Abstract
GATA2 deficiency was described in 2011, and shortly thereafter allogeneic hematopoietic stem cell transplantation (HSCT) was shown to reverse the hematologic disease phenotype. However, there remain major unanswered questions regarding the type of conditioning regimen, type of donors, and graft-versus-host disease (GVHD) prophylaxis. We report 59 patients with GATA2 mutations undergoing HSCT at National Institutes of Health between 2013 and 2020. Primary endpoints were engraftment, reverse of the clinical phenotype, secondary endpoints were overall survival (OS), event-free survival (EFS), and the incidence of acute and chronic GVHD. The OS and EFS at 4 years were 85.1% and 82.1% respectively. Ninety-six percent of surviving patients had reversal of the hematologic disease phenotype by one-year post-transplant. Incidence of grade III-IV aGVHD in matched related donor (MRD) and matched unrelated donor recipients (URD) patients receiving Tacrolimus/Methotrexate for GVHD prophylaxis was 32%. In contrast, in the MRD and URD who received post-transplant cyclophosphamide (PT/Cy), no patient developed grade III-IV aGVHD. Six percent of haploidentical related donor (HRD) recipients developed grade III-IV aGVHD. In summary, a busulfan-based HSCT regimen in GATA2 deficiency reverses the hematologic disease phenotype, and the use of PT/Cy reduced the risk of both aGVHD and cGVHD.
Keywords: GATA2, transplantation, donor, allogeneic, cyclophosphamide
Introduction
A decade ago, a new disease syndrome was described resulting from heterogeneous familial or sporadic mutations in the transcription factor GATA2.(1–4) The morbidity and mortality of the disease results not only from serious opportunistic infections, but from the proclivity of the disease to evolve into a myelodysplastic syndrome (MDS) with further progression into acute myelogenous leukemia (AML) or chronic myelomonocytic leukemia (CMML). Once infectious symptoms appear, or evidence of MDS or bone marrow failure occurs, the disease tends to display a progressive downhill course leading to death from infection or MDS/AML.
In our initial report we used a nonmyeloablative hematopoietic stem cell transplant (HSCT) conditioning regimen in 14 patients with the MonoMAC syndrome or GATA2 deficiency.(5) There was one relapse in the four matched related donor (MRD) recipients, and one graft rejection in the four unrelated donor (URD) recipients. Together, the one graft rejection, the one relapse, and the need for pre-transplant cycles of chemotherapy prior to the conditioning regimen in three patients, indicated that a more intensive conditioning regimen might obviate the need for chemotherapy preceding the conditioning regimen and result in an improved disease outcome by reducing the incidence of graft rejection and relapse.
In 2018 we reported our initial results of a clinical trial involving 22 patients with allogeneic HSCT for GATA2 deficiency using a busulfan-based conditioning regimen and noted a 86% disease-free survival.(6) With continuing accrual, subsequent analysis indicated that despite a 10/10 HLA match of MRD and URD, there was a 32% incidence of grade III-IV acute graft-versus-host disease (aGVHD) with Tacrolimus/Methotrexate (Tacro/MTX) GVHD prophylaxis. In contrast, there was no grade III-IV aGVHD in the haploidentical related donor (HRD) recipients, all of whom received GVHD prophylaxis with post-transplantation cyclophosphamide (PT/Cy) followed by tacrolimus/mycophenolate (Tacro/MMF). (7) We subsequently replaced Tacro/MTX with PT/Cy followed by Tacrolimus/Mycophenolate (Tacro/MMF) in the regimen for MRD and URD patients. We now report on this expanded cohort of 59 patients, representing the largest experience with HSCT for GATA2 deficiency to date, and demonstrate that a busulfan-based regimen coupled with PT/Cy results in the highest GVHD-free OS and EFS.
Methods
Study Design and Participants
This single-institution protocol 13-C-0132 was approved by the Institutional Review Board of the National Cancer Institute and conducted at the National Institutes of Health Clinical Center between August of 2013 and May 31st of 2021.(8) All patients > 18 years of age gave written informed consent. Minors gave assent. The study was monitored for safety and data accuracy (ClinicalTrials.gov number: NCT00923364, Pilot and Feasibility Study of Reduced-Intensity Hematopoietic Stem Cell Transplant for MonoMAC). All parents of pediatric patients (<18 years) provided written, informed consent to participate in this study.
Patients between 8 to 70 years of age were eligible if they met the following criteria: 1) at least one episode of a life-threatening infection; 2) mutation in the GATA2 gene with variant allele frequency consistent with germline transmission; 3) flow cytometry profile on peripheral blood demonstrating severe monocytopenia and CD19+ B-cell and CD3-CD56+ NK cell lymphopenia, and MDS; or 4) infections consistent with the MonoMAC phenotype (atypical mycobacterial infection AND flow cytometry profile on peripheral blood demonstrating severe monocytopenia and CD19+ B-cell and CD3-CD56+ NK cell lymphopenia,) (8); 5) 10/10 or 9/10 MRD or URD, or a haploidentical related donor.
Sixty patients were enrolled on this study and underwent allogeneic HSCT between 2013 and 2020; there were 55 patients with verified GATA2 mutations. One patient with MonoMAC syndrome was excluded when subsequent whole exome sequencing revealed a homozygous stop codon in the adenosine deaminase type 2 gene (DADA2).(9) There were 4 patients with the MonoMAC syndrome including two second cousins. This study includes the 22 patients previously reported. (6)
Statistical aspects of the trial design
The trial began as a limited size pilot study, with a total of 15 evaluable recipients permitted. With continuing successful results over time, additional patients were permitted by design to enroll on the trial to improve the precision of the estimate of the fraction engrafting and who experience reversal of the clinical survival as well as overall survival (OS) and event free survival (EFS). The trial was always a single arm trial, with provision made for stopping the trial based on development of severe adverse events.
Statistical Analysis
The primary endpoints were engraftment and reversal of the clinical phenotype with normalization of cytopenias characteristic of GATA2 deficiency. The secondary endpoints were OS, EFS, and incidence of aGVHD and cGVHD. EFS was calculated from the date of transplant until the date of the first among possible events: relapse, death, graft failure from any cause, or second transplant. Patients without an event had their EFS interval censored on May 31, 2021. Overall Survival was calculated from the transplantation date to the date of death or censored on May 31, 2021 for those who remained alive. The Kaplan-Meier method was used to calculate the probability of OS and EFS as a function of time, with the difference among the curves determined by a long-rank test. The cumulative incidence of development of grade III-IV aGVHD or moderate to severe cGVHD was determined using the inverse Kaplan-Meier method, censoring for death, secondary transplant, or lack of development of GVHD of the degree necessary to be considered an event. Descriptive statistics were used for chimerism, monocyte, NK and lymphocyte counts. All analyses were performed using GraphPad Prism or SAS version 9.4 software. The difference between binary variables between two groups was assessed using Fisher’s exact test, while the difference in binary variables across the three groups was assessed using Mehta’s modification to Fisher’s exact test. Continuous parameters were compared among the five groups using an exact Kruskal-Wallis test. Hazard ratios for paired comparisons between groups were determined using a Cox proportional hazards model.
HLA
HLA-matching was confirmed by molecular four-digit typing (HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA DQB1).
Exclusion Criteria for Donors
Exclusion criteria included mutation in GATA2, history of mycobacterial or other opportunistic infections, or abnormal monocyte, NK or B cell counts. If more than one donor was available, each donor was evaluated individually in terms of HLA-matching, age, overall health, ABO matching, CMV status, etc. to select the best donor.
Conditioning Regimen
MRD and URD recipients received 4 days busulfan with the dose adjusted to target an AUC between 3200 and 4800 μMol/L/min (cumulative busulfan of 52–79 mg*h/L for matched related and unrelated donors; cumulative busulfan exposure of 26–59 mg*h/L for haploidentical related donor recipients depending upon whether they received two or three days of busulfan. Busulfan dosing was based upon a test dose of busulfan administered at 0.8 mg/kg approximately one week before the start of conditioning). For matched related and unrelated donor recipients busulfan was given IV on days −6, −5, −4, and −3 and administered once daily over 3 hours as an infusion. Fludarabine 40 mg/m2 per day was given on days −6, −5, −4, and −3. HRD recipients received fludarabine 30 mg/m2 per day IV for 5 days (days −6 to −2), cyclophosphamide 14.5 mg/kg IV for 2 days (days −6 and −5), dose adjusted busulfan on days −4 and −3 (and day −2 if a clonal cytogenetic abnormality was present), and 200 cGy TBI on day −1.(7) Serotherapy with anti-thymocyte globulin or alemtuzumab was not used in the conditioning regimen for two reasons. First, most GATA2 patients have serious viral infections pre-transplant, and serotherapy would exacerbate viral reactivation and disease. Second, normal donor hematopoietic stem cells have a survival advantage over GATA2 bone marrow, thus serotherapy is not required to facilitate engraftment.
GVHD Prophylaxis
MRD and URD received Tacrolimus starting on day −3 and methotrexate (MTX) 5 mg/m2 intravenously (IV) on days +1, +3, +6, and +11. Tacrolimus was continued for 6 months and then stopped if no GVHD was present. Following an amendment in 2017, MRD and URD with normal or favorable cytogenetics including trisomy 8 received GVHD prophylaxis with PT/Cy 50 mg/kg/day IV for 2 days on days +3 and +4 followed by Tacrolimus and Mycophenolate Mofetil (Tacro/MMF) starting on day +5.(7) In the PT/Cy treatment arm, MMF was administered from days +5 to +35, and Tacrolimus was administered from day +5 to day +180, then stopped if no GVHD was present. Post-transplant immunosuppression for HRD consisted of PT/Cy 50mg/kg/day IV for 2 days on days +3 and +4 followed by Tacro/MMF starting on day +5.(7) Immunosuppression with MMF was stopped at day +35, and immunosuppression with tacrolimus was stopped at 6 months post-transplant if there was no evidence of GVHD.
Engraftment
Engraftment was defined as an absolute neutrophil count of greater than 500/μl for three successive days.
Analysis of Chimerism
Chimerism was analyzed by analysis of informative microsatellite DNA sequences. Peripheral blood CD14+, CD3-/CD56+, CD19+ and CD3+ subsets were isolated by flow cytometry at designated time points and analyzed for short-tandem repeats (STR). In addition, CD14+/CD15+ myeloid cells and CD3+ T-lymphocytes were selected using immunobeads and chimerism was assessed on the selected cells.
Immune reconstitution of T, B, and NK Cells and monocytes
CD14+ monocytes, CD3-/CD56+ NK cells, CD19+ B-lymphocytes, and CD3+, CD4+, and CD8+ T- lymphocytes were quantified by flow cytometry using subset specific monoclonal antibodies pre-transplant, and at designated intervals post-transplant.
Supportive Care
Standard guidelines for supportive care at the National Institutes of Health Clinical Center for patients undergoing allogeneic HSCT were used. These guidelines follow international guidelines for preventing infectious complications among HSCT recipients (Supplementary Methods).(10)
Results
Infectious disease co-morbidities
There were considerable infectious co-morbidities in this cohort of patients (Table I and Supplementary Table I). In total, twenty-five percent (15/59) of patients had history of non-tuberculous mycobacterial (NTM) infections, including three who came to transplant with active disease. However, there were no instances of reactivation of NTM infections following HSCT. Human papilloma virus (HPV) infections were especially frequent in this cohort with 32/59 (54%) of patients having HPV prior to HSCT.
Table I.
Patients and Pre-Transplant Characteristics
| Donor Type: Post-Transplant Conditioning | MRD/URD Tacro/MTX N=19 | MRD/URD PT/Cy N=23 | HRD PT/Cy N=17 |
|---|---|---|---|
| Average Age at Transplant years (range) | 28 (18–52) | 29 (14–45) | 28 (16–46) |
| Sex (%) | |||
| Male | 10 (53) | 6 (26) | 3 (18) |
| Female | 9 (47) | 17(74) | 14 (82) |
| Cytogenetics (%) | |||
| Normal | 8 (42) | 15 (65) | 11(65) |
| Abnormal | 11 (58) | 8 (35) | 6 (35) |
| Bone Marrow Cellularity (%) | |||
| Normocellular | 4 (17) | ||
| Hypocellular | 16 (84) | 18 (78) | 15 (88) |
| Hypercellular | 3 (16) | 1 (4) | 2 (12) |
| Viral Infection (%) | |||
| HPV | 8 (42) | 16 (70) | 8 (47) |
| Other Infections (%) | |||
| NTM | 4 (21) | 5 (22) | 6 (35) |
| Other Complications (%) | |||
| MDS | 14 (74) | 14 (61) | 13 (76) |
| Autoimmunity, Lymphedema, Deafness | 7 (37) | 10 (43) | 2 (12) |
HPV, Human Papilloma Virus; MRD, Matched Related Donor, URD, Unrelated Donor; HRD, Haploidentical Related Donor; MDS, Myelodysplastic Syndrome; NTM, Non-Tuberculous Mycobacterial Infection
Viral-driven malignancies were present in three patients, one with an HPV-driven squamous cell carcinoma of the rectum, one with an HPV-driven squamous cell carcinoma of the vulva, and one with a multifocal Epstein Barr Virus (EBV) spindle cell tumor of the liver and spine. This latter patient had complete eradication of the tumor in the liver and spine by imaging studies 100 days post-HSCT and is now 7 years post-transplant.(11) There was one HPV driven lesion that progressed to metastatic squamous cell carcinoma of the rectum two years following transplant; this resulted in a fatal outcome.
Myeloid abnormalities
Myeloid abnormalities were common in this cohort of patients prior to HSCT, especially MDS (Table I). Three patients had advanced myeloid malignancies, one with AML in remission after induction chemotherapy (Supplementary Table Ic, patient 7), one with a refractory AML (Supplementary Table Ic, patient 3), and one with CMML (Supplementary Table Ie, patient 13). One patient had a routine screening marrow which showed trisomy 8 and 10% myeloblasts (Supplementary Table Id, patient 15). Cytogenetic abnormalities were common in this group of patients: trisomy 8 was present in 25% (15/59) patients and monosomy 7 in 10% (6/59). Notably, 6 patients had translocations involving chromosome 1q; four patients had a der (1;7) (q10; p10) resulting in a 1q+ and 7q-, (Supplementary Table I), and two patients had other translocations involving chromosome 1q.
Myeloid progression post-HSCT was a major concern. In this regard, one patient with refractory AML who received an URD HSCT (Supplementary Table IIc, patient 3) relapsed with AML by day 30 and underwent a second transplant from the same donor; she died from persistent AML at day 100 following the second HSCT. One patient with AML in first complete remission remains in complete remission nearly 4 years after URD HSCT. A 15-year-old with CMML received two cycles of venetoclax and decitabine pre-HSCT; he remains in remission two years after HRD.(12) One asymptomatic patient with 10% myeloblasts and trisomy 8 on a routine screening bone marrow also was in remission following two cycles of venetoclax/decitabine and remains in remission nearly two years following URD HSCT. One HRD recipient with MDS relapsed with acute myelomonocytic leukemia (AMML) one and one-half years post-HSCT and was re-transplanted with an unrelated donor (Supplementary Table Ic, patient 16). She subsequently died of an immune reconstitution syndrome. No other patient has relapsed with a myeloid malignancy.
The transplant characteristics for the three treatment groups— MRD/URD Tacro/MTX, MRD/URD PT/Cy, and HRD— are shown in Tables I and II (Supplementary Tables I and II). Median potential follow-up for MRD/URD patients receiving Tacro/MTX was 5.4 years, for MRD/URD patients receiving PT/Cy was 3.5 years, and for HRD patients receiving PT/Cy was 4.2 years. The stem cell sources are shown in Table II. All but one of the MRD/URD HLA matches were 10/10; the only 9/10 HLA match was in the URD PT/Cy treatment arm.
Table II.
Transplant Information and Complications
| Donor Type: Post-Transplant Conditioning | MRD/URD Tacro/MTX N=19 | MRD/URD PT/Cy N=23 | HRD PT/Cy N=17 | Overall p-value |
|---|---|---|---|---|
| Donor Gender (%) | ||||
| Female | 7 (37) | 9 (39) | 10 (59) | |
| Male | 12 (63) | 14 (61) | 7 (41) | |
| Average--BU Target AUC μMol/L/min | ||||
| 4428.3 | 3852.2 | 4064.3 (2 days BU) | <0.0001 * | |
| 3866.7 (3 days BU) | <0.0001 ** | |||
| Cumulative Busulfan Exposure mg*h/L | ||||
| 72.6 | 63.1 | 33.3 ( 2 days BU) | <0.0001 * | |
| 49.2 (3 days BU) | <0.0001 ** | |||
| HLA match (%) | ||||
| 10/10 | 19 (100) | 22 (96) | ||
| 9/10 | 1 (4) | |||
| 8/10 | 2 (12) | |||
| 7/10 | 2 (12) | |||
| 6/10 | 2 (12) | |||
| 5/10 | 11 (65) | |||
| Stem-Cell Source (%) | ||||
| PBSC | 8 (42) | 3 (13) | 2 (12) | |
| BM | 11 (58) | 20 (87) | 15 (88) | |
| Average CD34+/Kg Infused | ||||
| 4.9 × 106 | 4.62 × 106 | 4.79 × 106 | 0.91 | |
| Average CD3 +/Kg Infused | ||||
| 6.5 × 107 | 6.17 × 107 | 4.82 × 107 | 0.99 | |
| Average Days of Engraftment | ||||
| Neutrophils | 12.53 | 16.22 | 16.2 | 0.0002 |
| Platelets | 16.9 | 23.45 | 20.71 | 0.0034 |
| aGVHD III-IV | ||||
| 6 (32) | 0 | 1 (6) | p=0.0014 | |
| cGVHD moderate-severe | ||||
| 8 (42) | 2 (9) | 4 (24) | p=0.042 | |
| Average Time after Transplant (years) | ||||
| 4.44 | 2.74 | 4.2 | 0.0049 | |
| Current Status (%) | ||||
| Off of immunosuppression | 9 (47) | 13 (57) | 10 (59) | |
| On immunosuppression | 5(26) | 7 (30) | 6 (35) | |
| Dead | 4 (21) | 3 (13) | 1 (6) | |
| Alive but lost to follow-up | 1 (5) | |||
= comparison with three groups, but limited to 2 BU days for HRD PT/Cy
=comparison with three groups, but limited to 3 BU days for HRD PT/Cy
aGVHD indicates acute graft vs. host disease; cGVHD, chronic graft vs. host disease; HLA, human leukocyte antigen; other abbreviations are explained in Table I.
Conditioning
The dose of busulfan was de-escalated during the study due to the high levels of engraftment and the absence of relapse. MRD/URD on the treatment arm receiving Tacro/MTX were transplanted earlier in the study. MRD/URD receiving Tacro/MTX had a cumulative busulfan exposure of 72.6 mg*h/L; In contrast, MRD/URD receiving PT/CY had a cumulative busulfan exposure of 63.1mg*h/L. HRD without clonal cytogenetic abnormalities received two days of busulfan for a cumulative busulfan exposure of 33.3 mg*h/L) and HRD with clonal cytogenetic abnormalities received three day of busulfan for a cumulative busulfan exposure of 49.2 mg*h/L) (Table II).
Donors and Cell Doses
The median donor age was 28 years (IQR 13). Donor sources included: 11 MRD, 31 URD, and 17 HRD. The CD34+ and CD3+ cell doses are shown in Table II and Supplementary Table II.
Chimerism
Only four patients had less than 50% donor CD3 chimerism at day 100 (Supplementary Tables IIIa, patient 2; Table IIId, patients 2 and 13; Table IIIe, patient 17). Two of these patients went on to have graft failure/rejection (Supplementary Tables IIId, patient 13; Table IIIe, patient 17); both patients were successfully re-transplanted.
GVHD
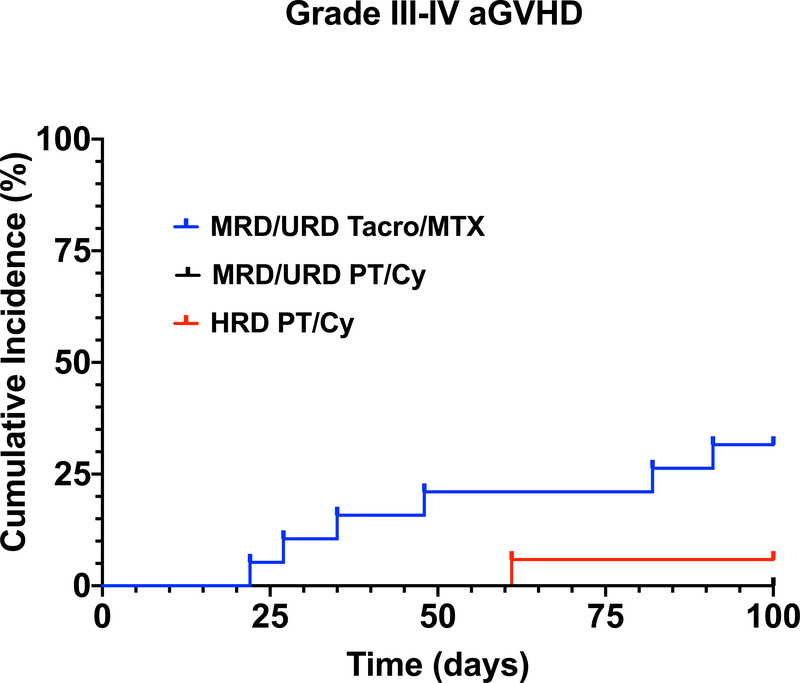
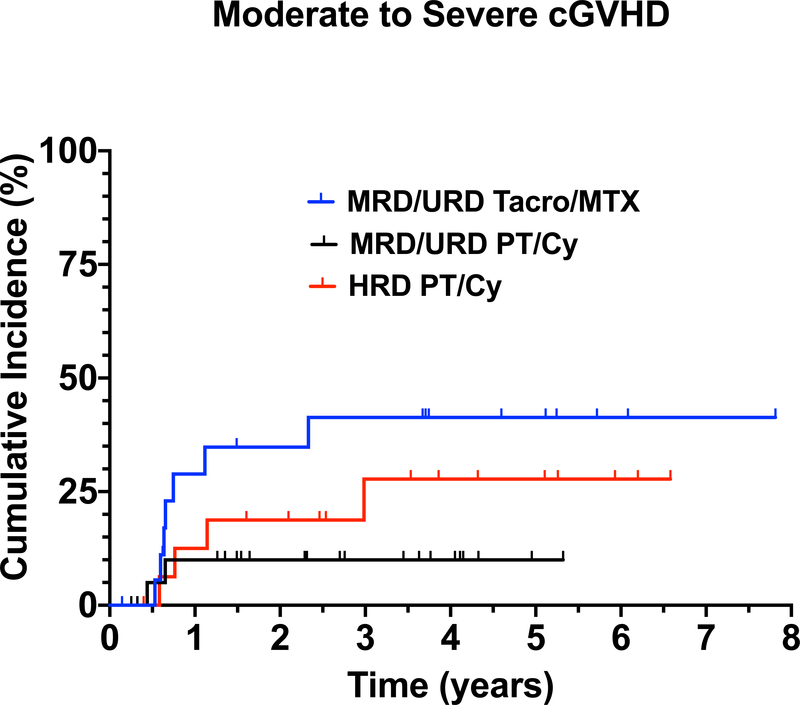
Initially, all MRD and URD received Tacro/MTX for GVHD prophylaxis. However, despite 10/10 HLA match of MRD and URD, there was a 32% (6/19) incidence of grade III-IV aGVHD in patients receiving Tacro/MTX for GVHD prophylaxis (Table III, Figure 2A). In contrast, in the 23 MRD/URD recipients who received PT/Cy followed by Tacro/MMF, none developed grade III-IV aGVHD (p=0.0014) (Table II,III) (Figure 2A). Only 6% (1/17) of HRD recipients developed grade III-IV aGVHD. Forty-two percent (8/19) of MRD/URD patients who received post-transplant Tacro/MTX developed moderate-to-severe cGVHD, 9% (2/23) of MRD/URD patients who received PT/Cy developed cGVHD, and 24% (4/17) of HRD patients developed cGVHD within the first 2 years post-transplant. (Figure 2B) (Table II, III)
Table III.
Outcome of HSCT in GATA 2 Deficiency Patients by Donor Source and GVHD Prophylaxis
| Outcome by Donor Source | |||
|---|---|---|---|
| MRD/URD (Tacro/MTX) | MRD/URD (PT/Cy) | HRD (PT/Cy) | |
| n | 19 | 23 | 17 |
| Engraftment | 19 (100%) | 22 (96%) | 16 (94%) |
| Relapse | 1 (5.2%) | 0 | 1 (6%) |
| Phenotype Reversal | 18 (95%) | 22 (96%) | 16 (94%) |
| aGVHD (grade III/IV) | 6 (32%) | 0 | 1 (6%) |
| cGVHD (moderate/severe) | 8 (42%) | 2 (9%) | 4 (24%) |
| Deaths | 4 (21%) | 3 (13%) | 1 (5.9%) |
| 4-year-OS probability (%) | 78.90% | 82.20% | 93.3%% |
| 4-year EFS probability (%) | 78.90% | 78.30% | 88.20% |
Figure 2.
(A) Cumulative Incidence of aGVHD (grade III-IV) in MRD/URD (Tacro/MTX) recipients, MRD/URD (PT/Cy), HRD (PT/Cy). (B) Cumulative Incidence of moderate/severe cGVHD in MRD/URD (Tacro/MTX), MRD/URD (PT/Cy), HRD (PT/Cy) recipients.
Outcomes
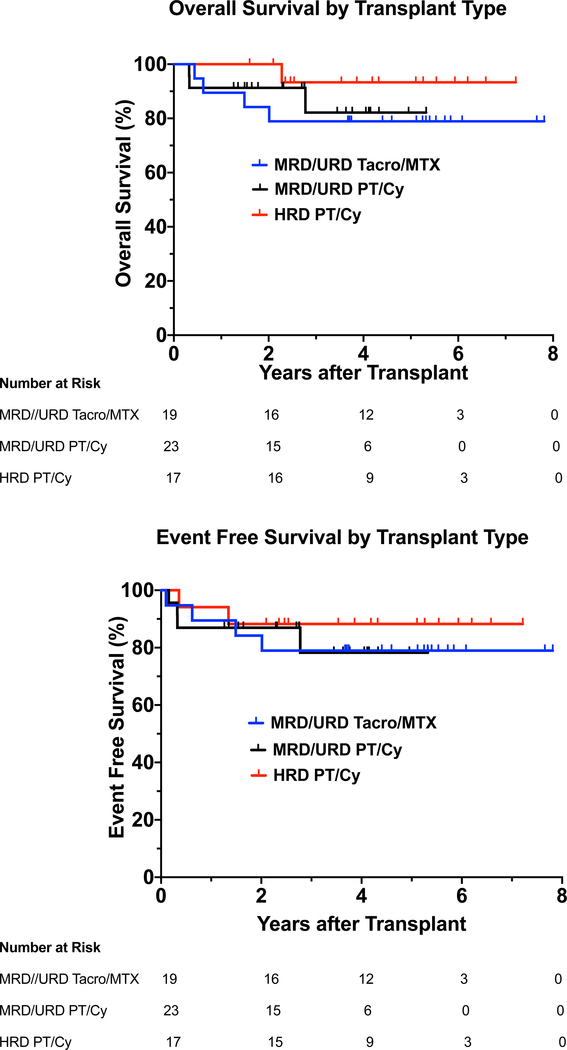
The 4-year OS probability was 85.1% (95% CI: 72.2–92.3%) with a 4-year EFS of 82.1% (95% CI: 69.1–90%) (Fig 2A,2B). Subgroup analysis (Table III) indicated that for patients in the MRD/URD Tacro/MTX group, the 4-year OS probability was 78.9%, and the 4-year EFS probability was 78.9%. For patients in the MRD/URD PT/Cy group, the 4-year OS probability was 82.2%, and the 4-year EFS probability was 78.3%. For patients in the HRD PT/Cy group, the 4-year OS probability was 93.3%, and the 4-year EFS probability was 88.2% (Table III and Figure 2A and 2B).
Complications
There were 4 deaths in the MRD/URD group receiving Tacro/MTX: two from complications of GVHD, one from sepsis, and one from refractory AML (Table III). There were three deaths in the MRD/URD group receiving PT/Cy, one from poor graft function and cardiac arrest, one from HPV-associated metastatic squamous cell carcinoma of the rectum two years post-transplant, and one from sepsis. There was one death in the HRD PT/Cy cohort due to immune reconstitution syndrome following a second transplant.
There was one primary graft failure in a recipient of an URD bone marrow transplant who received PT/Cy (Supplementary Table Id, patient 13), and one secondary graft rejection in a recipient of HRD bone marrow transplant (Supplementary Table I e, patient 17; both patients were successfully re-transplanted, the URD recipient receiving a different URD with PBSC, and the HRD received a second transplant with the same donor with PBSC. One URD who received PT/Cy had poor graft function requiring a stem cell boost from the same URD, which was unsuccessful (Supplementary Table Id, patient 2).
There were several other major complications. One URD patient had an immune hemolytic anemia (Supplementary Table II c, patient 6) which developed at 6 months post-transplant and required IVIG. Two patients who were transplanted with active NTM developed a syndrome consistent with an immune reconstitution inflammatory syndrome (IRIS) following engraftment (Supplementary Table IIb, patient 2; Table IId, patients 2 and 9).(13–15) This syndrome was responsive to high-dose corticosteroids. Surprisingly, there were no instances of venous occlusive disease of the liver, one case of acute kidney injury requiring hemodialysis (Supplementary Table II d, patient 13), and three cases of pneumonia post-transplant.
Viral reactivation remained a problem in all three cohorts with CMV reactivation in 26.3%, 47.8%, and 47% of the MRD/URD Tacro/MTX, MRD/URD PT/Cy, and HRD treatment groups, respectively. EBV reactivation occurred in 84.2%, 91.3%, and 94.1% of the same three groups. Only one case of adenovirus reactivation occurred in an MRD/URD Tacro/MTX patient. Lastly, BK viral reactivation occurred in 26.3%, 60.8%, and 35.2% of the same three treatment groups.
Reconstitution of monocytes and lymphocytes subsets after HSCT
Abnormalities of leukocyte subpopulations prior to transplant were characteristic of GATA2 deficiency including severely reduced monocyte count in 95% of the patients (56/59) (mean 0.03K/uL), very low levels of CD19+ B-lymphocytes in 92% of the patients (54/59) (mean 18.6 cell/uL), and low levels of Natural Killer (NK) cells in 95% of the patients (56/59) (mean 31.88 cells/uL) (Supplementary Figure 1).
Immune reconstitution was rapid post-HSCT. At one-hundred-days-post-transplant, 75% (42/56) of the patients had normal absolute monocyte counts (mean 0.35 K/uL), 48% (26/54) had normal absolute NK cell counts (mean 130 cells/uL) and 64% (34/53) had normal B cells counts (mean 113 cells/uL). By one-year post-transplant, 87% (39/45) had normal absolute monocyte counts (mean 0.43 K/uL), 93% (41/44) had normal CD19+ B-lymphocytes, 38% (17/45) had normal absolute NK cell counts (mean 141 cells/uL) (Supplementary Figure 1).
Immune Reconstitution
The numbers of CD4+ T cells were low in 49% (29/59) of patients (mean 432 cells/uL) six months prior to transplant. The number of CD4+ naïve T cells (CD4+ CD45RA+) was obtained in 50 patients, 46% of whom (23/50) had low numbers of naïve CD4+CD45RA+T cells (mean 224 cells/uL) six months prior to transplant. CD8+ T cell numbers were less affected in this cohort, 19% (11/59) of patients had CD8 lymphopenia (mean 439 cells/uL) and 22% of patients (11/50) had low CD8+ CD45RA+ naïve T cells (mean 286 cells/uL) during the same period of time (Supplementary Figure 1).
Discussion
In this single institution cohort of 59 patients with GATA2 deficiency (including 4 patients with the MonoMAC syndrome), HSCT using a targeted, busulfan-based conditioning regimen was safe and effective with a 4-year OS probability of 85.1% and a 4-year EFS probability of 82.1%. There were subgroup specific differences with the 4-year OS of 78.9%, 82.2%, and 93.3% in the MRD/URD Tacro/MTX arm, the MRD/URD PT/Cy arm, and the HRD PT/Cy arm, respectively.
The optimal timing of allogeneic HSCT in GATA2 deficiency remains challenging. This issue is compounded by the highly variable clinical course of individuals with mutations in GATA2, even in individuals in the same family with identical mutations. Patients with GATA2 deficiency or the MonoMAC syndrome were eligible for HSCT if they had at least one episode of a life-threatening opportunistic infection, including high-grade HPV disease. Progressive infections are a hallmark of the disease, and once opportunistic infections begin, the course is frequently progressive. HSCT was effective in reversing the infectious disease phenotype in GATA2 deficiency. There were no instances of reactivation of NTM infections after HSCT. Similarly, there was only one progression of HPV driven malignancy.
Patients with MDS with one or more peripheral blood cytopenia’s constituted nearly one-half of our cohort. Cytogenetic changes, particularly monosomy 7, or two or more cytogenetic changes, also prompted HSCT in a number of cases. Malignant myeloid progression is an ominous development in GATA2 deficiency since the malignant clones arise out of a background of MDS.
The optimal conditioning regimen for HSCT in GATA2 deficiency remains unclear, primarily due to the variable disease progression, and where the patient is in the disease trajectory. In a previous studies when there is a hypocellular marrow without overt dysplasia, or minimal/borderline dysplasia not meeting WHO criteria for MDS, and without cytogenetic abnormalities, or with trisomy 8 alone, engraftment can be achieved with a nonmyeloablative or reduced intensity conditioning regimen.(5),(16) A nonmyeloablative regimen can result in reliable engraftment because the GATA2-deficient marrow is at a proliferative disadvantage.(17, 18) However, as with other malignant myeloid diseases with clonal progression and adverse cytogenetic changes, such as monosomy 7, a higher dose regimen may be required to achieve reliable engraftment and eradication of the myeloid clone. (19) In a retrospective study of 4 patients with GATA2 deficiency, Tholoui et al. demonstrated that a T-cell depleted reduced-intensity regimen consisting of fludarabine, alemtuzumab, and an alkylating agent (Busulfan or melphalan or treosulfan) resulted in engraftment and reversal of the disease phenotype in four patients.(16) One of the patients had trisomy 8 pre-transplant, and this clone was eliminated with HSCT.
We have addressed this issue in our regimen by targeting a lower busulfan AUC when the marrow is hypocellular and lacks clonal cytogenetic abnormalities, the exception being trisomy 8, which in the background of GATA2 is eradicated by lower doses of conditioning chemotherapy. During this study from 2013 to 2020, we progressively decreased the targeted AUC for busulfan. The work of Bartelink et al.(20) is informative in this regard since with busulfan dosing there appears to be an optimum area under the concentration curve that resulted in the best event-free survival by reducing toxicity from the regimen, yet preventing graft failure or relapse. Of note, both the patient with primary graft failure and the patient with secondary graft rejection in our study received one of the lowest doses of busulfan with targeted AUCs of 3600 and 3400 μMol*min/L, respectively, suggesting that we may be approaching the lower limit of our busulfan dosing.
Initially, all MRD and URD received Tacro/MTX for GVHD prophylaxis. However, despite 10/10 HLA matching of MRD and URD, there was a high incidence of grade III-IV aGVHD (6 patients) in the 19 MRD/URD patients receiving Tacro/MTX for GVHD prophylaxis. The protocol was amended to incorporate PT/Cy for the subsequent 23 MRD/URD patients with normal or favorable cytogenetics. None of the MRD/URD patients receiving PT/Cy developed grade III-IV aGVHD, and only one of the 17 HRD recipients developed grade III-IV aGVHD. The aGVHD had consequences, in those two of the four deaths in the Tacro/MTX group resulted from GVHD. Although 87% of the PT/Cy MRD/URD patients received marrow as the stem cell source, only 58% of the MRD/URD Tacro/MTX group received marrow, the overall CD3+ dose/kg in the Tacro/MTX group was comparable to that in the PT/Cy group.
There was a progressive recovery to normal absolute monocytes counts, NK cell counts, and B cells counts in the majority of transplant recipients. Longitudinal analyses of the cells one-year post-transplant showed that monocytopenia resolved in more than 80% of the patients and B cell lymphopenia in 93% of the patients. Therefore, the characteristic cytopenias of GATA2 deficiency are readily corrected with HSCT, although some cells populations take longer to recover than others. There were 2 patients in the URD PT/Cy group that did not normalize their monocyte count after 2 years post-transplant, however, both patients had 100% myeloid chimerism by day 100 post-transplant. The mechanisms underlying differential rates of lymphoid reconstitution are unclear but represent an important area for future research.
Our study has several limitations of a retrospective analysis that could have influenced data interpretation. A relatively small number of patients in each transplant category could explain the lack of differences identified, as well as being a single center experience, the heterogeneity of conditioning regimens, donor type/source, GVHD regimens as well as the heterogeneity of the GATA 2 patient population in terms of clinical status, infections, etc.
In conclusion, allogenic HSCT represents the only definitive therapy for GATA2 deficiency. Although this study does not directly address the question, HSCT appears to have a better outcome the earlier in the disease course that it is performed and when co-morbidities are fewer. PT/Cy resulted in a considerably reduced incidence of both acute and chronic GVHD than Tacro/MTX. Also, PT/Cy did not appear to increase the risk of relapse in patient with MDS/AML. Opportunities for improvement in outcome are clear, and further adjustment of the preparative and prophylactic regimens will be critical for achieving these. However, these data also show that excellent results can be obtained in this otherwise inexorably progressive disease. The disease course is adversely affected by the development of other malignancies, but, surprisingly, not by ongoing infections. Our future studies of HSCT in GATA2 deficiency will involve targeting c-kit on the hematopoietic stem cell with monoclonal antibodies in patients with GATA2 deficiency with a hypocellular bone marrow without clonal chromosomal abnormalities.(21)
Supplementary Material
Figure 1.
(A) Overall Survival-OS in MRD/URD (Tacro/MTX), MRD/URD (PT/Cy), HRD (PT/Cy) recipients, as a function of years after transplant.
Hazard Ratios (HR): MRD/URD (Tacro/MTX) vs. MRD/URD (PT/Cy): HR= 0.723 (95% CI 0.161–3.251); MRD/URD (Tacro/MTX) vs. HRD (PT/Cy): HR= 0.258 (95% CI 0.029–2.313); MRD/URD (PT/Cy) vs. HRD (PT/Cy): HR= 0.372 (95% CI 0.038–3.596).
(B) Event Free Survival-EFS in MRD/URD (Tacro/MTX), MRD/URD (PT/Cy), HRD (PT/Cy) recipients, as a function of years after transplant.
Hazard Ratios (HR): MRD/URD (Tacro/MTX) vs. MRD/URD (PT/ Cy): HR= 0.940 (95% CI 0.234–3.785); MRD/URD (Tacro/MTX) vs. HRD (PT/Cy): HR = 0.547 (95% CI 0.100–2.985); MRD/URD (PT/Cy) vs. HRD (PT/Cy):HR= 0.590 (95% CI 0.108–3.233).
Acknowledgments
This research was supported (in part) by the Intramural Research program of the NIH including the National Cancer Institute, National Institutes of Health (Hematopoietic Stem Cell Transplant for GATA2 Deficiency, ZIA BC010870 Hickstein, Dennis – NCI), in part by the Division of Intramural Research, and (in part) under Contract No. HHSN261200800001E, and Contract No. 75N910D00024, Task Order No. 75N91019F00131. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Competing Interest Statement
The authors declare no competing financial interests.
References
- 1.Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118(10):2656–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43(10):1012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118(10):2653–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet. 2011;43(10):929–31. [DOI] [PubMed] [Google Scholar]
- 5.Grossman J, Cuellar-Rodriguez J, Gea-Banacloche J, Zerbe C, Calvo K, Hughes T, et al. Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Biol Blood Marrow Transplant. 2014;20(12):1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parta M, Shah NN, Baird K, Rafei H, Calvo KR, Hughes T, et al. Allogeneic Hematopoietic Stem Cell Transplantation for GATA2 Deficiency Using a Busulfan-Based Regimen. Biol Blood Marrow Transplant. 2018;24(6):1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115(8):1519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu AP, West RR, Calvo KR, Cuellar-Rodriguez J, Parta M, Kelly SJ, et al. Adenosine deaminase type 2 deficiency masquerading as GATA2 deficiency: Successful hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2016;138(2):628–30 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parta M, Cuellar-Rodriguez J, Freeman AF, Gea-Banacloche J, Holland SM, Hickstein DD. Resolution of Multifocal Epstein-Barr Virus-Related Smooth Muscle Tumor in a Patient with GATA2 Deficiency Following Hematopoietic Stem Cell Transplantation. J Clin Immunol. 2017;37(1):61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina JC, Asare JM, Tuschong L, West RR, Calvo KR, Persky R, et al. Venetoclax/decitabine for a pediatric patient with chronic myelomonocytic leukemia. Pediatr Blood Cancer. 2020:e28865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Airas L, Paivarinta M, Roytta M, Karhu J, Kauppila M, Itala-Remes M, et al. Central nervous system immune reconstitution inflammatory syndrome (IRIS) after hematopoietic SCT. Bone Marrow Transplant. 2010;45(3):593–6. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Iniguez A, Carrera-Villanueva KA, Leon-Rodriguez E, Cuellar-Rodriguez J. Recurrent arthritis and immune reconstitution inflammatory syndrome in hematopoietic stem cell transplantation (HSCT). Bone Marrow Transplant. 2018;53(3):374–6. [DOI] [PubMed] [Google Scholar]
- 15.Manion M, Dimitrova D, Pei L, Gea-Banacloche J, Zelazny A, Lisco A, et al. IRIS as a post-transplantation complication in primary immunodeficiency with disseminated M. avium. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tholouli E, Sturgess K, Dickinson RE, Gennery A, Cant AJ, Jackson G, et al. In vivo T-depleted reduced intensity transplantation for GATA2-related immune dysfunction. Blood. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickstein D HSCT for GATA2 deficiency across the pond. Blood. 2018;131(12):1272–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigues NP, Janzen V, Forkert R, Dombkowski DM, Boyd AS, Orkin SH, et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106(2):477–84. [DOI] [PubMed] [Google Scholar]
- 19.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol. 2017;35(11):1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartelink IH, Lalmohamed A, van Reij EM, Dvorak CC, Savic RM, Zwaveling J, et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. Lancet Haematol. 2016;3(11):e526–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318(5854):1296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.