Dear Editor,
With the recent emergence of new SARS-CoV-2 variants, many hematology teams are facing unconventional situations and are being asked to modify their practices in order to offer the best medical care for their patients [1], [2], [3]. In this context, hematopoietic stem cell transplantation (HSCT) for autoimmune disease patients represent one of the most vulnerable groups of patients due to preexisting comorbidities and the acquired immunodepression during conditioning with chemotherapy [4]. Despite all the protective measures (PCR tests at admission, social distancing and vaccination) and the proper use of personal protection equipment (e.g. mask, ventilators…etc.) at hospitalization time, SARS-CoV-2 transmission remains a concern in HSCT units early before admission and during chemotherapy administered for mobilization or at time of conditioning. COVID-19 management in this setting is a difficult challenge. Its diagnosis and the logistic adaptability of the HSCT procedure, due to a wide differential diagnosis for post-HSCT infections and the complexity of the graft handling process, are two of the most important factors affecting clinical outcomes in this setting [5, 6].
Here we present the case of a patient who underwent an autologous stem cell transplantation (ASCT) for multiple sclerosis (MS) with a long-lasting COVID-19 infection during transplantation.
A 28-year-old man suffering from relapsing-remitting MS, with clinical and radiological inflammatory activities, was admitted to our hematology department in January 2021 for an ASCT. He had been diagnosed 5 years ago with MS with Uhthoff's phenomenon and a sensory-motor deficit of the left side of the body, and balance and sphincter disorders. Between 2016 and 2018, first-line treatment with consecutive corticosteroid administrations resulted in a partial response, with an Expanded Disability Status Scale (EDSS) of 3.5. In 2018, the MRI showed cerebral lesions with important activity, and the John Cunningham virus (JCV) serological test was concomitantly positive (index value of 2.88). Therefore, a second-line treatment with rituximab was initiated at a total dose of 1000 mg at D1 and D15 of every cycle, 1 cycle/ 6 months, for a total of 3 cycles.
The clinical course was marked by intermittent phases of symptom improvement, interspersed with numerous crises, leading the medical team to introduce corticosteroids to control these events. These episodes finally led to the initiation of a third-line treatment with ocrelizumab in late 2019, without allowing clinical improvement after a few months of well-conducted treatment. The diagnosis of recurrent/refractory MS was reported during 2020 and the decision was made to perform an ASCT by the end of the year, after multidisciplinary evaluation based on confirmation by the French national autoimmune diseases committee (MATHEC: www.mathec.com).
A fertility preservation, a pre-ASCT Hematopoietic Cell Transplant Comorbidity Index (HCT-CI) of 0 and a negative SARS-CoV-2 PCR test were documented both at mobilization and ASCT admission. The hematopoietic stem cell graft was mobilized by cyclophosphamide (Endoxan® 1 g/m2 at the first and second day of mobilization and granulocyte-colony-stimulating factor (G-CSF). The conditioning regimen comprised cyclophosphamide (50 mg/kg from d-5 to d-2) and anti-thymocyte globulin (ATG, 0.5 mg/kg at d-5, then 1.5 mg/kg from d-4 to d-1). The graft was reinjected (viable CD34+ = 7.24 106/kg) on D 0 and the patient developed fever after a few hours.
Antibiotic therapy with cefepime was initiated, secondarily combined with daptomycin following a positive Gram-positive blood culture. The central line was ablated at D + 3, concomitant with a new SARS-CoV-2 PCR test. Central line cultures revealed methicillin-susceptible Staphylococcus aureus (MSSA) and a SARS-CoV-2 PCR test returned positive at D + 3. On day 4, The CT scan showed minimal lung involvement and COVID-19 convalescent plasma therapy was administered promptly on D + 4 and D + 5, accompanied by a curative anticoagulation with low molecular weight heparin (LMWH, Lovenox®, 7000 UI x 2/day) on account of high d-Dimer titers. A cardiac ultrasound revealed an ejection fraction (EF) of 44% with negative troponin, thus, a combination of β-blocker and angiotensin-converting enzyme inhibitor therapy was introduced, taking into consideration the differential diagnosis of SARS-CoV-2-induced myocarditis or stress-induced heart disease. The patient was maintained on intensive surveillance as became oxygen-dependent at D + 4, requiring oxygenation at 2 liters/minute. On day 6, as per protocol G-CSF was started from D + 6 until the engraftment.
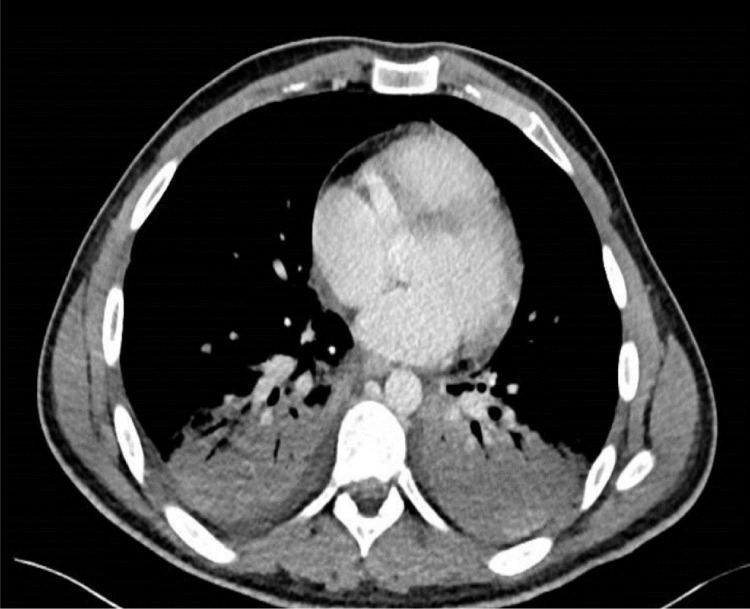
White blood cell engraftment was recorded at D + 10, while the patient presented with a pleuro-pneumonia, probably due to a bacterial non-documented superinfection one day after engraftment (Fig. 1 ), requiring the administration of meropenem for one week. The progress was slowly positive, and the patient was weaned from oxygenation at D + 21. The patient did not require corticosteroids or any other therapy for COVID-19. The patient was discharged from the department after 37 days of hospitalization with a strict follow-up program at our outpatient unit. Cardiac function rapidly normalized with an EF of 65% two months later. The PCR test remained positive until 2 months after transplant before the viral load turned negative conversion.
Fig. 1.
Computed tomography showing pleuro-pneumonia.
At 6 months post COVID-19 diagnosis, and in accordance with local guidelines, the patient was vaccinated with one dose of mRNA COVID-19 vaccine, the serological test revealed 917.9 UA/ml.
At 10 months after the ASCT, the patient is continuing to improve clinically without MS-specific treatment. His-neurological manifestations became considerably less frequent and less intense, except for cerebellar syndrome which remains stable.
Discussion
Infectious diseases are not uncommon in hematology patients, especially among HSCT recipients; nevertheless, the COVID-19 pandemic has considerably changed the practices of hematologists worldwide. While rapidly and efficiently treating the patients remains the optimal goal, repeated pre-admission PCR testing, reinforced isolation, use of personal protective equipment, and have become a standard-of-care prior to any new hospitalization or aplasia- inducing treatment [7].
Although our guidelines clearly state that, a SARS-CoV-2 PCR test should be performed at admission, even in the absence of active contact with an infected patient, this well-applied measure did not prevent this patient from presenting COVID-19 during hospitalization. In such situations, the infection may be due to an undocumented contact or a passive transmission by a visitor or staff member during the first few days of hospitalization. In our case, the hypothesis of community-transmitted SARS-CoV-2 seems stronger because of the narrow interval between admission and first symptom (6 days) and the fact that some of his confirmed pre-hospitalization contacts were diagnosed with COVID-19, 48 h before him.
To the best of our knowledge 3 cases were previously reported of COVID-19 in the context of ASCT [8], [9], [10], none were recorded in an autoimmune disease setting. Our case may show the impact of COVID-19 on ASCT recipients during the period of immune reconstitution. It may also illustrate the importance of the following combined strategy in the immunocompromised B-cell depleted population: early diagnosis and treatment of concomitant and secondary infections and early administration of SARS-CoV-2 antibodies by plasma infusion or anti-spike monoclonal antibodies. Our patient totally recovered after convalescent plasma and a course of antibiotics with no tocilizumab or dexamethasone administered.
The mortality rate in the HCT setting appears to be higher than in the general population, probably in relation to profound immunosuppression or because of the long exposure to immune-modulating and immune-compromising therapies (such as rituximab and dexamethasone) used to treat our patients [9].
Of note, is the importance of applying rigidly the proper indications and procedures [11] for the ASCT in the setting of autoimmune disease, as shown in this case, in order to obtain the best possible outcome [12]. This may also highlight the critical role of implementing strict preventive measures and outreach approaches developed to reduce the burden of COVID-19 infections in ASCT recipients.
As a consequence of the present observation, the MATHEC FRENC group for AHSCT in autoimmune disease now recommend a specific COVID-19 risk assessment before ASCT, as well as the implementation of a preventive scheme proposing a 7-day period of controlled contact prior to admission, excluding those with symptoms suggestive of COVID-19 and those who do not have a negative SARS-CoV-2 PCR test within 3 days of contact.
Patient and family education for all candidates to HSCT, as well as pre-transplant effective vaccination with at least 3 injection of RNA vaccines prior to mobilization and then careful monitoring of all patients after admission [13] will help to reduce current risk associated with COVD infection in such fragile patients.
Informed consent
An informed consent was obtained from the patient for the publication of this letter.
Declaration of interests
T.A. reports receiving honoraria from Biotest, outside the submitted work.
F.M. reports lecture honoraria from Therakos/Mallinckrodt, Janssen, Biocodex, Sanofi, JAZZ Pharmaceuticals, Gilead, Novartis, and Astellas, all outside the submitted work.
R.D. reports personal fees from Takeda, Biostest, and Novartis, and non-financial support from Gilead, all outside the submitted work.
References
- 1.Malard F., Genthon A., Brissot E., van de Wyngaert Z., Marjanovic Z., Ikhlef S., Banet A., Lapusan S., Sestilli S., Corre E., Paviglianiti A., Adaeva R., 'Hammedi-Bouzina F M., Labopin M., Legrand O., Dulery R., Mohty M. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020 Nov;55(11):2180–2184. doi: 10.1038/s41409-020-0931-4. Epub 2020 May 6. PMID: 32376969PMCID: PMC7201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsuliman T., Faict S., Malard F., Genthon A., Brissot E., Van de Wyngaert Z., Ikhlef S., Banet A., Lapusan S., Sestili S., Corre E., M'hammedi-Bouzina F., Schaeffer L., Legrand O., Dulery R., Mohty M., Marjanovic Z. Does Ibrutinib impact outcomes of viral infection by SARS-CoV-2 in mantle cell lymphoma patients? Curr Res Transl Med. 2021 Jan;69(1) doi: 10.1016/j.retram.2020.103273. Epub 2020 Nov 25. PMID: 33460953PMCID: PMC7687383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijenthira A., Gong I.Y., Fox T.A., Booth S., Cook G., Fattizzo B., Martín-Moro F., Razanamahery J., Riches J.C., Zwicker J., Patell R., Vekemans M.C., Scarfò L., Chatzikonstantinou T., Yildiz H., Lattenist R., Mantzaris I., Wood W.A., Hicks L.K. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020 Dec 17;136(25):2881–2892. doi: 10.1182/blood.2020008824. PMID: 33113551PMCID: PMC7746126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A., Bhatt N.S., St Martin A., Abid M.B., Bloomquist J., Chemaly R.F., Dandoy C., Gauthier J., Gowda L., Perales M.A., Seropian S., Shaw B.E., Tuschl E.E., Zeidan A.M., Riches M.L., Shah G.L. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021 Mar;8(3):e185–e193. doi: 10.1016/S2352-3026(20)30429-4. Epub 2021 Jan 19. Erratum in: Lancet Haematol. 2021 Jun;8(6):e393. PMID: 33482113PMCID: PMC7816949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohty B., Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J. 2011 Apr;1(4):e16. doi: 10.1038/bcj.2011.14. Epub 2011 Apr 29. PMID: 22829137PMCID: PMC3255242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama S., Hamdeh S., Micic D., Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2020 Oct 13 doi: 10.1136/annrheumdis-2020-218946. annrheumdis-2020-218946Epub ahead of print. PMID: 33051220PMCID: PMC7554412. [DOI] [PubMed] [Google Scholar]
- 7.Garnica M., Maiolino A. COVID and hematology: special considerations regarding patient safety, gold standard therapies and safety for health care professionals. Hematol Transfus Cell Ther. 2020 Apr-Jun;42(2):111–112. doi: 10.1016/j.htct.2020.04.001. Epub 2020 Apr 11. PMID: 32292885PMCID: PMC7151410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanellopoulos A., Ahmed M.Z., Kishore B., Lovell R., Horgan C., Paneesha S., Lloyd R., Salhan B., Giles H., Chauhan S., Venkatadasari I., Khakwani M., Murthy V., Xenou E., Dassanayake H., Srinath S., Kaparou M., Nikolousis E. COVID-19 in bone marrow transplant recipients: reflecting on a single centre experience. Br J Haematol. 2020 Jul;190(2):e67–e70. doi: 10.1111/bjh.16856. Epub 2020 Jun 23. PMID: 32469077PMCID: PMC7283684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malek A.E., Adachi J.A., Mulanovich V.E., Sassine J., Raad I.I., McConn K., Seiler G.T., Dhal U., Khawaja F., Chemaly R.F. Immune reconstitution and severity of COVID-19 among hematopoietic cell transplant recipients. Transpl Infect Dis. 2021 Aug;23(4):e13606. doi: 10.1111/tid.13606. Epub 2021 Apr 3. PMID: 33755273PMCID: PMC8250217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaus H.A., Rabitsch W., Buchtele N., Cserna J., Wohlfarth P. Autologous hematopoietic stem cell transplantation with concomitant SARS-CoV-2 infection. Ann Hematol. 2021 Oct 13:1–4. doi: 10.1007/s00277-021-04680-z. Epub ahead of print. PMID: 34643768PMCID: PMC8511851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zephir H., Puyade M., Gueguen A., Michel L., Terriou L., Dive D., Laureys G., Mathey G., Labauge P., Marjanovic Z., Pugnet G., Badoglio M., Lansiaux P., Yakoub-Agha I., Béguin Y., Farge D. Indications de l'autogreffe dans la sclérose en plaques : recommandations de la Société francophone de greffe de moelle et de thérapie cellulaire (SFGM-TC) en lien avec la Société francophone de la sclérose en plaques [Indications and follow-up for autologous hematopoietic stem cell transplantation in multiple sclerosis: guidelines from the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC) in association with the Francophone Society of Multiple Sclerosis] Bull Cancer. 2019 Jan;106(1S):S92–S101. doi: 10.1016/j.bulcan.2018.11.002. FrenchEpub 2018 Dec 5. PMID: 30527815. [DOI] [PubMed] [Google Scholar]
- 12.Burt R.K., Farge D., Ruiz M.A., Saccardi R., Snowden J.A. 1st ed. CRC Press; 2021. Hematopoietic stem cell transplantation and cellular therapies for autoimmune diseases. [DOI] [Google Scholar]
- 13.Brissot E., Labopin M., Baron F., et al. Management of patients with acute leukemia during the COVID-19 outbreak: practical guidelines from the acute leukemia working party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2021;56:532–535. doi: 10.1038/s41409-020-0970-x. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]