Abstract
The hematopoietic cell-specific protein Vav1 is a substrate of tyrosine kinases activated following engagement of many receptors, including FcɛRI. Vav1-deficient mice contain normal numbers of mast cells but respond more weakly than their normal counterparts to a passive systemic anaphylaxis challenge. Vav1-deficient bone marrow-derived mast cells also exhibited reduced degranulation and cytokine production, although tyrosine phosphorylation of FcɛRI, Syk, and LAT (linker for activation of T cells) was normal. In contrast, tyrosine phosphorylation of phospholipase Cγ1 (PLCγ1) and PLCγ2 and calcium mobilization were markedly inhibited. Reconstitution of deficient mast cells with Vav1 restored normal tyrosine phosphorylation of PLCγ1 and PLCγ2 and calcium responses. Thus, Vav1 is essential to FcɛRI-mediated activation of PLCγ and calcium mobilization in mast cells. In addition to its known role as an activator of Rac1 GTPases, these findings demonstrate a novel function for Vav1 as a regulator of PLCγ-activated calcium signals.
The early events following activation of the high-affinity receptor for immunoglobulin E (IgE) (FcɛRI) on mast cells are well studied (32). Like other immunoreceptors, FcɛRI contains multiple subunits, the IgE binding α chain, and the β and γ chains that function to transduce signals via the paired tyrosine residues of the immunoreceptor tyrosine-based activation motifs (ITAMs) that are found within (45). Aggregation of multiple IgE-occupied FcɛRI by polyvalent antigen (Ag) leads to transphosphorylation of the β- and γ-chain ITAMs by the associated Src family protein tyrosine kinase (PTK) Lyn (17, 41). This creates a new binding surface for Syk PTK, whose tandem Src homology 2 (SH2) domains facilitate the interaction with the ITAM, resulting in activation of this enzyme (4, 31). Activated Syk then phosphorylates multiple substrates, among which the linker for activation of T cells (LAT) is proximal and essential to FcɛRI-activated responses (48). LAT, the SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76), and phospholipase Cγ (PLCγ) have all been implicated in the regulation of calcium responses in mast cells (40, 48, 59) and in other cells (12, 59, 64). These proteins, along with the Rac GTPase-activating Vav1 protein, interact to form a functional macromolecular complex at the plasma membrane that regulates both Rac and Ras signaling (1, 14, 21, 37, 51).
Vav1 is a cytosolic protein primarily expressed in hematopoietic cells whose structure and function have been extensively examined (6). Its structure includes a calponin homology (CH) domain, a Dbl homology (DH) domain, a pleckstrin homology (PH) domain, a single SH2 domain, and two Src homology 3 (SH3) domains that flank the SH2 domain. Functional studies demonstrated that tyrosine phosphorylation activates Vav's guanine nucleotide exchange factor (GEF) activity for Rho family GTPases (15) with a clear preference for Rac GTPases. Vav1 also promotes intracellular signaling by its C-terminal Grb2-like adapter region, which contains an SH2 domain flanked by two SH3 domains (47). Thus, because of its GEF and adapter functions Vav1 influences multiple cellular processes (47). Among these processes, the mobilization of calcium is intimately linked with Vav1 (5), as this is the most dominant defect in T-cell receptor (TCR)-stimulated Vav1-deficient T cells. TCR signaling in general also appears significantly reduced in Vav1-deficient thymocytes, with the resulting impairment in positive and negative selection allowing very limited numbers of T cells to mature and migrate into the periphery (18, 55). Though peripheral B-cell numbers were unaffected by Vav1 deficiency, the B-1a B-cell population in the peritoneal cavity was almost completely absent (63). In addition, the existing peripheral B cells displayed a reduced functional capacity following B-cell receptor stimulation. Unlike that of T cells, mast cell development is unimpaired by Vav1 deficiency (66); hence, exploring Vav1 function in mast cells avoids the possible secondary effects caused by abnormal cell development. Given the known similarities in intracellular signaling among the TCR, the B-cell Ag receptor, and FcɛRI (29), investigation of Vav1 function in FcɛRI-stimulated mast cells may likely reveal mechanisms common to all.
MATERIALS AND METHODS
Mice and BMMC culture.
Vav1−/− mice and Vav1+/+ littermate controls on a mixed C57BL/6 and 129/Sv background were generated as described previously (57). Animals were maintained and used in accordance with National Institutes of Health (NIH) guidelines. Bone marrow mast cell (BMMC) cultures were established from 4- to 8-week-old mice as previously described (48). Mast cell differentiation was confirmed by toluidine blue staining and measurement of IgE receptor expression as determined by flow cytometry. Cultures were utilized only when ≥90% of the cells stained positive. IgE receptor expression was determined by incubating approximately 106 cells at 37°C with 1.5 μg of anti-2,4-dinitrophenol (DNP) IgE antibody (Ab) for 3 h. Following washing, the cells were incubated at 4°C for 5 min with a 0.5-μg concentration of Fc Block (PharMingen) followed by a 0.5-μg concentration of fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgE Ab in a standard staining buffer (45 min). After washing, the cells were analyzed for surface IgE on a flow cytometer and compared to an isotype control.
Reagents and Abs.
Anti-DNP IgE Ab was produced as previously described (34). DNP-human serum albumin (DNP-HSA; Ag; Sigma) was diluted in phosphate-buffered saline (PBS) prior to use. For virus-mediated gene transfer experiments, the pSFV1 expression system, Vav1 constructs, and infection procedure were as previously described (2, 51). Briefly, rat cDNAs of Vav1 and of a Vav1 with the Dbl domain (K194 to I405) deleted were subcloned into the AvrII and NsiI sites of a modified pSFV1 plasmid that contained a multiple cloning site and an in-frame green fluorescent protein (GFP) cassette. For virus production, mRNA was generated in vitro from the pSFV1-Vav1 constructs and from the helper pSFV2 (which encodes viral coat proteins) and electroporated into baby hamster kidney cells to generate virus (2). BMMCs (107) were infected, with all procedures performed 5 h following initial infection. The Vav1 with the Dbl domain deleted was previously shown to be unable to activate the Rac1-JNK pathway in mast cells (56) but able to translocate to the plasma membrane upon aggregation of FcɛRI, like wild-type Vav1 (51). Abs used for immunoprecipitation were as follows: mouse anti-FcɛRI β chain (46), chicken anti-FcɛRI γ chain (36), rabbit anti-Syk (gift of U. Blank, Institut Pasteur), rabbit anti-LAT, rabbit anti-Vav, mixed mouse monoclonal anti-PLCγ1, rabbit anti-PLCγ2, goat anti-SLP-76, goat anti-ERK2, and goat anti-JNK1 (Santa Cruz Biotechnology). Abs used for immunoblotting were as follows: mouse anti-FcɛRI β chain, chicken anti-FcɛRI γ chain, mouse anti-Syk (gift of P. Draber, Institute of Molecular Genetics, Prague, Czech Republic), rabbit anti-LAT serum (gift of L. E. Samelson, National Cancer Institute, NIH), mouse anti-Vav, mixed mouse monoclonal anti-PLCγ1, rabbit anti-PLCγ2, rabbit anti-SLP-76, rabbit anti-ERK2, rabbit anti-JNK1 (Santa Cruz), mouse anti-phospho-ERK2, rabbit anti-phospho-JNK (NEN), rabbit anti-Akt, rabbit anti-phospho-Akt (P-Ser 472/473/474; PharMingen), and mouse antiphosphotyrosine (4G10-biotin; Upstate Biotechnology). Secondary Abs used for blotting were as follows: sheep anti-mouse IgG–horseradish peroxidase (HRP), donkey anti-rabbit IgG–HRP (Amersham), mouse anti-rabbit Ig–biotin, rabbit anti-goat IgG–HRP (Sigma), and rabbit anti-chicken IgY–HRP (Jackson Immunoresearch). Extravidin peroxidase conjugate (Sigma) was used as a secondary reagent for all biotin-conjugated Abs.
Lysate preparation, immunoprecipitation, and immunoblotting.
For the lysate preparation, cells (106/ml) were incubated in interleukin-3 (IL-3)-deficient medium containing 0.5 μg of anti-DNP IgE Ab/ml (preincubation) for 4 h. After preincubation, washing, and resuspension in Tyrodes–0.05% bovine serum albumin (BSA) (Tyrodes consists of 10 mM HEPES, 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, and 5.6 mM glucose), the cells (30 × 106/ml) were stimulated for various times with 100 ng of DNP-HSA/ml. Reactions were terminated by the addition of ice-cold PBS containing pyrophosphate, orthovanadate, and 50 mM EGTA. After washing in the same PBS solution, cells were lysed on ice using 1% NP-40 containing Tris-HCl (pH 7.5), 60 mM β-octylglucoside, pyrophosphate, orthovanadate, aprotinin, leupeptin, and phenylmethylsulfonyl fluoride for 30 min as described previously (52). Immunoprecipitations and immunoblotting were previously described (1). Relative quantitation of immunoblots was performed by densitometry.
Systemic anaphylaxis and histology.
The anaphylaxis method used was described previously (40). Briefly, animals were sensitized with 3 μg of DNP-specific IgE Ab by intravenous tail vein injection. The animals were subsequently challenged (24 h) by intravenous tail vein injection with vehicle (PBS) or 500 μg of DNP-HSA. After 1.5 min, mice were sacrificed, blood samples were immediately obtained by cardiac puncture, and the serum was isolated. Serum histamine levels were determined according to the manufacturer's protocol using a histamine inhibition enzyme-linked immunosorbent assay kit (Immunotech). Determination of skin mast cell number was previously described (48).
Phosphatidylinositol-3,4,5-trisphosphate (PIP3), inositol-1,4,5-trisphosphate (IP3), and calcium measurements.
PIP3 measurements were performed by a modification of the previously described procedure (42). Briefly, BMMCs were incubated in the absence of IL-3 but in the presence of 1 μg of IgE/ml for 4 h at 37°C. Cells were washed twice with Tyrodes-BSA buffer and resuspended in the same buffer at 5 × 106 cells/ml in the presence of 120 μCi of [32P]orthophosphate/ml. After a 90-min incubation at 37°C, the cells were washed once with Tyrodes-BSA and resuspended at 107 cells/ml. Wortmannin (100 nM) was added to the corresponding tubes 15 min before Ag stimulation. Stimulation was performed with 200 ng of Ag/ml at 37°C for 1 or 5 min. The reaction was stopped by microcentrifuging the cells for 10 s at 4°C and resuspending the pellet in 750 μl of methanol–1.2 N HCl (1:1 [vol/vol]). Twenty micrograms of PIP3 and phosphoinositides from bovine brain was added as a carrier before the addition of 390 μl of chloroform. Tubes were vortexed, and the organic phase was collected after centrifugation and reextracted with 400 μl of methanol–0.1 M EDTA (pH 8.0). The new organic (lower) phase was recovered after centrifugation, and 200 μl was dried using a Savant evaporator. Phospholipids were resuspended in 25 μl of chloroform and applied to thin-layer chromatography (TLC) plates precoated with 1.2 M potassium oxalate. Phosphoinositides (Sigma) of known composition were used as a standard. The separation was performed in a mixture of chloroform-acetone-methanol-acetic acid-water (80/30/26/24/14 [vol/vol/vol/vol/vol]). Chromatography was performed for 4 h, and labeled phospholipids were detected using Molecular STORM. The migration of each phospholipid was confirmed by exposing the plates to iodine vapors.
IP3 production was measured as described by Choi et al. (10), with a minor modification. Briefly, 107 BMMCs (suspension cells) were stimulated in PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] buffer (500 μl) in the absence or presence of 100 ng of Ag, and the reaction was terminated by adding 100 μl of ice-cold 100% trichloroacetic acid. IP3 was then extracted from the samples and quantified using a commercially available assay kit (Dupont/NEN).
Calcium was measured as described previously (48). Briefly, cells (2 × 106) were dually loaded with either 16 μM Fluo-3-AM or 16 μM Fura Red (Molecular Probes) in RPMI–2% fetal calf serum for 45 min at 37°C. The cells were then incubated with IgE (1 μg/106 cells) on ice for 1 h and brought to room temperature for 20 min. The cells were resuspended in Tyrodes-BSA, and changes in dye fluorescence with time were determined by flow cytometry, after stimulation with 30 ng of Ag/ml followed by 1 μM thapsigargin at 37°C. Calcium mobilization is reported as the Fluo-3/Fura Red fluorescence intensity ratio over time. For the virus-mediated gene transfer experiments with GFP or Vav-GFP, 107 cells per sample were stained with Fura Red, and the GFP-expressing (GFP+) cells (based on a histogram gate compared to noninfected cells) were analyzed for Fura Red fluorescence over time. The percentage of GFP+ cells varied from 2.5 to 25%.
Degranulation.
Degranulation was determined by β-hexosaminidase release (44). Cells (106) were stimulated with indicated concentrations of DNP-HSA for up to 30 min at 37°C in 0.5 ml of Tyrodes–0.05% BSA and then placed on ice. Cells were centrifuged (280 × g at 4°C for 10 min), and supernatants were collected. Cell pellets were lysed (30 min) in 0.5% Triton X-100 (0.5 ml), and the supernatant and pellet (30-μl) samples were incubated for 1 h at 37°C with 1 mM p-nitrophenyl-N-acetyl-β-d-glucosamide (30 μl) in 0.1 M sodium citrate buffer on a 96-well plate. The incubation was terminated by the addition of 0.1 M Na2CO3-NaHCO3 buffer (200 μl), and the optical density was read on a plate reader at a 405-nm wavelength. Net degranulation is expressed as the percentage of total cellular β-hexosaminidase released to the medium following Ag stimulation minus that released prior to Ag stimulation (spontaneous release). Spontaneous release did not differ significantly among the phenotypes for all of the experiments.
RT-PCR.
After preincubation, washing, and resuspension in Tyrodes-BSA, the cells (30 × 106 total in 5 ml) were stimulated for various times with 1 ng of DNP-HSA/ml. The reaction was terminated by the addition of TRI reagent (Molecular Research Center, Inc.). The RNA was extracted using chloroform and precipitated with isopropanol. First-strand cDNA was synthesized using 5 μg of total RNA with the Life Technologies Superscript reverse transcription-PCR (RT-PCR) kit. Using 7.5 μl of the first-strand cDNA reaction, amplification of the individual cytokines was performed using specific primers (Clontech) under the following conditions: 96°C for 1 min; 2 cycles of 96°C for 1 min and 60°C for 4 min; 28 cycles (IL-4, IL-6, tumor necrosis factor alpha, and monocyte chemoattractant protein 1) or 35 cycles (IL-2, IL-3, IL-10, and gamma interferon [IFN-γ]) of 94°C for 1 min, 60°C for 2.5 min, and 72°C for 4 min; 72°C for 10 min; and 4°C for 6 h. Most cytokines amplified under these conditions show a linear increase in detectable levels with corresponding increases in cDNA. This allowed relative quantitation of cytokine levels when normalized to the mRNA levels of a housekeeping gene like glyceraldehyde-3-phosphate dehydrogenase or when a competitor cDNA was used, as we previously described (8, 54). The products were resolved by 2% agarose gel electrophoresis.
RESULTS
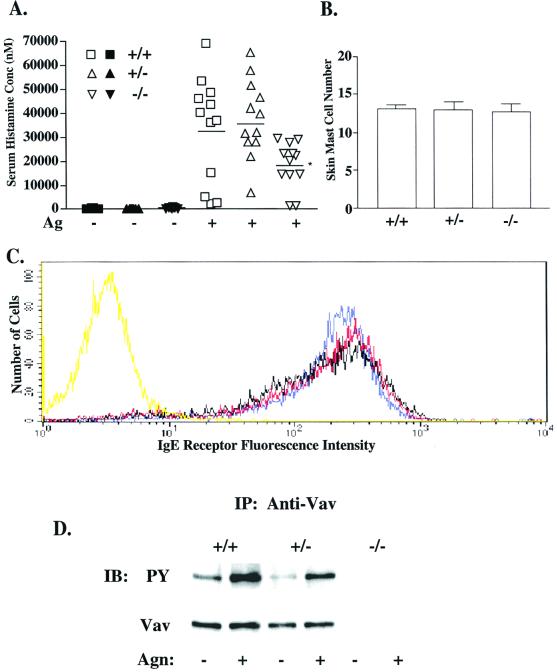
To explore the importance of Vav1 in mast cell responses, we tested Vav1-deficient (Vav1−/−) animals for their ability to undergo passive systemic anaphylaxis. Ag challenge of animals previously sensitized with DNP-specific IgE Ab showed that Vav1−/− mice displayed a reduced ability to undergo systemic anaphylaxis compared with that of Vav1+/+ mice (Fig. 1A). This decrease was not due to Vav1-deficient mice possessing fewer mast cells, as the number of skin mast cells and their granule phenotype, as determined by histochemical staining, were unchanged among Vav1+/+, Vav1+/−, and Vav1−/− mice (Fig. 1B and data not shown). This suggested that the decreased serum histamine levels in Vav1−/− mice likely resulted from an FcɛRI-mediated functional defect and not a defect in mast cell development.
FIG. 1.
Effect of Vav1 deficiency on passive systemic anaphylaxis, IgE receptor expression, and Vav tyrosine phosphorylation. (A) Vav+/+ (squares), Vav+/− (triangles), and Vav−/− (inverted triangles) mice (n = 12 per dose group) were passively sensitized and challenged to induce systemic anaphylaxis as described in Materials and Methods. The serum histamine concentration (nanomolar concentration) was determined via inhibition enzyme-linked immunosorbent assay and compared among PBS (filled symbols)- and Ag (open symbols)-challenged animals, with the mean for each dose group indicated by a line. The asterisk indicates a significance of P ≤ 0.03 (t test) for Vav−/− responders compared to Vav+/+ mice. No significant difference was found between Vav+/+ and Vav+/− mice. (B) A section of depilated dorsal skin was removed from each animal (n = 2 per phenotype), and three sections per skin sample were stained with toluidine blue for mast cells. Mast cell number was assessed at a magnification of ×200 in random fields. (C) Vav+/+ (blue), Vav+/− (red), and Vav−/− (black) BMMC cultures (4 weeks) were sensitized with IgE and stained with an FITC-conjugated anti-IgE Ab to indirectly determine BMMC surface IgE receptor expression by flow cytometry. Nonspecific binding was determined by an FITC-conjugated isotype control (yellow). (D) Vav was immunoprecipitated from lysates of BMMCs (30 × 106) stimulated or not with 100 ng of Ag for 2 min, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane. Tyrosine phosphorylation was determined by blotting with the antiphosphotyrosine Ab (PY, upper panel). After stripping, protein levels were determined by reblotting with anti-Vav Ab (lower panel). IP, immunoprecipitation; IB, immunoblotting.
To investigate the underlying mechanism responsible for this physiological defect, all subsequent experiments were performed on BMMCs cultured from the bone marrow of Vav1−/−, Vav1+/−, and Vav1+/+ mice, all of which developed normally. By 4 to 5 weeks of culture, the fraction of cells expressing FcɛRI (Fig. 1C) was more than 95% and the staining of mast cell granule content by toluidine blue or alcian blue-safranin (data not shown) was comparable among Vav1−/−, Vav1+/−, and Vav1+/+ cells. A gene dose-dependent decrease in FcɛRI-induced tyrosine phosphorylation of Vav1 corresponded with the loss of Vav1 protein (Fig. 1D). To correlate the previous systemic anaphylaxis results with mast cell degranulation in vitro, BMMC degranulation was compared among the Vav1+/+, Vav1+/−, and Vav1−/− phenotypes (Fig. 2A). FcɛRI-mediated degranulation (indicated by β-hexosaminidase release) was significantly reduced in Vav1−/− cells compared to Vav1+/+ cells, although the total amount of β-hexosaminidase per cell did not differ among phenotypes. The most dramatic reduction (63%) was at suboptimal concentrations of Ag (3 ng/ml), where the response was linear, but even at optimal Ag concentrations a reduction of more than 30% was detected. This decrease was due, in part, to slower kinetics of release by Vav1−/− cells (Fig. 2B). Interestingly, stimulation with thapsigargin (Fig. 2A, inset) or ionomycin (data not shown) did not reconstitute the degranulation response in Vav−/− cells.
FIG. 2.
Vav1 deficiency affects mast cell degranulation and cytokine mRNA levels. (A) Degranulation (percentage of total β-hexosaminidase release) was measured following 30 min of stimulation with various doses of Ag among IgE-sensitized Vav+/+ (squares), Vav+/− (triangles), and Vav−/− (inverted triangles) BMMCs. Values represent an average of six independent dose response experiments per phenotype. Degranulation was also measured following 30 min of stimulation with 250 ng of thapsigargin (inset). (B) Cells of all three phenotypes (as described above) were stimulated with 9 ng of Ag for 0 to 30 min, and the percentage of total β-hexosaminidase release was determined. Values represent an average of four independent kinetic experiments for Vav+/+ and Vav−/− cells and three experiments for Vav+/− cells. For panels A and B, the single asterisks represent P ≤ 0.05 and the double asterisks represent P ≤ 0.01 for Vav+/+ cells compared to Vav−/− cells at that Ag concentration (A) or time point (B), as determined by t test. (C) The kinetics of BMMC cytokine mRNA levels among Vav+/+, Vav+/−, and Vav−/− cells were determined from unstimulated cells (0 h) or cells stimulated for up to 2 h with 1 ng of Ag. Cytokine mRNA levels were determined by RT-PCR as described in Materials and Methods. One representative of five experiments is shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MCP-1, monocyte chemoattractant protein 1; TNFα, tumor necrosis factor alpha.
Mast cell cytokine production is another important component of the mast cells' physiological role in health and disease (see reference 20 for a review). Since activated Vav1−/− splenic T cells have a reduced capacity to produce IL-2 (55), there was an interest in determining whether Vav1 regulates expression of similar genes in mast cells. The mRNA profile of a variety of cytokines was examined by RT-PCR following FcɛRI stimulation, as we previously demonstrated that cytokine mRNA levels correlate with cytokine production and secretion (48). While Vav1 deficiency did not appreciably alter the kinetics of mRNA expression, the changes observed in mRNA levels were gene dose dependent. IL-2 and IFN-γ were the most significantly affected; relative quantitation (8, 54) revealed an 85.4% ± 6.9% inhibition of IL-2 mRNA and a 60.4% ± 15.0% inhibition of IFN-γ mRNA in Vav1−/− cells compared to that in Vav1+/+ cells (Fig. 2C). IL-3, IL-4, IL-6, and tumor necrosis factor alpha mRNA levels were also decreased, though not to the extent of IL-2 and IFN-γ. In most experiments, IL-10 mRNA was increased while the chemokine monocyte chemoattractant protein 1 was only minimally affected in Vav1−/− cells (Fig. 2C). That Vav1 deficiency inhibited IL-2 and IFN-γ mRNA levels more dramatically than those of other cytokines suggested that Vav's influence on FcɛRI-mediated cytokine mRNA expression is rather selective.
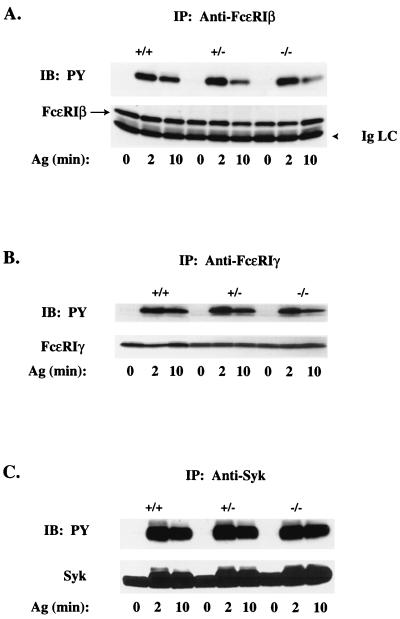
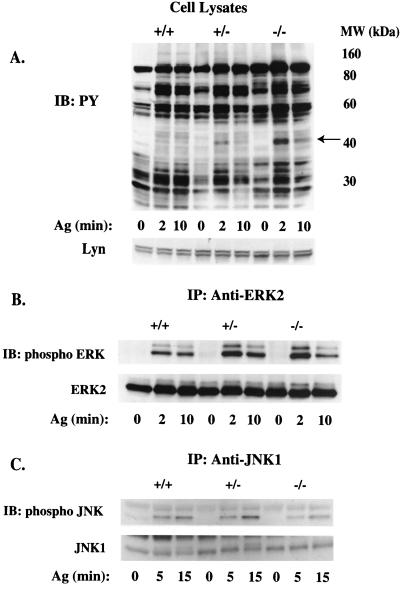
Vav's role in FcɛRI signaling was explored by analyzing the tyrosine phosphorylation of proteins from Vav1+/+, Vav1+/−, and Vav1−/− BMMCs following FcɛRI stimulation. For all proteins investigated, no change in the overall expression level was observed among the three phenotypes. Following FcɛRI stimulation, tyrosine phosphorylation of FcɛRI β and γ chains or of Syk was unaffected by Vav1 deficiency at 2 min (the peak time of receptor phosphorylation [Fig. 3]). However, in Vav1−/− cells, phosphorylation of FcɛRI β and γ chains was partly decreased (33.1% ± 7.7%) at 10 min poststimulation compared to that in Vav1+/+ cells, suggesting a more transient phosphorylation of FcɛRI in the absence of Vav1. No significant difference in FcɛRI phosphorylation was observed for Vav+/− cells, and Syk phosphorylation appeared to be identical for all three genotypes at the indicated time. The profile of FcɛRI-induced tyrosine-phosphorylated proteins for each phenotype demonstrated that the loss of Vav1 led to the increased tyrosine phosphorylation of a 42-kDa protein (Fig. 4A). Because this approximates the molecular mass of the mitogen-activated protein kinases ERK2 and JNK1, which are known to regulate gene expression (53), we investigated their FcɛRI-dependent activation. ERK2 phosphorylation was significantly increased (approximately twofold) in Vav1+/− and Vav1−/− cells compared to that in Vav1+/+ cells, suggesting that it was the 42-kDa protein that was detected in the previous antiphosphotyrosine experiment (Fig. 4B). In contrast, JNK1 phosphorylation was decreased by up to 53% (inhibition ranged from 29 to 53% at the peak phosphorylation time of 15 min) in the absence of Vav1 (Fig. 4C).
FIG. 3.
Tyrosine phosphorylation of the IgE receptor and Syk is Vav1 independent. Lysates were prepared from IgE-sensitized Vav+/+, Vav+/−, and Vav−/− BMMCs (30 × 106/ml) stimulated for 0, 2, or 10 min with 100 ng of Ag. FcɛRI β chain (A), FcɛRI γ chain (B), and Syk (C) were immunoprecipitated, resolved on sodium dodecyl sulfate-polyacrylamide gels, and transferred to nitrocellulose membranes. Tyrosine phosphorylation (PY) was determined by blotting with the antiphosphotyrosine Ab (upper panels). Individual protein levels were determined by reblotting with protein-specific Abs (lower panels). FcɛRI β protein is identified with an arrow to distinguish it from Ig light chain (IgLC). One representative of three experiments is shown. IP, immunoprecipitation; IB, immunoblotting.
FIG. 4.
Tyrosine phosphorylation of cell lysate proteins, ERK2, and JNK1 is differentially affected by Vav1 deficiency. Lysates were prepared from IgE-sensitized Vav+/+, Vav+/−, and Vav−/− BMMCs (30 × 106/ml) stimulated for 0, 2, or 10 min with 100 ng of Ag. Total cell lysates (A) for the three phenotypes were resolved on a sodium dodecyl sulfate-polyacrylamide gel and transferred to a nitrocellulose membrane. ERK2 (B) and JNK1 (C) were immunoprecipitated and resolved as described above. Tyrosine phosphorylation (PY) was determined by blotting with the antiphosphotyrosine Ab (upper panel, A) or with phosphospecific Abs to ERK2 and JNK (upper panels, B and C, respectively). Individual protein levels were determined by reblotting with protein-specific Abs (lower panels) for ERK2 and JNK1, while the cell lysates were probed for protein content with anti-Lyn Ab. One representative of a minimum of three experiments is shown. IB, immunoblotting; IP, immunoprecipitation.
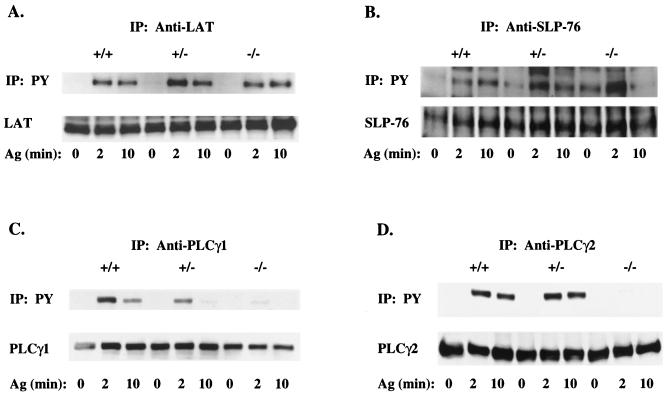
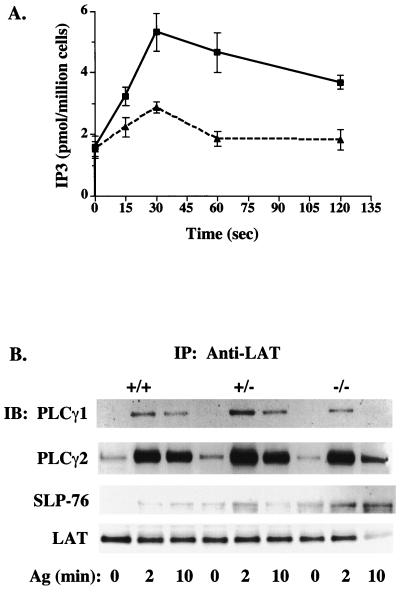
The inhibition of degranulation, cytokine production, and JNK1 activation by Vav1 deficiency suggested that Vav1 is likely to regulate an event common to all these responses. Therefore, because we previously demonstrated the presence of a Vav1-containing FcɛRI-induced LAT-organized signaling complex that is critical to mast cell responses (1, 48), we hypothesized that the defect should result from impaired complex formation and/or activation. LAT phosphorylation was unchanged in Vav1−/− cells (Fig. 5A). Because Vav and SLP-76 were previously shown to interact and synergize in the production of IL-2 in T cells (62), we examined the activation of SLP-76 as measured by its phosphorylation. SLP-76 phosphorylation was significantly enhanced in nonactivated and activated Vav1+/− and Vav1−/− cells compared to their wild-type counterpart (Fig. 5B). Because Syk kinase phosphorylates both Vav1 and SLP-76, the absence of Vav1 could allow more SLP-76 to interact with Syk, thus causing enhanced phosphorylation. Nevertheless, it is clear that the phosphorylation of LAT and SLP-76 occurs in the absence of Vav1. The tyrosine phosphorylation of PLCγ1 and PLCγ2 was also investigated, since PLCγ was previously demonstrated to associate with LAT (65). Tyrosine phosphorylation of PLCγ1 decreased in a gene dose-dependent manner with the loss of Vav1. Little or no detectable phosphorylated protein was evident 10 min following stimulation of Vav1−/− cells (Fig. 5C). Tyrosine phosphorylation of PLCγ2 was also affected, although differences were less apparent between Vav1+/+ and Vav1+/− cells, whereas a dramatic decrease was observed in Vav1−/− cells (Fig. 5D). This demonstrated that the absence of Vav1 protein caused a dramatic inhibition in the FcɛRI-induced tyrosine phosphorylation of both PLCγ1 and PLCγ2. Thus, as might be expected, PLCγ activity was significantly reduced, with peak IP3 levels inhibited by approximately 50% in the absence of Vav1 (Fig. 6A). To determine if the loss of PLCγ1 and PLCγ2 phosphorylation and activity resulted from a decreased association with LAT, we immunoprecipitated LAT from mast cells of all three phenotypes and immunoblotted for PLCγ1 and PLCγ2. Figure 6B demonstrates that PLCγ1, PLCγ2, and SLP-76 were recruited to LAT even in the absence of Vav1. The association was dependent on FcɛRI stimulation with no significant differences in association of PLCγ1 and PLCγ2 with LAT observed at the peak of phosphorylation (2 min). At 10 min poststimulation, all three experiments showed slightly less (10.2 to 33.5%) of PLCγ1 and PLCγ2 coimmunoprecipitating with LAT derived from Vav1−/− mast cells, suggesting a slightly more transient association of PLCγ1 and PLCγ2 with LAT in the absence of Vav1. Thus, Vav1 is not required for PLCγ1 and PLCγ2 interaction with LAT but is required for PLCγ tyrosine phosphorylation.
FIG. 5.
Tyrosine phosphorylation of LAT and SLP-76 is intact in Vav1-deficient mast cells, whereas tyrosine phosphorylation of PLCγ1 and PLCγ2 is inhibited. Lysates were prepared from IgE-sensitized Vav+/+, Vav+/−, and Vav−/− BMMCs (30 × 106/ml) stimulated for 0, 2, or 10 min with 100 ng of Ag. LAT (A), SLP-76 (B), PLCγ1 (C), and PLCγ2 (D) were immunoprecipitated, resolved on sodium dodecyl sulfate-polyacrylamide gels, and transferred to nitrocellulose membranes. Tyrosine phosphorylation (PY) was determined by blotting with the antiphosphotyrosine Ab (upper panels). Individual protein levels were determined by reblotting with protein-specific Abs (lower panels). A representative of a minimum of three experiments is shown. IP, immunoprecipitation.
FIG. 6.
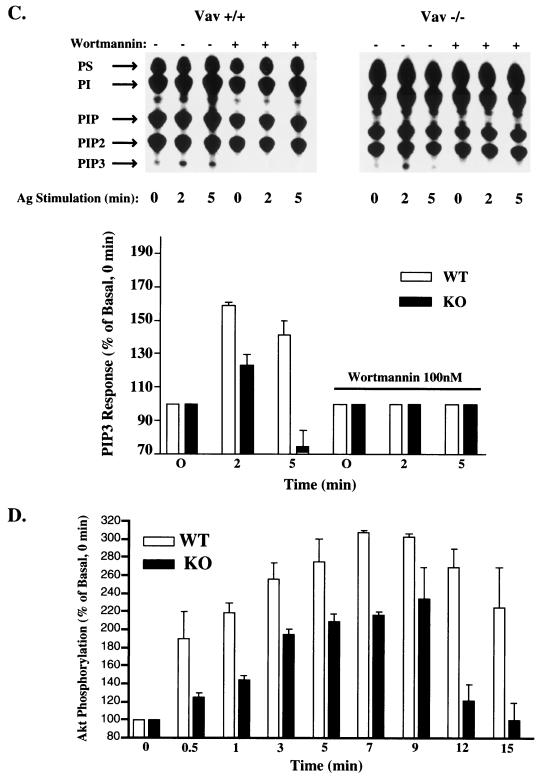
Vav1 deficiency inhibits IP3 and PIP3 production but does not affect the association of PLCγ1, PLCγ2, and SLP-76 with LAT. (A) IP3 levels from Vav1+/+ (squares) and Vav−/− (triangles) BMMCs were measured prior to and after stimulation with 100 ng of Ag. Samples were collected at the indicated times. (B) Lysates were prepared from IgE-sensitized Vav+/+, Vav+/−, and Vav−/− BMMCs (30 × 106/ml) stimulated for 0, 2, or 10 min with 100 ng of Ag. LAT was immunoprecipitated, resolved on a sodium dodecyl sulfate-polyacrylamide gel, and transferred to a nitrocellulose membrane. PLCγ and SLP-76 coimmunoprecipitating with LAT were determined by immunoblotting with Abs to PLCγ1, PLCγ2, and SLP-76. The amount of LAT was subsequently determined by reblotting with anti-LAT Abs (lower panel). One representative of three experiments is shown. IP, immunoprecipitation; IB, immunoblotting. (C) PIP3 levels in [32P]orthophosphate-labeled Vav+/+ or Vav−/− mast cells were measured prior to and following Ag stimulation (200 ng/ml) for the indicated time in the absence (−) or presence (+) of wortmannin. One representative experiment for TLC is shown. The TLC separation pattern shown was determined from a phosphoinositide standard of known composition. A prolonged exposure is shown to detect PIP3 signals. Results of the quantitation of all experiments are shown in the graph. PIP3 levels were normalized to nonstimulation conditions (where 0 min = 100%). PIP3 levels in the presence of wortmannin were arbitrarily set to the levels of nonstimulated cells (100%), as no signal was detectable. WT, wild type; KO, knockout; PS, phosphatidylserine; PI, phosphatidylinositol. (D) Akt activation is more transient in Vav−/− than in Vav+/+ mast cells. Cells were activated as described above, and Akt phosphorylation was measured from whole-cell lysates with an anti-Akt phosphospecific Ab. One representative of three experiments is shown. WT, wild type; KO, knockout.
Because phosphatidylinositol 3-kinase (PI3K) activity is required for membrane recruitment and activation of PLCγ as well as for calcium responses (3, 43), we determined the PI3K activity in Vav+/+ and Vav−/− mast cells by measuring the amount of PIP3 generated in response to FcɛRI. That Vav1 could have an effect on PI3K activity is supported by various studies, including the previously described interaction between these proteins (50). Measurement of PIP3 levels in Ag-stimulated cells, in the presence or absence of the PI3K inhibitor wortmannin, revealed a more transient production of PIP3 in the Vav1−/− mast cells than in Vav+/+ cells (Fig. 6C). At 5 min poststimulation, almost complete loss of PIP3, compared to that in Vav+/+ cells, was observed in Vav−/− cells (Fig. 6C). Because PI3K activity is required for the activation of protein kinase B/Akt (19), we measured the activation of Akt in Vav+/+ and Vav−/− cells by use of a phosphospecific Ab. Figure 6D shows that Akt activation in Vav−/− mast cells is more transient than that observed in Vav+/+ cells, thus confirming a role for Vav1 in the sustained activation of PI3K and Akt.
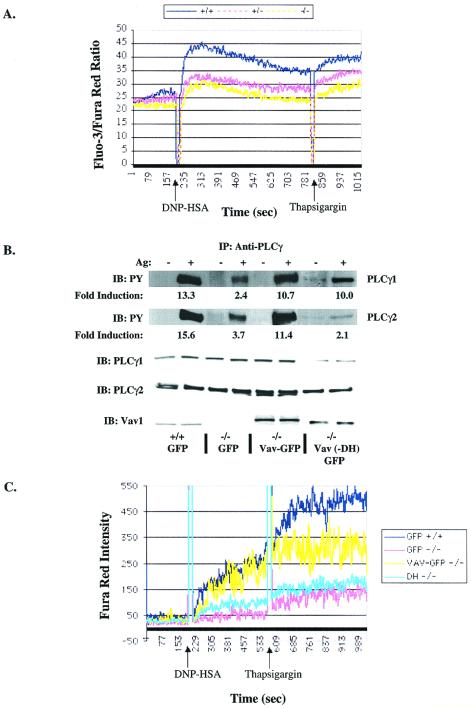
Activation of PLCγ was demonstrated to be critical for its function in generating the second messenger IP3 (39), which regulates calcium mobilization; thus, we next investigated calcium responses in mast cells. This line of investigation was supported by previous reports of reduced calcium mobilization in Vav1−/− T cells and B cells (18, 57). Calcium mobilization was monitored following stimulation with Ag, and a gene dose-dependent decrease in calcium mobilization was found, with the lowest calcium response being observed for Vav1−/− cells (Fig. 7A). Interestingly, subsequent stimulation with thapsigargin also showed lowered calcium responses from Vav1−/− cells. Consistent with a decrease in IP3 production, the decreased mobilization of calcium also correlated with the reduced degranulation following Ag or thapsigargin stimulation of Vav1−/− BMMCs (Fig. 2A). Depletion of extracellular calcium and addition of EGTA to the medium did not cause a change in the observed differences of the calcium profiles of all three phenotypes, although the extent of the response for each phenotype was reduced (data not shown). This suggested that the mobilization of calcium from intracellular stores was reduced in the absence of Vav1, consistent with the observed defect in PLCγ activation.
FIG. 7.
Calcium mobilization and tyrosine phosphorylation of PLCγ1 and PLCγ2 are Vav1 dependent. (A) IgE-sensitized Vav1+/+ (blue), Vav+/− (red), and Vav−/− (yellow) cells (2 × 106) in Tyrodes–0.05% BSA were loaded with Fluo-3-AM and Fura Red AM for determination of calcium mobilization by flow cytometry. Cells were stimulated with 30 ng of Ag followed by 1 μM thapsigargin (as indicated). Data shown are ratios of Fluo-3 and Fura Red dye fluorescence measured over time. One representative of four experiments is shown. (B and C) For reconstitution of PLCγ phosphorylation (B) and the calcium response (C), cells were infected with a GFP-expressing viral vector or one containing Vav1-GFP and loaded with Fura Red AM only (C). (B) Lysates were prepared from Vav1+/+ BMMCs expressing GFP (GFP; lanes 1 and 2), Vav1−/− BMMCs expressing GFP (GFP; lanes 3 and 4), Vav1−/− BMMCs expressing Vav1-GFP (lanes 5 and 6), or Vav1−/− BMMCs expressing Vav1-GFP with the Dbl domain deleted (−DH) (lanes 7 and 8) following 0 or 2 min of stimulation with 100 ng of Ag. PLCγ1 and PLCγ2 were immunoprecipitated, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. Tyrosine phosphorylation (PY) was determined by blotting with the antiphosphotyrosine Ab (upper panels). Individual protein levels were determined by reblotting with protein-specific Abs (lower panels). One representative experiment is shown. The fold induction was determined by densitometric analysis of a minimum of three experiments, and the intensity of the phosphotyrosine signal was normalized to the amount of immunoprecipitated protein. Standard deviations ranged from 7 to 22% of the values shown. IP, immunoprecipitation; IB, immunoblotting. (C) Infected BMMCs were identified by GFP fluorescence. The calcium response of Vav1+/+ cells expressing GFP (blue), Vav1−/− cells expressing GFP (pink), Vav1−/− cells expressing −DH Vav1-GFP (teal), or Vav1−/− cells expressing Vav1-GFP (yellow) was assessed following stimulation as described above. Data are presented as Fura Red intensities for GFP+ cells over time.
We tested the postulate that reconstitution of Vav1 in Vav1−/− cells would lead to reconstitution of the tyrosine phosphorylation and activation of PLCγ1 and PLCγ2. We reasoned that Vav could contribute to the tyrosine phosphorylation of PLCγ by facilitating the interaction of a tyrosine kinase with PLCγ in this complex, as Syk and other tyrosine kinases (16, 35) interact with Vav1. Since activation of PLCγ1 and PLCγ2 is required for normal mast cell calcium responses (40, 48, 59), we also measured calcium responses following reconstitution with Vav1. We introduced Vav1 with an enhanced GFP tag (Vav1-GFP) or the GFP vector alone (as a control) into Vav+/+ or Vav1−/− cells using a previously described Semliki Forest virus vector (2). Ag stimulation of GFP-expressing Vav+/+ cells showed an increase of 13.3- and 15.6-fold in the tyrosine phosphorylation of PLCγ1 and PLCγ2, respectively. Expression of GFP alone in Vav1−/− cells followed by Ag stimulation showed some increased tyrosine phosphorylation of PLCγ1 and PLCγ2 (2.4- and 3.7-fold increase, respectively) compared to that for nonstimulated conditions. However, when PLCγ1 and PLCγ2 were immunoprecipitated from Vav-GFP-reconstituted Vav1−/− cells, a dramatic increase in the FcɛRI-stimulated tyrosine phosphorylation of PLCγ1 and PLCγ2 (10.7- and 11.4-fold increase, respectively) was detected (Fig. 7B). This demonstrates that Vav1 is critical to the tyrosine phosphorylation of PLCγ1 and PLCγ2. To further address the underlying mechanism, we asked if the GEF activity of Vav1 is required for tyrosine phosphorylation of PLCγ1 and PLCγ2. Vav−/− mast cells were reconstituted with a Vav-GFP construct in which only the DH domain (K194 to I405) was deleted (−DH). Immunoprecipitation of PLCγ1 and PLCγ2 from nonstimulated and stimulated cells, in three individual experiments, revealed that tyrosine phosphorylation of PLCγ1 is relatively independent of the GEF activity of Vav1 (average 10.0-fold increase in three experiments) whereas that of PLCγ2 (average 2.1-fold increase in three experiments) is dependent (Fig. 7B). Thus, the results show that Vav1 regulates the tyrosine phosphorylation of PLCγ1 and PLCγ2 differentially.
Because GFP fluoresces at the same wavelength as does Fluo-3, for calcium studies, we preloaded cells with Fura Red alone as the indicator for increased free calcium. Thus, the results are expressed as an increase in intensity of Fura Red fluorescence rather than the fluorescence ratio of two dyes (Fig. 7A versus C). Following reconstitution with Vav1-GFP, the ability of the infected (GFP+) cells to mobilize calcium upon FcɛRI activation was examined. As expected, and comparable to dual dye experiments, Vav1−/− (GFP+) cells showed a significantly diminished calcium response compared to that of Vav1+/+ cells (Fig. 7C). Additionally, a test of whether the GEF activity of Vav1 is required for the reconstitution of the calcium response revealed that the absence of this activity results in a defective calcium response identical to that in Vav1−/− (GFP+) cells. However, when the Vav1−/− BMMCs were reconstituted with Vav1-GFP, the calcium mobilization following Ag stimulation was comparable to that in GFP-infected wild-type mast cells (Fig. 7C). Unlike the Vav1−/− (GFP+) or Vav (DH−)-GFP+ reconstituted cells, reconstitution of Vav1-GFP in Vav−/− mast cells reconstituted the thapsigargin-induced calcium response. However, although all experiments showed reconstitution of the calcium response activated by thapsigargin, the level of the reconstituted response was more variable than that seen with Ag stimulation. Preliminary experiments also showed that, in the absence of extracellular calcium, and in the presence of EGTA, Vav1 reconstitution of the calcium response still occurred (data not shown) consistent with reconstitution of release from intracellular stores. Collectively, these data demonstrate that decreased PLCγ activation and calcium mobilization are reversed by the introduction of competent Vav1.
DISCUSSION
The present study shows that Vav1 deficiency in mast cells results in a well-defined phenotype. We found no obvious role for Vav1 in the development of mast cells. Activation of FcɛRI-proximal PTKs was also intact, as demonstrated by normal levels of β and γ chains and Syk tyrosine phosphorylation. Most signals downstream of Syk PTK activation were also intact, in the absence of Vav1, as the tyrosine phosphorylation of LAT was unaffected and tyrosine phosphorylation of SLP-76 and ERK2 was enhanced. Signaling deficiencies were observed for molecules that regulate calcium responses or are regulated by intracellular calcium; thus, tyrosine phosphorylation of PLCγ1, PLCγ2, and JNK1 was defective.
Vav1 was reported to activate JNK and gene expression in mast cells (52, 56). Because JNK activity was implicated in the regulation of IL-2 mRNA stability and expression (9), our observation of a dramatic loss of IL-2 mRNA is consistent with the observed partial loss of JNK1 activity. The loss of calcium responses is also a likely explanation for reduced IL-2 responses, as calcium is required for synergy of calcineurin and PKCθ in activation of JNK1 and IL-2 production (60). That Vav1 deficiency mainly affected IL-2 and IFN-γ was also not surprising. IL-2 and IFN-γ expression are regulated by NF-AT, whose activation is exquisitely sensitive to calcium (7, 58), and Vav1 regulates the activation of NF-AT in T cells (61). In contrast, an increased IL-10 mRNA level was observed in Vav1-deficient cells. This correlated with increased phosphorylation of ERK2 and SLP-76; however, a causal relationship remains to be established.
Genetic studies of mast cell function have revealed overlapping phenotypes in the absence of LAT (48), SLP-76 (40), PLCγ2 (59), and now Vav1 expression. As all of these proteins are linked by participation in a macromolecular signaling complex (1), it is not surprising that the absence of one may impinge on the function of others. A theme common to all is the regulation of calcium responses. Little is known of the phosphorylation status of the other signaling proteins in the absence of PLCγ1 (27) or PLCγ2 (59), although, for the former, activation of ERK2 was found to be prolonged in murine embryonic fibroblasts (26), consistent with our observation of enhanced ERK2 activation in the absence of Vav1. However, it should be noted that an opposite effect has been reported previously for PLCγ2-deficient B cells (22), thus suggesting cell-specific differences in the regulation of ERK2. In Vav1-deficient mast cells, phosphorylation of LAT and SLP-76 was not adversely affected, whereas phosphorylation of PLCγ1 and PLCγ2 was inhibited. In SLP-76-deficient mast cells, tyrosine phosphorylation of PLCγ1 and Vav1 was inhibited; however, the state of LAT phosphorylation in these cells was not reported (40). In LAT-deficient mast cells (48), phosphorylation of Vav1 was normal, whereas that of SLP-76, PLCγ1, and PLCγ2 was inhibited, and calcium responses were also decreased. Thus, it appears that failure to activate Vav1 and/or to target activated Vav1 to a LAT-organized signaling complex (1), where it activates Rac1, contributes to stable activation of PI3K, and promotes phosphorylation and activation of PLCγ1 and PLCγ2, is a reasonable explanation for the calcium response defect in LAT-, SLP-76-, or Vav1-deficient cells (40, 48). Our present findings strongly support this premise, because, in the absence of Vav1, association of PLCγ1, PLCγ2, and SLP-76 with LAT was not significantly altered. Thus, all components (with the exception of Vav1) shown to regulate calcium responses localized appropriately, but Vav−/− cells showed transient PI3K activation, loss of PLCγ1 and PLCγ2 phosphorylation, reduced IP3 levels, and diminished calcium responses. Furthermore, rescue of the defective tyrosine phosphorylation of both PLCγ isoforms and calcium responses was achieved by expression of Vav1. Thus, we conclude that Vav1 association with a LAT-organized complex regulates the activity of PLCγ1 and PLCγ2 in membranes.
Interestingly, in CD3/CD28-stimulated Vav−/− T cells PLCγ1 phosphorylation appeared normal, although IP3 calcium responses were reduced (13). The diminished IP3 response suggests that activation or function of PLCγ is defective in Vav1-deficient T cells; thus, we have no clear explanation for the seemingly normal phosphorylation of Vav1 in T cells. Nevertheless, it is possible that Vav1 functions differently in T cells than in mast cells or that it regulates PLCγ activity by other means in these cells. Our data show that Vav1 contributes to prolonged PI3K activation and thus to the presence of the PI3K product, PIP3, which regulates the membrane recruitment and activity of PLCγ (43). Thus, the absence of Vav1 could inhibit calcium mobilization by affecting the activity of PI3K and thus the recruitment or, more likely (based on our findings of normal association with LAT), the activation of PLCγ, consistent with the previously described role of PI3K in calcium responses (3). Our finding that thapsigargin did not reconstitute calcium responses in Vav1-deficient mast cells also supports a connection between Vav1 and PI3K, as the thapsigargin-induced calcium response was previously demonstrated to be PI3K dependent in mast cells (11, 25). Interestingly, the GEF activity of Vav1 was found to be critical for Vav1-mediated calcium responses and for the tyrosine phosphorylation of PLCγ2 but less essential for PLCγ1 phosphorylation. Therefore, because wild-type Vav1 reconstitutes the phosphorylation of both PLCγ isoforms, our findings support the view that Vav1 has both GEF-dependent and -independent functions (33) and suggest that both functions may contribute to the calcium response. Because of Vav's multiple protein-protein interaction domains, it is not surprising that Vav1 may facilitate the juxtaposition of a (as yet unidentified) tyrosine kinase with its substrate, namely, PLCγ1, resulting in its phosphorylation independent of the Vav1 GEF activity. Moreover, preliminary experiments support a role for Vav's adapter function in the regulation of the calcium response, as Vav−/− mast cells reconstituted with an SH2 domain-mutated Vav1 (21) showed a dramatic delay in calcium responses (data not shown). Nevertheless, our results show that the apparently normal phosphorylation of PLCγ1 alone is not sufficient for the calcium response and that the GEF activity of Vav1 is required. In fact, recent studies demonstrate that the Rho family members Rac1 and Cdc42 serve as regulators of mast cell degranulation by activating the IP3-calcium pathway (23). Additionally, these small GTPases can reconstitute calcium responses and degranulation in a response-deficient RBL mast cell line (24). Since Vav1 is an upstream effector controlling Rac1 and possibly Cdc42 activation (15, 38), and these small GTPases are important in PI3K activation (30), Vav1's adapter and GEF activities appear to function synergistically to regulate PLCγ1 and PLCγ2 activity and calcium responses.
The prominent phenotype of Vav1-deficient mice is a defect in the development of T cells and the B1 B-cell subset. The present study of normally developing mast cells provides the suggestion that Vav-1-mediated PLCγ activation may be involved in ontogeny of T cells and of certain B cells. This is supported by the recent observation that PLCγ2-deficient mice demonstrated a selective absence of the B1 B-cell subset (59) akin to the defect seen in Vav1−/− mice (63). However, PLCγ2-deficient mice showed normal T-cell development; thus, PLCγ2 does not appear to play a critical role in T-cell development (59). Whether PLCγ1 activation plays a role in T-cell development remains to be determined, but PLCγ1 has been demonstrated elsewhere to play an important role in mammalian growth and development, as homozygous deletion of this gene results in embryonic lethality (28). It is clear, however, that development of different cell types is variably dependent on activation of PLCγ and calcium responses (28, 49, 59).
Our findings demonstrate Vav1's ability to activate PLCγ by phosphorylation- and GEF-dependent pathways as critical for normal calcium responses in mast cells. Vav1 is selectively expressed in hematopoietic cells, but other homologs are ubiquitously expressed and can serve as GEFs for members of the Rho family of GTPases (6). Thus, beyond their role as GEFs in the remodeling of the cell's cytoskeleton, it remains to be determined whether all members of this GEF family function in the regulation of calcium signals and whether the mechanisms involved are common to all members.
ACKNOWLEDGMENT
This work was partly supported by a Pan American Fellowship award (CONACYT/NIH) to C.G.-E.
REFERENCES
- 1.Arudchandran R, Brown M J, Peirce M J, Song J S, Zhang J, Siraganian R P, Blank U, Rivera J. The Src homology 2 domain of Vav is required for its compartmentation to the plasma membrane and activation of c-jun NH(2)-terminal kinase 1. J Exp Med. 2000;191:47–60. doi: 10.1084/jem.191.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arudchandran R, Brown M J, Song J S, Wank S A, Haleem-Smith H, Rivera J. Polyethylene glycol-mediated infection of non-permissive mammalian cells with Semliki Forest virus: application to signal transduction studies. J Immunol Methods. 1999;222:197–208. doi: 10.1016/s0022-1759(98)00161-6. [DOI] [PubMed] [Google Scholar]
- 3.Barker S A, Caldwell K K, Hall A, Martinez A M, Pfeiffer J R, Oliver J M, Wilson B S. Wortmannin blocks lipid and protein kinase activities associated with PI 3-kinase and inhibits a subset of responses induced by FcɛRI cross-linking. Mol Biol Cell. 1995;6:1145–1158. doi: 10.1091/mbc.6.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benhamou M, Ryba N J P, Kihara H, Nishikata H, Siraganian R P. Protein-tyrosine kinase p72syk in high affinity receptor signaling. Identification as a component of pp72 and association with the receptor γ chain after receptor aggregation. J Biol Chem. 1993;268:23318–23324. [PubMed] [Google Scholar]
- 5.Brown A M, O'Sullivan A J, Gomperts B D. Induction of exocytosis from permeabilized mast cells by the guanosine triphosphatases Rac and Cdc42. Mol Biol Cell. 1998;9:1053–1063. doi: 10.1091/mbc.9.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustelo X R. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell P M, Pimm J, Ramassar V, Halloran P F. Identification of a calcium-inducible, cyclosporine sensitive element in the IFN-gamma promoter that is a potential NFAT binding site. Transplantation. 1996;61:933–939. doi: 10.1097/00007890-199603270-00016. [DOI] [PubMed] [Google Scholar]
- 8.Chang E-Y, Szallasi Z, Acs P, Raizada V, Wolfe P C, Fewtrell C, Blumberg P M, Rivera J. Functional effects of overexpression of protein kinase C-α, -β, -δ, -ɛ, and -η in the mast cell line RBL-2H3. J Immunol. 1997;159:2624–2632. [PubMed] [Google Scholar]
- 9.Chen C-Y, Del Gatto-Konczak F, Wu Z, Karin M. Stabilization of interleukin-2 mRNA by the c-jun NH2-terminal kinase pathway. Science. 1998;280:1945–1949. doi: 10.1126/science.280.5371.1945. [DOI] [PubMed] [Google Scholar]
- 10.Choi O H, Kim J H, Kinet J P. Calcium mobilization via sphingosine kinase in signalling by the FcɛRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 11.Cissel D S, Fraundorfer P F, Beaven M A. Thapsigargin-induced secretion is dependent on activation of a cholera toxin-sensitive and phosphatidylinositol-3-kinase-regulated phospholipase D in a mast cell line. J Pharmacol Exp Ther. 1998;285:110–118. [PubMed] [Google Scholar]
- 12.Clements J L, Yang B, Ross-Barta S E, Eliason S L, Hrstka R F, Williamson R A, Koretzky G A. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 13.Costello P S, Walters A E, Mee P J, Turner M, Reynolds L F, Prisco A, Sarner N, Zamoyska R, Tybulewicz V L J. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-kappaB pathways. Proc Natl Acad Sci USA. 1999;96:3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crespo P, Bustelo X R, Aaronson D S, Coso O A, Lopez-Barahona M, Barbacid M, Gutkind J S. Rac-1 dependent stimulation of the JNK/SAPK signaling pathway by Vav. Oncogene. 1996;13:455–460. [PubMed] [Google Scholar]
- 15.Crespo P, Schuebel K E, Ostrom A A, Gutkind J S, Bustelo X R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 16.Deckert M, Tartare-Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- 17.Eiseman E, Bolen J B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 18.Fischer K-D, Zmuidzinas A, Gardner S, Barbacid M, Bernstein A, Guidos C. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+ CD8+ thymocytes. Nature. 1995;374:474–477. doi: 10.1038/374474a0. [DOI] [PubMed] [Google Scholar]
- 19.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 20.Galli S J, Gordon J R, Wershil B K. Cytokine production by mast cells and basophils. Curr Opin Immunol. 1991;3:865–872. doi: 10.1016/s0952-7915(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 21.Gulbins E, Coggeshall K M, Baier G, Katsav S, Burn P, Altman A. Tyrosine kinase-stimulated guanine nucleotide exchange activity of Vav in T cell activation. Science. 1993;260:822–825. doi: 10.1126/science.8484124. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto A, Okada H, Jiang A, Kurosaki M, Greenberg S, Clark E A, Kurosaki T. Involvement of guanosine triphosphatases and phospholipase C-gamma2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J Exp Med. 1998;188:1287–1295. doi: 10.1084/jem.188.7.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong-Geller E, Cerione R A. Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP(3)/calcium pathway in RBL-2H3 cells. J Cell Biol. 2000;148:481–494. doi: 10.1083/jcb.148.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong-Geller E, Holowka D, Siraganian R P, Baird B, Cerione R A. Activated Cdc42/Rac reconstitutes FcɛRI-mediated Ca2+ mobilization and degranulation in mutant RBL mast cells. Proc Natl Acad Sci USA. 2001;98:1154–1159. doi: 10.1073/pnas.98.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber M, Hughes M R, Krystal G. Thapsigargin-induced degranulation of mast cells is dependent on transient activation of phosphatidylinositol-3 kinase. J Immunol. 2000;165:124–133. doi: 10.4049/jimmunol.165.1.124. [DOI] [PubMed] [Google Scholar]
- 26.Ji Q S, Carpenter G. Role of basal calcium in the EGF activation of MAP kinases. Oncogene. 2000;19:1853–1856. doi: 10.1038/sj.onc.1203517. [DOI] [PubMed] [Google Scholar]
- 27.Ji Q S, Ermini S, Baulida J, Sun F L, Carpenter G. Epidermal growth factor signaling and mitogenesis in Plcγ1 null mouse embryonic fibroblasts. Mol Biol Cell. 1997;9:749–757. doi: 10.1091/mbc.9.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji Q S, Winnier G E, Niswender K D, Horstman D, Wisdom R, Magnuson M A, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development. Proc Natl Acad Sci USA. 1997;94:2999–3003. doi: 10.1073/pnas.94.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keegan A D, Paul W E. Multichain immune recognition receptors: similarities in structure and signaling pathways. Immunol Today. 1992;13:63–68. doi: 10.1016/0167-5699(92)90136-U. [DOI] [PubMed] [Google Scholar]
- 30.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 31.Kihara H, Siraganian R P. Src homology 2 domains of Syk and Lyn bind to tyrosine-phosphorylated subunits of the high affinity IgE receptor. J Biol Chem. 1994;269:22427–22432. [PubMed] [Google Scholar]
- 32.Kinet J P. The high-affinity IgE receptor (FcɛRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 33.Kuhne R M, Ku G, Weiss A. A guanine nucleotide exchange factor-independent function of Vav1 in transcriptional activation. J Biol Chem. 2000;275:2185–2190. doi: 10.1074/jbc.275.3.2185. [DOI] [PubMed] [Google Scholar]
- 34.Liu F T, Bohn J W, Ferry E L, Yamamoto H, Molinaro C A, Sherman L A, Klinman N R, Katz D H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980;124:2728–2737. [PubMed] [Google Scholar]
- 35.Michel F, Grimaud L, Tuosto L, Acuto O. Fyn and ZAP-70 are required for Vav phosphorylation in T cells stimulated by antigen-presenting cells. J Biol Chem. 1998;273:31932–31938. doi: 10.1074/jbc.273.48.31932. [DOI] [PubMed] [Google Scholar]
- 36.Moriya K, Rivera J, Odom S, Sakuma Y, Muramato K, Yoshiuchi T, Miyamoto M, Yamada K. ER-27319, a novel acridone-related compound, inhibits release of antigen-induced allergic mediators from mast cells by selective inhibition of FcɛRI-mediated activation of Syk. Proc Natl Acad Sci USA. 1997;94:12539–12544. doi: 10.1073/pnas.94.23.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nimnual A S, Yatsula B A, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 38.Olson F M, Pasteris N G, Gorski J L, Hall A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- 39.Park D J, Min H K, Rhee S G. IgE-induced tyrosine phosphorylation of phospholipase C-gamma1 in rat basophilic leukemia cells. J Biol Chem. 1991;266:24237–24240. [PubMed] [Google Scholar]
- 40.Pivniouk V I, Martin T R, Lu-Kuo J M, Katz H R, Oettgen H C, Geha R S. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J Clin Investig. 1999;103:1737–1743. [PMC free article] [PubMed] [Google Scholar]
- 41.Pribluda V S, Pribluda C, Metzger H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc Natl Acad Sci USA. 1994;91:11246–11250. doi: 10.1073/pnas.91.23.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ptasznik A, Prossnitz E R, Yoshikawa D, Smrcka A, Traynor-Kaplan A E, Bokoch G M. A tyrosine kinase signaling pathway accounts for the majority of phosphatidylinositol 3,4,5-trisphosphate formation in chemoattractant-stimulated human neutrophils. J Biol Chem. 1996;271:25204–25207. doi: 10.1074/jbc.271.41.25204. [DOI] [PubMed] [Google Scholar]
- 43.Rameh L E, Rhee S G, Spokes K, Kazlauskas A, Cantley L C, Cantley L G. Phosphoinositide 3-kinase regulates phospholipase Cgamma-mediated calcium signaling. J Biol Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- 44.Razin E, Mencia-Huerta J M, Stevens R L, Lewis R A, Liu F T, Corey E, Austen K F. IgE-mediated release of leukotriene C4, chondroitin sulfate E proteogylcan, beta-hexosaminidase, and histamine from cultured bone marrow-derived mouse mast cells. J Exp Med. 1983;157:189–201. doi: 10.1084/jem.157.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 46.Rivera J, Kinet J-P, Kim J, Pucillo C, Metzger H. Studies with a monoclonal antibody to the beta subunit of the receptor with high affinity for immunoglobulin E. Mol Immunol. 1988;25:647–661. doi: 10.1016/0161-5890(88)90100-9. [DOI] [PubMed] [Google Scholar]
- 47.Romero F, Fischer S. Structure and function of vav. Cell Signal. 1996;8:545–553. doi: 10.1016/s0898-6568(96)00118-0. [DOI] [PubMed] [Google Scholar]
- 48.Saitoh S, Arudchandran R, Manetz T S, Zhang W, Sommers C L, Love P E, Rivera J, Samelson L E. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- 49.Shearer J, De Nadai C, Emily-Fenouil F, Gache C, Whitaker M, Ciapa B. Role of phospholipase C gamma at fertilization and during mitosis in sea urchin eggs and embryos. Development. 1999;126:2273–2284. doi: 10.1242/dev.126.10.2273. [DOI] [PubMed] [Google Scholar]
- 50.Shigematsu H, Iwasaki H, Otsuka T, Ohno Y, Arima F, Niho Y. Role of the vav proto-oncogene product (Vav) in erythropoietin-mediated cell proliferation and phosphatidylinositol 3-kinase activity. J Biol Chem. 1997;272:14334–14340. doi: 10.1074/jbc.272.22.14334. [DOI] [PubMed] [Google Scholar]
- 51.Song J S, Gomez J, Stancato L F, Rivera J. Association of a p95 Vav-containing signaling complex with the FcɛRI gamma chain in the RBL-2H3 mast cell line—evidence for a constitutive in vivo association of Vav with Grb2, Raf-1, and ERK2 in an active complex. J Biol Chem. 1996;271:26962–26970. doi: 10.1074/jbc.271.43.26962. [DOI] [PubMed] [Google Scholar]
- 52.Song J S, Haleem-Smith H, Arudchandran R, Gomez J, Scott P M, Mill J F, Tan T H, Rivera J. Tyrosine phosphorylation of Vav stimulates IL-6 production in mast cells by a Rac/c-Jun N-terminal kinase-dependent pathway. J Immunol. 1999;163:802–810. [PubMed] [Google Scholar]
- 53.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 54.Swann P G, Odom S, Zhou Y-J, Szallasi Z, Blumberg P M, Draber P, Rivera J. Requirement for a negative charge at threonine 60 of the FcRγ for complete activation of Syk. J Biol Chem. 1999;274:23068–23077. doi: 10.1074/jbc.274.33.23068. [DOI] [PubMed] [Google Scholar]
- 55.Tarakhovsky A, Turner M, Schaal S, Mee P J, Duddy L P, Rajewsky K, Tybulewicz V L J. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 1995;374:467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 56.Teramoto H, Salem P, Robbins K C, Bustelo X R, Gutkind J S. Tyrosine phosphorylation of the vav proto-oncogene product links FcɛRI to the Rac1-JNK pathway. J Biol Chem. 1997;272:10751–10755. doi: 10.1074/jbc.272.16.10751. [DOI] [PubMed] [Google Scholar]
- 57.Turner M, Mee J, Walters A E, Quinn M E, Mellor A L, Zamoyska R, Tybulewicz V L J. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–460. doi: 10.1016/s1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- 58.Viola J P, Rao A. Role of the cyclosporine-sensitive transcription factor NFAT1 in the allergic response. Mem Inst Oswaldo Cruz. 1997;92(Suppl. 2):147–155. doi: 10.1590/s0074-02761997000800020. [DOI] [PubMed] [Google Scholar]
- 59.Wang D, Feng J, Wen R, Marine J C, Sangster M Y, Parganas E, Hoffmeyer A, Jackson C W, Cleveland J L, Murray P J, Ihle J N. Phospholipase Cγ2 is essential in the functions of B cells and several Fc receptors. Immunity. 2000;13:25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 60.Werlen G, Jacinto E, Xia Y, Karin M. Calcineurin preferentially synergizes with PKC-θ to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 1998;17:3101–3111. doi: 10.1093/emboj/17.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu J, Katzav S, Weiss A. A functional T-cell receptor signaling pathway is required for p95vav activity. Mol Cell Biol. 1995;15:4337–4346. doi: 10.1128/mcb.15.8.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu J, Motto D G, Koretsky G A, Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- 63.Zhang R, Alt F W, Davidson L, Orkin S H, Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 1995;374:470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W, Sommers C L, Burshtyn D N, Stebbins C C, DeJarnette J B, Trible R P, Grinberg A, Tsay H C, Jacobs H M, Kessler C M, Long E O, Love P E, Samelson L E. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 65.Zhang W, Trible R P, Zhu M, Liu S K, McGlade C J, Samelson L E. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J Biol Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 66.Zmuidzinas A, Fischer K-D, Lira S A, Forrester L, Bryant S, Bernstein A, Barbacid M. The Vav proto-oncogene is required early in embryogenesis but not for hematopoietic development in vitro. EMBO J. 1995;14:1–11. doi: 10.1002/j.1460-2075.1995.tb06969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]