Abstract
In South Korea, all 12th grade students (highs school seniors) were offered BNT162b2 vaccine starting July 19, 2021; while 10th–11th grade students were not eligible. We conducted a nationwide retrospective cohort study by to determine the safety and effectiveness of BNT162b2 mRNA Covid-19 vaccine in adolescents against SARS-CoV-2 infection. Among 444,313 persons who received the first dose of vaccine, reporting rate for myocarditis and/or pericarditis was 1.8 per 100,000 (95% C.I. 0.8–3.5) among first-dose recipients and 4.3 per 100,000 (95% C.I. 2.6–6.7) in second-dose recipients. Vaccine effectiveness against symptomatic/asymptomatic SARS-CoV-2 infection 14 days post-first dose vaccination was 91.1% (95% C.I. 89.6–92.5), and 14 days post-second dose was 99.1% (95% C.I. 98.5–99.5). In this retrospective cohort study, BNT162b2 vaccination was safe and was associated with a significantly lower risk of SARS-CoV-2 infection, suggesting that vaccination in adolescent may reduce the burden of Covid-19.
Keywords: Coronavirus disease 2019, Covid-19, SARS-CoV-2, Vaccine, Vaccination, Adolescent
1. Introduction
Children and adolescents appear to have milder form of coronavirus disease 2019 (Covid-2019) than adults but remain at risk for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection across all ages [1]. Since its development and licensure, the Covid-19 vaccine studies have been conducted mainly in adults, and data on adolescent population is relatively scarce. Although high immunogenicity and efficacy in adolescents had been reported in controlled trials, data to inform the vaccine safety and effectiveness in the real-world setting are limited [2].
In South Korea, Covid-19 vaccination was initially prioritized for older adults and healthcare workers, with subsequent rollout to younger population.[3] As Covid-19 vaccine distribution continued, all 12th grade students (highs school seniors) were offered BNT162b2 mRNA Covid-19 vaccine starting July 19, 2021; while 10th and 11th grade students were not eligible to receive the vaccines. This Covid-19 vaccination program targeting the 12th grade students provide a unique opportunity to study the real-world impact of vaccination program in adolescents.
2. Methods
We conducted a retrospective cohort study within the Korean education system, a three-year high school system, from 10th grade to 12th grade (around 16–18 years of age). We linked the student Covid-19 confirmed case reports from the Ministry of Education and immunization registry from the Korea Disease Control and Prevention Agency, to determine the effectiveness of BNT162b2 mRNA Covid-19 vaccine in adolescents against SARS-CoV-2 infection (both symptomatic and asymptomatic). Korean education system has a three-year high school system, from 10th grade to 12th grade (around age 16–18 years of age). Laboratory testing, epidemiologic investigation, and surveillance practices did not change over the course of vaccination campaign.
Passive surveillance for adverse events was conducted through the medical facilities across the country, in accordance with COVID-19 Vaccination Adverse Events Management Guideline [4]. Anyone, including vaccine recipients and health care providers, were encouraged to report a suspected adverse event following immunization to the government. Serious adverse events were defined as cases with diagnoses of myocarditis and/or pericarditis, anaphylaxis reaction, convulsion/seizure, acute paralysis, encephalopathy/encephalitis, thrombocytopenia, Guillain-Barre syndrome, and any other diagnosis deemed by doctors that require close medical attention. History of hospitalization due to the symptoms following immunizations were recorded. All other adverse events were classified as nonserious adverse event. The presence of adverse events was monitored between the first and the second dose of BNT162b2 mRNA Covid-19 vaccines (post-first dose), and 30 days following the second dose (post-second dose).
Daily number of COVID-19 polymerase-chain-reaction (PCR) confirmed cases were obtained, and incidence per 100,000 was calculated per respective grades. Weekly incidence rate ratio was compared between the 10th–11th grade students vs. 12th grade students. We estimated vaccine effectiveness by calculating the reduction in cases in vaccinated students (≥14-day post 1st dose and ≥ 14-days post 2nd dose vaccination) to unvaccinated students. Weekly Covid-19 vaccination coverage rate (%) was derived from the national immunization registry.
This study was conducted as a legally mandated public health investigation under the authority of the Korean Infectious Diseases Control and Prevention Act (No. 12,444 and No. 13,392) and was not research that was subject to institutional review board approval; therefore, written informed consent was not required.
3. Results
The vaccination campaign targeted 45,4876 12th grade high school students across the country (out of 1,299,965 total high school students), reaching 82.2% of one-dose vaccine coverage rate during the first 2 weeks, and 95.4% of two-dose vaccine coverage rate within the next 3 weeks.
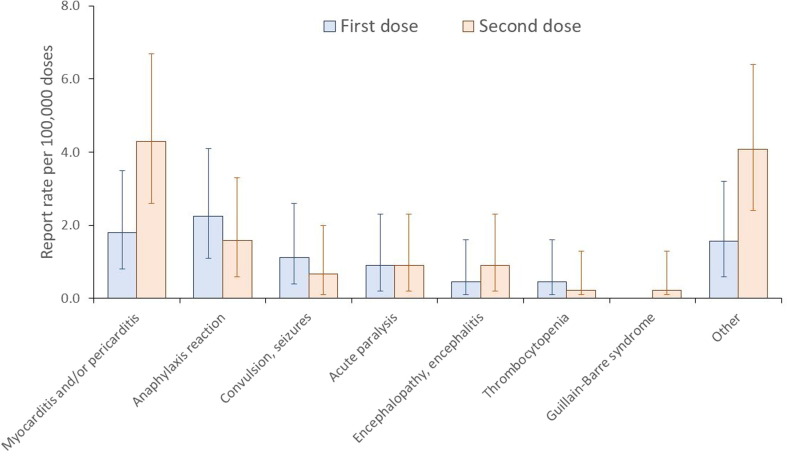
Among 444,313 persons who received the first dose of BNT162b2 vaccine, 1,287 (0.29%) reported to have adverse events, while 2,694/442,025 (0.61%) of second-dose recipients had reported the events (Table 1 ). Among the first-dose recipients and the second-dose recipients, 37 (0.01%) and 57 (0.01%) serious adverse events were reported, respectively. The reporting rate for myocarditis and/or pericarditis was the most common serious adverse event reported, with 1.8 per 100,000 (95% C.I. 0.8–3.5) among first-dose recipients and 4.3 per 100,000 (95% C.I. 2.6–6.7) in second-dose recipients (Fig. 1 ). Other adverse events include anaphylaxis reaction (1.6–2.3 per 100,000), convulsion (0.7–1.1 per 100,000), seizure (0.7–1.1 per 100,000), and acute paralysis (0.9 per 100,000).
Table 1.
Safety profile of BNT162b2 by number of doses in high school students following a nationwide 12th grade students vaccination campaign in Korea.
| Variables | First dose |
Second dose |
Total |
|||
|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | |
| Total vaccinations | 444,313 | 442,025 | 886,338 | |||
| Adverse event reports | 1287 | (0.29) | 2694 | (0.61) | 3981 | (0.45) |
| Nonserious adverse event | 1250 | (0.28) | 2637 | (0.60) | 3887 | (0.44) |
| Serious adverse event | 37 | (0.01) | 57 | (0.01) | 94 | (0.01) |
| Hospitalization | 36 | (0.01) | 71 | (0.02) | 107 | (0.01) |
* Patients in the “nonserious adverse event” and “serious adverse event” are mutually exclusive, whereas patients in the “hospitalization” are mutually inclusive with the aforementioned groups.
Fig. 1.
Report rate of serious adverse events following BNT162b2 by number of doses in high school students following a nationwide 12th grade students vaccination campaign in Korea. * Others (mutually inclusive with other adverse events): chest pain, dyspnea, chest discomfort.
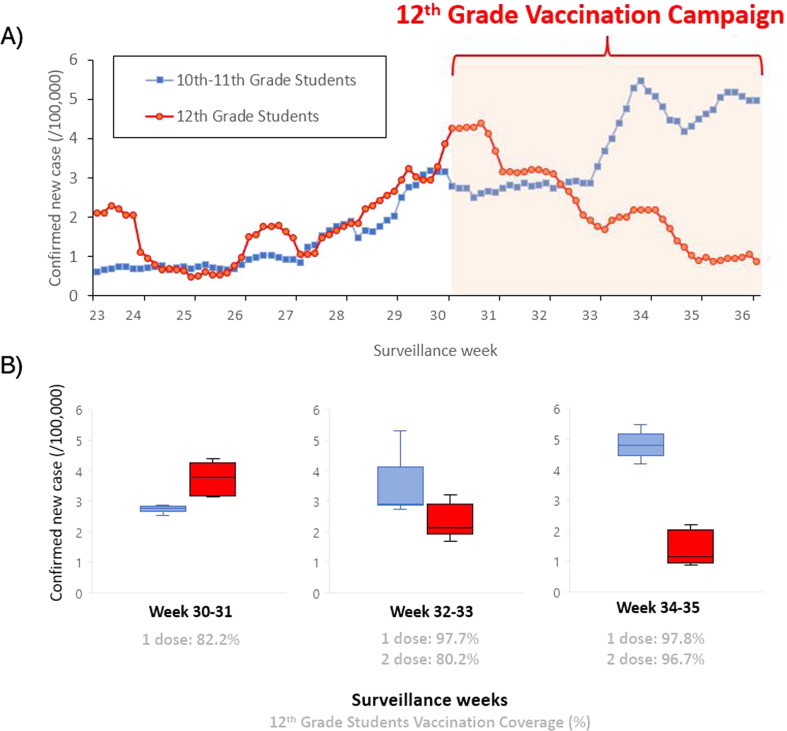
Following the 12th grade student vaccination program, there was a decrease in 12th grade student cases (Fig. 2 A), from 3.73 per 100,000 (95% C.I. 3.47–4.00) during 30–31st week to 2.35 per 100,000 (95% C.I. 2.08–2.63) during 32–33rd week, to 1.39 per 100,000 (95% C.I. 1.12–1.66) after 34–35th week (Fig. 2B). The incidence of 10-11th grade students increased from 2.78 per 100,000 (95% C.I. 2.69–2.88) during 30–31st week to 3.34 per 100,000 (95% C.I. 3.02–3,87) during 32–33rd week to 4.80 per 100,000 (95% C.I. 4.60–5.00) after 34–35th week. Incidence rate ratio for 12th grade students vs. 10-11th grade students has decreased from 1.34 (95% C.I. 1.29–1.39) during 30-31st week to 0.29 (95% C.I. 0.24–0.33) after 34–35th week. Vaccine effectiveness against symptomatic/asymptomatic SARS-CoV-2 infection ≥ 14 days post-first dose vaccination was 91.1% (95% C.I. 89.6–92.5), and ≥ 14 days post-second dose vaccination in adolescent was 99.1% (95% C.I. 98.5–99.5) (Table 2 ).
Fig. 2.
A) Daily laboratory-confirmed SARS-CoV-2 infections among high school students following a nationwide 12th grade students vaccination campaign in Korea; B) Bi-weekly laboratory-confirmed SARS-CoV-2 infections and vaccination coverage rate.
Table 2.
Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection in high school students following a nationwide 12th grade students vaccination campaign in Korea.
| Vaccine Status | High school students |
Vaccine Effectiveness |
|||
|---|---|---|---|---|---|
| Case | Total | Attack Rate (%) | V.E. % | (95% C.I.) | |
| No vaccine | 3,358 | 863,341 | (0.39) | ||
| One dose vaccination (post 14 days) | 153 | 444,322 | (0.03) | 91.1 | (89.6–92.5) |
| Two doses vaccination (post 14 days) | 15 | 439,079 | (0.01) | 99.1 | (98.5–99.5) |
4. Discussion
Among adolescents, receipt of the BNT162b2 vaccine was safe, and was associated with a lower risk of laboratory-confirmed Covid-19 in South Korea. The rate of serious adverse events was low in both vaccination doses, and no potentially vaccine-associated deaths were identified. Our data on safety of BNT162b2 vaccine in adolescents were strengthened by study from Israel, which showed the risk of myocarditis for 16–19 years of age within 21 days of vaccination to be 1.34 per 100,000 (first dose) and 15.07 per 100,000 (second dose) [5]. In the U.S., myocarditis/pericarditis rates were around 12 cases per million doses of second-dose mRNA vaccine among individuals 12–39 years of age [6].
Our finding extends previous clinical trial data on 2,260 adolescent aged 12–15 years resulting vaccine efficacy of 100% (95% C.I., 75.3–100) [2]. Evidence among adolescents on vaccine effectiveness against Covid-19 in real-world settings are limited. A nationwide retrospective cohort study from Israel on vaccine effectiveness of BNT162b2 in adolescents during SARS-CoV-2 delta variant outbreak showed 91.5% (95% CI 88.2–93.9%) post two-dose vaccination [7]. In a test-negative, case-control study at U.S. pediatric hospitals, the effectiveness of 2 doses of BNT162b2 against Covid-19 hospitalization in children and adolescents aged 12–18 years was 93% (95% CI, 83–97) [8]. Note that the BNT162b2 mRNA vaccine was effective against PCR-confirmed SARS-CoV-2 infection in Korean adolescents, despite the B.1.617.2 (delta) variant emerged as the predominant strain in the country [9]. The schools reopened for the fall semester after 34th week, albeit showing continued reduction in cases among 12th grade students. Our finding complements other reported estimates of the effectiveness of the BNT162b2 mRNA vaccine [10], [11], [12], and it confirms that the public health impact in adolescents.
Study limitations include possible confounded result because our estimates are unadjusted because of the lack of demographic data for individual cases.10–11th grade students might be more prone to present for testing than 12th grade students because of concerns about their vulnerability. However, SARS-CoV-2 testing was offered to all suspected persons regardless of presence of symptoms or vaccination status.
As of November 13, 2021, >75% of Korean adult population aged 18 years and older were fully vaccinated against Covid-19, while the vaccination is now open to children and adolescents aged 12 years and older. In this retrospective cohort study of high school students in South Korea, BNT162b2 mRNA vaccination was safe and was associated with a significantly lower risk of SARS-CoV-2 infection. Our finding suggests that vaccination in children and adolescent may reduce the burden of Covid-19 in the community.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We would like to thank the COVID-19 Vaccination Task Force and Division of National Immunization, Korea Disease Control and Prevention Agency for their dedication to provide public health services in mitigating COVID-19 pandemic. Additionally, we are grateful to the staff at the local health departments for their dedication.
Disclaimers
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Korea Disease Control and Prevention Agency, Ministry of Education, or the institutions with which the authors are affiliated.
References
- 1.Bhopal S.S., Bagaria J., Olabi B., Bhopal R. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolescent Health. 2021;5(5):e12–e13. doi: 10.1016/S2352-4642(21)00066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frenck R.W., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. New Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi M.J., Choi W.S., Seong H., Choi J.Y., Kim J.-H., Kim Y.-J., et al. Developing a Framework for Pandemic COVID-19 Vaccine Allocation: a Modified Delphi Consensus Study in Korea. J Korean Med Sci. 2021;36(23) doi: 10.3346/jkms.2021.36.e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh H.-K., Kim E.K., Hwang I., Kim T.E., Lee Y.-K., Lee E., et al. COVID-19 vaccine safety monitoring in the Republic of Korea: February 26, 2021 to April 30, 2021. Osong Public Health Res Perspectives. 2021;12(4):264–268. doi: 10.24171/j.phrp.2021.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mevorach D., Anis E., Cedar N., Bromberg M., Haas E.J., Nadir E., et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. New Engl J Med. 2021;385(23):2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozkurt B., Kamat I., Hotez P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glatman-Freedman A., Hershkovitz Y., Kaufman Z., Dichtiar R., Keinan-Boker L., Bromberg M. Effectiveness of BNT162b2 Vaccine in Adolescents during Outbreak of SARS-CoV-2 Delta Variant Infection, Israel, 2021. Emerg Infect Dis. 2021;27(11):2919–2922. doi: 10.3201/eid2711.211886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson S.M., Newhams M.M., Halasa N.B., Price A.M., Boom J.A., Sahni L.C., et al. Effectiveness of Pfizer-BioNTech mRNA Vaccination Against COVID-19 Hospitalization Among Persons Aged 12–18 Years - United States, June-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(42):1483–1488. doi: 10.15585/mmwr.mm7042e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang H., Lim J.S., Song S.A., Achangwa C., Sim W., Kim G., et al. Transmission dynamics of the Delta variant of SARS-CoV-2 infections in South Korea. J Infectious Dis. 2021 doi: 10.1093/infdis/jiab586. [DOI] [PubMed] [Google Scholar]
- 10.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. New Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H., Yoon S.K., Meece J., et al. Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. New Engl J Med. 2021;385(4):320–329. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson M.G., Stenehjem E., Grannis S., Ball S.W., Naleway A.L., Ong T.C., et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. New Engl J Med. 2021;385(15):1355–1371. doi: 10.1056/NEJMoa2110362. [DOI] [PMC free article] [PubMed] [Google Scholar]