Abstract
Objective
Vaccine-preventable human papillomavirus (HPV) infection is common, especially in sub-Saharan Africa where HIV risk is also high. However, unlike other sexually transmitted infections (STIs), HPV’s role in HIV acquisition is unclear. We evaluated this relationship using data from MTN-003, a clinical trial of HIV chemoprophylaxis among cisgender women in sub-Saharan Africa.
Design
Case-control study
Methods
We matched 138 women who acquired HIV (cases) to 412 HIV-negative controls. Cervicovaginal swabs collected within 6 months before HIV seroconversion were tested for HPV DNA. We estimated the associations between carcinogenic (high-risk) and low-risk HPV types and types targeted by HPV vaccines and HIV acquisition, using conditional logistic regression models adjusted for time-varying sexual behaviors and other STIs.
Results
Mean age was 23 (+/− 4) years. Any, high-risk, and low-risk HPV was detected in 84%, 74%, and 66% of cases, and 65%, 55%, and 48% of controls. Infection with ≥2 HPV types was common in cases (67%) and controls (49%), as was infection with nonavalent vaccine-targeted types (60% and 42%). HIV acquisition increased with any (aOR 2.5, 95% CI 1.3-4.7), high-risk (aOR 2.6, 95% CI 1.5-4.6), and low-risk (aOR 1.8, 95% CI 1.1-2.9) HPV. Each additional type detected increased HIV risk by 20% (aOR 1.2, 95% CI 1.1-1.4). HIV acquisition was associated with HPV types targeted by the nonavalent (aOR 2.1, 95% CI 1.3-3.6) and quadrivalent vaccines (aOR 1.9, 95% CI 1.1-3.2).
Conclusions
HPV infection is associated with HIV acquisition in sub-Saharan African women. In addition to preventing HPV-associated cancers, increasing HPV vaccination coverage could potentially reduce HIV incidence.
Keywords: HIV acquisition, Human Papillomavirus, cervical cancer, adolescent girls and young women
Introduction
Despite the encouraging decline in global HIV incidence in the past decade, adolescent girls and young women (AGYW) living in sub-Saharan Africa continue to be disproportionately affected by the HIV epidemic.1 Although they represent only 10% of the population, AGYW aged 14-25 years accounted for 25% of new HIV infections in 2017.2 Unlike the global HIV incidence trend, the risk of HIV acquisition among AGYW in the highest risk communities has not declined.3
AGYW in sub-Saharan Africa experience a number of biological, social, and structural factors that increase their susceptibility to HIV. One highly prevalent biological factor may be human papillomavirus (HPV) infection, which causes cervical cancer and is associated with other anogenital and oropharyngeal cancers.4,5 HPV is one of the most common sexually transmitted infections,6,7 and individuals are highly susceptible shortly after sexual debut.8 Globally, HPV prevalence is highest among women younger than 25 years compared to older age groups; although in some regions, including Africa, HPV prevalence peaks again in women over 45.9,10 In Africa specifically, the prevalence of infection with any HPV type is 36% in AGYW.11
While the role of other sexually transmitted infections (STIs) in increasing HIV risk is well established,12,13 data on the association between HPV and HIV acquisition is relatively scarce. A meta-analysis of the studies published thus far found a 2-3 fold increase in the risk of subsequent HIV acquisition among women with an HPV infection.14,15 However, several of these studies were limited by small sample size, uncontrolled confounding, and misclassification bias related to HPV exposure.14,15 In nearly half of the nine studies included in the meta-analyses, the time interval between the ascertainment of HPV infection and HIV seroconversion was >1 year. As most HPV infections are transient and clear within one year,16,17 the HPV types detected in those studies are potentially not etiologically relevant to the outcome of HIV seroconversion >1 year later. Further, since both infections are acquired through sexual activity, disentangling behavioral and biological cofactors is not trivial. Consequently, sexual behaviors and coinfection with other STIs were not adequately controlled for in most of the studies.14,15
To minimize biases present in previous studies, we estimated the risk of subsequent HIV acquisition in women with HPV infection or cervical precancerous lesions, leveraging data from MTN-003 Vaginal and Oral Interventions to Control the Epidemic (VOICE), a randomized controlled trial of pre-exposure prophylaxis (PrEP) against HIV infection, while accounting for the temporal pattern of sexual behaviors and STI coinfections. Due to low adherence to study products, PrEP did not reduce HIV incidence among VOICE participants.18
Methods
Study participants and design
Our study was nested in VOICE, a multicenter randomized placebo-controlled trial in South Africa, Uganda, and Zimbabwe.18 VOICE aimed to assess the efficacy of HIV oral and topical PrEP in sexually active cisgender women at risk for acquiring HIV. The VOICE trial was conducted between 2009 and 2015, and enrolled non-pregnant, contracepting, HIV-uninfected women aged 18-45 years who provided written informed consent. The VOICE study was approved by the institutional review boards and ethics committees at participating institutions. Participants were randomized to receive daily placebo or tenofovir-based PrEP in the forms of oral tablets or vaginal gel. Participants underwent HIV testing monthly with a rapid test, which, if positive, was followed by a confirmatory Western blot. HIV seroconversion was dated at the first positive rapid test. Endocervical and vaginal swabs were collected every six months, though the swabs were not tested for HPV in real time. Additionally, participants were assessed for other STIs and vaginal infections at enrollment, at annual follow-up visits, and when clinically indicated. Strand displacement amplification of urine samples was used to detect Chlamydia trachomatis and Neisseria gonorrhoeae (BD ProbeTec; Becton Dickinson). Serological testing was conducted for syphilis. Vaginal fluid swabs were used for rapid tests for bacterial vaginosis and Trichomonas vaginalis (BVBlue Test and OSOM, Genzyme), and for wet mount for candidiasis. Wet mount slides were read by local clinical staff or lab staff. Tests for herpes simplex virus type 2 (HSV-2) antibody was conducted using a HSV-2–specific enzyme immunoassay (EIA; Focus Diagnostics) with a cutoff value of <3.5 on stored plasma collected at enrollment and at quarterly follow up visits if they were negative at enrollment.19 Participants reported their sexual activity in the previous three months using a computer-assisted self-interview platform at enrollment and quarterly during follow-up. Details of the VOICE study procedures were published previously.18 During the VOICE trial, HPV vaccines were not widely available South Africa, Zimbabwe, and Uganda, therefore participants were not offered the vaccines.
To establish a clear temporal relationship between the detection of HPV and HIV acquisition, selection criteria for our case-control analysis were:
Having a stored cervical or vaginal swab specimen available for testing, and
Cervical or vaginal swab was collected between one to six months before HIV seroconversion.
We excluded swabs collected within one month of HIV seroconversion to minimize the possibility of reverse association, as the detection of HPV infection is increased immediately following HIV seroconversion.20 Of the 312 participants who seroconverted during the VOICE study, 138 met inclusion criteria. Each case was matched to 3 controls from VOICE based on study visit, age within 5 years, and study site. Using a risk-set sampling method, HIV-negative women who met matching criteria and had a stored cervical or vaginal swab that was collected within six months before the study visit during which cases seroconverted were randomly selected to serve as controls. Our selection criteria were based on the availability of stored cervical swabs. If cervical swabs were not available, we substituted with vaginal swabs. In our final analytic sample, 1% of the specimens tested were from vaginal swabs.
To assess control selection bias, we compared the baseline characteristics of controls in our study sample to that of VOICE participants overall18 (Table 1). On average, the participants serving as controls in our study were younger than the VOICE population, thus less likely to be married at the time of enrollment and had fewer children. While 95% of our controls were from South Africa, 81% of VOICE participants were from South Africa, 6% from Uganda, and 13% from Zimbabwe. However, controls in our study were similar to VOICE participants overall in terms of education, condom use, number of male sex partners in the three months before enrollment, and proportion reporting anal sex in the three months before enrollment. We also assessed internal validity by comparing the cases to VOICE participants who seroconverted but were not included in our study (Table 1). At enrollment, the cases and the other VOICE participants who seroconverted were nearly identical in terms of age, country of residence, and the number of live births, but slightly fewer cases had secondary school education or higher (88% vs. 92%). The proportion of cases who were married, use condoms during last vaginal sex, had ≥2 male sex partners in the past three months, and reported anal sex in the past three months were also similar compared to other VOICE participants who seroconverted.
Table 1.
Baseline demographic characteristics and sexual behaviors among cases and controls compared to VOICE participants overall.
| Controls* (n = 412) | VOICE participants (n = 5029)14 | Cases (n = 138) | VOICE participants who seroconverted, but were not included (n=174) | |
|---|---|---|---|---|
| Mean age (SD) | 23.6 (3.9) | 25.5 (5.1) | 23.2 (4.1) | 23.2 (4.1) |
| Some secondary school education or higher | 95% | 92% | 88% | 92% |
| Country | ||||
| South Africa | 391 (95%) | 4077 (81%) | 131 (95%) | 165 (95%) |
| Uganda | 12 (3%) | 322 (6%) | 4 (3%) | 5 (3%) |
| Zimbabwe | 9 (2%) | 630 (13%) | 3 (2%) | 4 (2%) |
| Currently married | 34 8% | 1052 (21%) | 7 (5%) | 5 (3%) |
| Mean number of live births (SD) | 1.2 (0.9) | 1.5 (1.1) | 1.2 (0.9) | 1.2 (0.9) |
| Condom use during last vaginal sex | 325/373 (87%) | 3766/4420 (85%) | 109/126 (87%) | 137/161 (85%) |
| ≥2 male sex partners in the past 3 months | 92/410 (22%) | 1104/4991 (22%) | 39/137 (28%) | 43/173 (25%) |
| Anal sex in the past 3 months | 70/407 (17%) | 868/4991 (17%) | 27/136 (20%) | 38/171 (22%) |
Abbreviation: SD, standard deviation
Controls were HIV-negative VOICE participants matched to cases based on study visit, age within 5 years, and study site.
Laboratory procedures
For our nested case-control study, we utilized HIV test results and STI data provided by VOICE. We tested the cervical and vaginal swabs, which were stored at −70C in cryovials containing 400 uL phosphate buffered saline, for presence of HPV DNA at the University of Washington (UW) Pathology Laboratory at Harborview Medical Center. HPV DNA was detected using a Luminex-based liquid bead micro array assay with qualitative detection for 37 HPV types.21 Following the International Agency for Research on Cancer classification scheme, we defined HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 as high-risk types and HPV 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 67, 69, 70, 71, 72, 73, 81, 82, 82 subtype (IS39), 83, 84, and 89 (CP6108) as low-risk types.22 The women were considered positive for HPV if either the cervical or vaginal swabs tested positive for HPV DNA.
Statistical analysis
We examined HPV infection in several ways: infection with any HPV type, infection with any of the high-risk types, infection with any of the low-risk types, and infection with any high-risk type other than HPV 16 and 18. We additionally defined the exposure as infection with either of the types targeted by the bivalent vaccine (HPV 16 or 18), infection with any type targeted by the nonavalent vaccine (HPV 6, 11, 16, 18, 31, 33, 45, 52, 58), and infection with any type targeted by the quadrivalent vaccine (HPV 6, 11, 16, 18) to examine the association of vaccine-preventable HPV types on HIV risk. The comparison group to all of the above exposure definition is no HPV infection or infection with HPV types not included in the exposure definition. We also evaluated dose-response relationship between HIV acquisition and the number of HPV types detected by examining the latter as a categorical variable as well as a continuous variable; in both cases, the comparison group is people negative for all HPV types.
A priori, we considered the following covariates as potential confounders: demographics (age, country of residence, and education attainment), sexual behaviors (condom use, number of recent sex partners, having a current primary partner, the participant or her partner having a concurrent relationship outside of their partnership, and having transactional sex in the past year), any recent history of STIs (syphilis, gonorrhea, chlamydia, or trichomoniasis), HSV-2 positivity, and any recent history of other vaginal infections (bacterial vaginosis or candidiasis). Participants’ study product assignment in VOICE was considered a confounder if assignment was different by case or control status in our analysis. Data on demographic covariates were extracted from enrollment questionnaires. Sexual behavior data were extracted from quarterly behavioral questionnaires. We used data provided at any time between three months before the cervical or vaginal swab collection visit and the HIV seroconversion visit in cases. If participants answered multiple behavioral questionnaires during that time, we used data from the questionnaire closest to swab collection visit. Similarly, we considered participants to have had a recent history of STI or other vaginal infections if they were diagnosed with a STI between three months before the cervical or vaginal swab collection visit and the HIV seroconversion visit in cases.
We conducted univariate comparisons by case and control status on the covariates listed above using analysis of variance tests for continuous variables and chi-square tests for categorical variables. Using conditional logistic regression models, we estimated odds ratios and 95% confidence intervals (CI) quantifying the association between HPV infection and HIV acquisition. Because controls were matched to cases based on study visit (i.e., follow-up time in study), the resulting effect measures from the conditional logistic regression approximate the incidence rate ratio. We performed data management and analyses in R (Version 4.0.3) and SAS 9.4 (SAS Institute, Cary, NC).
Results
Cases and controls were on average 23 years old at enrollment, with standard deviation of 4 years (Table 2). While most participants (93%) had at least some secondary school education, the controls were more likely than cases to have attended university or college (p = 0.02). The vast majority of the women in the case-control study were enrolled at South African sites (Table 2). Although cases were more likely to have been randomized to the vaginal PrEP and placebo arms and the controls to the oral PrEP and placebo arms, randomization assignment was not significantly different by case status.
Table 2:
Demographic characteristics and sexual behaviors of cases and controls at enrollment and during follow-up.
| Case (N=138) | Control (N=412) | Total (N=550) | p-value* | |
|---|---|---|---|---|
| Characteristics at enrollment | ||||
| Mean age (SD) | 23.2 (4.1) | 23.6 (3.9) | 23.5 (4.0) | 0.353 |
| Education | 0.022 | |||
| No schooling | 3 (2.2%) | 1 (0.2%) | 4 (0.7%) | |
| Primary | 13 (9.4%) | 20 (4.9%) | 33 (6.0%) | |
| Secondary | 113 (81.9%) | 356 (86.4%) | 469 (85.3%) | |
| College/University | 9 (6.5%) | 35 (8.5%) | 44 (8.0%) | |
| Country | 1.000 | |||
| South Africa | 131 (94.9%) | 391 (94.9%) | 522 (94.9%) | |
| Uganda | 4 (2.9%) | 12 (2.9%) | 16 (2.9%) | |
| Zimbabwe | 3 (2.2%) | 9 (2.2%) | 12 (2.2%) | |
| Study product | 0.067 | |||
| Oral FTC/TDF | 27 (19.6%) | 89 (21.6%) | 116 (21.1%) | |
| Oral placebo | 22 (15.9%) | 98 (23.8%) | 120 (21.8%) | |
| Oral TDF | 21 (15.2%) | 75 (18.2%) | 96 (17.5%) | |
| Vaginal placebo | 40 (29.0%) | 79 (19.2%) | 119 (21.6%) | |
| Vaginal TFV gel | 28 (20.3%) | 71 (17.2%) | 99 (18.0%) | |
| Time-varying sexual behaviors, STIs, and vaginal infections | ||||
| Had transactional sex in the past year | 0.422 | |||
| Yes | 6 (4.4%) | 18 (4.4%) | 24 (4.4%) | |
| No | 131 (95.6%) | 392 (95.6%) | 523 (95.6%) | |
| Had a primary partner in the past 3 months | 0.020 | |||
| Yes | 130 (94.2%) | 403 (98.1%) | 533 (97.1%) | |
| No | 8 (5.8%) | 8 (1.9%) | 16 (2.9%) | |
| Mean number of sex partners in the past 3 months | 1.4 (0.7) | 1.4 (2.4) | 1.4 (2.1) | 0.980 |
| Primary partner has other partners | 0.006 | |||
| Yes | 99 (71.7%) | 282 (68.4%) | 381 (69.3%) | |
| No | 20 (14.5%) | 101 (24.5%) | 121 (22.0%) | |
| Don’t know | 19 (13.8%) | 29 (7.0%) | 48 (8.7%) | |
| Had other partners besides primary partner | 0.033 | |||
| Yes | 35 (26.3%) | 84 (20.7%) | 119 (22.1%) | |
| No | 100 (73.5%) | 337 (82.0%) | 437 (79.9%) | |
| Condom used at last sex | 0.987 | |||
| Yes | 84 (66.1%) | 249 (66.2%) | 333 (66.2%) | |
| No | 43 (33.9%) | 127 (33.8%) | 170 (33.8%) | |
| Positive for syphilis, gonorrhea, chlamydia, or trichomoniasis | 0.051 | |||
| Yes | 42 (31.3%) | 93 (22.9%) | 135 (25.0%) | |
| No | 92 (68.7%) | 313 (77.1%) | 405 (75.0%) | |
| Positive for herpes simplex virus 2 | 0.014 | |||
| Yes | 80 (58.0%) | 189 (45.9%) | 269 (48.9%) | |
| No | 58 (42.0%) | 223 (54.1%) | 281 (51.1%) | |
| Positive for bacterial vaginosis or candidiasis | 0.448 | |||
| Yes | 9 (6.5%) | 20 (4.9%) | 29 (5.3%) | |
| No | 129 (93.5%) | 392 (95.1%) | 521 (94.7%) | |
Abbreviation: SD, standard deviation; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate; TFV, tenofovir
Estimated from analysis of variance tests for continuous variables and chi-square tests for categorical variables.
Time-varying sexual behaviors, STIs, and other vaginal infections are also described in Table 2. Approximately 4% of cases and controls had a history of transactional sex in the past year. Over 90% of the women in our sample reported having a primary partner in the past three months. However, cases were significantly less likely to have a primary partner compared to controls (94% vs. 98%, p = 0.020). While the average number of sex partners in the 3 months was the same between cases and controls, cases were more likely to report that their primary partners were non-monogamous (p = 0.006) and that they themselves had sex partners other than their primary partner (p = 0.033). About 66% of both cases and controls reported using condoms the last time they had sex. Compared to controls, cases had a higher prevalence of recent STI (31% vs 23%, p = 0.051), higher seroprevalence of HSV-2 (58% vs 46%, p = 0.014), and similar prevalence of other vaginal infections (7% vs 5%, p = 0.448).
The prevalence of HPV infection was high in our study sample regardless of HIV status (Table 3), with infection with any HPV type detected in 84% of cases and 65% of controls (p < 0.001). The prevalence of any high-risk HPV infection was 74% among cases and 55% among controls (p < 0.001). Cases were also more likely to be positive for HPV infection with any low-risk type compared to controls (66% vs 48%, p < 0.001). However, the prevalence of infection with either HPV 16 or 18 was not significantly different by case or control status. Infection with multiple HPV types was also common in our study (67% in cases and 49% in controls, p < 0.001). Cases had an average of 3 HPV infections whereas controls had an average of 1.9 infections (p<0.001).
Table 3.
Crude and adjusted associations between HPV types detected and HIV acquisition.
| Prevalence | Crude OR (95% CI) | Adjusted OR1 (95% CI) | |||
|---|---|---|---|---|---|
| Cases (n = 138) | Controls (n = 412) | P-value2 | |||
| Any HPV 3 | 116 (84.1%) | 269 (65.3%) | <0.001 | 3.0 (1.8-5.2) | 2.6 (1.4-4.9) |
| High-risk HPV 4 | 102 (73.9%) | 227 (55.1%) | <0.001 | 2.5 (1.6-4.0) | 2.7 (1.5-4.9) |
| Low-risk HPV 5 | 91 (65.9%) | 196 (47.6%) | <0.001 | 2.1 (1.4-3.2) | 1.8 (1.1-2.9) |
| HPV 16 or18 | 34 (24.6%) | 73 (17.7%) | 0.098 | 1.5 (1.0-2.4) | 1.6 (0.9-2.8) |
| Non-HPV 16/18 high risk HPV 6 | 94 (68.1%) | 201 (48.8%) | <0.001 | 2.4 (1.6-3.7) | 2.6 (1.5-4.4) |
| Nonavalent vaccine HPV 7 | 83 (60.1%) | 173 (42.0%) | <0.001 | 2.2 (1.5-3.4) | 2.2 (1.3-3.7) |
| Quadrivalent vaccine HPV 8 | 50 (36.2%) | 90 (21.8%) | 0.001 | 2.0 (1.3-3.1) | 2.0 (1.2-3.23 |
| Number of HPV types detected 9 | 3.0 (2.6) | 1.9 (2.1) | <0.001 | 1.3 (1.1-1.4) | 1.2 (1.1-1.4) |
Abbreviations: OR, odds ratio; HPV, human papillomavirus
Adjusted for age, education, study product arm, having transactional sex in the past year, having a primary partner in the past 3 months, number of sex partners in the past 3 months, having partners other than the primary partner, the primary partner having other partners, condom use at last sex, STI, and vaginal infections. 59 observations were dropped from adjusted conditional logistic regressions for HPV infections due to missing data on covariates. 72 observations were dropped from the adjusted conditional logistic regression for abnormal Pap results due to missing data on Pap results and covariates.
Estimated from chi-square tests comparing prevalence by case-control status. P-value for number of HPV types detected was estimated using analysis of variance test.
Defined as infection with any of the 37 HPV types.
Defined as infection with any of the following: HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, or 68.
Defined as infection with any of the following: HPV 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 67, 69, 70, 71, 72, 73, 81, 82, 82 subtype (IS39), 83, 84, and 89 (CP6108).
Defined as infection with any of the following: HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, or 68.
Defined as infection with any of the following: HPV 6, 11, 16, 18, 31, 33, 45, 52, or 58.
Defined as infection with any of the following: HPV 6, 11, 16, or 18.
Data entered under the Prevalence columns represent mean number of HPV types detected and standard deviation
Adjusting for age, education, condom use, number of sex partners, having a current primary partner, the participant and her partner having concurrent relationships outside of their partnership, and having transactional sex in the past year, STIs, HSV-2, and other vaginal infections, women with any HPV infection had 2.6 times higher risk of HIV seroconversion compared to women with no HPV infection (95% CI 1.4 – 4.9, Table 3). Risk of HIV seroconversion was also 2.7 times higher with a high-risk HPV infection (95% CI 1.5-4.9) compared to no high-risk HPV infection and 1.8 times higher with a low-risk HPV infection (95% CI 1.1-2.9) compared to no low-risk infection. For vaccine-preventable HPV infections specifically, we found that HIV acquisition was associated with infection with any of the types targeted by the nonavalent vaccine (aOR 2.2, 95% CI 1.3-3.7) and the quadrivalent vaccine (aOR 2.0, 95% CI 1.2–3.3), but not the bivalent vaccine after adjusting for covariates (aOR 1.6, 95% CI 0.9-2.8). The risk of HIV seroconversion was elevated with infection with a single HPV type (aOR 1.9, 95% CI 0.9-4.2) and increased with the number of HPV types detected (aOR 2.2, 95% CI 1.1-4.6 for 2-3 types and aOR 4.1, 95% CI 1.9-8.7 for 4 or more types; (Figure 1)). With each additional HPV type detected, HIV risk increased by 20% (aOR 1.2, 95% CI 1.1-1.4).
Figure 1.
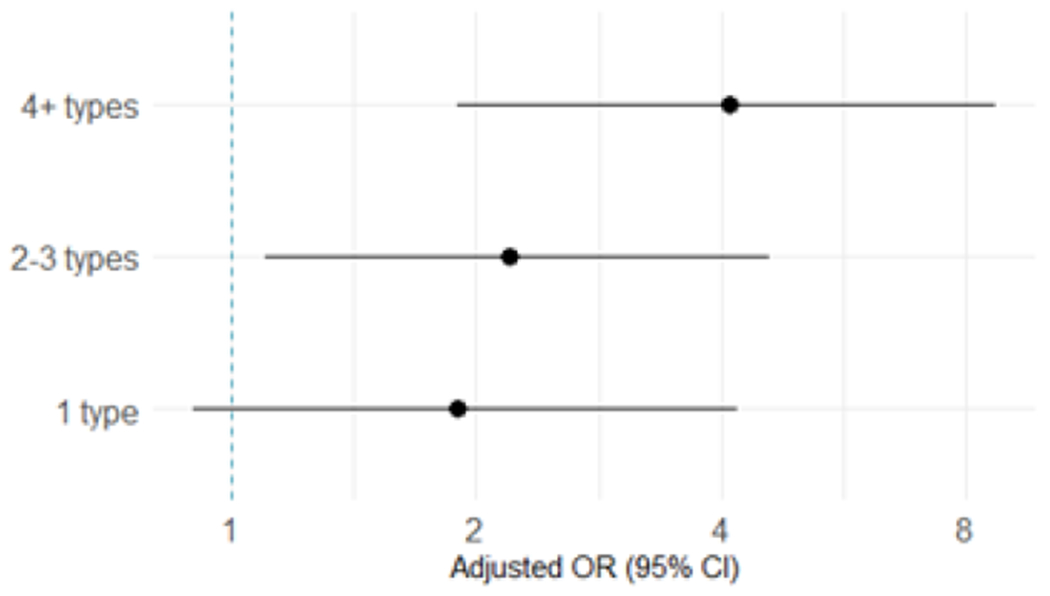
Associations between the number of HPV types detected and HIV acquisition. Odds ratios and 95% CI compared number of HPV types detected to no HPV infection and were adjusted for age, education, study product arm, having transactional sex in the past year, having a primary partner in the past 3 months, number of sex partners in the past 3 months, having partners other than the primary partner, the primary partner having other partners, condom use at last sex, STI, and vaginal infections. The dotted line vertical line indicate null.
Discussion
In this case-control study of young women at risk for HIV, infection with any HPV type, high-risk HPV types, and low-risk HPV types were associated with increased risk of HIV infection during follow-up, after adjusting for sexual behaviors and recent history of other STIs. Infection with HPV types targeted by the nonavalent and the quadrivalent HPV vaccines also significantly increased HIV acquisition. We additionally found that the risk of HIV acquisition increased with the number of HPV types detected.
These findings are consistent with previous epidemiological studies and meta-analyses on this topic that controlled for confounding.14,15,23,24 However, our findings disagreed with those from a similar case-control study conducted by Gallagher et al,25 which found that HPV infections, regardless of type groupings, were not associated with HIV acquisition.25 The high prevalence of HSV-2 among both cases and controls (>80%) in that study could have masked the effect of HPV on HIV acquisition, as HSV-2 is strongly associated with HIV acquisition.26,27 In contrast, 58% and 46% of the cases and controls in our study were positive for HSV-2.
We additionally found that infection with any HPV types included in the nonavalent vaccine and the quadrivalent vaccine was associated with a 2-fold increase in HIV acquisition. HPV vaccination is highly efficacious in reducing cervical cancer risk and is being implemented in an increasing number of countries.28,29 Our results suggest that HPV vaccination, particularly with the nonavalent vaccine, could potentially have the added benefit of protecting women from HIV infection. However, other HIV prevention measures should also be maintained, as treatment or prevention of STIs did not always result in population-level reduction of HIV incidence.30
Vaccination with the bivalent vaccine, however, may not impact HIV acquisition, as infection with HPV 16 and/or 18 did not significantly increase HIV risk. One potential explanation is that with the relatively lower prevalence of HPV 16/18 in this population, our power was limited to detect a statistically significant association. However, it is also possible that HPV 16 and 18 are less likely than other types to activate a mucosal T-cell response and increase cervical vulnerability to HIV infection. HPV 16/18 infections are the most likely to persist and progress to cervical cancer,31 and the ability of HPV 16/18 to persist may be correlated with adeptness to evade the immune system. Cytotoxic T lymphocyte responses to HPV oncoproteins E6 and E7 were less likely to be detected in HPV positive women with squamous intraepithelial neoplasia (SIL) than in HPV positive women without SIL, which suggests that persistent HPV infections were less likely to generate target cells for HIV infection.32 Consistent with this explanation, previous epidemiological studies found no association between abnormal Pap results and HIV infection.24
Infection with multiple HPV types was common in our study sample, as well as in the general population of women.11 We found that concurrent infection with multiple HPV types significantly increased HIV risk. Specifically, the risk of HIV acquisition increased by 20% with each additional HPV type detected. This confirms a previous finding of a dose-response relationship between HPV infection and HIV acquisition among South African women.23 In that study, relative to no HPV infection, infection with 2 HPV types was associated with 2-fold increase in HIV acquisition and infection with >4 HPV types was associated with almost a 6-fold increase in HIV acquisition.23 Since both our study and the South African study adjusted for sexual behaviors, the positive association implies this finding is due to an increased biological susceptibility to HIV among women with multiple HPV infections rather than confounding. As individual HPV infections progress and regress independently of each other,33,34 having multiple infections may prolong periods of susceptibility to HIV infection.
Our study contributes to the still limited body of research on the effect of HPV infection and HIV acquisition. With a relatively large sample size, the ability to adjust for important time-varying confounders, and short interval between ascertainments of HPV and HIV infections, our study addressed some of the limitations of previously published studies and provided additional evidence of a potential biological interaction between HPV and HIV infections. However, further studies, particularly ones capturing the incidence and clearance of HPV infections, are required to definitively establish causality.
Our study has several limitations. As with many observational studies assessing the association between STIs and HIV acquisition, residual confounding by unmeasured sexual behaviors is possible.35 The significant associations we observed could potentially be the result of a time lag between HPV and HIV acquisitions from HPV and HIV co-infected partners, because HIV has a lower per-coital act transmission probability compared to HPV.36,37 Since we do not have data on the HPV and HIV status of the participants’ partners, we cannot definitively eliminate this explanation. However, we were able to adjust for partnerships outside the primary partner to control for concurrent partnerships that could have led to HPV infection and HIV acquisition. Although 312 women acquired HIV over the course of the VOICE trial, we were only able to include 138 women who had stored cervical or vaginal swab specimens collected one to six months prior to HIV seroconversion, which could limit internal validity. However, we found that demographic and behavioral characteristics at baseline were similar between cases and controls and the overall VOICE population and between cases and the other VOICE participants who acquired HIV but were not included. Ascertainment of STI and vaginal infections in VOICE was done at the enrollment visit, annually, and at presentation of symptoms, which could have led some women with asymptomatic STIs or vaginal infections between enrollment and the annual visits to be misclassified as negative. Residual confounding by unmeasured or misclassified STI may have biased the associations away from the null.
In August 2020, the World Health Organization announced an ambitious initiative to eliminate cervical cancer as a public health problem in all countries.38 The initiative has a strong focus on increasing the coverage of HPV vaccination in sub-Saharan African countries, which bear almost 70% of the global HIV burden and have the highest cervical cancer incidence rates in the world.1,38,39 In countries with high burden of both HPV and HIV infections, wide-spread implementation of HPV vaccination with the nonavalent or the quadrivalent vaccines may potentially have the dual benefit of concurrently reducing risk for HIV and cervical cancer among women.
Acknowledgement
The authors gratefully acknowledge the immense contribution of the late Dr. Gita Ramjee to MTN-003 and the field of HIV prevention for women.
The authors declare no conflict of interest. This research was funded by the University of Washington King K Holmes Endowed Professorship in STDs and AIDS. Funding for the VOICE Study was through a grant to the Microbicide Trials Network from the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. The data has not been previously presented.
References
- 1.UNAIDS. Global AIDS update 2019 — Communities at the centre. Geneva, Switzerland: UNAIDS;2019. [Google Scholar]
- 2.UNAIDS. Women and HIV — A spotlight on adolescent girls and young women. Geneva, Switzerland: UNAIDS;2019. [Google Scholar]
- 3.Birdthistle I, Tanton C, Tomita A, et al. Recent levels and trends in HIV incidence rates among adolescent girls and young women in ten high-prevalence African countries: a systematic review and meta-analysis. The Lancet Global Health. 2019;7(11):e1521–e1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of pathology. 1999;189(1):12–19. [DOI] [PubMed] [Google Scholar]
- 5.Gillison ML, Shah KV. Chapter 9: Role of Mucosal Human Papillomavirus in Nongenital Cancers. JNCI Monographs. 2003;2003(31):57–65. [DOI] [PubMed] [Google Scholar]
- 6.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2008;43(4 Suppl):S5–25, S25.e21–41. [DOI] [PubMed] [Google Scholar]
- 7.Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sexually transmitted diseases. 2014;41(11):660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157(3):218–226. [DOI] [PubMed] [Google Scholar]
- 9.de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. The Lancet Infectious diseases. 2007;7(7):453–459. [DOI] [PubMed] [Google Scholar]
- 10.Franceschi S, Herrero R, Clifford GM, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. International journal of cancer. 2006; 119(11):2677–2684. [DOI] [PubMed] [Google Scholar]
- 11.Ogembo RK, Gona PN, Seymour AJ, et al. Prevalence of human papillomavirus genotypes among African women with normal cervical cytology and neoplasia: a systematic review and meta-analysis. PloS one. 2015;10(4):e0122488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Røttingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis. 2001;28(10):579–597. [DOI] [PubMed] [Google Scholar]
- 14.Houlihan CF, Larke NL, Watson-Jones D, et al. Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. AIDS (London, England). 2012;26(17):2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Looker KJ, Rönn MM, Brock PM, et al. Evidence of synergistic relationships between HIV and Human Papillomavirus (HPV): systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J Int AIDS Soc. 2018;21(6):e25110–e25110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winer RL, Hughes JP, Feng Q, et al. Early natural history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(4):699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanley M. Pathology and epidemiology of HPV infection in females. Gynecologic Oncology. 2010;117(2, Supplement):S5–S10. [DOI] [PubMed] [Google Scholar]
- 18.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. New England Journal of Medicine. 2015;372(6):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrazzo JM, Rabe L, Kelly C, et al. Tenofovir Gel for Prevention of Herpes Simplex Virus Type 2 Acquisition: Findings From the VOICE Trial. J Infect Dis. 2019;219(12): 1940–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Wright TC, Denny L, Kuhn L. Rapid rise in detection of human papillomavirus (HPV) infection soon after incident HIV infection among South African women. J Infect Dis. 2011;203(4):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Q, Cherne S, Winer RL, et al. Development and evaluation of a liquid bead microarray assay for genotyping genital human papillomaviruses. Journal of clinical microbiology. 2009;47(3):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. The Lancet Oncology. 2009;10(4):321–322. [DOI] [PubMed] [Google Scholar]
- 23.Averbach SH, Gravitt PE, Nowak RG, et al. The association between cervical human papillomavirus infection and HIV acquisition among women in Zimbabwe. AIDS (London, England). 2010;24(7): 1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith-McCune KK, Shiboski S, Chirenje MZ, et al. Type-specific cervico-vaginal human papillomavirus infection increases risk of HIV acquisition independent of other sexually transmitted infections. PloS one. 2010;5(4):e10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher KE, Baisley K, Grosskurth H, et al. The Association Between Cervical Human Papillomavirus Infection and Subsequent HIV Acquisition in Tanzanian and Ugandan Women: A Nested Case-Control Study. J Infect Dis. 2016;214(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menezes LJ, Pokharel U, Sudenga SL, et al. Patterns of prevalent HPV and STI coinfections and associated factors among HIV-negative young Western Cape, South African women: the EVRI trial. Sex Transm Infect. 2018;94(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. The Lancet Infectious diseases. 2017;17(12):1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo F, Cofie LE, Berenson AB. Cervical Cancer Incidence in Young U.S. Females After Human Papillomavirus Vaccine Introduction. American Journal of Preventive Medicine. 2018;55(2): 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.PATH. Global HPV Vaccine Introduction Overview. 2020; https://path.azureedge.net/media/documents/Global_HPV_Vaccine_Intro_Overview_Slides_webversion_2020May.pdf. Accessed Dec. 9, 2020.
- 30.Hayes R, Watson-Jones D, Celum C, van de Wijgert J, Wasserheit J. Treatment of sexually transmitted infections for HIV prevention: end of the road or new beginning? AIDS. 2010;24 Suppl 4(0 4):S15–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demarco M, Hyun N, Carter-Pokras O, et al. A study of type-specific HPV natural history and implications for contemporary cervical cancer screening programs. EClinicalMedicine. 2020;22:100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KH, Greenfield WW, Cannon MJ, Coleman HN, Spencer HJ, Nakagawa M. CD4+ T-cell response against human papillomavirus type 16 E6 protein is associated with a favorable clinical trend. Cancer immunology, immunotherapy : CII. 2012;61(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wentzensen N, Schiffman M, Dunn T, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. International journal of cancer. 2009;125(9):2151–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaturvedi AK, Katki HA, Hildesheim A, et al. Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. The Journal of infectious diseases. 2011;203(7):910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zetola NM, Bernstein KT, Wong E, Louie B, Klausner JD. Exploring the relationship between sexually transmitted diseases and HIV acquisition by using different study designs. Journal of acquired immune deficiency syndromes (1999). 2009;50(5):546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burchell AN, Richardson H, Mahmud SM, et al. Modeling the sexual transmissibility of human papillomavirus infection using stochastic computer simulation and empirical data from a cohort study of young women in Montreal, Canada. Am J Epidemiol. 2006;163(6):534–543. [DOI] [PubMed] [Google Scholar]
- 37.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet (London, England). 2001;357(9263):1149–1153. [DOI] [PubMed] [Google Scholar]
- 38.WHO. Global strategy to accelerate the elimination of cervical cancer as a public health problem. Geneva: World Heath Organization;2020. [Google Scholar]
- 39.Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. The Lancet Global Health. 2020;8(2):e191–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]


