Abstract
Objective
A higher premature ventricular complex (PVC) frequency is associated with incident congestive heart failure (CHF) and death. While certain PVC characteristics may contribute to that risk, the current literature stems from patients in medical settings and is therefore prone to referral bias. This study aims to identify PVC characteristics associated with incident CHF in a community-based setting.
Methods
The Cardiovascular Health Study (CHS) is a cohort of community-dwelling individuals that underwent prospective evaluation and follow-up. We analyzed 24-hour Holter data to assess PVC characteristics and used multivariable logistic and Cox proportional hazards models to identify predictors of a left ventricular ejection fraction (LVEF) decline and incident CHF, respectively.
Results
Of 871 analyzed participants, 316 participants exhibited at least 10 PVCs during the 24-hour recording. For participants with PVCs, the average age was 72 ± 5 years, 41% were women, and 93% were white. Over a median follow up of 11 years, 34% developed CHF. After adjusting for demographics, cardiovascular comorbidities, antiarrhythmic drug use, and PVC frequency, a greater heterogeneity of the PVC coupling interval was associated with an increased risk of LVEF decline and incident CHF. Of note, neither PVC duration nor coupling interval duration exhibited a statistically significant relationship with either outcome.
Conclusions
In this first community-based study to identify Holter-based features of PVCs that are associated with LVEF reduction and incident CHF, the fact that coupling interval heterogeneity was an independent risk factor suggests that the mechanism of PVC generation may influence the risk of heart failure.
Keywords: premature ventricular complexes (PVCs), heart failure, Holter monitoring, coupling interval
INTRODUCTION
Congestive heart failure (CHF) affects more than five million Americans, and the prevalence is expected to increase by 25% within the next fifteen years [1]. Although conventional cardiovascular risk factors, such as advancing age, male sex, diabetes, and hypertension, are important, prediction models for this disease remain incomplete, with an ongoing effort to refine optimal approaches to identifying those at risk [2, 3, 4]. Improved prediction models for this debilitating disease would facilitate early therapies and primary preventive measures.
The concept of premature ventricular complexes (PVCs) as a potential cause of CHF has been largely relegated to electrophysiologists who generally perform ablations only after CHF has developed. Indeed, multiple studies have demonstrated that, among patients with a high PVC burden, successful PVC ablation can result in normalization of left ventricular ejection fraction (LVEF) and resolution of CHF [5, 6]. Various features of PVCs, including frequency [7], morphology [7], duration [8, 9, 10], coupling interval [8], and anatomic exit site [11], have been investigated, but those characteristics are constrained to primarily cross-sectional contexts among those who already have or have not developed CHF, making disentangling cause and effect difficult.
Although we previously demonstrated that an increased PVC frequency among community-dwelling individuals was associated with both the CHF development and a decline in LVEF [12], PVC characteristics (apart from frequency) that predict CHF in the community, specifically those in the general population without clinical indications for continuous rhythm monitoring or PVC ablation, remain unknown [13]. Therefore, when encountering an individual with PVCs in the absence of reduced LVEF, risk stratification is limited. It is also unclear if pre-emptive PVC eradication with ablation would be prudent, and in particular whether patients with certain PVC characteristics might be selected as appropriate candidates. Therefore, we sought to identify Holter-based PVC characteristics associated with a decline in LVEF and the new onset of CHF in a community-based cohort.
METHODS
Study Design and Subjects
We utilized data from the prospective, longitudinal, cohort study of community-based individuals, the Cardiovascular Health Study (CHS). Details of the relevant methods were described previously [14, 15, 16]. In brief, 5,201 adults aged ≥ 65 randomly selected from a list of Medicare beneficiaries in four geographic locations (enrolled by Johns Hopkins University, Wake Forest University, University of Pittsburgh, and University of California, Davis) were recruited between 1989 and 1990. Semi-annual contact alternating between telephone interviews and annual clinical examinations was conducted to obtain information about potential events until 1998–99. Beginning in 2000, participants or their proxies were contacted every 6 months by telephone.
Initially, 1,429 participants were randomly selected to undergo 24-hour Holter monitor. Participants with prevalent CHF or any evidence of ventricular systolic dysfunction on the baseline echocardiogram were excluded. The current analysis included those with available raw Holter data and ≥ 10 PVCs during the 24-hour recording.
Holter Data Assessment
Raw data from Holter monitors were initially analyzed at the Washington University School of Medicine Heart Rate Variability Laboratory using a MARS-8000 Holter scanner (GE Medical Systems, Milwaukee, WI). Secondary quality control was performed on analyzed Holter files to ensure that all RR intervals of the analyzed files aligned with those of the raw data files. Only subjects with mean a difference in RR intervals between raw and analyzed files < 20 ms were included in the analysis. Holter monitors exhibiting atrial fibrillation or paced rhythms were excluded.
For this study, the Holter data was also analyzed using a VivoSense software (Vivonetics, Inc, Newport Coast, CA) that was custom-designed to automatically detect morphologies of all QRS complexes and group each QRS into bins of similar morphologies. An independent analyzer, blinded to the outcomes of the studies, inspected each bin and designated each as representing a sinus or PVC beat. Within the PVC bins, particular PVCs were considered to have the same morphologies if they exhibited the same sequence of deflection with amplitude of each deflection differing < 25% [8].
PVC characteristics, selected based on previous literature, were analyzed to identify predictors independent of PVC frequency: PVC duration, coupling interval, and coupling interval heterogeneity [8, 11, 17, 18, 19]. The frequency of PVCs was calculated as the total number of PVCs divided by the total number of cardiac cycles for the 24-hour recording. PVC characteristics were measured from PVCs with the most prevalent morphology. PVC duration was measured from the averaged tracing of every beat within a bin and was defined as the interval between the complex’ first deflection from an isoelectric line and the end of the last deflection from an isoelectric line. The coupling interval of each PVC was measured from the first deflection from an isoelectric line of the QRS complex of the sinus beat preceding the PVC to the first deflection from an isoelectric line of the QRS complex of the PVC. Coupling intervals of PVCs preceded by PVCs were excluded from analysis. Coupling interval heterogeneity was defined as the SD divided by the mean.
Covariate Ascertainment
Race and biological sex were self-identified. Hypertension was defined as either a reported history of physician-diagnosed hypertension and the use of antihypertensive medications or systolic blood pressure ≥ 140 mmHg or diastolic pressure ≥ 90 mmHg at the baseline visit. Diabetes was defined as a reported use of an antihyperglycemic medications or a fasting glucose level ≥ 126 mg/dL. Myocardial infarctions were identified by participant self-report and were verified through medical record review [14]. Use of Vaughan-Williams class Ia, Ib, Ic, II, III, and IV antiarrhythmics was determined by a medication inventory of prescriptions filled and taken by the patient within 2 weeks prior to the baseline visit [20].
Echocardiographic Evaluation
The echocardiographic assessment of participants in the CHS has been described previously [21, 22]. In brief, 2-dimensional (2D) echocardiography imaging was performed on each participant at baseline and 5 years after enrollment using Toshiba SSH-160A echocardiogram machines (Toshiba Medical Systems, Tustin, CA) [22]. LVEF was qualitatively assessed from the 2D imaging views. Function was qualitatively categorized as normal, borderline, or abnormal, with 94% inter-reader agreement and 98% intra-reader agreement of paired studies [21].
Outcome Ascertainment
A decline in LVEF was defined as a reduction in the qualitative assessments between the 5-year compared to the baseline echocardiograms. To ascertain incident CHF, medical records were reviewed from all hospitalizations after study enrollment. Hospitalized and outpatient episodes of potential CHF were investigated based on initial identification through the International Classification of Diseases (ICD) diagnostic codes, or presence of an endpoint on the hospital face sheet, discharge summary, or outpatient procedure report [16].
Statistical Analyses
Distributions of continuous variables was assessed using both graphical (Q-Q plot) and numerical (Shapiro-Wilk test) methods. Normally distributed continuous variables were reported as means ± SD while those that were not normally distributed are presented as medians and interquartile ranges (IQR). Linear association between continuous variables was measured by Pearson correlation coefficients. Multivariable logistic regression models were used to assess predictors of the 5-year reduction which was defined as a categorial change either from normal to borderline or abnormally reduced LVEF, or from borderline to abnormally reduced LVEF. Predictors of incident CHF were analyzed using multivariable Cox proportional hazard models. Participants were censored upon the time of CHF diagnosis, death, or at the end of follow-up. A sensitivity analysis treating overall mortality as a competing risk was conducted. Orthogonal contrasts were used to assess linear trends in change in LVEF and incident CHF across quartiles of coupling interval heterogeneity [6]. Covariates included in multivariable models were demographics and cardiovascular comorbidities previously shown to be associated with the outcome. Incident CHF analyses were also adjusted for use of class Ia, Ib, Ic, II, and IV antiarrhythmics (no participants took class III drugs); these were not included in the change in LVEF analyses due to a 0 in one of the resulting cells. Model discrimination was measured by calculating C-statistics for LVEF reduction at 5 years and incident CHF at 10 years. Evaluation of the statistical models demonstrated that they were adequate (Supplementary Notes). All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
After exclusion criteria were applied, data from 871 participants were available for analyses. Among those excluded, 39 exhibited abnormally reduced left ventricular (LV) systolic function or had a history of CHF (Supplementary Table I). Three hundred and sixteen participants had ≥ 10 PVCs during the 24-hour Holter monitor recording. Baseline characteristics of these participants are shown in Table 1. No correlation between PVC frequency and coupling interval heterogeneity was observed (Supplementary Figure I). Baseline characteristics of excluded participants can be found in Supplementary Table I.
Table 1:
Baseline characteristics of participants stratified by PVC frequency
| (Total participants = 316) | |
|---|---|
| Mean age (yrs) | 72.2 ± 5.1 |
| Female sex | 131 (41%) |
| Race | |
| - white | 294 (93%) |
| - black | 22 (7%) |
| Mean BMI (kg/m2) | 26.8 ± 4.0 |
| Diabetes | 52 (17%) |
| Hypertension | 179 (57%) |
| History of myocardial infarction | 45 (14%) |
| Smoking | |
| - non-smoker | 117 (37%) |
| - ex-smoker | 164 (52%) |
| - current smoker | 33 (11%) |
| Taking class Ia, Ib, and Ic antiarrhythmics | 10 (3%) |
| Taking class II antiarrhythmics | 1 (0.3%) |
| Taking class III antiarrhythmics | 0 (0%) |
| Taking class IV antiarrhythmics | 20 (6%) |
| PVC frequency (%) | 0.11 ± 0.28 |
| Mean PVC duration (ms) | 155.9 ± 24.3 |
| Mean coupling interval (ms) | 482.5 ± 73.5 |
| Mean coupling interval heterogeneity | 0.11 ± 0.06 |
BMI denotes Body Mass Index
Reduction in LVEF
Two hundred and nine individuals (66%) underwent both a baseline and a 5-year echocardiographic evaluation. Over the course of 5 years, 29 participants (14%) experienced a reduction in their LVEF (Supplementary Table II). In unadjusted analyses, only coupling interval heterogeneity was statistically significantly associated with an LVEF decline (Supplementary Table III). The number of PVC morphologies was not statically associated with LVEF reduction (unadjusted odd ratio 0.82, 95% CI 0.28–2.38, p-value 0.71). After adjusting for age, sex, race, body mass index (BMI), diabetes, hypertension, myocardial infarction, smoking status, and PVC burden, coupling interval heterogeneity remained the only measured PVC characteristic that was statistically significantly associated with an LVEF decline (Figure 1A, Supplementary Table III, and Table 2). Of note, PVC frequency was not statistically significantly associated with an LVEF decline before or after multivariable adjustment. The C-statistic for this multivariable model excluding coupling interval heterogeneity utilizing a 5-year LVEF reduction as the outcome was 0.73 (95% CI 0.62–0.83). The C-statistic was higher after adding coupling interval heterogeneity (0.78, 95% CI 0.69–0.87) although this was not statistically significantly different (p-value 0.14).
Figure 1:
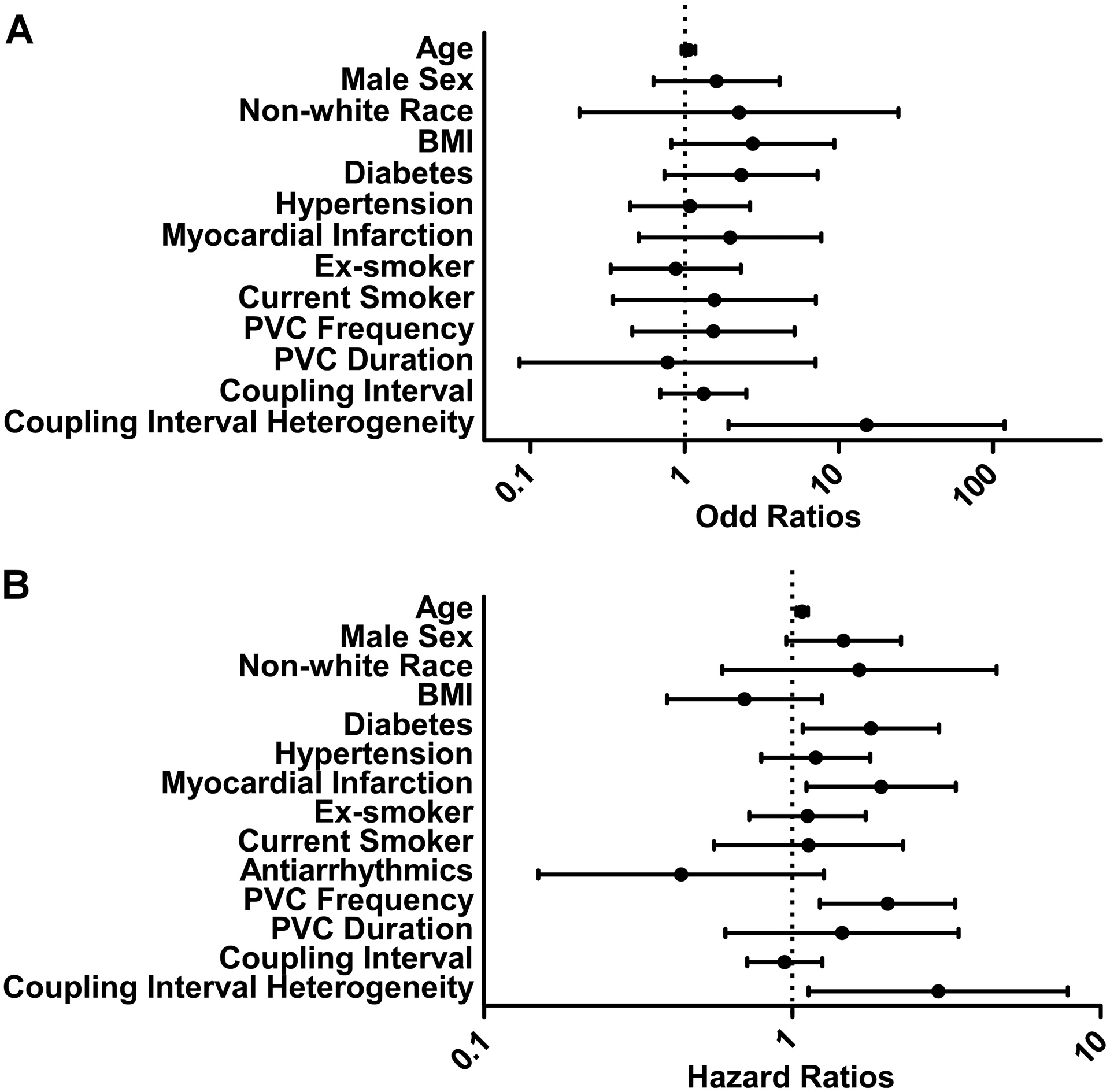
Predictors of LVEF reduction and incident CHF.
Figure 1A: (A) Adjusted OR for predictors of LVEF decline at 5-year follow-up.
Figure 1B: (B) Adjusted HR of incident CHF. BMI (by 10 kg/m2); the reference for former and current smoker is never smoker; antiarrhythmics include Vaughan-Williams class Ia, Ib, Ic, II and IV antiarrhythmics; PVC frequency is per 0.1%; PVC duration is per 100 ms; coupling interval is per 100 ms; coupling interval heterogeneity is log10 transformed. BMI, body mass index; CHF, congestive heart failure; LVEF, left ventricular ejection fraction; PVC, premature ventricular complex.
Table 2:
Association between quartiles of coupling interval heterogeneity and change in LVEF
| Variable | Unadjusted OR | 95% CI | p-value | Adjusted OR* | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Quartile 1 | Reference | Reference | ||||
| Quartile 2 | 2.05 | 0.46 to 9.04 | 0.68 | 1.72 | 0.34 to 8.58 | 0.51 |
| Quartile 3 | 2.2 | 0.52 to 9.29 | 0.81 | 1.78 | 0.38 to 8.45 | 0.47 |
| Quartile 4 | 7.64 | 2.04 to 28.6 | 0.0004 | 7.92 | 1.84 to 34.0 | 0.005 |
| Test of Trend | 0.005 | 0.009 |
Adjusted for age, gender, race, body mass index, history of diabetes, hypertension, myocardial infarction, smoking, PVC frequency, PVC duration, and coupling interval duration
Incident CHF
Over a median follow-up of 11 years (IQR 6–16 years), 108 participants (34%) developed incident CHF. The median time to incident CHF was 9 years (IQR 5–13 years). The number of PVC morphologies was not statically associated with incident CHF (unadjusted hazard ratio 1.03, 95% CI 0.68–1.55, p-value 0.90). In unadjusted analyses, older age, male sex, diabetes, history of a myocardial infarction, and PVC frequency (Supplementary Table IV) were associated with incident CHF. After adjusting for age, sex, race, BMI, diabetes, hypertension, myocardial infarction, smoking status, and use of antiarrhythmics, every 0.1% increase in baseline PVC frequency was associated with a more than two-fold increased risk of incident CHF (Figure 1B). Older age, diabetes, and a history of myocardial infarction also remained significantly associated with incident CHF in the multivariable model (Supplementary Table IV). After adjusting for the same covariates, each increasing quartile of PVC coupling interval heterogeneity was associated with a higher risk of incident CHF (Figure 2). C-statistics for this multivariable model using 10-year incident CHF risk as the outcome without and with coupling interval heterogeneity included were neither meaningfully nor statistically different (0.72, 95% CI 0.64–0.80) and 0.72 (95% CI0.64–0.80), respectively, (p-value 0.84). In a sensitivity analysis treating death as a competing risk, both PVC frequency and coupling interval heterogeneity remained statistically associated with a higher risk of incident CHF (Supplementary Figure II).
Figure 2:
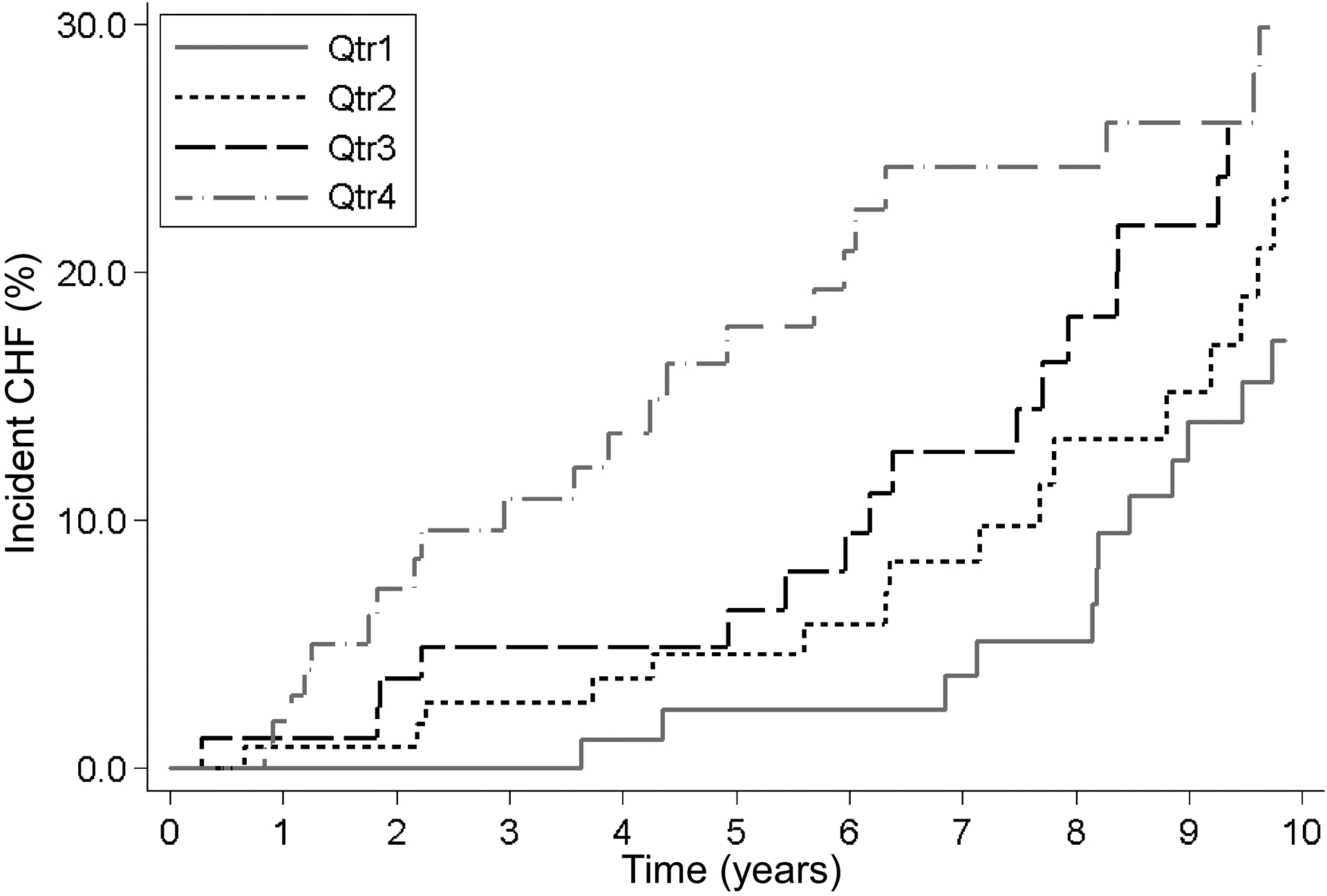
Association between quartiles of coupling interval heterogeneity and incident CHF over time. Kaplan-Meier curves adjusted for age, gender, race, body mass index, history of diabetes, hypertension, myocardial infarction, smoking, use of Vaughan-Williams class Ia, Ib, Ic, II and IV antiarrhythmics, PVC frequency, PVC duration and coupling interval duration. CHF, congestive heart failure; PVC, premature ventricular complex.
DISCUSSION
In a prospective, community-based cohort, we found that PVC coupling interval heterogeneity was associated with both LVEF reduction and incident CHF after taking participant characteristics and PVC frequency into account.
PVCs are known to be associated with development of CHF. For instance, a high PVC burden has consistently been shown to predict incident CHF [7]. Other PVC characteristics beyond frequency, including PVC duration, morphology, and coupling interval duration, may help refine risk prediction. These characteristics are determined by several factors, including the anatomic location of PVC exit site and the electrophysiologic mechanism of arrhythmogenesis. For example, a wider PVC complex generally represents a PVC with increased ventricular conduction time, such as from the lateral LV, which may more likely lead to LV dyssynchrony and a consequent reduction in LVEF [23]. Three known mechanisms for PVC generation include automaticity, re-entry, and triggered activity. Coupling intervals of PVCs caused by automaticity or parasystole tend to vary, while coupling intervals of PVCs caused by re-entry and triggered activity tend to be fairly constant [17].
Previous studies that investigated PVC characteristics have been cross-sectional, either in a population with clinical indications for continuous cardiac monitoring or patients with PVCs being referred for ablation [9, 17, 18, 19]. These studies have shown an association between increased PVC coupling interval heterogeneity and cardiomyopathy [17, 18, 19]. In these populations, extant cardiac dysfunction makes it difficult, if not impossible, to determine the temporal relationships between PVC characteristics and CHF, and referral bias may hinder accurate extrapolations to the general population. Our prospective study, performed in a community-dwelling cohort without cardiomyopathy at baseline, has shown, for the first time, that PVC coupling interval heterogeneity (and not the coupling interval duration itself) forecasts a reduction in LVEF and incident CHF. Given the expected greater variability in coupling interval among PVCs generated by automaticity or parasystole, it appears such PVCs, as opposed to those arising from triggered activity or potentially reentry, are more likely to be associated with future LV dysfunction and ultimately the development of heart failure.
An irregular ventricular rhythm, as would be expected with PVCs with more variable coupling intervals, has been shown to promote LV dysfunction both in vitro and in observational clinical studies. Pacing monolayers of neonatal rat ventricular cardiomyocytes at irregular cycle lengths adversely altered expression of calcium handling proteins and dynamics of calcium cycling compared to pacing at regular cycle length at the same average frequency [24]. Additionally, PVCs with higher coupling interval heterogeneity may promote LV dysfunction via a ‘cardio-cardiac reflex:’ in a porcine model, right ventricular outflow tract PVCs with variable coupling intervals elicited more responses from intrinsic cardiac neurons compared to PVCs with fixed coupling intervals [25]. In observational studies, patients with rate-controlled atrial fibrillation have been shown to experience an improved LVEF after maintenance of normal sinus rhythm [26, 27].
In the most recent guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society, therapies for PVCs, whether medication or catheter ablation, are only recommended in patients with symptoms or those who have already developed reduced LV systolic function [28]. There is no current consensus regarding optimal methods to identify patients at risk for a decline in LV systolic function related to PVCs. At the same time, enhanced convenience of continuously recording ECG devices and growing use of direct-to-consumer wearables with ECG capabilities will likely lead to more questions among patients found to have remarkable PVC burdens. Ultimately, well-designed randomized controlled trials will be needed before establishing a clear guideline to inform the best approaches in these patients. Our results, based on a community-dwelling population not prone to referral bias, should add to the literature that itself can contribute to the hypotheses which can be tested in such a trial, specifically to determine whether greater coupling internal heterogeneity plays an important role in predicting a reduction in LV systolic dysfunction.
Study limitations
Several limitations of this study should be acknowledged. Because the current analysis focused on a community-based population and not patients selected for high burdens of PVCs (such as those seen in cardiac electrophysiology practices), we acknowledge that our observations may not fully apply to those selected individuals. Only individuals aged ≥ 65 were included. Therefore, these findings might not be generalizable to younger individuals. Exclusion of participants with baseline CHF or cardiomyopathy could have inadvertently created a selection bias against those who have already demonstrated some propensity to maintain normal cardiac function despite frequent PVCs, and it is possible that our current findings may not generalize to those with existing CHF. Moreover, our analyses do not include interval changes of predictors, for example, new diagnosis of myocardial infarction, initiation or cessation of antiarrhythmic usage, which could have either attenuated or accentuated our observed results. Furthermore, PVC characteristics may change over time [29], and it is important to acknowledge that this current study reports on a cross-sectional evaluation of one Holter per participant in time. Additionally, we did not include every covariate that may be associated with the risk of CHF, such as lack of physical activity or alcohol consumption [30], and future research may identify relationships between such modifiable risk factors and PVC characteristics that are relevant to CHF risk. In addition, we cannot exclude insufficient power as an explanation for any of our negative (non-statistically significant) results. It is also possible that coupling interval heterogeneity is an epiphenomenon that travels with certain PVCs from a particular location that might be evident from 12-lead ECGs. However, the absence of a relationship between PVC duration (as a crude differentiator of different PVC morphologies) and either an LVEF decline or incident CHF may make this less likely. Finally, as this was an observational study, we cannot exclude residual or unmeasured confounders and therefore cannot confidently comment on causal effects.
CONCLUSION
In this first community-based study to identify Holter-based features of PVCs related to prospectively ascertained changes in ventricular function, greater PVC coupling interval heterogeneity was associated with both LVEF reduction and incident CHF. These findings suggest that the mechanism of PVC generation may influence the risk of CHF. This knowledge might contribute to understanding the pathophysiologic process underlying PVC-induced cardiomyopathy and help to identify those at higher risk of developing the disease.
Supplementary Material
KEY QUESTIONS.
What is already known about this subject?
Multiple studies have explored premature ventricular complexes (PVCs) as a potential cause of congestive heart failure (CHF). Although various features of PVCs have been investigated, those characteristics are constrained to primarily cross-sectional contexts among those who may already have CHF, making disentangling cause and effect difficult.
What does this study add?
Here, we analyzed features of PVCs from baseline Holter-based electrocardiograms in a cohort of community-dwelling adults without CHF to prospectively ascertain changes in left ventricular (LV) function and incident CHF. In this first community-based study, we demonstrated that greater PVC coupling interval heterogeneity was associated with both LV function reduction and an increase risk of developing CHF.
How might this impact on clinical practice?
This knowledge might contribute to understanding the pathophysiologic process underlying PVC-induced cardiomyopathy, and ultimately help to risk stratify adults with PVC burden.
Funding
In addition to a Resident Clinical and Translational Research Funding (RRF) and a Cardiology Innovation Award from the University of California, San Francisco, this research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Footnotes
Prior Publication and Presentation
Part of the data and the abstract included in this manuscript were electronically presented at the Heart Rhythm Society Scientific Sessions in San Diego, May of 2020.
Competing interests
None.
Ethics approval
The University of California, San Francisco Institutional Review Board provided a certificate of approval to conduct these analyses of de-identified data.
Patient and Public Involvement statement
Patient and public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review
Not commissioned; externally peer reviewed.
Data availability statement
Data availability is subjected to the Cardiovascular Health Study policy.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal SK, Chambless LE, Ballantyne CM, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation Heart failure 2012;5:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chahal H, Bluemke DA, Wu CO, et al. Heart failure risk prediction in the Multi-Ethnic Study of Atherosclerosis. Heart 2015;101:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JG, Newton-Cheh C, Almgren P, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. Journal of the American College of Cardiology 2010;56:1712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takemoto M, Yoshimura H, Ohba Y, et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. Journal of the American College of Cardiology 2005;45:1259–65. [DOI] [PubMed] [Google Scholar]
- 6.Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009;95:1230–7. [DOI] [PubMed] [Google Scholar]
- 7.Ephrem G, Levine M, Friedmann P, et al. The prognostic significance of frequency and morphology of premature ventricular complexes during ambulatory holter monitoring. Annals of noninvasive electrocardiology : the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc 2013;18:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher MM, Padula M, Sgueglia M, et al. Electrocardiographic markers of structural heart disease and predictors of death in 2332 unselected patients undergoing outpatient Holter recording. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 2007;9:1203–8. [DOI] [PubMed] [Google Scholar]
- 9.Carballeira Pol L, Deyell MW, Frankel DS, et al. Ventricular premature depolarization QRS duration as a new marker of risk for the development of ventricular premature depolarization-induced cardiomyopathy. Heart rhythm 2014;11:299–306. [DOI] [PubMed] [Google Scholar]
- 10.Del Carpio Munoz F, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. Journal of cardiovascular electrophysiology 2011;22:791–8. [DOI] [PubMed] [Google Scholar]
- 11.Aktas MK, Mittal S, Kutyifa V, et al. The Burden and Morphology of Premature Ventricular Contractions and their Impact on Clinical Outcomes in Patients Receiving Biventricular Pacing in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Annals of noninvasive electrocardiology : the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc 2016;21:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dukes JW, Dewland TA, Vittinghoff E, et al. Ventricular Ectopy as a Predictor of Heart Failure and Death. Journal of the American College of Cardiology 2015;66:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus GM. Evaluation and Management of Premature Ventricular Complexes. Circulation 2020;141:1404–18. [DOI] [PubMed] [Google Scholar]
- 14.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Annals of epidemiology 1995;5:278–85. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Annals of epidemiology 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 16.Psaty BM, Delaney JA, Arnold AM, et al. Study of Cardiovascular Health Outcomes in the Era of Claims Data: The Cardiovascular Health Study. Circulation 2016;133:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vries LJ, Martirosyan M, van Domburg RT, et al. Coupling interval variability of premature ventricular contractions in patients with different underlying pathology: an insight into the arrhythmia mechanism. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing 2018;51:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamura M, Badhwar N, Vedantham V, et al. Coupling interval dispersion and body mass index are independent predictors of idiopathic premature ventricular complex-induced cardiomyopathy. Journal of cardiovascular electrophysiology 2014;25:756–62. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu T, Ikeda K, Tomoike H. Assessment of the variability in coupling intervals of ventricular premature contractions. Japanese circulation journal 1993;57:781–8. [DOI] [PubMed] [Google Scholar]
- 20.Psaty BM, Lee M, Savage PJ, et al. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. Journal of clinical epidemiology 1992;45:683–92. [DOI] [PubMed] [Google Scholar]
- 21.Gardin JM, Siscovick D, Anton-Culver H, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation 1995;91:1739–48. [DOI] [PubMed] [Google Scholar]
- 22.Gardin JM, Wong ND, Bommer W, et al. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography 1992;5:63–72. [DOI] [PubMed] [Google Scholar]
- 23.Walters TE, Rahmutula D, Szilagyi J, et al. Left Ventricular Dyssynchrony Predicts the Cardiomyopathy Associated With Premature Ventricular Contractions. Journal of the American College of Cardiology 2018;72:2870–82. [DOI] [PubMed] [Google Scholar]
- 24.Ling LH, Khammy O, Byrne M, et al. Irregular rhythm adversely influences calcium handling in ventricular myocardium: implications for the interaction between heart failure and atrial fibrillation. Circulation Heart failure 2012;5:786–93. [DOI] [PubMed] [Google Scholar]
- 25.Hamon D, Rajendran PS, Chui RW, et al. Premature Ventricular Contraction Coupling Interval Variability Destabilizes Cardiac Neuronal and Electrophysiological Control: Insights From Simultaneous Cardioneural Mapping. Circulation Arrhythmia and electrophysiology 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kieny JR, Sacrez A, Facello A, et al. Increase in radionuclide left ventricular ejection fraction after cardioversion of chronic atrial fibrillation in idiopathic dilated cardiomyopathy. European heart journal 1992;13:1290–5. [DOI] [PubMed] [Google Scholar]
- 27.Stulak JM, Dearani JA, Daly RC, et al. Left ventricular dysfunction in atrial fibrillation: restoration of sinus rhythm by the Cox-maze procedure significantly improves systolic function and functional status. The Annals of thoracic surgery 2006;82:494–500; discussion −1. [DOI] [PubMed] [Google Scholar]
- 28.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018;138:e210–e71. [DOI] [PubMed] [Google Scholar]
- 29.Lee AKY, Andrade J, Hawkins NM, et al. Outcomes of untreated frequent premature ventricular complexes with normal left ventricular function. Heart 2019;105:1408–13. [DOI] [PubMed] [Google Scholar]
- 30.Del Gobbo LC, Kalantarian S, Imamura F, et al. Contribution of Major Lifestyle Risk Factors for Incident Heart Failure in Older Adults: The Cardiovascular Health Study. JACC Heart failure 2015;3:520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability is subjected to the Cardiovascular Health Study policy.


