Abstract
Objective:
The purpose of this article is to report the translational process of an implantable microdevice platform with an emphasis on the technical and engineering adaptations for patient use, regulatory advances, and successful integration into clinical workflow.
Methods:
We developed design adaptations for implantation and retrieval, established ongoing monitoring and testing, and facilitated regulatory advances that enabled the administration and examination of a large set of cancer therapies simultaneously in individual patients.
Results:
Six applications for oncology studies have successfully proceeded to patient trials, with future applications in progress.
Conclusion:
First-in-human translation required engineering design changes to enable implantation and retrieval that fit with existing clinical workflows, a regulatory strategy that enabled both delivery and response measurement of up to 20 agents in a single patient, and establishment of novel testing and quality control processes for a drug/device combination product without clear precedents.
Significance:
This manuscript provides a real-world account and roadmap on how to advance from animal proof-of-concept into the clinic, confronting the question of how to use research to benefit patients.
Keywords: Biomedical engineering, biomaterials, biomarkers, cancer, clinical trials, drug delivery, drug discovery, implants, in vivo, tumor microenvironment
I. Introduction
A KEY question facing biomedical engineers is how to translate developments made in the preclinical realm into the patient setting. This translational process involves two major areas, one being the regulatory review (primarily by the U.S. Food and Drug Administration (FDA) in the United States, and institutional review boards) and two, the clinical translation process, consisting mainly of engineering design and testing to integrate the novel technology into existing clinical workflow and to reduce risks to the patient to a point where a potentially beneficial yet unproven intervention is justified. For a novel technology for which clear precedents do not exist, a translational path needs to be newly developed.
Implantable microdevices (IMD) that perform diagnostic and other measurements within the patient’s body play an important role in the implementation of precision medicine but are still rare in clinical practice and in investigational clinical use [1]–[4]. In addition to technical and engineering challenges, their development also encounters regulatory and clinical implementation challenges associated with translating an engineering innovation into the patient setting [5]. This article describes the translational process of an IMD platform to perform up to 20 drug response measurements simultaneously in a cancer patient, with a focus on technical and engineering adaptation for patient use, regulatory advances, and successful integration into clinical workflow.
The IMD platform consists of a micro-implant, roughly the size of a grain of rice, constituted by 20 micro-reservoirs, each containing a unique drug or drug combination (Fig. 1) [6]. The IMD is placed directly into a patient’s tumor percutaneously using a conventional thin interventional needle and remains in situ for a pre-determined duration of several hours to days. During this time, drugs from the micro-reservoirs are released into spatially separate and confined regions of the tumor in a time and concentration-dependent manner controlled by the formulation of the drugs in a polymer matrix within each microreservoir. Up to 3 days later (the incubation time depends on the surgical modality chosen for each patient’s specific cancer), the IMD is retrieved from the patient tumor with surrounding tissue by minimally invasive biopsy or surgical means [6]. The device/tissue specimen is then analyzed by a variety of methods to characterize drug response in the tissue, ranging from standard histopathology to cutting-edge spatial transcriptomics, metabolomics, or highly multiplexed immunofluorescence [7]–[9].
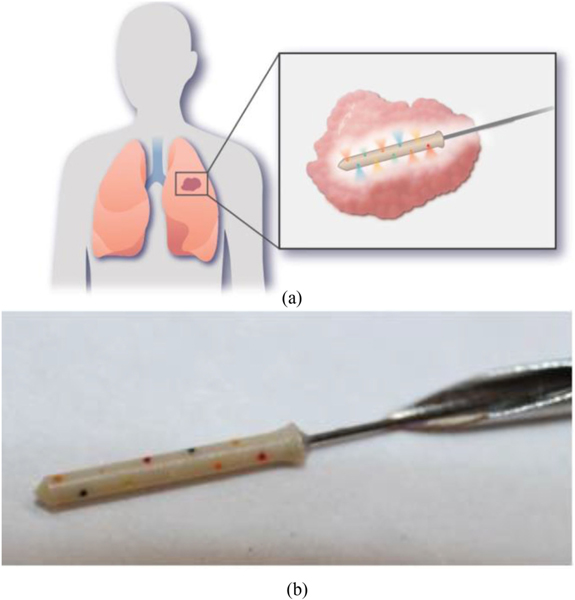
Fig. 1.
The IMD. The IMD is inserted into tumors where it releases microdoses of drugs at controlled time points (a). There are 20 microreservoirs, each containing a different drug or drug combination (colorcoded in the photograph to visualize each reservoir’s pore). The IMD is attached to a guidewire and is deployed via a needle (b).
The resulting dataset provides deep phenotyping of each drug effect on the tumor and its microenvironment, which may serve as predictive biomarkers to match individual patients with the most effective agent(s) for personalized treatment [10]. Major advantages over conventional approaches [11] are that drug effects are examined within days, and response measurement includes the effects of tissue architecture, immune microenvironment, and other effects on drug response that cannot be recapitulated outside of the body [12], [13]. In addition, because drug quantities per reservoir are ~1/100,000th or less of systemic doses, up to 20 therapies are examined without exposing patients to the toxicity of systemic drug administration [6]. In clinical oncology, few tools currently exist for oncologists to identify effective therapeutic treatments a priori, and therapy selection in some clinical contexts remains largely empirical (especially in recurrent disease) [11]. In this setting, the drug response data obtained with the IMD may be used to identify the most effective treatment regimen for individual patients or to test novel therapies more rapidly and safely within patients.
The clinical translation of the IMD technology involved several key innovations: First, the IMD platform leverages existing favorable regulations for microdosing that were intended to enable studies of drug distribution in vivo, and applies them for the measurement of drug response in the targeted tissue of interest [3]. The FDA micro-dosing concept provides a pathway for early testing of drugs in humans at a significantly reduced regulatory burden [14]. The framework was originally conceived as part of the Critical Path Initiative to enable earlier studies of drug distribution of novel therapies in patients to determine pharmacokinetic (PK) properties of a compound, and this has been the case for virtually all microdose applications to date [15]. The regulations establish a total dose of less than 1% of systemic therapeutic dose as the metric that determines micro-dose status (In the IMD platform, the total amount of drug in the patient is ~2 ng, and thus ~factor 1000 lower than the cutoff for micro-dose status) [16]. Pharmacodynamic (PD) measurements, however, require therapeutic dose levels in the diseased tissue. This is accomplished by performing intra-target micro-dosing where nanogram quantities of drugs are released controllably into very small and confined regions of a tumor where their local concentrations are at therapeutic levels.
Second, instead of studying one therapy per patient, the IMD approach enables the simultaneous study of up to 20 compounds in a single patient [6]. This represents the first instance in which such a large set of different therapies are administered to a single patient, and this brought unique challenges to the developmental and regulatory processes. The key technical advancement involved is the ability to release the drugs into distinct non-overlapping regions (which is needed to enable a separate assessment of each drug’s efficacy) in heterogeneous patient tumors, which necessitates formulating the drugs in an appropriate polymer matrix. This process renders each compound/polymer mixture a new chemical entity per FDA guidelines. To advance this concept to clinical trials, a novel regulatory path was charted, which provided the FDA and clinicians with safety assurance and streamlined integration into existing clinical workflows through a set of documentation and quality control measures described in the Methods section. Third, the IMD needs to be implanted and retrieved with intact surrounding tissue so that tumor response to drugs can be measured. Though similarly sized micro-implants are routinely used clinically, such as radiological markers or brachytherapy seeds, they are virtually never removed selectively from the patient and do not require surrounding tissue for clinically relevant measurements. A nexus of clinicians, engineers, and scientists collaborated to develop a device that satisfied the technical demands and could also be integrated into the clinical workflow without disruption to the patient’s course of treatment. Throughout this iterative process, many structural, material, and other modifications were made to the IMD, and each one was documented, inspected, and tested, leading to the development of a Design History File (described below)
To date, the FDA has approved six clinical studies covering 93 drugs (includes small molecule drugs, biologics, and novel combinations) for the IMD platform. As early clinical data become available, a feedback loop between engineers, clinicians and regulators emerged, which has shaped the development and translation of the technology. We outline how regulatory considerations and FDA feedback affected engineering design and adaptations throughout the clinical implementation phase. This article describes the steps taken to advance the IMD from preclinical concept to clinical innovation and provides lessons for innovators in the field of combination products and novel implantable technologies.
II. FDA Designation of a Regulatory Pathway
From a regulatory perspective, the IMD platform is a drug/device combination product with diagnostic intent [17]. FDA jurisdiction for such products typically spans both the Center for Drug Evaluation and Research (CDER) and the Center for Devices and Radiological Health (CDRH), with one assuming a lead role [18]. While internal guidelines exist to determine lead authority, these are often not transparent to inventors and researchers. As the IMD does not distinctly fall into “drug” or “device” categories, regulatory consultations were required to determine eligibility criteria prior to submission. Thus, a pre-submission inquiry was made to the FDA to request enhanced transparency of the review process.
Detailed background information, including a device description, product development, indications for use, and previous submissions, were submitted as part of a Request for Designation (RFD) to determine the regulatory pathway and FDA jurisdiction [19]. The first element of the RFD was determining the risk classification for the product. FDA requested that investigators identify and catalogue all presumed potential risks to patients related to the IMD. This was conducted in extensive consultation with the oncologists, interventional radiologists, and surgeons involved in each of the clinical trials. Later, these potential risks are presented during trial enrollment to patients as part of the consent process. Specific remedies to mitigate and minimize each of the potential risks were developed with clinicians and presented to FDA for approval as part of the regulatory review process.
The FDA designated the IMD as a combination product that would fall under the Investigational New Drug (IND) category and be reviewed by the CDER. This guided us towards the review departments and provided necessary guidelines for approval [20]. With the IND route and lead reviewer established, the IND package was prepared. Clinical trial protocols and consent forms were developed along with appendices that outlined performance status criteria, patient instructions, pharmacy manuals, and required FDA forms.
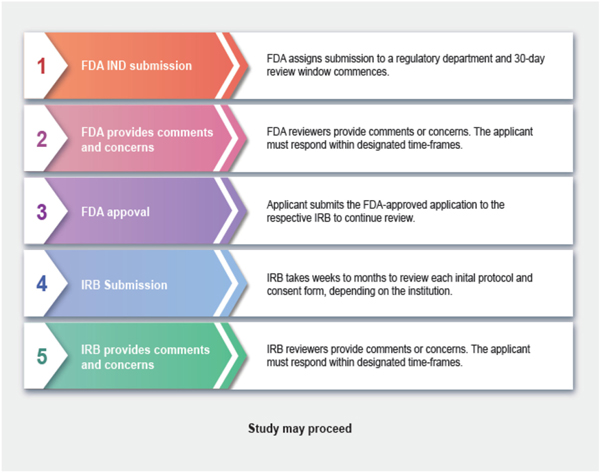
Upon original IND submission, the FDA begins a 30-day review period to respond with one of three potential outcomes: study approval, full clinical hold, or non-hold [21]. Submitters are required to respond to any concerns within a designated time frame. If non-hold comments are provided, the submitter may proceed with the recruitment of patients with the understanding that the concerns must be addressed throughout the course of the study [22]. Fig 2. provides an overview of the approval process, from original IND submission to IRB approval. It has been our experience that the number and severity of concerns decreased significantly with each additional protocol, indicating increased familiarity and comfort by the FDA with the technology.
Fig. 2.
Timeline of regulatory review process. After FDA IND submission, the FDA has 30 days to review the application and provide any concerns that must be addressed. The IRB submission process is similar, but typically has a longer timeline.
A. Master Access File (MAF) for Regulatory Review Across Multiple Clinical Studies
After multiple applications were discussed with the FDA, a Master Access File (MAF) was suggested in order to establish uniformity across clinical protocols [23]. A MAF was subsequently developed as a central database for all relevant information pertaining to the IMD, such as device description, labeling, sterilization and shelf life information, biocompatibility testing, animal performance testing, and risk assessment.
Any descriptive information of the IMD is included in the MAF. We submitted a dimensional drawing of the device, SOPs for the device manufacturing and preparation process, a process flow diagram, manufacturing batch record, and a list of supplies required for device and drug preparation.
The FDA sets strict labeling requirements for INDs in 21 CFR 312.6, and were a required element of the MAF [24]. Also included is information regarding bioburden testing, sterility testing, validation results, gamma irradiation dosimetry reports, endotoxin testing, and a chemical integrity study conducted using mass spectrometry. We reference the MAFs, with permission, from manufacturers of materials we use in the manufacturing process of IMDs[23]. Benchtop, animal and human performance testing were also provided in the MAF to demonstrate the safety and efficacy of the IMD. The establishment of the MAF leveraged FDA feedback to establish uniformity across clinical protocols and efficient regulatory review.
B. Design History File
In accordance with 21 CFR 820.30(j), a Design History File (DHF) is compiled to document design changes to the IMD and its intended use [24]. In our case, the DHF outlined changes to the IMD manufacturing, materials, and design since its inception and provided detailed ex-vivo and in-vivo animal studies, as well as benchtop performance testing, to assure that user needs were met.
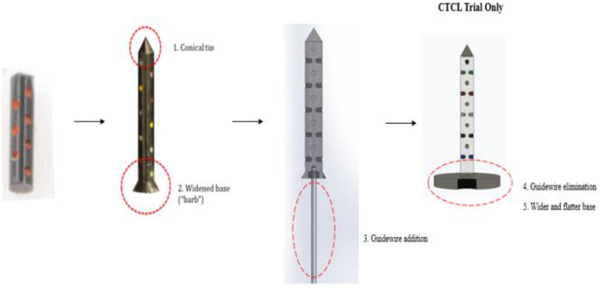
The initial IMD prototype was of cylindrical shape with a flat top. However, the early pathological evaluation identified significant tissue damage upon implantation in animal experiments. This led to engineering changes, including the design of a conical tip to reduce tissue stress during implantation. Additional studies demonstrated a necessity for a widened base in order to lock the device from migrating backwards in the needle track and provide solid anchoring for consistent drug release within a tissue. Further, a guidewire was attached by press-fit into the bottom base of the device to provide better visualization within tissue and an improved ability to retrieve the IMD. Lastly, the material choice was optimized by switching from poly-ether-ether-ketone (PEEK) to poly-ether-ketone-ketone (PEKK), both plastic materials commonly used in artificial joints [25]. PEKK had the advantage of being available as a barium sulfate doped polymer, which is radio-opaque, thus facilitating visibility on CT, mammogram, and ultrasound. The biocompatible properties and long-term safety in the human body of these materials eliminated valid safety concerns. Each adaptation was recorded by Quality Assurance personnel and thoroughly documented in the DHF to demonstrate optimal IMD design. An overview of the design changes is provided in Fig. 6.
Fig. 6.
Iterative design changes to the IMD. The initial IMD prototype was a basic cylinder with reservoirs. A conical tip, widened base, and guidewire were later added. For the CTCL trial, the IMD was re-designed to include a wider and flatter base and elimination of the guidewire.
C. Preclinical Safety and Efficacy Studies
Ex-vivo and in-vivo animal studies were provided to demonstrate safety and efficacy of the IMD [26], [27]. Ex-vivo studies clearly showed an effect of reservoir size on drug release, with smaller reservoir sizes significantly decreasing lateral drug diffusion and moderately reducing radial drug diffusion. The simultaneous release of different compounds from the same reservoir was also demonstrated through ex vivo and in vivo studies, enabling the testing of drug combinations clinically. In-vivo animal studies showed an effective integration between device and tissue and identified methods for imaging drug distribution [28]. A spatial and temporal distribution of marker compound elution from the device was proven possible and led to developing methods for controlling drug release [6]. We performed lab testing of various polymers that can be co-formulated with a range of drugs with varying chemical properties. We identified Polyethylene glycol (PEG) as the most appropriate polymer for IMD use, because of its wide utility, for instance as an excipient in many pharmaceutical products; Generally-Regarded-As-Safe (GRAS) designation; tolerability in large quantities; and for favorable chemical properties, such as the ability to create high osmotic pressure; and high solubility or complete miscibility with water [29] which facilitates controlled uptake in tissue. Importantly, PEG is also available in many molecular weights, making it tunable to desired release kinetics across a range of time points. An example from human glioblastoma is shown in Fig. 7a, which compares release kinetics for drugs with different chemical properties and solubility, demonstrating the ability to obtain uniform release profiles across drugs.
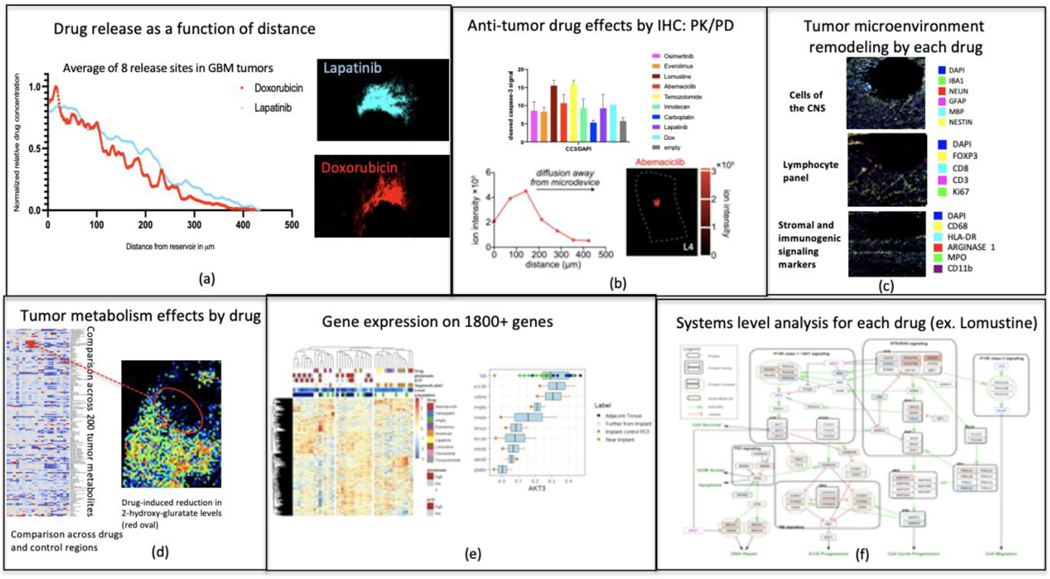
Fig. 7.
Tumor drug response readouts. Drug concentration is measured by autofluorescence or mass spectrometry (a). Drug effects are visualized by immunohistochemistry (b) and TME remodeling (c). MALDI can be used to analyze changes in the local tumor metabolism (d) and spatial transcriptomics focuses on the expression of over 1800 genes (e). Ultimately, a systems-level analysis is conducted for each drug (f).
In addition, other documented changes such as altering reservoir size, or placement of reservoirs to create spatially separate or overlapping regions of drug distribution, were made in the DHF and provided to the FDA to demonstrate safety and efficacy of the IMD with variable features optimized for each tissue type and application.
III. Production and Process Control
A. Microdevice Preparation
Strict endotoxin and sterility limits for implants exist depending on the intended use of the device and the tissue which the device directly or indirectly contacts. Endotoxin limits vary based on whether the IMD contacts the cardiovascular system, lymphatic system, cerebrospinal fluid, or is permanently implanted or implanted subcutaneously [30]. For microdevices that directly or indirectly contact the cardiovascular and lymphatic system, which includes a majority of our IMD applications, the limit is 20EU. For devices in contact with cerebrospinal fluid, as in our glioblastoma trial, the limit is 2.15 EU. Because our trials involve multiple devices implanted in each patient, the EU limit is divided between the number of devices implanted.
The IMD is unique in that it is implanted for a short amount of time relative to other commonly used implants such as radiological seeds or drug-eluting stents. However, clear guidance on such short-term implants was not provided and we had to account for the remote possibility that the device could not be retrieved and resides in the body long-term. Without clear data on the most effective approach to endotoxin removal, multiple methods were tested which would be effective for a variety of materials, such as metals and plastic, while also not interfering with the drugs’ efficacy.
In addition to the endotoxin test, devices must also demonstrate zero growth on a sterility test after gamma irradiation [31]. With endotoxin removal and sterilization steps finalized, an overall step-by-step procedure for device preparation was developed that would demonstrate a high level of safety assurance to regulators and clinicians.
B. Microdevice Loading
The detailed pharmaceutical information for each agent included in the IMD is provided in the appendices of each IND packet. All agents currently used are FDA-approved for use in cancer patients and are purchased commercially. Microdevices are loaded with the formulated agents in a research pharmacy clean room. Loading is a multi-step process, which varies if biological agents are used. If only small-molecule agents are used, devices are loaded, packaged, and gamma irradiated. Immediately after, they undergo endotoxin and sterility testing. If biological agents are used, these are not subjected to terminal gamma sterilization per FDA’s guidance and are loaded after sterilization and prior to testing.
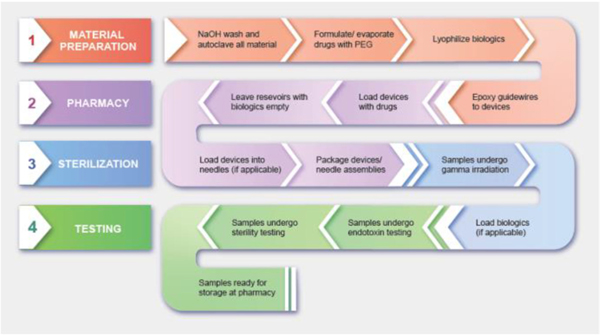
The following graphic outlines the preparation of microdevices from initial cleaning steps to storage at the pharmacy (Fig. 3). While the specifications are unique to the IMD, the individual processes and SOPs can be applied to a broad spectrum of implants and other diagnostic or measurement tools for use in the human body.
Fig. 3.
IMD preparatory steps for clinical trial. Preparation of the IMD for clinical trial involves a thorough material disinfection and drug formulation process, drug loading at the pharmacy, sterilization of samples, and endotoxin and sterility testing.
C. Ongoing Monitoring and Testing
The FDA identified long-term stability of the biologic agents stored in the IMDs prior to implantation as a potential concern. As a result, the stability of the biologic agents is assessed every 90 days using a series of tests considered to be the industry standard. In these assays the biologics are stored in IMDs and their integrity is compared to the initial compound provided at the initial release date.
Another barrier to approval was concern over potential toxicity risk due to systemic presence of drug after IMD retrieval. Therefore, blood samples are taken at a follow-up procedure after implantation and retrieval and analyzed by HPLC and MALDI to determine whether any detectable amount drug can be measured in the patient’s blood.
D. Reporting Requirements
The FDA requires annual reports for all ongoing IND studies. If annual reports are not received within 60 days the FDA has the right to terminate the study [32]. In addition to the amendment and adverse event notifications, updates regarding study results, patient demographics, and any adverse events or amendments from the previous year are sent to the FDA on an annual basis.
IV. Results
A. First Human Testing
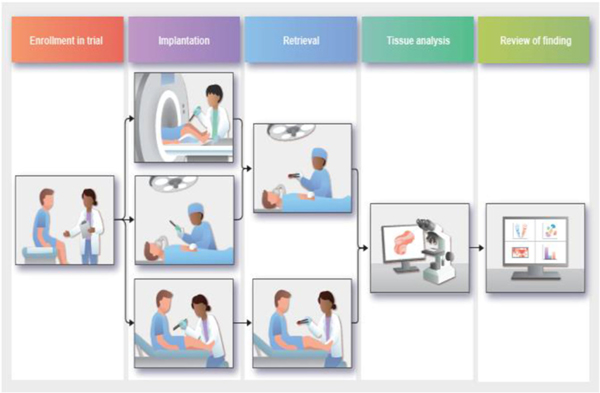
Clinical trials began following regulatory approval (DFCI protocols 18–639 approved Oct. 12, 2018; 18–623 approved Sept. 4, 2019; 19–599 approved Dec. 12, 2019; 20–357 approved Oct. 28, 2019; Mass General Brigham 2017P002402 approved Oct. 1, 2018). Fig. 4 outlines the overall patient journey throughout the microdevice trial. The IMD is intended to be used in solid cancers throughout the body, and therefore procedures for implantation and retrieval had to be developed that are amenable to different anatomical sites. In addition, there are differences in the standard of care and clinical workflow across different cancers. An important aspect of the translational process therefore became integration of IMD applications with both surgical and minimally invasive standard of care procedures.
Fig. 4.
The patient journey. After confirming eligibility, patients undergo either an intra-operative, image-guided, or cutaneous IMD implantation. Depending on which implantation method was used, the patient then undergoes an intra-operative or cutaneous retrieval. The tissue is then analyzed and findings are presented to the involved clinicians and the FDA.
1). Cutaneous T-cell Lymphoma (CTCL) Trial:
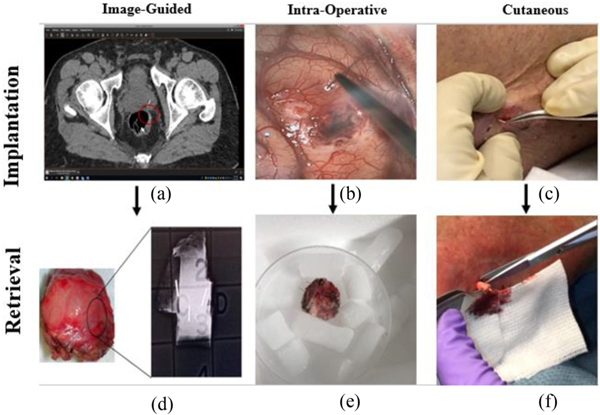
IMD implantation in the CTCL trial occurs entirely in an outpatient setting. Using a 19-gauge needle, a puncture is created in the desired tumor location, and the physician inserts the device using forceps (Fig. 5c). The IMD is left in the tumor with the base of the device fully inserted, and a guidewire protruding slightly from the skin. IMDs are retrieved using a standard skin punch biopsy tool 72 hours after implantation. The 4–5mm biopsy tool is placed coaxially around the microdevice and guidewire, and the device is retrieved in a column of surrounding tissue (Fig. 5f).
Fig. 5.
IMD implantation and retrieval. In prostate, ovarian, and breast cancer trials, IMDs are implanted using an image-guided approach, visualized in the red circle on the prostate CT-scan (a). IMDs are retrieved during surgery 48–72 hours later, with confirmation using a specimen X-ray (d). In the glioblastoma trial, IMDs are implanted at the beginning of surgery (b) and retrieved at the end (e). In the CTCL trial, devices are implanted using a cutaneous method in an outpatient setting (c) and retrieved using a punch biopsy tool (f).
2). Lung Cancer Trial:
In the lung cancer trial, we have demonstrated intraoperative implantation and retrieval methods. Each subject undergoes standard-of-care surgery for tumor resection, during which the devices are placed at the beginning of surgery and stay in the tumor microenvironment (TME) for the duration of surgery (Fig. 5b). The device is placed under direct visualization into the tumor via a thoracotomy, video-assisted thoracoscopy, or robotic assistance. The operation proceeds per standard clinical procedure with the guidewires used to ensure that the device has not migrated.
Resection is completed per clinical standard-of-care, and the intact lung specimen is carefully removed from the thoracic cavity to avoid dislodgement of the devices. The tumor is examined by a pathologist prior to device retrieval to assure that standard pathologic parameters are collected and that tumor and margin status are determined as needed for clinical oncologic care of the patient. After pathologic assessment is complete, a section of tumor encasing the device is removed from the specimen via sectioning or coring around the guidewires. A specimen X-ray is obtained after surgery to confirm successful device retrieval.
3). Glioblastoma Trial:
Similarly to the lung trial, devices in the glioblastoma trial are implanted and retrieved intraoperatively. Devices remain in the tumor throughout surgery, and are removed at the time of tumor resection (Fig. 5e). Tumor resection is performed as per standard-of-care, and the portion of the tumor containing the microdevice is resected last to maximize the length of time it is in contact with the tissue. Tissue is cored out surrounding the guidewires similar to in the lung trial.
4). Prostate Cancer Trial:
For deep tumors such as in the prostate, we have demonstrated image-guided percutaneous needle placements for IMD delivery. An interventional radiologist guides an interventional IMD implantation needle into a tumor under MRI guidance. Once the tumor is reached, the physician deploys the IMD and conducts further imaging to confirm device placement. A CT-scan is commonly used after implantation to confirm IMD location, as IMDs are currently visible on CT but not yet on MRI (Fig. 5a). MRI-visible devices are currently being developed by our group.
IMDs are removed surgically approximately 24 hours after implantation, during which patients undergo standard-of-care surgery. Surgeons localize the IMDs using anatomic landmarks and by reviewing post-implantation imaging, after which a specimen X-ray is used to confirm successful retrieval of all implanted IMDs (Fig. 5d).
5). Ovarian Cancer Trial:
The ovarian cancer trial involves a similar implantation procedure to the prostate trial, but uses CT/CT-fluoroscopy for image guidance instead of MRI. A thin (<18 gauge) needle is percutaneously inserted into the tumor location and the IMD is deployed upon localization of the desired lesion. Multiple (3–6) microdevices can be deployed into a tumor during a single procedure. This process is similar to current interventional biopsy and fiducial marker placement procedures and is performed as a short outpatient procedure. IMDs are retrieved surgically approximately 72 hours later with a similar method to the prostate cancer trial.
6). Breast Cancer Trial:
Implantation and retrieval occur nearly identically to those of the ovarian and prostate cancer trials, except that ultrasound is used for image guidance.
B. Iterative Design Changes Based on Clinical Feedback
Initial clinical deployment revealed necessary modifications that could not have been identified in benchtop and animal testing. Key insights included a change of IMD shape to enable better anchoring, varying guidewire length or removal of guidewire altogether depending on the anatomical location, improvements to the pathology workflow across all trials, and lessons from interventional radiology about implanting and retrieving from multiple anatomic sites for image-guided procedures. Each modification is submitted to regulators prior to implementation, and along with benchtop testing to demonstrate increased safety and efficacy.
1). IMD Modifications for Cutaneous Applications:
The originally approved IMD design in the CTCL IND package included a nitinol guidewire attached to the microdevice, intended to assist the physician in device localization upon retrieval. Upon initiating the clinical trial, it became evident that pressure on the guidewire caused the device to be pushed into the deep dermis or subcutis, making retrieval difficult. We developed a new microdevice design specifically for this trial and formulated a hypothesis: eliminating the guidewire and creating a wider and flatter base to the IMD would create a physical barrier to pushing the device too deeply within the skin. A series of benchtop tests were performed using ex-vivo tissues to test this hypothesis, and results were compared to the original IMD design. We observed that the wider and flatter base created an anchor at the top of the epidermis layer, similar to a thumbtack, which greatly reduced the risk of dislodgement. A statistically significant lower likelihood of device dislodgement was demonstrated when using the new IMD design, leading to a lower risk of device migration. This finding was submitted as an amendment to the FDA to eliminate the guidewire for the CTCL trial and to change the microdevice design in the DHF. The final design, including all the previous IMD designs, are illustrated in Fig. 6.
2). Varying Guidewire Length:
Progression through trials revealed that varying guidewire lengths were required for different anatomical sites, with some benefiting from elimination of the guidewire altogether. In glioblastomas, a longer guidewire (approximately 10cm) was preferred by the surgical team to better visualize the device throughout the procedure. For CTCL and prostate tumors, the guidewire was eliminated altogether to prevent device dislodgment. In lung tumors, the guidewire was customized to the depth of the lesion within the patient’s chest, thereby allowing longer or shorter wires to minimize dislodgement and maximize ease of retrieval.
3). Microdevice Visibility:
Although PEKK devices are biocompatible, IMD visibility within the body after implantation was a potential concern. Clinicians performing device implantation sought confirmation that devices were implanted into the desired location, and surgeons desired assurance that devices were entirely retrieved during excisions. To ensure device visibility, we identified PEKK infused with 20% barium sulfate for radiopacity as a potentially superior material. This material was incorporated into the machining process to manufacture the IMDs, allowing us to obtain visualization on CT, MRI, and X-Ray [33]. This application would also assist in localizing the devices after specimen retrieval, prior to embedding and sectioning the tissue/device specimen.
4). Tissue Retrieval Modifications:
Our original plan for processing of samples following large excisions containing tumor was to retrieve tissue surrounding the IMDs from the tumor by using a standard coring device, such as common punch biopsy needles used in dermatology procedures. However, in surgical settings such as lobectomy for lung tumors, fresh tissue can show considerable hemorrhage during the intraoperative procedure and may be quite soft and pliable in its consistency. Retrieval of IMDs from fresh specimens can therefore be complicated by hemorrhage that obscures the visualization of the IMDs, along with intrinsic tissue softness that can lead to migration of the IMDs during retrieval.
To minimize the likelihood of device migration during tissue retrieval, we implemented a technique in which the entire resected specimen would be fixed in formalin immediately after surgical excision, which is in keeping with the pre-existing histopathology laboratory processing and workflow. After fixation, coring out tissue surrounding the IMDs was consistently robust due to firming of the tissue and better visualization of the underlying tumor in the formalin-fixed state as compared to the fresh state. The tissue was then further fixed in formalin for 24 hours, followed by storage in 70% ethanol. Altogether, this adjustment maximized the chance of success in retrieving IMDs, while reducing the time needed for retrieval and minimizing any potential disruptions to the diagnostic pathology workflow. These clinical workflow adaptations enabled streamlined adoption of IMD analysis across cancers.
5). Lessons From Interventional Radiology:
Trials from varying anatomic sites revealed key modifications to the interventional radiology workflow. The post-implantation CT scan for prostate tumors became necessary after device visualization was difficult during MRIguided procedures. Additionally, multiple practice runs were needed to determine a method for singular insertion of several device implantations to maximize patient safety and comfort. Through this process, an iterative process of clinician feedback and regulatory approvals was established to adapt the IMD technology to regulatory requirements and the established confines of the clinical workflow.
C. Laboratory Analysis
For each IMD retrieved, and following specimen processing as previously described, a multiplexed readout is used to measure local drug concentration and interrogate intratumor drug responses for each therapy on the IMD, including phenotypic markers of cell death and proliferation (by immunofluorescence staining) spatial transcriptomics and proteomics, and MALDI mass spectrometry (Fig. 7). These readouts analyze the antitumor drug effects, TME remodeling by drugs, changes in gene expression, and tumor metabolism effects, ultimately providing a systems level analysis for each drug.
Tumor heterogeneity is an important factor to consider which may affect variability of the assay readouts. We examined the effect of heterogeneity in patient derived xenografts of tumors, where we measured CC3 expression and incidence of necrotic areas across 16 identically loaded reservoirs in various parts of tumors [6]. We found that the coefficient of variation (CV) in these measurements ranged from 19–25%. The CV was shown to be lower in IMD measurements than in systemically dosed studies in the same tumor model, which may be due to inhomogeneous drug penetration in tumors during systemic dosing [34].
An important technical aspect is to achieve similar release kinetics for various drugs on a given IMD, so that their effects on a tumor can be compared fora single timepoint of implantation. A PEG matrix was found to control release so as to standardize the release distance for given timepoints across drugs with different molecular weight and solubilities. For example, Lapatinib and Doxorubicin, which have nearly identical molecular weights yet drastically different solubilities, are released at similar rates when formulated with PEG (Fig. 7a).
Cyclical immunofluorescence conducts a phenotype readout of over 20 markers to examine cell-type specific drug effects [35]. Staining for biomarkers such as Ki67, CC3, CD45, Foxp3, and many more, allows us to quantify cell response to each chemotherapy agent and analyze the pathway enrichment or suppression. By characterizing proliferation and apoptosis, leukocytes, immune suppression, regulatory T cells, and a multitude of other cell functions, we can also examine the effect of the TME on each drug.
Spatial transcriptomics provide readouts of drug effect on ~1800 genes for each reservoir. Using this, we examine the release of cancer cell antigens, stromal factors, common signaling pathways, and many other crucial genes [35]. MALDI mass spectrometry enables spatially defined metabolomic readouts to examine drug effects on ~170 metabolites for each drug and drug combination [35]. Wedges are created that correspond to drug exposure regions in the stained images to probe concentration-dependent pathways. Tissue adjacent to the IMD are selected based on drug concentration gradient and are then compared to a control region, and quantitively analyzed by custom algorithms. Two key advances have been made with this approach: one, drug effects are analyzed in a concentration-dependent manner in a single patient, where previously only one dose of drug can be measured with a given intervention. And two, we have demonstrated measurement of drug effects and associated biomarkers using multiple state-of-the-art phenotypic, molecular, and metabolic profiling techniques in a spatially conserved manner.
A quality control step is employed to confirm reservoir orientation and whether device migration may have occurred: During tumor device/specimen sectioning, reservoir orientation at each device level is visualized by brightfield imaging and recorded. For tissue/device cross-sections containing reservoirs with marker compounds (e.g., Doxorubicin), sections are imaged to confirm that drug release matches the reservoir orientation. If there is a mismatch between the orientation of reservoir opening and the release side of the drug, then it is likely that device rotation or migration occurred after device implantation.
We envision that these results will enable more efficient biomarker discovery for existing and novel therapies. The field has lacked a clear understanding of the expression of biomarkers of drug response across time. This is important because certain markers appear at different times and thus could be missed if a suboptimal time point is chosen for their measurement. Our findings impact biomarker-driven clinical trials of drugs such as novel immunotherapies, epigenetic agents, and targeted kinase inhibitors that increasingly include biopsy measurements of intratumor drug effects at specified time points. Combining readouts enables the examination of drug effects in a variety of methods. Firstly, many changes occur in a time-dependent manner, with metabolic changes typically occurring before changes in gene expression.
Limiting ourselves to only one readout would overlook the interactive changes that occur to drug response throughout time. Secondly, one use of the IMD is drug and biomarker discovery. Therefore, overlapping readout methods can examine drug effects that are not yet known, paving the way for new studies. Combining this with the other readouts establishes correlations between cell phenotype, gene expression, and metabolite levels. This systems-biology approach to understanding heterogeneous tumor and microenvironment response to drugs produces an individualized readout to quantify systemic response to the chemotherapy drug.
VI. Discussion
Our team designed an implantable microdevice that releases an array of therapeutic agents in a time-dependent manner and used the IND process to bring this concept from preclinical development to human use. With the technology used in six cancer indications, a pathway was crafted that incorporated both existing FDA guidance and newly developed methods and validation, such that subsequent applications could be effectively reviewed and approved and to further advance the safety and efficacy of ongoing studies.
We envision that the IMD platform will be utilized for two major purposes: one, to define optimal therapy regimens among approved agents for patients on a personalized basis; and two, to study early compounds in patients before traditional Phase 1/2/3 studies to examine target engagement, pharmacodynamic effects, effective concentrations, and optimal combination strategies [15]. Discussions with the FDA are currently underway to establish more formal guidance to broadly enable these applications for drug developers. Major applications may include early studies of lead identification, target validation, dose dependency, and combinatorial synergy.
The developed mechanisms using investigator-initiated IND may also be used by clinical investigators to further develop existing drugs, novel combinations, or new formulations such as thorough exploration of new combinations, for a compound in vivo, studying how to combine a drug product optimally with standard-of-care treatments, expanding use in new cancer indications, and establishing a correlation between “omic” tumor markers and drug response to approved therapies and experimental agents. This may be of particular interest in rare cancers where it is difficult to perform comparative studies of different therapies due to low numbers of available patients; and in pediatric applications, where the safety benefits of intra-target microdosing are particularly compelling compared to potential long-term risks associated with toxicity of systemic drug exposure to cytotoxic and targeted agents in children.
The utility of the IMD to be used for optimized selection of therapy for patients will depend on the correlation between short-term drug responses as measured by the IMD, and longer-term clinical outcomes such as tumor shrinkage, time to recurrence, or patient survival. Correlating IMD readouts with these longer-term outcomes is the subject of ongoing trials, and we hope to report on this aspect in the near future.
Additionally, future applications benefit from a less invasive approach that could dramatically increase the applicability of the IMD, for instance to the neoadjuvant setting. With that goal in mind, we have begun developing a percutaneous minimally invasive IMD retrieval method that could eliminate the need for surgical retrieval [36]. This method consists of a next- generation percutaneously retrievable IMD, which can be removed along with adjacent tissue using a custom retrieval device similar to existing core biopsy needles. Large animal studies are underway to establish the safety and feasibility of this approach, which would allow IMD-based assessment to be performed entirely in a low-risk, outpatient setting.
VII. CONCLUSION
We described the translational development of our IMD platform across multiple oncology applications. Even though the IMD is a drug/device combination with diagnostic intent, the FDA’s investigator-initiated IND pathway was utilized. When appropriate procedures were established, the focus of IND review became the safety of the interventional procedures associated with each anatomical site and optimization of these procedures with respect to established clinical workflows.
From IMD ideation and design to clinical implementation, obstacles were encountered that required consultations with clinicians, engineers, and regulators. From a regulatory standpoint, a process was developed to efficiently obtain IND approval by anticipating the measurements and safety features the FDA would require. From a translational standpoint, the process demonstrated that the regulatory process allows for iterations and evolution of the technology platform based on acquired clinical data and user feedback, and is therefore never a finished product; each patient application provides an opportunity for design and procedural improvements.
Although initial clinical applications are focused on FDA-approved drugs, we plan to expand IMD use to test the safety and efficacy of investigational and preclinical agents, as well as non-cancer indications. The established translational and regulatory processes described here should facilitate and streamline trials for these and other purposes in the future.
Acknowledgment
The authors thank Caroline Harvey for extensive help in developing formulation, sterilization, and pharmacy procedures, as well as Drs. Ann Farrell, Anuja Patel, Julia Beaver, and Alice Lee from the FDA for their guidance throughout the review process.
This work was supported by the U.S. National Institute of Health under Grants R37CA224144, R21CA216796, and R01CA232174, and in part by the Brigham Research Institute Director’s Transformative Award.
Contributor Information
Christine Dominas, Brigham and Women’s Hospital in Boston, MA USA.
Sharath Bhagavatula, Brigham and Women’s Hospital in Boston, MA USA.
Elizabeth H. Stover, Brigham and Women’s Hospital, and also with Dana-Farber Cancer Institute
Kyle Deans, Brigham and Women’s Hospital in Boston, MA USA.
Cecilia Larocca, Brigham and Women’s Hospital, and also with Dana-Farber Cancer Institute.
Yolanda L. Colson, Massachusetts General Hospital
Pierpaolo Peruzzi, Brigham and Women’s Hospital in Boston, MA USA.
Adam S. Kibel, Brigham and Women’s Hospital in Boston, MA USA
Nobuhiko Hata, Brigham and Women’s Hospital in Boston, MA USA.
Lillian L. Tsai, the Massachusetts General Hospital
Yin P. Hung, the Massachusetts General Hospital
Robert Packard, Medical Device Academy in Shrewsbury.
Oliver Jonas, Brigham and Women’s Hospital in Boston, MA USA.
References
- [1].Campbell R et al. , “Implantable cisplatin synthesis microdevice for regional chemotherapy,” Adv. Healthcare Mater., 2020, to be published, doi: 10.1002/adhm.202001582. [DOI] [PubMed] [Google Scholar]
- [2].Pons-Faudoa FP et al. , “Advanced implantable drug delivery technologies: Transforming the clinical landscape of therapeutics for chronic diseases,” Biomed. Microdevices, vol. 21, no. 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gundle KR et al. , “Multiplexed evaluation of microdosed antineoplastic agents in situ in the tumor microenvironment of patients with soft tissue sarcoma,” Clin. Cancer Res.: Official J. Amer. Assoc. Cancer Res., vol. 26, no. 15, 2020. [DOI] [PubMed] [Google Scholar]
- [4].Gurman P et al. , “Clinical applications of biomedical microdevices for controlled drug delivery,” Mayo Clinic Proc., vol. 90, no. 1, 2015. [DOI] [PubMed] [Google Scholar]
- [5].Pammolli F, Magazzini L, and Riccaboni M, “The productivity crisis in pharmaceutical R&D,” Nat. Rev. Drug Discov, vol. 10, no. 6, 2011. [DOI] [PubMed] [Google Scholar]
- [6].Jonas O et al. , “Erratum: An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors (Science translational medicine doi: 10.1126/scitranslmed.3010564),” Sci. Transl. Med., vol. 11, no. 520, 2019, doi: 10.1126/scitranslmed.aba1552. [DOI] [Google Scholar]
- [7].Wu D et al. , “Significance of single-cell and spatial transcriptomes in cell biology and toxicology,” Cell Biol. Toxicol., to be published, doi: 10.1007/s10565-020-09576-8. [DOI] [PubMed] [Google Scholar]
- [8].Balashova EE, Maslov DL, and Lokhov PG, “A metabolomics approach to pharmacotherapy personalization,” J. Personalized Med, vol. 8, no. 3, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Goulart BHL et al. , “Trends in the use and role of biomarkers in phase i oncology trials,” Clin. Cancer Res, vol. 13, no. 22, 2007. [DOI] [PubMed] [Google Scholar]
- [10].Meric-Bernstam F and Mills GB, “Overcoming implementation challenges of personalized cancer therapy,” Nat. Rev. Clin. Oncol, vol. 9, no. 9, 2012. [DOI] [PubMed] [Google Scholar]
- [11].Yamane N et al. , “Cost-effectiveness analysis of microdose clinical trials in drug development,” Drug Metab. Pharmacokinetics, vol. 28, no. 3, 2013. [DOI] [PubMed] [Google Scholar]
- [12].Burt T et al. , “Phase-0/microdosing studies using PET, AMS, and LCMS/MS: A range of study methodologies and conduct considerations. Accelerating development of novel pharmaceuticals through safe testing in humans– a practical guide,” Expert Opin. Drug Del, vol. 14, no. 5, 2017. [DOI] [PubMed] [Google Scholar]
- [13].Rani PU and Naidu MUR, “Phase 0-microdosing strategy in clinical trials,” Indian J. Pharmacol, vol. 40, no. 6, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burt T et al. , “Phase 0/microdosing approaches: Time for mainstream application in drug development?,” Nat. Rev. Drug Discov, vol. 19, no. 11, 2020. [DOI] [PubMed] [Google Scholar]
- [15].“Guidance for industry, investigators, and reviewers exploratory IND studies contains nonbinding recommendations guidance for industry, investigators, and reviewers exploratory IND studies,” Rockville, MD, USA, Jan. 2006. [Online]. Available: http://www.fda.gov/cder/guidance/index.htm [Google Scholar]
- [16].Farra R et al. , “First-in-human testing of a wirelessly controlled drug delivery microchip,” Sci. Transl. Med, vol. 4, no. 122, 2012. [DOI] [PubMed] [Google Scholar]
- [17].D. Administration, “How to write a request for designation (RFD): Guidance for industry,” Silver Spring, Apr. 2011. [Online]. Available: http://www.fda.gov/combinationproducts/ [Google Scholar]
- [18].Fijalkowska I, “The pre-submission how to efficiently communicate with FDA about planned applications,” July. 2019. [Google Scholar]
- [19].Haakenson C et al. , “The investigator-sponsored IND in clinical trials,” Controlled Clin. Trials, vol. 8, no. 2, 1987. [DOI] [PubMed] [Google Scholar]
- [20].Holbein MEB, “Understanding FDA regulatory requirements for investigational new drug applications for sponsor-investigators,” J. Invest. Med, vol. 57, no. 6, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].“Guidance for industry submitting and reviewing complete responses to clinical holds guidance for industry submitting and reviewing complete responses to clinical holds user fees revision 1 guidance for industry 1 submitting and reviewing complete responses to clinical holds,” Tel, Rockville, October. 2000. [Online]. Available: http://www.fda.gov/cder/guidance/index.htm or http://www.fda.gov/cber/guidelines.htm [Google Scholar]
- [22].“Introduction to master files for devices (MAFs),” November. 14, 2017. [Google Scholar]
- [23].“CFRCode of federal regulations title 21, ” April. 01, 2020. [Google Scholar]
- [24].Kurtz SM and Devine JN, “PEEK biomaterials in trauma, orthopedic, and spinal implants,” Biomaterials, vol. 28, no. 32, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jonas O et al. , “Parallel in vivo assessment of drug phenotypes at various time points during systemic BRAF inhibition reveals tumor adaptation and altered treatment vulnerabilities,” Clin. Cancer Res, vol. 22, no. 24, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jonas O et al. , “First in vivo testing of compounds targeting group 3 medulloblastomas using an implantable microdevice as a new paradigm for drug development,” J. Biomed. Nanotechnol, vol. 12, no. 6, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Davidson SM et al. , “Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors,” Nat. Med, vol. 23, no. 2, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fda C and Omalleys, “Radiation programs division of small manufacturers, international and consumer assistance center for devices and radiological health; food and drug administration 10903 new hampshire ave,” Tel, Silver Spring, June. 2012. [Online]. Available: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htmhttp://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm [Google Scholar]
- [29].Knowles D et al. , “Chemical interactions of polyethylene glycols (PEGs) and glycerol with protein functional groups: Applications to effects of PEG and glycerol on protein processes,” Biochemistry, vol. 54, no. 22, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fda and Cber, “Guidance for FDA reviewers and sponsors: Content and review of chemistry, manufacturing, and control (CMC) information for human somatic cell therapy investigational new drug applications (INDs),” Rockville, April. 2008. [Online]. Available: http://www.fda.gov/cber/guidelines.htm [Google Scholar]
- [31].“IND application reporting: Annual reports,” December. 2015. [Google Scholar]
- [32].Lusic H and Grinstaff MW, “X-ray-computed tomography contrast agents,” Chem. Rev, vol. 113, no. 3, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Aldridge S and Teichmann SA, “Single cell transcriptomics comes of age,” Nat. Commun, vol. 11, no. 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Minchinton A and Tannock I, “Drug penetration in solid tumors,” Nat. Rev. Cancer, vol. 6, no. 8, 2006. [DOI] [PubMed] [Google Scholar]
- [35].Johnson CH, Ivanisevic J, and Siuzdak G, “Metabolomics: Beyond biomarkers and towards mechanisms,” Nat. Rev. Mol. Cell Biol, vol. 17, no. 7, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bhagavatula SK et al. , “An interventional image- guided microdevice implantation and retrieval method for in-vivo drug response assessment,” Med. Phys, vol. 46, no. 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]