Abstract
Purpose of review:
Nonalcoholic fatty liver disease (NAFLD) is a common comorbidity and has wide ranging extrahepatic manifestations, including through cardiometabolic pathways. As such, there is growing interest in the impact of NAFLD on cerebrovascular disease and brain health more broadly. In this review, we assess recent research into understanding the association between NAFLD and brain health while highlighting potential clinical implications.
Recent findings:
Mechanistically, NAFLD is characterized by both a proinflammatory and proatherogenic state, which results in vascular inflammation and neurodegeneration, potentially leading to clinical and subclinical cerebrovascular disease. Mounting epidemiological evidence suggests an association between NAFLD and an increased risk and severity of stroke, independent of other vascular risk factors. Studies also implicate NAFLD in subclinical cerebrovascular disease, such as carotid atherosclerosis and microvascular disease. In contrast, there does not appear to be an independent association between NAFLD and cognitive impairment.
Summary:
The current literature supports the formulation of NAFLD as a multisystem disease that may also have implications for cerebrovascular disease and brain health. Further prospective studies are needed to better assess a temporal relationship between the two diseases, confirm these early findings, and decipher mechanistic links.
Keywords: nonalcoholic fatty liver disease, hepatic steatosis, metabolic syndrome, brain health, cerebrovascular disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease with an increasing prevalence of macrovascular and microvascular hepatic and extrahepatic complications (1–3). NAFLD encompasses a disease spectrum that ranges from isolated hepatic steatosis to steatosis with inflammation and hepatocyte injury (nonalcoholic steatohepatitis, NASH) in the absence of excessive alcohol use or other causes of liver disease. Ongoing inflammation with hepatocyte injury leads to disease progression with liver fibrosis and cirrhosis.
Over the last decade, there has been growing evidence that NAFLD is a multisystem disease affecting multiple extra-hepatic organ systems (2). NAFLD has been linked with the development of cardiometabolic complications, including cardiovascular disease (CVD), diabetes, and chronic kidney disease (2). In fact, the most common cause of death among NAFLD patients is CVD (3). Studies have demonstrated an association between NAFLD and CVD independent of established risk factors for CVD, such as age, sex, body mass index (BMI), waist circumference, smoking status, prevalent hypertension, or dyslipidemia (4–7). This body of literature suggests that the increased risk of CVD among NAFLD patients is beyond that conferred solely by traditional CVD risk factors or metabolic syndrome features.
NAFLD has been associated with both clinical and subclinical atherosclerosis regardless of the presence of CVD risk factors such as hypertension and dyslipidemia. Specifically, NAFLD has independently been linked with coronary artery disease, cardiac arrhythmias, and structural heart disease such as myocardial remodeling, resulting in systolic and/or diastolic dysfunction (8–10). Moreover, NAFLD has been associated with increased prevalence of unstable coronary plaques (2), increased arterial wall stiffness (11), increased carotid intima-media thickness (12), and impaired flow-mediated vasodilation (13).
While mounting data support the association between NAFLD and cardiometabolic complications, limited evidence is available on the association between NAFLD and cerebrovascular disease and brain health more broadly. Given the public health and economic burden of NAFLD and its potential impact on brain health, we conducted a review to better understand this relationship.
Association between NAFLD and cerebrovascular disease: plausible mechanisms
NAFLD and the development of cerebrovascular disease share common metabolic risk factors, such as hypertension, diabetes and insulin resistance, dyslipidemia, and obesity (8). These factors have also been reported to accelerate cerebral small vessel disease, leading to white matter lesions, cerebral microbleeds, smaller brain volumes and subsequent cerebral atrophy (14). Potential mechanisms linking NAFLD and cerebrovascular disease are shown in Figure 1.
Figure 1.
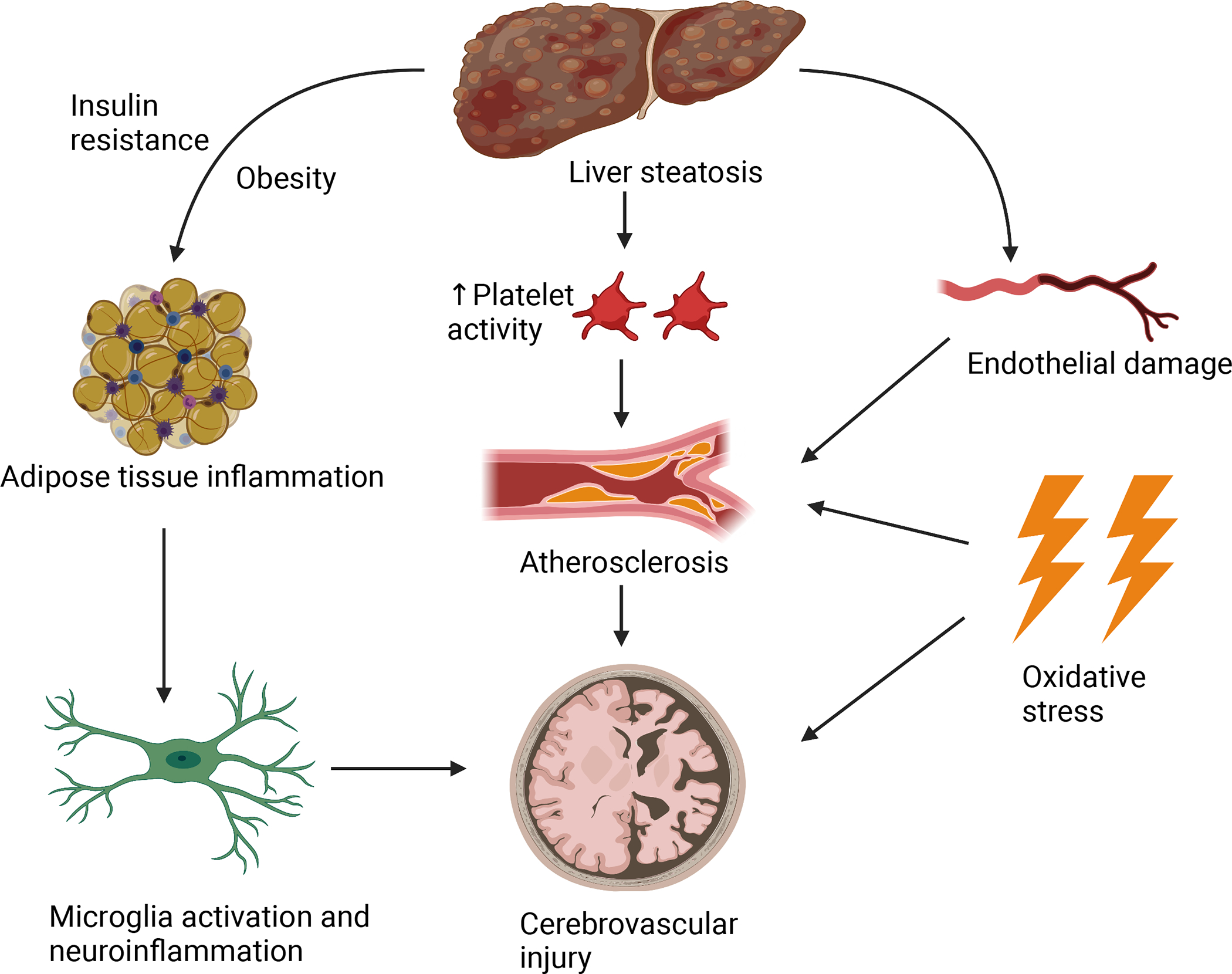
Schematic figure depicting potential mechanisms underlying the association between NAFLD and cerebrovascular disease.
NAFLD is characterized by a proinflammatory state that consists of chronic low-grade inflammation (15, 16). This inflammation is initially localized to adipose tissue and hepatocytes, however, has the potential to spread and become systemic and impact other organs, including the brain (15). Obesity and insulin resistance propagate adipose tissue inflammation, leading to lipid accumulation in the liver. Following hepatic steatosis, adipocytokines induce macrophage activation and recruitment to the liver (17). Macrophages subsequently express high levels of proinflammatory cytokines, producing high amounts of reactive oxygen species, facilitating NASH progression (17). Hence, adipocytokines create a positive feedback loop by constant macrophage recruitment and subsequent cytokine release, leading to systemic, persistent inflammation (17–19). This systemic inflammation may spread to the brain as cytokines activate receptors on peripheral endothelial cells, leading to the release of proinflammatory factors inside the CNS, causing neuroinflammation (20, 21). These processes activate microglia, stimulating release of proinflammatory cytokines, leading to further neuroinflammation, neuronal loss, and brain damage (22–25).
The inflammatory cascade surrounding NAFLD leads to increased oxidative stress, increased platelet activity, endothelial dysfunction and subsequent enhanced atherosclerosis (8). Hepatic inflammation promotes systemic proinflammatory and procoagulant factors, leading to increased atherosclerosis (26). NAFLD also has been independently associated with increased carotid intima-media thickness, leading to further development of atherosclerosis (27). Such changes may increase the risk of cerebrovascular insults, such as subcortical infarcts, resulting in white matter lesions, and cerebral infarcts, resulting in ischemic strokes (28–30). Additionally, vascular alterations, such as the increased carotid stiffness present in NAFLD patients, may also lead to alterations in cerebral perfusion (11). Increased carotid stiffness leads to higher pulsatile pressure and flow load to the brain, which may cause eventual microvascular damage and cerebral ischemia (11, 31).
In summary, NAFLD is characterized by both a proinflammatory and proatherogenic state that may also impact the cerebrovascular circulation, leading to cerebrovascular disease.
NAFLD and clinical cerebrovascular disease: epidemiological evidence
Several studies have addressed the association between NAFLD and cerebrovascular disease, though data has been inconclusive and often conflicting (Table 1). In a meta-analysis including seven studies with 6,183 participants, NAFLD was associated with 2.3 times higher risk for stroke than those without NAFLD (32). NAFLD was associated with increased risk of both cerebral hemorrhage and ischemic strokes (32). Importantly, these associations were independent of cardiovascular risk factors such as dyslipidemia, diabetes, and obesity. However, the methods by which NAFLD was diagnosed were not reported and multivariable models were variably and incomplete adjusted. Therefore, unintentional bias might have been introduced despite low overall heterogeneity. In a separate meta-analysis of six studies, NAFLD, diagnosed by ultrasound with the exclusion of other causes of chronic liver disease, was associated with a two-fold increase in ischemic stroke risk compared to the non-NAFLD group (33). However, variability in outcome definition, heterogeneity of studies, duration of follow up, and publication bias were highly prevalent.
Table 1.
Major studies that evaluated the relationship between NAFLD and cerebrovascular disease
| Study name (Year) | Study design | Population (N) | NAFLD definition | Main outcome definition | Key findings |
|---|---|---|---|---|---|
|
| |||||
| Clinical Cerebrovascular Disease | |||||
|
| |||||
| Hu et al. (2018) | Meta-analysis (2 case-control, 7 cohort studies) | 6,183 | NR | CVA (including ischemic stroke and cerebral hemorrhage); NR | NAFLD was associated with increased risk of CVA (2.32, 95% CI 1.84–2.93, P < 0.001), including both hemorrhagic stroke (OR = 1.85, 95% CI 1.05–3.27, P = 0.034) and ischemic stroke (OR = 2.51, 95% CI 1.92–3.28, P < 0.001). Results confirmed when analysis also stratified by ethnicity, study design, and CVA classification. |
| Haddad et al. (2017) | Meta-analysis (6 prospective studies) | 25,837 | Ultrasound Excluded: excessive alcohol consumption, viral hepatitis, and chronic hepatotoxic drug use |
Cardiovascular events, including subgroup analysis of ischemic stroke and TIA (cerebral hemorrhage was excluded); NR | NAFLD was associated with higher risk of ischemic stroke (RR: 2.09; 95% CI 1.46–2.98, P < 0.001). |
| Moshayedi et al. (2014) | Cross-sectional | 110 | Ultrasound Excluded: excessive alcohol consumption and viral hepatitis |
Ischemic stroke by CT and verified by MRI to exclude tumor, hemorrhage, or previous ischemic stroke | NAFLD was associated with increased risk of ischemic stroke (OR 2.15 95% CI 1.25–3.71, P = 0.006) compared to sex and age-matched controls. Multivariate analysis that adjusted for metabolic risk factors revealed no association between NAFLD and ischemic stroke. |
| Parikh et al. (2019) | Cross-sectional | 27,040 | NFS > 0.676 and FIB-4 score > 3.25 Excluded: pregnant participants, viral hepatitis, possible acute liver injury, excessive alcohol consumption, use of medications associated with steatosis |
Stroke (ischemic and hemorrhagic stroke unable to be differentiated) by self-reported survey | NAFLD was associated with increased risk of stroke in unadjusted models when using NFS and FIB-4 composite score (OR 5.75, 95% CI 4.66–7.09), FIB-4 (OR 6.54, 95% 3.81–11.22) score and NFS (OR 5.79, 95% 4.79–7.01). Multivariate analysis that adjusted for metabolic factors revealed an association between NAFLD and stroke only when NAFLD was defined by FIB-4 score (OR 1.87, 95% CI 1.00–3.50). |
| Xu et al. (2021) | Prospective cohort | 79,905 | Ultrasound Excluded: excessive alcohol consumption and other liver diseases |
Ischemic stroke, defined based on signs, symptoms, and brain CT or MRI findings | NAFLD was associated with increased risk of stroke (HR 1.16, 95% CI 1.07–1.26) after adjusting for metabolic risk factors. Higher severity of NAFLD was associated with increasing risk of stroke (mild: HR 1.15, 95% CI 1.05–1.25; moderate: 1.19, 95% CI 1.06–1.34; severe: 1.21; 95% 1.08–1.50). |
| Abdeldyem et al. (2017) | Prospective cohort | 242 | Ultrasound Excluded: chronic viral hepatitis, drug toxicity, and excessive alcohol consumption |
Severity of acute ischemic stroke by NIHSS score at admission and functional outcome by mRS score at discharge | At admission, the severity of stroke at admission was higher in participants with NAFLD (NIHSS 8.7±7.4) than those without NAFLD (NIHSS 5.5±6.5; P = 0.013). The functional outcome after stroke was worse in participants with NAFLD (mRS 3.6±2.3) than those without NAFLD (mRS 1.8±2.4). Multivariate analysis adjusting for potential confounders was not performed. |
| Li et al. (2018) | Retrospective | 306 | Ultrasound Excluded: chronic viral hepatitis, drug toxicity, excessive alcohol consumption, cholestatic diseases, and rhabdomyolysis |
Severity of acute ischemic stroke by NIHSS score at admission, progression of stroke by increase in NIHSS score, and functional outcome by mRS score at discharge | NAFLD was associated with severity independent of metabolic risk factors (HR 2.327, 95% CI 1.252–4.324). The risk of progression of stroke was higher in those with NAFLD than those without, after adjustment for potential confounders (HR 2.378, 95% CI 1.260–4.486). Multivariate analysis revealed no association between NAFLD and functional outcome after stroke at discharge. |
| Seo et al. (2016) | Cross-sectional | 4,472 | Ultrasound Excluded: excessive alcohol consumption and viral hepatitis |
Cognitive learning, memory, attention, and concentration (SDLT), psychomotor speed (SRTT), visuospatial function (SDST) | NAFLD was associated with reduced cognitive learning, poor memory, attention, and concentration independent of metabolic risk factors (β = 0.726, 95% CI 0.105–1.347). There was no association between NAFLD and psychomotor speed or visuospatial function after adjustments for metabolic covariates. |
| Gerber et al. (2021) | Cross-sectional | 2,809 | CT using LA ≤ 51 Excluded: pregnant participants, viral hepatitis, cirrhosis, excessive alcohol consumption, use of medications associated with steatosis |
Processing speed (DSST), verbal memory (RAVLT), executive function (Stroop Test) | After adjustments for metabolic covariates, there was no association between NAFLD and performance on cognitive tests. |
|
| |||||
| Subclinical Cerebrovascular Disease | |||||
|
| |||||
| Airaghi et al. (2018) | Case-control | 34 | Liver biopsy | Cerebral perfusion by MRI spectroscopy | NAFLD was associated with reduced cerebral perfusion confined to limited brain areas, such as left semioval center and posterior cingulate cortex (beta coefficient -5.7, 95% CI -11, -0.08). |
| Sinn et al. (2016) | Retrospective cohort | 8,020 | Ultrasound Excluded: excessive alcohol consumption, viral hepatitis, history of cirrhosis |
Carotid plaque defined as CIMT ≥0.5mm by carotid artery ultrasound | NAFLD was associated with the presence of subclinical carotid atherosclerosis compared to age-adjusted controls (HR 1.23, 95% CI 1.13–1.35, P < 0.001). Multivariate analysis adjusted for metabolic variables, such as diabetes, hypertension, and dyslipidemia revealed no association. |
| Zhang et al. (2020) | Cross-sectional | 12,990 | Ultrasound Excluded: excessive alcohol consumption, viral/autoimmune/drug-induced hepatitis |
Carotid plaque defined as CIMT ≥1.5mm by carotid artery ultrasound | NAFLD was associated with the presence of subclinical carotid atherosclerosis independent of metabolic risk factors, such as glucose levels, lipid profiles, and BMI (OR 1.89, 95% CI 1.59–2.24). |
| Weinstein et al. (2018) | Cross-sectional | 766 | CT using liver to phantom ratio ≤0.33 Excluded: excessive alcohol consumption |
Cerebral brain volume, hippocampal and white matter hyperintensity volumes, covert brain infarcts by brain MRI | NAFLD was associated with smaller total cerebral brain volume after adjustment for vascular risk factors, including hypertension, dyslipidemia, diabetes, and cardiovascular disease (beta coefficient -0.26 (0.11), P =0.02). There was no relationship between NAFLD and hippocampal or white matter hyperintensity volumes or covert brain infarcts. |
| Jang et al. (2019) | Cross-sectional | 1,260 | Ultrasound FIB-4 ≥1.45: high-intermediate probability of advanced fibrosis FIB-4 < 1.45: low probability of advanced fibrosis Excluded: excessive alcohol consumption, viral hepatitis, history of cirrhosis |
WMH, lacunes, and microbleeds by brain MRI | NAFLD was associated with moderate to severe WMH independent of other cardiometabolic risk factors (OR 1.64, 95% CI 1.10–2.42). Participants with high-intermediate probability of advanced fibrosis had odds of WMH (OR 1.77, 95% CI 1.13–2.78) than those with low probability of advanced fibrosis (OR 1.14, 95% 0.72–1.82). There was no relationship between NAFLD and the presence of lacunes or microbleeds. |
Abbreviations: NR: not reported; CVA: cerebrovascular accident; NAFLD: non-alcoholic fatty liver disease; TIA: transient ischemic attack; CT: computed tomography; MRI: magnetic resonance imaging; NFS: nonalcoholic fatty liver disease fibrosis score; FIB-4: fibrosis-4 score, NIHSS: NIH stroke scale; mRS: modified Rankin score; SDLT: serial digit learning test; SRTT: simple reaction time; SDST: symbol digit substitution test; LA: liver attenuation; HU: Hounsfield Units; DSST: digit symbol substitution test; RAVLT: Rey Auditory Verbal Learning Test; CIMT: carotid intima-media thickness; BMI: body mass index; WMH: white matter hyperintensities
In a cross-sectional study of 110 participants, NAFLD participants had a 2.15 times increased odds of ischemic stroke compared to sex and age-matched controls (34). However, in multivariate analysis including metabolic factors, such as hypertension and diabetes status, dyslipidemia, smoking status, and heart disease, there was no association between NAFLD and stroke (34). It is possible that the small sample size of this study may account for this finding. Another cross-sectional study of 27,040 health survey participants demonstrated significant associations between NAFLD and increased odds of stroke, but only when advanced fibrosis was also present as defined using the Fibrosis-4 (FIB-4) score (35). There was no association when NAFLD with advanced fibrosis was defined using the NAFLD fibrosis score. In terms of longitudinal data, a case-cohort study using data from a prospective stroke study found that liver steatosis and liver fibrosis scores were associated with incident ischemic stroke in women but not in men after adjustments for metabolic covariates (36, 37). The use of surrogate markers for NAFLD and liver fibrosis limits the conclusions that can be drawn. However, in the largest study to date (n=80,000), NAFLD was defined using liver ultrasound, and people with NAFLD had a 16% higher risk of stroke, after adjusting for age, sex, and metabolic risk factors (38).
The literature regarding the impact of NAFLD on stroke severity and outcomes is limited. In a small prospective study of patients admitted with acute ischemic stroke, NAFLD was associated with the severity of stroke at admission as assessed with the National Institutes of Health Stroke Scale (39). Patients with NAFLD had more severe strokes and worse functional outcomes, as assessed by the modified Rankin scale (39). While prevalent hypertension, smoking status, and BMI were similar between patients with and without NAFLD, prevalent diabetes and waist circumference were significantly higher in patients with NAFLD. Additionally, patients with NAFLD had significantly higher glucose, triglycerides, and LDL (39). These differences in vascular risk factors were not accounted for in the comparison of stroke severity and functional outcome between NAFLD and non-NAFLD patients, possibly impacting the results. A retrospective study of 306 patients demonstrated that those with NAFLD experienced more severe strokes and were at higher risk for neurological deterioration during hospitalization but had no difference in functional outcomes (40). These associations remained significant after adjustment of other traditional risk factors, suggesting the presence of NAFLD has a negative impact in the outcome of patients affected with cerebrovascular disease.
NAFLD and subclinical cerebrovascular disease
The literature surrounding NAFLD and subclinical cerebrovascular disease is sparse. Reports regarding the association between NAFLD and carotid atherosclerosis have been conflicting. A retrospective cohort study of approximately 8,000 men demonstrated an association between NAFLD and subclinical carotid atherosclerosis development when adjusted for age, smoking status, alcohol consumption, BMI, and weight change (41). However, when also adjusted for metabolic factors, such as diabetes, hypertension, and dyslipidemia, the association was attenuated, suggesting that these factors possibly mediate the association between NAFLD and development of subclinical carotid atherosclerosis (41). However, a recent study of approximately 13,000 men and women demonstrated that NAFLD participants were more likely to develop carotid plaque, even after adjustments for metabolic factors (27). These disparate results may be due to the differences in carotid plaque assessment between the two studies. The first defined carotid plaque by carotid intima-media thickness greater than 0.5mm while the latter used a cut-off of 1.5mm (27, 41).
Cerebral white matter hyperintensity volume, considered a reflection of cerebral small vessel disease, is associated with an increased risk of stroke, cognitive decline, dementia, disability, and mortality (42). A study of 1,260 participants found NAFLD was associated with white matter hyperintensities (WMH) independent of cardiometabolic risk factors, such as hypertension, obesity, diabetes, hyperlipidemia, and smoking status (43). Further, participants with an intermediate to high FIB-4 score had higher odds of WMH compared to those with a low FIB-4 score (43), suggesting NAFLD may be an independent risk factor for the development of WMH in a dose-dependent manner. Last, there is evidence linking NAFLD-associated genetic variants with cerebral microvascular disease. Most notably, the rs738408 C>G polymorphism of the PNPLA3 gene is strongly associated with NAFLD (3). In one study, the PNPLA3 variant was associated with carotid atherosclerosis and intima-media thickening progression among a Sicilian cohort of biopsy-proven NAFLD patients (44). Additionally, in a cross-sectional analysis, PNPLA3 GG homozygosity was associated with greater WMH volume compared to non-carriers, even after adjusting for metabolic risk factors such as diabetes, hypertension, and BMI (45). Homozygous carriers have an increased risk for progressive liver disease and these studies suggest they also have an increased risk of subclinical cerebrovascular disease (3, 44, 45).
Finally, NAFLD may also impair cerebral perfusion. A small case-control study demonstrated reduced cerebral perfusion confined to limited brain areas in NAFLD patients compared to controls (46). Notably, NAFLD participants were normotensive with a mean BMI of 26.5 kg/m2 and normal levels of triglycerides and fasting plasma glucoses, suggesting subclinical cerebrovascular disease can occur in NAFLD patients even before other extrahepatic manifestations or atherosclerotic risk factors of metabolic syndrome are present (46).
While there is growing evidence linking NAFLD with various forms of subclinical cerebrovascular disease, available data are limited by their small size, cross-sectional study design, and variable ascertainment of NAFLD and cerebrovascular disease. These studies do not support a temporal relationship between NAFLD and subclinical atherosclerosis or other cerebrovascular disease, highlighting the need for further prospective studies.
NAFLD and cognition
In addition to clinical stroke and subclinical cerebrovascular disease, there is emerging but limited evidence that NAFLD may impact cognition (26). A large cross-sectional study of 4,472 participants below the age of 59 investigated the association between NAFLD and cognitive performance measures (47). NAFLD was defined as steatosis detected on ultrasound in the absence of other causes of chronic liver disease or steatosis. NAFLD was associated with reduced cognitive learning, poor memory, attention, and concentration (Serial Digit Learning Test; SDLT) independent of metabolic risk factors (47). However, there was no association between NAFLD and psychomotor speed (Simple Reaction Time Test; SRTT) or visuospatial function (Symbol Digit Substitution Test; SDST) after adjustments for metabolic covariates (47). This variation in association between different cognitive tests may suggest that NAFLD might affect cognitive function through region-specific processes rather than diffuse cortical dysfunction. A recent study that evaluated the relationship between NAFLD and processing speed, verbal memory, and executive function demonstrated similar results (48). NAFLD was defined according to liver attenuation on CT examination after exclusion for other causes of steatosis (48). After adjustment for metabolic covariates, there was no relationship between NAFLD and any of the cognitive performance tests (48). Rather, it may be that more advanced forms of NAFLD, such as forms including liver fibrosis, impact cognition. In the Framingham Study, computed tomography evidence of NAFLD was not assisted with cognitive function; however, participants with higher fibrosis scores had worse performance in tests of executive function and reasoning (49). Similarly, in a study of a population-based sample of older Americans (age 60–80), noninvasive measures of liver fibrosis were associated with worse cognitive performance on multiple domains (50). A major limitation of the above studies is the use of varied measures of NAFLD and the use of neuropsychological screening tools that may not be sensitive for the cognitive phenotype of NAFLD. Further, there are sparse and conflicting neuroimaging data regarding brain volumes and other imaging markers of brain health to corroborate these data (30).
Conclusion
The current literature promotes the idea that the clinical burden of NAFLD extends beyond the liver and encompasses a multisystem disease that may have implications for stroke, subclinical cerebrovascular disease, and cognitive brain health. However, prospective studies using precise measures of liver disease and stroke are limited. Prospective studies using consistent diagnostic criteria, such as liver biopsy or high sensitivity cross-sectional imaging (e.g., MR elastography) for assessment of NAFLD disease severity and MRI for stroke, are needed to better evaluate for evidence of a causal relationship between NAFLD and cerebrovascular disease.
Further research regarding the risk of stroke in NAFLD, and the impact of NAFLD on stroke outcomes, may have important clinical implications. Confirmation of prior findings may support investigation of stroke prevention strategies in patients with NAFLD, such as the use of lipid-lowering medications or anti-platelet agents. Additional research may also evaluate interventions to mitigate the deleterious impact of NAFLD on stroke outcomes. Last, confirmation of preliminary findings about the role of NAFLD in cognitive brain health may yield opportunities to improve cognition in a subset of people.
Key points:
NAFLD may be associated with increased risk and severity of stroke, independent of other cardiometabolic risk factors.
NAFLD may contribute to carotid atherosclerosis and cerebral microvascular disease, as forms of subclinical cerebrovascular disease.
NAFLD and associated conditions may contribute to cognitive impairment.
High quality prospective studies are needed to better understand whether these associations are casual.
Acknowledgements
The authors thank Corinne Miller and the Galter Health Sciences Library staff for their assistance with this project.
Dr. VanWagner is supported by the National Heart, Lung and Blood Institute (K23 HL136891). Dr. Parikh is supported by funding from the Leon Levy Foundation, the NY State Empire Clinical Research Investigator Program, and the Florence Gould foundation.
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Younossi ZM, Henry L, Bush H, Mishra A. Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Clin Liver Dis. 2018;22(1):1–10. [DOI] [PubMed] [Google Scholar]
- 2.VanWagner LB, Rinella ME. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Curr Hepatol Rep. 2016;15(2):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotter TG, Rinella M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology. 2020;158(7):1851–64. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–53. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Mingolla L, Rigolon R, Pichiri I, Cavalieri V, Zoppini G, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular disease in adult patients with type 1 diabetes. Int J Cardiol. 2016;225:387–91. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65(3):589–600. [DOI] [PubMed] [Google Scholar]
- 7.Zeb I, Li D, Budoff MJ, Katz R, Lloyd-Jones D, Agatston A, et al. Nonalcoholic Fatty Liver Disease and Incident Cardiac Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2016;67(16):1965–6. [DOI] [PubMed] [Google Scholar]
- 8.Kasper P, Martin A, Lang S, Kutting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology. 2015;62(3):773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanWagner LB, Wilcox JE, Ning H, Lewis CE, Carr JJ, Rinella ME, et al. Longitudinal Association of Non-Alcoholic Fatty Liver Disease With Changes in Myocardial Structure and Function: The CARDIA Study. J Am Heart Assoc. 2020;9(4):e014279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaruvongvanich V, Chenbhanich J, Sanguankeo A, Rattanawong P, Wijarnpreecha K, Upala S. Increased arterial stiffness in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017;29(9):e28–e35. [DOI] [PubMed] [Google Scholar]
- 12.Tana C, Ballestri S, Ricci F, Di Vincenzo A, Ticinesi A, Gallina S, et al. Cardiovascular Risk in Non-Alcoholic Fatty Liver Disease: Mechanisms and Therapeutic Implications. Int J Environ Res Public Health. 2019;16(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–64. [DOI] [PubMed] [Google Scholar]
- 14.Bizino MB, Sala ML, de Heer P, van der Tol P, Smit JW, Webb AG, et al. MR of multi-organ involvement in the metabolic syndrome. Magn Reson Imaging Clin N Am. 2015;23(1):41–58. [DOI] [PubMed] [Google Scholar]
- 15.Fricker ZP, Pedley A, Massaro JM, Vasan RS, Hoffmann U, Benjamin EJ, et al. Liver Fat Is Associated With Markers of Inflammation and Oxidative Stress in Analysis of Data From the Framingham Heart Study. Clin Gastroenterol Hepatol. 2019;17(6):1157–64 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–85. [DOI] [PubMed] [Google Scholar]
- 17. Alharthi J, Latchoumanin O, George J, Eslam M. Macrophages in metabolic associated fatty liver disease. World J Gastroenterol. 2020;26(16):1861–78. * This article reviews recent advances in our understanding of macrophage involvement in the propogation of inflammation related to NAFLD and extra-hepatic involvement.
- 18.Catrysse L, van Loo G. Adipose tissue macrophages and their polarization in health and obesity. Cell Immunol. 2018;330:114–9. [DOI] [PubMed] [Google Scholar]
- 19.Lefere S, Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Rep. 2019;1(1):30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antel JP, Becher B, Ludwin SK, Prat A, Quintana FJ. Glial Cells as Regulators of Neuroimmune Interactions in the Central Nervous System. J Immunol. 2020;204(2):251–5. [DOI] [PubMed] [Google Scholar]
- 21.Cai D, Liu T. Inflammatory cause of metabolic syndrome via brain stress and NF-kappaB. Aging (Albany NY). 2012;4(2):98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balzano T, Forteza J, Borreda I, Molina P, Giner J, Leone P, et al. Histological Features of Cerebellar Neuropathology in Patients With Alcoholic and Nonalcoholic Steatohepatitis. J Neuropathol Exp Neurol. 2018;77(9):837–45. [DOI] [PubMed] [Google Scholar]
- 23.Balzano T, Forteza J, Molina P, Giner J, Monzo A, Sancho-Jimenez J, et al. The Cerebellum of Patients with Steatohepatitis Shows Lymphocyte Infiltration, Microglial Activation and Loss of Purkinje and Granular Neurons. Sci Rep. 2018;8(1):3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Cheng X, Wang X, Liu Q, Ma H, Li M. Dyslipidemic Diet Induces Mobilization of Peripheral Neutrophils and Monocytes That Exacerbate Hemorrhagic Brain Injury and Neuroinflammation. Front Cell Neurosci. 2020;14:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang QQ, Zhou JW. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia. 2019;67(6):1017–35. [DOI] [PubMed] [Google Scholar]
- 26. Kjærgaard K, Mikkelsen ACD, Wernberg CW, Grønkjær LL, Eriksen PL, Damholdt MF, et al. Cognitive Dysfunction in Non-Alcoholic Fatty Liver Disease-Current Knowledge, Mechanisms and Perspectives. J Clin Med. 2021;10(4). ** This article summarizes the current evidence for NAFLD cognitive dysfunction and reviews possible mechanisms implicated in the development of brain dysfunction in NAFLD as well the current challenges in understanding this relationship further.
- 27. Zhang Y, Xu R, Ai L, Fan Z. Association between non-alcoholic fatty liver disease and silent carotid plaque in Chinese aged population: a cross-sectional study. Ann Palliat Med. 2020;9(2):182–9. * This study uses carotid plaque development to directly evaluate the association between NAFLD and subclinical atherosclerosis.
- 28.Cermakova P, Ding J, Meirelles O, Reis J, Religa D, Schreiner PJ, et al. Carotid Intima-Media Thickness and Markers of Brain Health in a Biracial Middle-Aged Cohort: CARDIA Brain MRI Sub-study. J Gerontol A Biol Sci Med Sci. 2020;75(2):380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Della-Morte D, Dong C, Markert MS, Elkind MSV, Sacco RL, Wright CB, et al. Carotid Intima-Media Thickness Is Associated With White Matter Hyperintensities: The Northern Manhattan Study. Stroke. 2018;49(2):304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinstein G, Zelber-Sagi S, Preis SR, Beiser AS, DeCarli C, Speliotes EK, et al. Association of Nonalcoholic Fatty Liver Disease With Lower Brain Volume in Healthy Middle-aged Adults in the Framingham Study. JAMA Neurol. 2018;75(1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW, et al. Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation. 2017;136(8):719–28. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Xu Y, He Z, Zhang H, Lian X, Zhu T, et al. Increased risk of cerebrovascular accident related to non-alcoholic fatty liver disease: a meta-analysis. Oncotarget. 2018;9(2):2752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, Alla VM. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab Syndr. 2017;11 Suppl 1:S209–S16. [DOI] [PubMed] [Google Scholar]
- 34.Moshayedi H, Ahrabi R, Mardani A, Sadigetegad S, Farhudi M. Association between non-alcoholic fatty liver disease and ischemic stroke. Iran J Neurol. 2014;13(3):144–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Parikh NS, VanWagner LB, Elkind MSV, Gutierrez J. Association between nonalcoholic fatty liver disease with advanced fibrosis and stroke. J Neurol Sci. 2019;407:116524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parikh NS, Koh I, VanWagner LB, Elkind MSV, Zakai NA, Cushman M. Liver Fibrosis is Associated with Ischemic Stroke Risk in Women but not Men: The REGARDS Study. J Stroke Cerebrovasc Dis. 2021;30(7):105788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander KS, Zakai NA, Lidofsky SD, Callas PW, Judd SE, Tracy RP, et al. Non-alcoholic fatty liver disease, liver biomarkers and stroke risk: The Reasons for Geographic and Racial Differences in Stroke cohort. PLoS One. 2018;13(3):e0194153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu J, Dai L, Zhang Y, Wang A, Li H, Wang Y, et al. Severity of Nonalcoholic Fatty Liver Disease and Risk of Future Ischemic Stroke Events. Stroke. 2021:103–10. ** This paper features one of the largest cohort studies in the current literature that evalutes the association between NAFLD and the incidence and severity of stroke.
- 39.Abdeldyem SM, Goda T, Khodeir SA, Abou Saif S, Abd-Elsalam S. Nonalcoholic fatty liver disease in patients with acute ischemic stroke is associated with more severe stroke and worse outcome. J Clin Lipidol. 2017;11(4):915–9. [DOI] [PubMed] [Google Scholar]
- 40.Li H, Hu B, Wei L, Zhou L, Zhang L, Lin Y, et al. Non-alcoholic fatty liver disease is associated with stroke severity and progression of brainstem infarctions. Eur J Neurol. 2018;25(3):577–e34. [DOI] [PubMed] [Google Scholar]
- 41.Sinn DH, Cho SJ, Gu S, Seong D, Kang D, Kim H, et al. Persistent Nonalcoholic Fatty Liver Disease Increases Risk for Carotid Atherosclerosis. Gastroenterology. 2016;151(3):481–8 e1. [DOI] [PubMed] [Google Scholar]
- 42.Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurol. 2019;76(1):81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang H, Kang D, Chang Y, Kim Y, Lee JS, Kim KW, et al. Non-alcoholic fatty liver disease and cerebral small vessel disease in Korean cognitively normal individuals. Sci Rep. 2019;9(1):1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petta S, Valenti L, Marchesini G, Di Marco V, Licata A, Camma C, et al. PNPLA3 GG genotype and carotid atherosclerosis in patients with non-alcoholic fatty liver disease. PLoS One. 2013;8(9):e74089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parikh NS, Dueker N, Varela D, Del Brutto VJ, Rundek T, Wright CB, et al. Association between PNPLA3 rs738409 G variant and MRI cerebrovascular disease biomarkers. J Neurol Sci. 2020;416:116981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Airaghi L, Rango M, Maira D, Barbieri V, Valenti L, Lombardi R, et al. Subclinical cerebrovascular disease in NAFLD without overt risk factors for atherosclerosis. Atherosclerosis. 2018;268:27–31. [DOI] [PubMed] [Google Scholar]
- 47.Seo SW, Gottesman RF, Clark JM, Hernaez R, Chang Y, Kim C, et al. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology. 2016;86(12):1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerber Y, VanWagner LB, Yaffe K, Terry JG, Rana JS, Reis JP, et al. Non-alcoholic fatty liver disease and cognitive function in middle-aged adults: the CARDIA study. BMC Gastroenterol. 2021;21(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinstein G, Davis-Plourde K, Himali JJ, Zelber-Sagi S, Beiser AS, Seshadri S. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: The Framingham Study. Liver Int. 2019;39(9):1713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parikh NS, Kumar S, Rosenblatt R, Zhao C, Cohen DE, Iadecola C, et al. Association between liver fibrosis and cognition in a nationally representative sample of older adults. Eur J Neurol. 2020;27(10):1895–903. [DOI] [PubMed] [Google Scholar]


