis failed to identify a unique genetic cause but rather detected several variants in genes belonging to signaling pathways involved in cell cycle and proliferation (Phosphoinositide 3-kinase/AKT/mTOR, MAPK/ERK, and Hippo), whose functional role deserves further investigations (Supplemental Digital Content Table S1, http://links.lww.com/MD2/A783).
The Copy Number Variations identified in our cohort of patients, mainly duplications, involved genes showing biological functions not directly related to the “lipomatous” phenotype, apart from one comprising the Long-chain fatty acid transport protein 1 gene (∗600691), encoding a transporter involved in long chain fatty acids metabolism.[29]
4. Discussion
Our results suggest that FREMS therapy may be used to alleviate pain and improve general quality of life in patients with DD. Overall, FREMS therapy reduced pain as measured by VAS at the limit of statistical level, and significantly ameliorated physical functioning, role limitation because of impaired physical health, body pain, vitality and social functioning as documented by the SF-36 score changes. These results confirmed what previously observed in our initial patient, where a reduction of pain and improvement in functional and health status was observed. In that single case we also observed a reduction in subcutaneous adipose tissue thickness, which we did not observe in this series of patients.[16] However, in our previous case the patient was a male with the generalized diffuse form of DD, while all the patients included in this study were females with the diffuse nodular form of DD.
As anticipated in that report,[16] the mechanism of action of FREMS has still to be elucidated. It has been demonstrated that it enhances microvascular blood flow,[30] increases vasomotor activity mediated by smooth cells,[31] releases vascular endothelial growth factor[32] and changes the amplitude of Hoffmann (H)-reflex.[33] We can postulate that the interaction of subcutaneous blood flow with painful adipose tissue may help reducing the perception of pain.
It is difficult to determine whether the positive effect on pain is due exclusively to FREMS therapy or if additional factors may play a role. Almost all patients assumed analgesic drug to cope with pain, and many of them also presented comorbidities and/or intercurrent factors (i.e., orthopedic surgery). We cannot exclude that the improvement in health status could be due, at least in part, to the participation to this clinical trial, as a sort of placebo effect.
It is important to mention that FREMS therapy required good patient compliance for the treatment sessions to be delivered on consecutive days and FREMS cycles to be delivered with the right time intervals. A low compliance may explain the withdrawals recorded in our study. Our study included a small number of patients but, as DD is a rare disease, we encountered difficulties in recruiting a larger sample of patients in a relatively short period of time.
Molecular analysis confirmed the clonality of lipomatous lesions obtained from different anatomical regions in all patients. Moreover, the presence of the MT-TK m.8344A>G variant, the only genetic cause that has been associated with lipomatosis up to now, has not been identified in any of our patients. On the other hand, whole-exome sequencing identified variants in multiple genes belonging to signaling pathways involved in cell cycle and proliferation, including genes encoding NFAT transcription factors. These proteins regulate the Hippo and Wnt signaling pathways, which are involved in the homeostasis maintenance through regulating cell proliferation, progenitor renewal and differentiation, and stress-induced cell apoptosis.[34,35] Although further observations on larger cohorts of patients and functional in vitro investigations are needed, our preliminary results indicate genetic heterogeneity of lipomas in patients with DD.
We can conclude that FREMS therapy may represent a safe therapeutic option in patients with DD together with analgesic therapy. Larger multicenter studies are needed to determine the efficacy of FREMS in DD. In the context of future clinical trial design, a more thorough biological characterization of DD, possibly comprising the genetic, immunohistochemical and transcriptional profiles of its associated lesions, will arguably help identify the most appropriate therapeutic option for each patient.
Author contributions
Conceptualization: Amelia Caretto, Sabina Martinenghi.
Data curation: Amelia Caretto, Edoardo Errichiello, Maria Grazia Patricelli, Sabina Martinenghi.
Formal analysis: Edoardo Errichiello, Marina Scavini.
Investigation: Amelia Caretto, Edoardo Errichiello, Maria Grazia Patricelli, Giulia Cristel, Silvia Ravelli, Marcella Sirtori, Sabina Martinenghi.
Methodology: Edoardo Errichiello, Sabina Martinenghi.
Supervision: Emanuele Bosi.
Writing – original draft: Amelia Caretto, Edoardo Errichiello, Sabina Martinenghi.
Writing – review & editing: Amelia Caretto, Edoardo Errichiello, Maria Grazia Patricelli, Orsetta Zuffardi, Marina Scavini, Emanuele Bosi, Sabina Martinenghi.
Footnotes
Abbreviations: AKT = protein kinase B, BAM = binary alignment map, DD = Dercum's disease, DEXA = dual-energy X-ray absorptiometry, D-loop = displacement loop, FREMS = frequency rhythmic electrical modulation system, gDNA = genomic deoxyribonucleic acid, mTOR = mammalian target of rapamycin, MT-TK = mitochondrially encoded tRNA-Lysine, NGS= Next-Generation Sequencing, OMIM = online mendelian inheritance in man, VAS = visual analogue scale.
How to cite this article: Caretto A, Errichiello E, Patricelli MG, Zuffardi O, Cristel G, Ravelli S, Sirtori M, Scavini M, Bosi E, Martinenghi S. Transcutaneous electrical stimulation therapy and genetic analysis in Dercum's disease: a pilot study. Medicine. 2021;100:51(e28360).
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].Hansson E, Svensson H, Brorson H. Review of Dercum's disease and proposal of diagnostic criteria, diagnostic methods, classification and management. Orphanet J Rare Dis 2012;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Campen R, Mankin H, Louis DN, Hirano M, Maccollin M. Familial occurrence of adiposis dolorosa. J Am Acad Dermatol 2001;44:132–6. [DOI] [PubMed] [Google Scholar]
- [3].Kyllerman M, Brandberg G, Wiklund LM, Månsson JE. Dysarthria, progressive parkinsonian features and symmetric necrosis of putamen in a family with painful lipomas (Dercum disease variant). Neuropediatrics 2002;33:69–72. [DOI] [PubMed] [Google Scholar]
- [4].Holme E, Larsson NG, Oldfors A, Tulinius M, Sahlin P, Stenman G. Multiple symmetric lipomas with high levels of mtDNA with the tRNA(Lys) A-->G(8344) mutation as the only manifestation of disease in a carrier of myoclonus epilepsy and ragged-red fibers (MERRF) syndrome. Am J Hum Genet 1993;52:551–6. [PMC free article] [PubMed] [Google Scholar]
- [5].Gámez J, Playán A, Andreu AL, et al. Familial multiple symmetric lipomatosis associated with the A8344G mutation of mitochondrial DNA. Neurology 1998;51:258–60. [DOI] [PubMed] [Google Scholar]
- [6].Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum's disease (adiposis dolorosa). Pain Med 2011;12:942–52. [DOI] [PubMed] [Google Scholar]
- [7].Hansson E, Manjer J, Svensson H, Brorson H. Quality-of-life in patients with Dercum's disease – before and after liposuction. J Plast Surg Hand Surg 2012;46:252–6. [DOI] [PubMed] [Google Scholar]
- [8].Lange U, Oelzner P, Uhlemann C. Dercum's disease (Lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int 2008;29:17–22. [DOI] [PubMed] [Google Scholar]
- [9].Singal A, Janiga JJ, Bossenbroek NM, Lim HW. Dercum's disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venereol 2007;21:717. [DOI] [PubMed] [Google Scholar]
- [10].Gonciarz Z, Mazur W, Hartleb J, et al. Interferon alfa-2b induced long-term relief of pain in two patients with adiposis dolorosa and chronic hepatitis C. J Hepatol 1997;27:1141. [DOI] [PubMed] [Google Scholar]
- [11].Reggiani M, Errani A, Staffa M, Schianchi S. Is EMLA effective in Dercum's disease? Acta Derm Venereol 1996;76:170–1. [DOI] [PubMed] [Google Scholar]
- [12].Petersen P, Kastrup J. Dercum's disease (adiposis dolorosa). Treatment of the severe pain with intravenous lidocaine. Pain 1987;28:77–80. [DOI] [PubMed] [Google Scholar]
- [13].Atkinson RL. Intravenous lidocaine for the treatment of intractable pain of adiposis dolorosa. Int J Obes 1982;6:351–7. [PubMed] [Google Scholar]
- [14].Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res 2010;3:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ibarra M, Eekema A, Ussery C, Neuhardt D, Garby K, Herbst KL. Subcutaneous adipose tissue therapy reduces fat by dual X-ray absorptiometry scan and improves tissue structure by ultrasound in women with lipoedema and Dercum disease. Clin Obes 2018;8:398–406. [DOI] [PubMed] [Google Scholar]
- [16].Martinenghi S, Caretto A, Losio C, Scavini M, Bosi E. Successful treatment of Dercum's disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore) 2015;94:e950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bosi E, Conti M, Vermigli C, et al. Effectiveness of frequency-modulated electromagnetic neural stimulation in the treatment of painful diabetic neuropathy. Diabetologia 2005;48:817–23. [DOI] [PubMed] [Google Scholar]
- [18].Bosi E, Bax G, Scionti L, et al. FREMS European Trial Study Group. Frequency-modulated electromagnetic neural stimulation (FREMS) as a treatment for symptomatic diabetic neuropathy: results from a double-blind, randomised, multicentre, long-term, placebo-controlled clinical trial. Diabetologia 2013;56:467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Janković A, Binić I. Frequency rhythmic electrical modulation system in the treatment of chronic painful leg ulcers. Arch Dermatol Res 2008;300:377–83. [DOI] [PubMed] [Google Scholar]
- [20].Santamato A, Panza F, Fortunato F, et al. Effectiveness of the frequency rhythmic electrical modulation system for the treatment of chronic and painful venous leg ulcers in older adults. Rejuvenation Res 2012;15:281–7. [DOI] [PubMed] [Google Scholar]
- [21].Gandolfi A, Pontara A, Di Terlizzi G, et al. Improvement in clinical symptoms of scleredema diabeticorum by frequency-modulated electromagnetic neural stimulation: a case report. Diabetes Care 2014;37:e233–4. [DOI] [PubMed] [Google Scholar]
- [22].Farina S, Casarotto M, Benelle M, et al. A randomized controlled study on the effect of two different treatments (FREMS AND TENS) in myofascial pain syndrome. Eura Medicophys 2004;40:293–301. [PubMed] [Google Scholar]
- [23].Geurts-Giele WR, Gathier GH, Atmodimedjo PN, Dubbink HJ, Dinjens WN. Mitochondrial D310 mutation as clonal marker for solid tumors. Virchows Arch 2015;467:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Errichiello E, Venesio T. Mitochondrial DNA variants in colorectal carcinogenesis: drivers or passengers? J Cancer Res Clin Oncol 2017;143:1905–14. [DOI] [PubMed] [Google Scholar]
- [25].Zhou XP, Waite KA, Pilarski R, et al. Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am J Hum Genet 2003;73:404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lindhurst MJ, Sapp JC, Teer JK, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med 2011;365:611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Martinez-Lopez A, Blasco-Morente G, Perez-Lopez I, et al. CLOVES syndrome: review of a PIK3CA-related overgrowth spectrum (PROS). Clin Genet 2017;91:14–21. [DOI] [PubMed] [Google Scholar]
- [28].Madsen RR, Vanhaesebroeck B, Semple RK. Cancer-associated PIK3CA mutations in overgrowth disorders. Trends Mol Med 2018;24:856–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Qiu F, Xie L, Ma JE, et al. Lower expression of SLC27A1 enhances intramuscular fat deposition in chicken via down-regulated fatty acid oxidation mediated by CPT1A. Front Physiol 2017;8:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Conti M, Peretti E, Cazzetta G, et al. Frequency-modulated electromagnetic neural stimulation enhances cutaneous microvascular flow in patients with diabetic neuropathy. J Diabetes Complications 2009;23:46–8. [DOI] [PubMed] [Google Scholar]
- [31].Bocchi L, Evangelisti A, Barrella M, Scatizzi L, Bevilacqua M. Recovery of 0.1 Hz microvascular skin blood flow in dysautonomic diabetic (type 2) neuropathy by using Frequency Rhythmic Electrical Modulation System (FREMS). Med Eng Phys 2010;32:407–13. [DOI] [PubMed] [Google Scholar]
- [32].Bevilacqua M, Dominguez LJ, Barrella M, Barbagallo M. Induction of vascular endothelial growth factor release by transcutaneous frequency modulated neural stimulation in diabetic polyneuropathy. J Endocrinol Invest 2007;30:944–7. [DOI] [PubMed] [Google Scholar]
- [33].Barrella M, Toscano R, Goldoni M, Bevilacqua M. Frequency rhythmic electrical modulation system (FREMS) on H-reflex amplitudes in healthy subjects. Eura Medicophys 2007;43:37–47. [PubMed] [Google Scholar]
- [34].Zecca M, Struhl G. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol 2010;8:e1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev 2013;27:355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]