Abstract
Cancer cells acquire a diverse range of metabolic adaptations that support their enhanced rates of growth and proliferation. While these adaptations help tune metabolism to support higher anabolic output and bolster antioxidant defenses, they can also decrease metabolic flexibility and increase dependence on nutrient uptake versus de novo synthesis. Diet is the major source of nutrients that ultimately support tumor growth, yet the potential impact of diet is currently underutilized during the treatment of cancer. Here, we review several forms of dietary augmentation therapy including those that alter the content of food, such as energy or macronutrient restriction, and those that alter the timing of food consumption, like intermittent fasting regimens. We discuss how these dietary strategies can be combined with pharmacologic therapies to exaggerate the metabolic liabilities of different cancer types.
Cancer Metabolism
Cancer cells can acquire a diverse range of metabolic adaptations that support their enhanced rates of growth and proliferation, and changes to cellular metabolism are recognized as a key hallmark of cancer 1. These metabolic changes are driven directly and indirectly by gene mutations which activate a variety of cellular metabolic pathways. While these adaptations help tune metabolism to support higher anabolic output and bolster antioxidant defenses, they can also decrease metabolic flexibility and increase dependence on nutrient uptake versus de novo synthesis. Cancer cells consume a wide range of nutrients, including sugars, amino acids and fatty acids. An adequate supply of exogenous nutrients is critical to provide for energy production, macromolecule precursors – both of which support anabolism – and the ability to combat stresses such as reactive oxygen species. Diet is the major source of nutrients that ultimately support tumor growth 2,3, yet dietary interventions are currently underutilized during the treatment of cancer.
Dietary Augmentation Therapy
Estimates suggest that 10–35% of cancer initiation can be attributed to diet 4,5. There are three primary ways in which diet promotes tumor development and progression. (1) Diet may introduce nutrients like sugars and lipids that can be used by tumor cells for anabolic growth 6. (2) Diet may strongly impact systemic signaling factors like leptin, insulin, insulin-like growth factor 1 (IGF1), and estradiol, which stimulate tumor cell growth and survival 7. (3) Diet may predispose to obesity, which creates a milieu that favors tumor initiation and growth. All three mechanisms are innately intertwined so dietary strategies that focus on one approach may broadly benefit the host.
The manipulation of foods in order to exaggerate the metabolic liabilities of cancer cells is called dietary augmentation therapy. Currently, several forms exist. Some focus on content, such as energy or macronutrient restriction (e.g. calorie restriction or low carbohydrate diets), while others are defined by timing, like intermittent fasting regimens that involve intervals of complete or partial energy restriction, regardless of meal composition. There are even hybrid models that alter both content and timing by delivering protein- and carbohydrate-restricted meals in-between periods of fasting 8. Most of these interventions impose a caloric deficiency, which induces a standard physiologic response including weight loss, a reduction in leptin, fatty acid mobilization, and reduced blood glucose variability. Other dietary approaches impact metabolism and systemic hormone levels without changing weight. Ultimately, the best approach depends on the biology of the host and metabolism of the tumor.
Modulating Dietary Content
Calorie-restriction (CR) is the most commonly used dietary augmentation strategy. CR reduces the total daily energy intake while maintaining a well-balanced macronutrient ratio. In a prospective, randomized, controlled trial of healthy, sedentary men and women (n=48), a 6 month exposure to a 25% CR diet reduced weight, fasting insulin, core temperature, and total energy expenditure 9 without affecting the somatotropic axis (GH and IGF1) 10. These metabolic benefits persist through 2-years of intervention 11, and similar results have been observed in trials where CR is added to additional lifestyle interventions in obese subjects with pre-existing metabolic dysfunction (~40% of the adult population in Western countries) 12,13. The effects of CR in subjects with cancer has not been robustly studied because of concerns regarding cachexia, quality of life, and compliance.
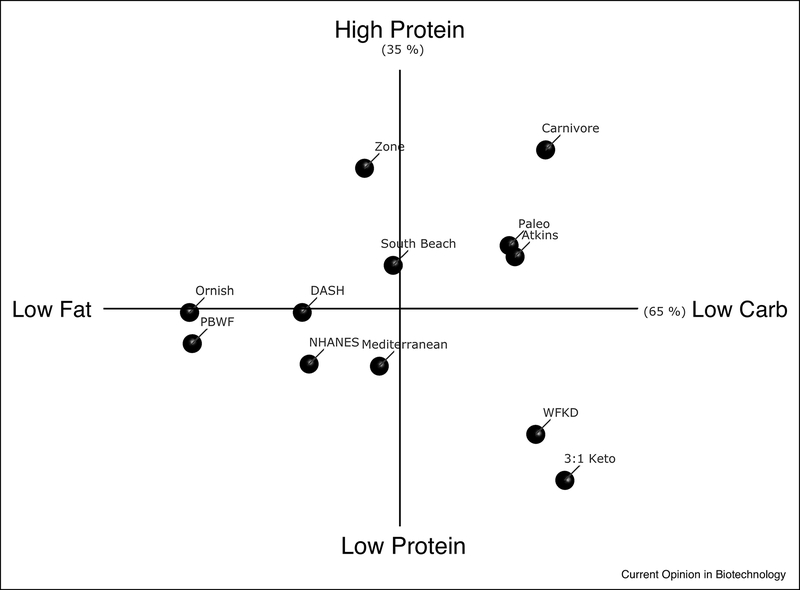
Some effects of CR can be recapitulated by eucaloric feeding of diets with altered macronutrient ratios. Diets with large proportions of fat and carbohydrate influence tumor development and progression in large, observational clinical studies 6. Therefore, dietary interventions that restrict these macronutrients may improve anti-cancer therapy (Figure 1). Low fat (less than 30% kcal per day, LFD) and very low fat (less than 10% kcal per day, VLFD) diets are popular clinical interventions, and there are various iterations of this style of eating including the Ornish, Plant-based whole food (PBWF), Dietary Approaches to Stop Hypertension (DASH), and American Heart Association (AHA) diets. Carbohydrate restricted diets also come in a variety of options including those low (less than 100g per day, LCD) and very low (less than 30–40g per day, VLCD) in carbohydrate. VLCDs potently induce ketosis by suppressing insulin so are referred to as ketogenic diets. However, this term encapsulates a wide variety of dietary eating patterns that vary in fat content, fat composition, and protein content 14.
Figure 1. Dietary Interventions that Modify Content.
A principle component analysis (PCA) was performed using the average macronutrient content (% carbohydrate, fat, and protein) of various dietary interventions. PC-1 (x-axis) segregated the diets based on carbohydrate and fat content, whereas PC-2 (y-axis) separated based on protein content. Ketogenic diets are found on the right side of the y-axis. Abbreviations: Plant-based whole food (PBWF), Dietary Approaches to Stop Hypertension (DASH), the average American diet from The Third National Health and Nutrition Examination Survey (NHANES III), WFKD (Well-formulated ketogenic diet), 3:1 classic ketogenic diet (3:1 Keto).
Although most dietary interventions fail to demonstrate superiority of one diet over another for weight loss14–17, the different content approaches may have unique metabolic effects that can be utilized for specific cancer types. For example, carbohydrate restriction using a VLCD lowers insulin quickly especially when insulin resistance is present 18–20. Therefore, these diets may be best in the setting of breast and endometrial tumors with an amplified insulin-PI3K pathway 21,22, but should be avoided in metastatic ovarian and prostate cancers that appear to feed on lipid 23,24. Dietary protein also impacts circulating mitogens. Protein intake is a key determinant of circulating levels of IGF125,26 so diets including a protein restriction (e.g. CR, “classic” 3:1 ketogenic, and the Well-Formulated Ketogenic (WFKD) diet) may be more effective against IGF1-sensitive tumors such as those in the prostate and colon 27–30. Furthermore, LFDs reduce circulating estrogens and/or progestins, which may explain the reduced deaths observed in the Women’s Health Initiative Dietary Modification trial, a prospective, randomized, controlled study in postmenopausal women designed to examine the long-term benefits and risks of a LFD on breast and colorectal cancers and cardiovascular disease 31–33.
Modulating Dietary Timing
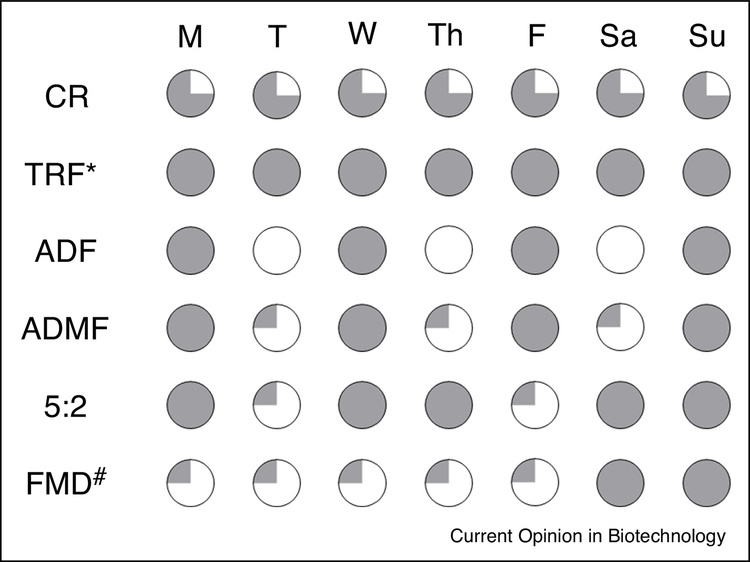
Intermittent fasting is a broad term that encompasses a variety of timing regimens that restrict energy intake for distinct periods. The five most common are time-restricted feeding (TRF), alternate-day fasting (ADF), alternate-day modified fasting (ADMF), the 5:2 schedule, and the fasting mimicking diet (FMD) (Figure 2)34. TRF prolongs the daily nocturnal fast by restricting intake to a pre-defined interval per day (e.g. 8 consecutive hours). ADF increases the fasting period further by requiring a complete fast every other day. ADMF is like ADF except that a small amount of food (about 25% of the daily kcal requirement) is allowed during the fasting day. The 5:2 program and FMD also utilize these days with minimal calorie intake occurring 2 days per week and 5 days per month, respectively.
Figure 2. Dietary Interventions that Modify Timing.
The schedule of different intermittent fasting approaches as compared to a daily 25% calorie restriction (CR). Time-restricted feeding (TRF*) does not restrict energy intake. Instead, food consumption is limited to certain hours per day to create an extended period of daily fasting. As its name implies, alternate-day fasting (ADF) alternates between unrestricted access and no food intake every other day. Alternate-day modified fasting (ADMF) is a version of ADF that allows a small amount (~25% of normal daily intake) of food on the fasting days. The 5:2 schedule is a less stringent version of ADMF where the low calorie days occur 2 days per week. The fasting mimicking diet (FMD) is a program where the low calorie days occur for 5 consecutive days each month and food is not restricted the remainder of the time (#). The shaded areas represent the daily % intake of total caloric needs, e.g. the fully shaded circles represent daily consumption of 100% of total caloric needs.
The estimated degree of calorie restriction and the associated weight loss varies among the timing approaches, but the effects of systemic growth factors appear consistent. ADF and ADMF both show similar amounts of weight loss as compared to CR35, but with better insulin suppression 36–39. TRF has no effect on weight loss over 12 months40 despite rapid changes to insulin sensitivity 41. FMD did not reduce body weight in women with breast cancer, however still reduced insulin, IGF1, and leptin 42,43.
Engineering Diets to Improve Cancer Outcomes
While many agree that dietary factors can contribute to the development of certain tumors types44, it is unlikely that diet alone will combat tumor progression. In this setting, diet should be combined with pharmacologic approaches to enhance anti-cancer therapy. There are several promising strategies that show efficacy in pre-clinical models. For example, a VLCD enhanced the effects of multiple PI3K inhibitors against 12 different tumor models, including genetically engineered mouse models, traditional xenografts, and patient-derived xenografts 21. FMD led to durable remission of breast tumors in mice for over 160 days when combined with fulvestrant and CDK4/6 inhibition 43. Other approaches like CR2 and depletion of specific sugars45 or amino acids such as serine & glycine 46,47, cysteine 48, methionine 49–51 from the diet show prominent effects alone and are likely to enhance standard of care treatment2,46. Supplementing the diet with certain sugars (e.g. mannose 52), vitamins53,54, or amino acids (e.g. histidine 55) also have anti-tumour properties in pre-clinical models.
These dietary augmentation strategies are now being translated to humans with cancer. This process should be treated with the same care and rigor that we use in developing pharmacologic therapies including proper adjustment for the differences between mouse and human metabolism. We need well-designed and adequately powered phase I safety and feasibility studies, phase II therapeutic exploration trials, and, if needed, larger phase III studies for therapeutic confirmation. The first step is to design menus that are safe, cost-effective, tolerable, and efficacious. The best formulations arise from multidisciplinary collaboration amongst dieticians, chefs, food industry experts, and medical professionals familiar with patient behavior and potential side effects of medication that in some cases could alter food consumption and absorption (i.e. dysgeusia, anorexia, nausea, vomiting, stomatitis, dysphagia, autonomic dysfunction, pancreatic insufficiency, diarrhea, constipation, abdominal pain). For diets being prescribed for long periods of time, we recommend starting with a base of 25–30 kcal/kg of energy, 4 kcal/kg of protein, and splitting the rest of the energy using a 2.5 to 1 proportion carbohydrate to fat by kcal 56. For a 70 kg subject, this formulation yields about 1900 kcal, 70g protein, 50g fat, and 290g carbohydrate. This base can then be modified to meet the prescribed energy and macronutrient content. The menu should not contain simple sugars, processed meat, or ultra-processed foods 44. Thought should be given to the composition of saturated to unsaturated fats and the total fiber content as these factors may alter the growth of specific tumor types6. When it is not possible to provide a well-formulated diet, the missing vitamins, minerals, and other components should be supplemented. In addition to designing an effective intervention diet, care should be taken where possible to use an appropriate control arm, which could be relatively ‘normal’ diet (but within defined macronutrient & caloric ranges), or potentially a calorie matched defined diet.
Compliance with diet – as with any therapeutic agent – is an important consideration. While many cancer patients have high motivation to comply, the quality and taste of the food should be optimized. Preferably, the menu contains both fresh and frozen options and caters to a range of individual patient preferences. The use of ingredients like garlic, hot sauce, and curry powder can help make meals more appealing and acceptable to different cultural backgrounds. Having a wide range of meal options has helped compliance with commercial CR programs for weight loss 57. In addition to tasty food, dietary augmentation programs can be supported by dietary counseling and communication. Subject interaction through telephone follow up and video improves adherence to dietary interventions for chronic conditions such as diabetes and cardiovascular disease 58. Providing similar contact through smart phone apps has been shown to improve adherence to diet in healthy volunteers 59. Food is a source of joy for many people, and the emotional journey through cancer therapy is not easy. Counselors help to motivate and counsel patients through difficult times and teach subjects how to modify the dietary menu during special events and trips that inevitably arise.
Metabolic monitoring is the third core component to an effective dietary intervention. Metabolic monitoring is typically performed using metabolite or hormone measures in biofluids like venous and capillary blood, urine, saliva, or subcutaneous interstitial fluid. These measures ensure the efficacy of the intervention and the safety of the subject. For example, levels of capillary β-hydroxybutyrate are a reliable readout for the VLCD-induced suppression of insulin and safety monitoring for ketoacidosis when levels are too high 60. It is important to note that hormonal and metabolic changes that occur in biofluids do not always translate to tissue level effects 61,62. However, we lack convenient methods to easily access tissue level biomarkers. This may be possible using cells from freshly plucked hair or mucosal surfaces.
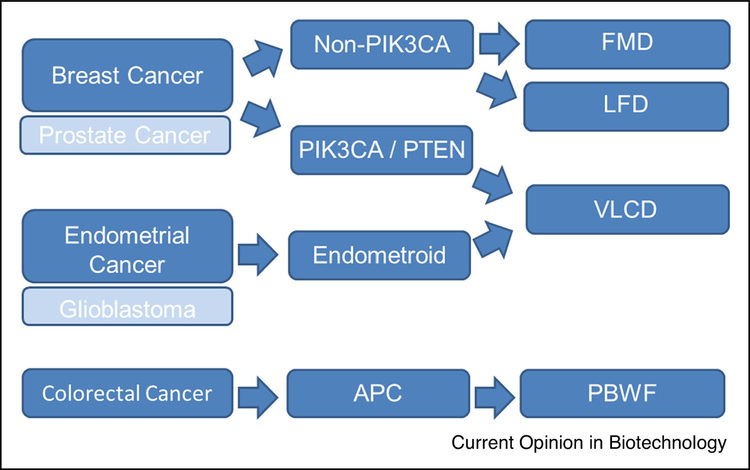
We are already quite advanced in the process to bring CR, VLCD, and FMD approaches to phase II and III clinical trials in cancer patients. In addition to being safe and feasible in subjects with cancer 63, a recent randomized, controlled trial provided evidence that a VLCD reduces tumor size in patients with locally advanced breast cancer22. Also, FMD also has very strong feasibility and safety data with hints of therapeutic efficacy when combined with CDK4/6 inhibition 43. With these data in mind, we have started to outline an approach for Precision Nutrition in cancer (Figure 3). In this algorithm, tumor tissue types are further delineated using histologic and molecular features that have data supporting their use in animal models. The dietary interventions are then prescribed and monitored for efficacy. One limitation of this approach is that we are currently ignoring host factors (obesity, insulin resistance, pre-menopause, etc) that are known to influence tumor progression. We look forward to designing more advanced designs that account for this issue.
Figure 3. Precision Nutrition Algorithm for Cancer.
In this algorithm, tumor tissue types are further delineated using histologic and molecular features that have data supporting their use in animal models and early phase clinical studies. Breast cancer is first delineated by the presence of alterations in the PI3K pathway, which are predicted to grow in response to systemic insulin. The treatment of these tumors, and endometroid subtypes of endometrial cancer that also have high PI3K activity, should be paired with a very low carbohydrate diet (VLCD). Other types of breast cancer may respond best to combination therapies using the fasting mimicking diet (FMD) or low fat diets (LFD). We speculate that this paradigm can also be used for prostate cancer and glioblastoma, however the specific subtypes that respond well to each diet have not been demonstrated. APC-mutated colorectal cancers develop and progress in response to a variety of dietary components like red meat, processed meat, fructose-containing sugars, and low fiber diets so a plant-based whole food (PBWF) approach may be best in this setting. PIK3CA: gene for phosphatidylinositol 3-kinase p110 alpha; PTEN: gene for Phosphatase and tensin homolog; APC: gene for adenomatous polyposis coli; FMD: fasting mimicking diet; LFD: low fat diet; VLCD: very low carbohydrate diet; PBWF: plant-based whole food diet.
We are on the verge of a revolution in way we implement diet in patients during cancer therapy. Several rigorous preclinical studies have demonstrated that multiple forms of dietary augmentation therapy can improve cancer outcomes. Over the next 5 years, we will learn if these beneficial effects persist in prospective, controlled, randomized clinical trials64. It has become clear that a one-sized-fits-all approach is not appropriate, and we need to clarify the algorithms we use to implement dietary interventions.
Acknowledgments
Funding
This work was supported by NIH K08 CA230318 (M.D.G.), NIH P50 CA211024 (M.D.G.), and Cancer Research UK Fellowship C53309/A19702 (O.D.K.M.).
Conflict of interest
M.D.G. is a co-founder, shareholder, and consultant of Faeth Therapeutics Inc., which is developing nutritional therapies for cancer. M.D.G. has received speaking and/or consulting fees from Pfizer Inc., Novartis AG, Petra Pharmaceuticals, and TruMacro nutrition. M.D.G.’s laboratory receives financial support from Pfizer, Inc. O.D.K.M. is a co-founder and shareholder of Faeth Therapeutics Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 2.Lien EC, Westermark AM, Li Z, Sapp KM, Vander Heiden MG. Caloric restriction alters lipid metabolism to contribute to tumor growth inhibition. bioRxiv 2020; [Google Scholar]
- 3. Kanarek N, Petrova B, Sabatini DM. Dietary modifications for enhanced cancer therapy. Nature 2020;579(7800):507–17. * A more comprehensive review of cancer cell metabolism and the promise of dietary augmentation therapy.
- 4.Blot WJ, Tarone RE. Doll and Peto’s quantitative estimates of cancer risks: holding generally true for 35 years. J Natl Cancer Inst 2015;107(4). [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer 2011;105(2):S77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goncalves MD, Hopkins BD, Cantley LC. Dietary Fat and Sugar in Promoting Cancer Development and Progression. Annu Rev Cancer Biol 2019;3:255–73. [Google Scholar]
- 7.Hopkins BD, Goncalves MD, Cantley LC. Obesity and Cancer Mechanisms: Cancer Metabolism. J Clin Oncol Off J Am Soc Clin Oncol 2016;34(35):4277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voss M, null Marlies Wagner, von Mettenheim N, et al. ERGO2: A prospective randomized trial of calorie restricted ketogenic diet and fasting in addition to re-irradiation for malignant glioma. Int J Radiat Oncol Biol Phys 2020; [DOI] [PubMed] [Google Scholar]
- 9.Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-mo. calorie restriction on biomarkers of longevity, metabolic adaptation and oxidative stress in overweight subjects. JAMA J Am Med Assoc 2006;295(13):1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redman LM, Martin CK, Williamson DA, Ravussin E. Effect of Caloric Restriction in Non-Obese Humans on Physiological, Psychological and Behavioral Outcomes. Physiol Behav 2008;94(5):643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kraus WE, Bhapkar M, Huffman KM, et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol 2019;7(9):673–83. ** CALERIE was a phase 2, multicenter, randomized controlled trial in 218 young and middle-aged, healthy non-obese men and women that were randomly assigned (2:1) to a 25% calorie restriction (CR) diet or an ad libitum control diet. CR reduced all measured conventional cardiometabolic risk factors. Although not in a cancer population, CALERIE is the first systematic investigation of CR in nonobese humans and it serves to define the long-term physiologic effects of this dietary approach.
- 12.Look AHEAD Research Group, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mudaliar U, Zabetian A, Goodman M, et al. Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis. PLoS Med 2016;13(7):e1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza RJ, Bray GA, Carey VJ, et al. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the POUNDS LOST trial. Am J Clin Nutr 2012;95(3):614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makris A, Foster GD. Dietary approaches to the treatment of obesity. Psychiatr Clin North Am 2011;34(4):813–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA 2014;312(9):923–33. [DOI] [PubMed] [Google Scholar]
- 17.Atallah R, Filion KB, Wakil SM, et al. Long-term effects of 4 popular diets on weight loss and cardiovascular risk factors: a systematic review of randomized controlled trials. Circ Cardiovasc Qual Outcomes 2014;7(6):815–27. [DOI] [PubMed] [Google Scholar]
- 18.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. N Engl J Med 2008;359(3):229–41. [DOI] [PubMed] [Google Scholar]
- 19.Hall KD, Chen KY, Guo J, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr 2016;104(2):324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nuttall FQ, Almokayyad RM, Gannon MC. Comparison of a carbohydrate-free diet vs. fasting on plasma glucose, insulin and glucagon in type 2 diabetes. Metabolism 2015;64(2):253–62. [DOI] [PubMed] [Google Scholar]
- 21. Hopkins BD, Pauli C, Du X, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 2018;560(7719):499–503. * This is a mouse study showcasing an improvement in efficacy when PI3K inhibitors are paired with very low carbohydrate diets.
- 22.Khodabakhshi A, Akbari ME, Mirzaei HR, Seyfried TN, Kalamian M, Davoodi SH. Effects of Ketogenic metabolic therapy on patients with breast cancer: A randomized controlled clinical trial. Clin Nutr Edinb Scotl 2020; [DOI] [PubMed] [Google Scholar]
- 23.Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 2011;17(11):1498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labbé DP, Zadra G, Yang M, et al. High-fat diet fuels prostate cancer progression by rewiring the metabolome and amplifying the MYC program. Nat Commun 2019;10(1):4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovannucci E, Pollak M, Liu Y, et al. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2003;12(2):84–9. [PubMed] [Google Scholar]
- 26.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 2008;7(5):681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen NE, Key TJ, Appleby PN, et al. Serum insulin-like growth factor (IGF)-I and IGF-binding protein-3 concentrations and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2007;16(6):1121–7. [DOI] [PubMed] [Google Scholar]
- 28.Wolk A, Mantzoros CS, Andersson SO, et al. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst 1998;90(12):911–5. [DOI] [PubMed] [Google Scholar]
- 29.Knuppel A, Fensom GK, Watts EL, et al. Circulating insulin-like growth factor-I (IGF-I) concentrations and incidence of 30 cancers: prospective analyses in UK Biobank. Cancer Res 2020; [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res 2002;62(4):1030–5. [PubMed] [Google Scholar]
- 31.Chlebowski RT, Aragaki AK, Anderson GL, et al. Low-Fat Dietary Pattern and Breast Cancer Mortality in the Women’s Health Initiative Randomized Controlled Trial. J Clin Oncol Off J Am Soc Clin Oncol 2017;35(25):2919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chlebowski RT, Aragaki AK, Anderson GL, et al. Association of Low-Fat Dietary Pattern With Breast Cancer Overall Survival: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trial. JAMA Oncol 2018;4(10):e181212. ** This is a secondary analysis of the Women’s Health Initiative randomized clinical trial where 48 835 postmenopausal women were randomized to a dietary intervention (N = 19 541) with goals to reduce fat intake to 20% of energy and increase fruit, vegetable, and grain intake as compared to a usual-diet group (N = 29 294). In the dietary intervention group, breast cancer overall survival was significantly greater (HR 0.78; 95% CI, 0.65–0.94; P = .01), and there were fewer deaths from breast cancer, other cancers, and cardiovascular disease.
- 33.Rose DP, Connolly JM, Chlebowski RT, Buzzard IM, Wynder EL. The effects of a low-fat dietary intervention and tamoxifen adjuvant therapy on the serum estrogen and sex hormone-binding globulin concentrations of postmenopausal breast cancer patients. Breast Cancer Res Treat 1993;27(3):253–62. [DOI] [PubMed] [Google Scholar]
- 34.Hoddy KK, Marlatt KL, Çetinkaya H, Ravussin E. Intermittent Fasting and Metabolic Health: From Religious Fast to Time-Restricted Feeding. Obes Silver Spring Md 2020;28 Suppl 1:S29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr 2017;37:371–93. [DOI] [PubMed] [Google Scholar]
- 36.Gabel K, Kroeger CM, Trepanowski JF, et al. Differential Effects of Alternate-Day Fasting Versus Daily Calorie Restriction on Insulin Resistance. Obes Silver Spring Md 2019;27(9):1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutchison AT, Regmi P, Manoogian ENC, et al. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obes Silver Spring Md 2019;27(5):724–32. [DOI] [PubMed] [Google Scholar]
- 38.Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes 2005 2011;35(5):714–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harvie M, Wright C, Pegington M, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr 2013;110(8):1534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Oliveira Maranhão Pureza IR, da Silva Junior AE, Silva Praxedes DR, et al. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: A 12-month randomized clinical trial. Clin Nutr Edinb Scotl 2020; [DOI] [PubMed] [Google Scholar]
- 41.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab 2018;27(6):1212–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandhorst S, Choi IY, Wei M, et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab 2015;22(1):86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Caffa I, Spagnolo V, Vernieri C, et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020;583(7817):620–4. * This is a mouse study showcasing an improvement in efficacy when CDK4/6 inhibitors are paired with fuvestrant and a fasting mimicking diet. The authors also present robust clinical feasibility data.
- 44.World Cancer Research/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report 2018. available at dietandcancerreport.org: [Google Scholar]
- 45.Goncalves MD, Lu C, Tutnauer J, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 2019;363(6433):1345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maddocks ODK, Athineos D, Cheung EC, et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 2017;544(7650):372–6. [DOI] [PubMed] [Google Scholar]
- 47.Baksh SC, Todorova PK, Gur-Cohen S, et al. Extracellular serine controls epidermal stem cell fate and tumour initiation. Nat Cell Biol 2020;22(7):779–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang T, Bauer C, Newman AC, et al. Polyamine pathway activity promotes cysteine essentiality in cancer cells. Nat Metab 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poirson-Bichat F, Gonçalves RA, Miccoli L, Dutrillaux B, Poupon MF. Methionine depletion enhances the antitumoral efficacy of cytotoxic agents in drug-resistant human tumor xenografts. Clin Cancer Res Off J Am Assoc Cancer Res 2000;6(2):643–53. [PubMed] [Google Scholar]
- 50.Epner DE, Morrow S, Wilcox M, Houghton JL. Nutrient intake and nutritional indexes in adults with metastatic cancer on a phase I clinical trial of dietary methionine restriction. Nutr Cancer 2002;42(2):158–66. [DOI] [PubMed] [Google Scholar]
- 51.Gao X, Sanderson SM, Dai Z, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 2019;572(7769):397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez PS, O’Prey J, Cardaci S, et al. Mannose impairs tumour growth and enhances chemotherapy. Nature 2018;563(7733):719–23. [DOI] [PubMed] [Google Scholar]
- 53.Agathocleous M, Meacham CE, Burgess RJ, et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017;549(7673):476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cimmino L, Dolgalev I, Wang Y, et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 2017;170(6):1079–1095.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanarek N, Keys HR, Cantor JR, et al. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 2018;559(7715):632–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr Edinb Scotl 2017;36(1):11–48. [DOI] [PubMed] [Google Scholar]
- 57.Gudzune KA, Doshi RS, Mehta AK, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med 2015;162(7):501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desroches S, Lapointe A, Ratté S, Gravel K, Légaré F, Turcotte S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst Rev 2013;(2):CD008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Ortiz L, Recio-Rodriguez JI, Agudo-Conde C, et al. Long-Term Effectiveness of a Smartphone App for Improving Healthy Lifestyles in General Population in Primary Care: Randomized Controlled Trial (Evident II Study). JMIR MHealth UHealth [Internet] 2018. [cited 2020 Aug 1];6(4). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5948409/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brooke J, Stiell M, Ojo O. Evaluation of the Accuracy of Capillary Hydroxybutyrate Measurement Compared with Other Measurements in the Diagnosis of Diabetic Ketoacidosis: A Systematic Review. Int J Environ Res Public Health [Internet] 2016;13(9). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5036670/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harvie MN, Sims AH, Pegington M, et al. Intermittent energy restriction induces changes in breast gene expression and systemic metabolism. Breast Cancer Res BCR 2016;18(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schübel R, Nattenmüller J, Sookthai D, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr 2018;108(5):933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klement RJ. Beneficial effects of ketogenic diets for cancer patients: a realist review with focus on evidence and confirmation. Med Oncol Northwood Lond Engl 2017;34(8):132. [DOI] [PubMed] [Google Scholar]
- 64.Lévesque S, Pol JG, Ferrere G, Galluzzi L, Zitvogel L, Kroemer G. Trial watch: dietary interventions for cancer therapy. Oncoimmunology [Internet] 2019. [cited 2020 Aug 1];8(7). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6527263/ [DOI] [PMC free article] [PubMed] [Google Scholar]