Abstract
Objective:
To assess the association of cardiovascular disease (CVD) risk scores and coronary artery plaque (CAP) progression in HIV-infected participants.
Methods:
We studied men with and without HIV-infection enrolled in the Multicenter AIDS Cohort Study (MACS) CVD study. Coronary artery plaque (CAP) at baseline and follow-up was assessed with cardiac computed tomography angiography (CCTA). We examined the association between baseline risk scores including pooled cohort equation (PCE), Framingham risk score (FRS) and Data collect of Adverse effects of anti-HIV drugs equation (D:A:D) and CAP progression.
Results:
We studied 495 men (211 HIV-uninfected, 284 HIV-infected). The adjusted odds ratio (aOR) of total plaque volume (TPV) and non-calcified plaque volume (NCPV) progression in the highest relative to lowest tertile was 9.4 (95% CI 2.4, 12.1, p<0.001) and 7.7 (3.1,19.1, p<0.001) times greater, respectively, among HIV-uninfected men in the PCE atherosclerotic cardiovascular disease (ASCVD) high vs. low risk category. Among HIV-infected men, the association for TPV and NCPV progression for the same PCE risk categories, OR 2.8 (1.4, 5.8, p<0.01) and OR 2.4 (1.2, 4.8, p<0.05) respectively (p-values for interaction by HIV= 0.02 and 0.08, respectively). Similar results were seen for the FRS risk scores. Among HIV-uninfected men, PCE high risk category identified the highest proportion of men with plaque progression in the highest tertile. While, in HIV-infected men, high risk category by D:A:D identified the greatest percentage of men with plaque progression albeit with lower specificity than FRS and PCE.
Conclusions:
PCE and FRS categories predict CAP progression better in HIV-uninfected compared to HIV-infected men. Improved CVD risk scores are needed to identify high risk HIV-infected men for more aggressive CVD risk prevention strategies.
Introduction:
There have been dramatic decreases in AIDS related mortality due to advances in the treatment of human immunodeficiency virus (HIV).1,2 Nonetheless, HIV-infected individuals have more subclinical coronary artery disease (CAD) as compared to HIV-uninfected individuals.3 Higher prevalence of traditional cardiovascular risk factors, inflammation, immune activation from the HIV virus and metabolic abnormalities (dyslipidemia and insulin resistance) due to antiretroviral medications are likely responsible for higher subclinical atherosclerosis.3–5 Furthermore, due to reduced AIDS events, HIV infected individuals are living longer, it is imperative that more CVD would be encountered in this population. Pooled cohort equation (PCE) risk score is widely used in clinical practice to classify individuals in high, moderate or low risk categories for the prediction of future cardiovascular disease (CVD) events5. A previous cross-sectional study from the Multicenter AIDS Cohort Study (MACS) reported that 36% of HIV-infected men in the low risk category by PCE risk score and 41% of HIV-infected men in low risk group per Framingham Risk Score (FRS) had coronary artery calcium (CAC) >06. In another study, FRS underestimated the prevalence of subclinical atherosclerosis as measured by carotid intima media thickness (CIMT); 56% of HIV-infected participants classified as low risk had evidence of carotid subclinical atherosclerosis7. Another study using cardiac computed tomography angiography (CTA) reported that despite similar FRS10-year risk, family history of coronary artery disease (CAD), and smoking status, HIV-infected individuals had a higher prevalence of CAC>0 and greater number of coronary segments involved compared with HIV uninfected (HIV-) individuals8. Investigators from the Data Collection on Adverse Effects of Anti-HIV drugs study (D:A:D) developed a risk equation using traditional cardiovascular risk factors and exposure to different antiretroviral drug therapies9. D:A:D estimated subclinical atherosclerosis and CVD outcomes better as compared to FRS10,11 in some studies, however, was not found to be a better predictor of coronary atherosclerosis in the MACS6. Since progression of subclinical coronary atherosclerosis is associated with CVD events12,13, we sought to assess the association of subclinical atherosclerosis and CVD risk scores including PCE, FRS and D:A:D risk scores in a prospective cohort of men living with HIV (HIV+) and similar at risk HIV-uninfected (HIV−) men.
METHODS:
Study Population:
The MACS is an ongoing prospective cohort study of the natural and treated histories of HIV+ and HIV− at risk men. It was initiated in 1984 as a study of gay and bisexual men with and without HIV, conducted at four study sites in Baltimore/Washington, DC, Chicago, Los Angeles, and Pittsburgh. Enrollment into the MACS occurred over four time periods (1984–85, 1987–90, 2001–03, and 2010–13) and study participants are followed through semiannual visits which include history, physical examinations, and collection of blood samples. As part of the MACS cardiovascular ancillary study eligible participants, underwent non-contrast cardiac computed tomography (CT) scanning for CAC scoring and coronary CT angiography (CTA) for plaque outcomes between January 2010 and August 2013 with follow-up CT scanning between January 2015 and October 2017.
Selection Criteria:
Inclusion and exclusion criteria for the MACS Cardiovascular ancillary study have been described in a previous report3,14. This analysis includes participants who undergo CT with contrast. Men aged 40–70 years and weighing less than 136 kg (300 pounds) were eligible while men who had a history of cardiac surgery or coronary intervention, atrial fibrillation, chronic kidney disease as defined by an estimated glomerular filtration rate less than 60 ml/min/1.73m2, or allergy to contrast agents were excluded from CT with contrast. Participants with missing covariate data for risk score formulae, statin use and HCV status were also excluded (Figure-1).
Fig-1.
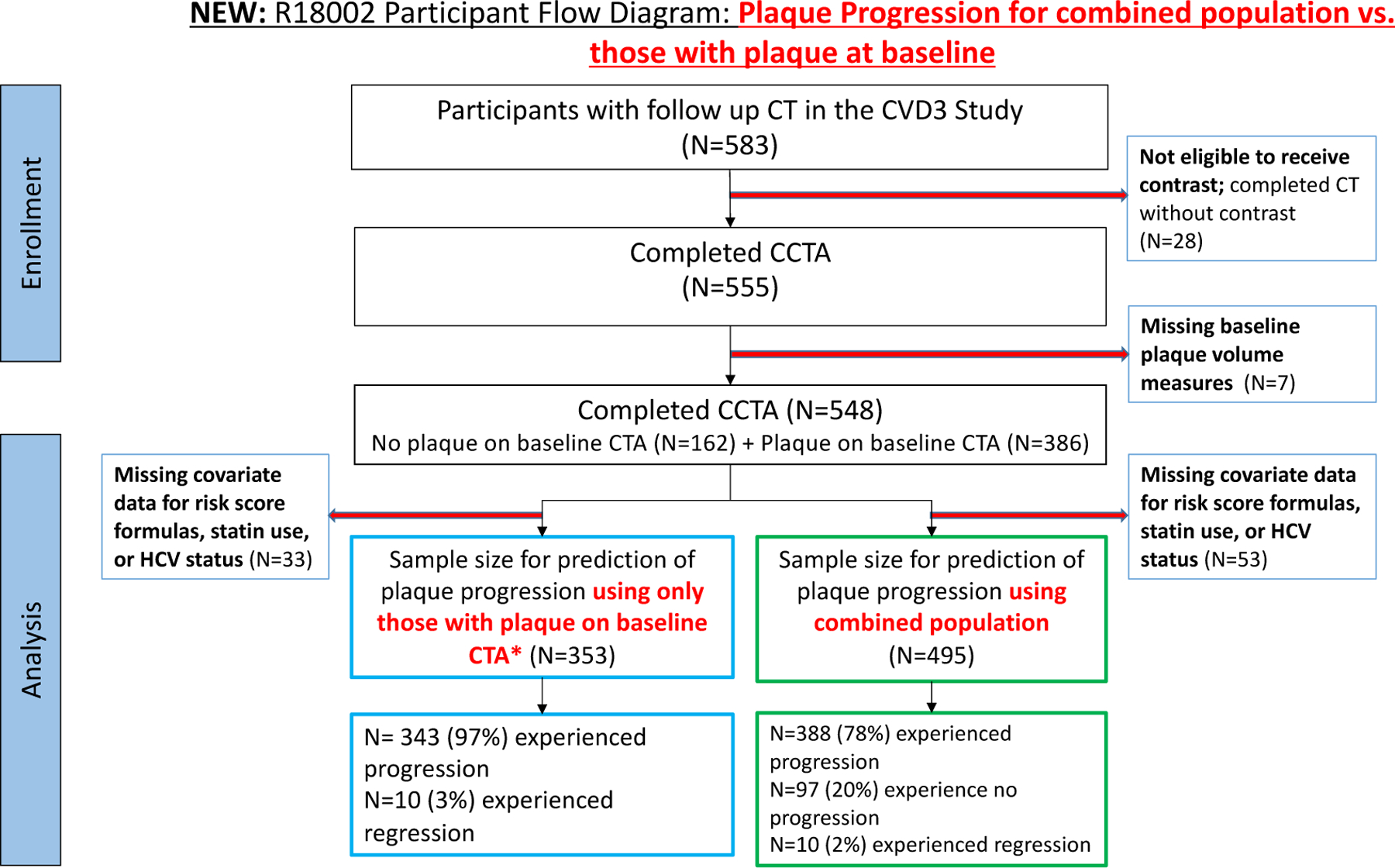
flow diagram of participants.
CAC Agatston score
CAC is defined as a plaque of at least 3 contiguous pixels with a density >130 HU. CAC score was calculated from the non-contrast images using the Agatston method15. A total CAC score was determined by summing the scores from each of 4 anatomic sites (left main, left anterior descending, circumflex, and right coronary).
CT scanning and Plaque Analysis:
Details regarding plaque analysis were described in a previous report14. In brief, CT images were transferred to the core CT reading center (The Lunquist Institute for Biomedical Innovation at Harbor-UCLA Medical, Torrance, California, USA) and analyzed by trained, experienced readers blinded to participant characteristics and HIV serostatus. Coronary CTA scans were assessed using a 17-segment American Heart Association coronary tree model in accordance with the Society of Cardiovascular Computed Tomography guidelines16.Coronary CTAs were assessed for presence, volume, and characteristics of coronary plaques using a semiautomated quantitative plaque analysis software (QAngioCT Research Edition version 3.0.37; Medis Medical Imaging Systems, Leiden, The Netherlands). Plaque volumes were measured in segments with a lumen diameter of at least 1.5 mm and adequate image quality. Vessel length was defined as the length of coronary arteries in measured segments. To increase the accuracy and precision of plaque progression on serial CTA scans, the location and length of each segment were matched between baseline and follow-up at a per-patient, per-vessel and per-segment levels14.
Risk Score Calculation
For each participant, the following CVD risk scores were calculated using their values at the baseline scan: (1) PCE (2) FRS, and (3) D:A:D for HIV-infected participants only.2 FRS was calculated using code on the FRS score sheets in the Adult Treatment Panel III (https://www.nhlbi.nih.gov/files/docs/guidelines/atglance.pdf).
Based on their PCE and FRS scores, participants were categorized into high risk, moderate risk, and low risk categories. For the D:A:D score, the low and moderate risk categories were combined because there was only one participant in the low risk category. Risk score categories were created as follows: for the FRS, individuals with > 20% estimated risk of myocardial infarction (MI) and coronary death within 10 years were classified as high risk, 10–20% as moderate risk, and < 10% as low risk. All diabetic participants were classified as high risk, regardless of FRS score. For the pooled cohort equation, individuals with > 7.5% risk of coronary death or nonfatal MI, or fatal or nonfatal stroke within 10 years were classified as high risk, 5–7.5% as moderate risk, and < 5% as low risk. HIV-infected individuals only were classified based on D:A:D risk scores. Participants with ≥ 5% risk of nonfatal and fatal MI within 5 years were classified as high risk, 1–5% as moderate risk, and < 1% as low risk.
Statistical Analysis
Distribution of demographic and clinical characteristics in HIV-infected and HIV-uninfected men were compared using chi-square and Wilcoxon rank sum test for categorical and continuous covariates, respectively. The progression of coronary artery plaque volume and CAC were defined as the absolute change in plaque volume or Agatston score, respectively, from baseline to follow-up, annualized to account for differences in inter-scan duration. Different analytic approaches were used to analyze these outcomes, based on their distributions (see below).
Total and Noncalcified Coronary Artery Plaque Volume Progression
Due to the highly skewed distribution of annualized change in total plaque volume (TPV) and noncalcified plaque volume (NCPV) and failure to meet the assumptions for a linear regression, these outcomes were categorized into tertiles of progression (low, moderate, and high), and multinomial logistic regression was used to assess the association between CVD risk score categories and tertiles of coronary artery plaque volume progression, with separate models for total and noncalcified plaque. The primary analyses of plaque progression were performed among the total sample, including participants with prevalent plaque (plaque volume > 0 mm3) and no prevalent plaque (plaque volume = 0 mm3) present on their baseline coronary CTA scans. Preliminary analyses provided little evidence to treat these as separate plaque outcomes, as no differences were found in the association between CVD risk score categories and plaque incidence and progression. HIV-stratified models were included for each outcome to evaluate differences in progression by HIV serostatus. To see if these differences were statistically meaningful (P < 0.05), models with interaction terms between HIV serostatus and each category of the CVD risk scores were added. Each model adjusted for recruitment center, HCV serostatus, and statin use. Fifty-three participants with missing covariate data for risk score calculation, or missing data for adjustment covariates were excluded.
CAC Incidence and Progression:
The annualized change in Agatston score was log-transformed and linear regressions were used to evaluate the association between increasing CVD risk score categories and CAC progression among men with CAC present on their baseline coronary CTA scans (CAC > 0). Normality was assessed using the non-normality Anderson-Darling test and model assumptions and fit were evaluated using leverage-versus-squared-residual plots and the R-squared statistic. Poisson regressions were used to determine the association between CVD risk score category and incident CAC among participants with no prevalent CAC on baseline coronary CTA (Agatston score = 0). Models were stratified by HIV serostatus and adjusted for recruitment center, HCV serostatus, and statin use. The time between scans (interscan interval) was used as the offset. An interaction between CVD risk score categories and HIV was included to evaluate difference in CAC incidence by HIV status. Incident CAC was defined as having a total Agatson score > 0 at follow-up. Participants with missing covariate data for risk score calculation, or missing data for adjustment covariates were excluded. Fig-1 shows the population with and without CAC at baseline.
ROC Analysis:
ROC curves were used to compare the discrimination of the CVD risk scores by HIV serostatus for the progression of TPV, NCPV, and CAC using pre-determined cutoff values. The cut-off value for having total and noncalcified coronary artery plaque progression was at the minimum plaque volume in the third tertile of annualized total and noncalcified plaque volume change, respectively. The ROC analyses for CAC progression and incidence were separated. The cut-off value for having CAC progression, was at > 20% change of the annualized Agatston score, while the cut-off for having CAC incidence was defined as the presence of any CAC (Agatston score > 0) at follow-up.17 The area under the curve (AUC) measured how well the CVD risk scores distinguished those with or without (1) plaque progression, (2) CAC progression, and (3) CAC incidence within each category.
All analyses were performed in Stata 15.1 and statistical significance was evaluated at P < 0.05.
Results:
Demographic and clinical characteristics:
Table 1 shows the distribution of clinical features and demographics by HIV serostatus. HIV-infected individuals were younger compared to HIV-uninfected men. HIV-infected men had higher triglycerides but lower LDL and HDL cholesterol. Majority of HIV-infected men (91%) were receiving HAART, most of them had (82%) undetectable HIV RNA levels. A small percentage of participants were taking abacavir, indinavir or lopinavir (2.1%, 0.7%,8.5%, respectively); antiretroviral drugs used in D:A:D risk score calculation. The distribution of risk score categories for HIV-infected and HIV-uninfected participants is shown in Fig-2 for various risk score algorithms. For the total population, more HIV-uninfected men were classified as high risk according to pooled cohort risk equation calculation (54% vs 42%). However, FRS classified lower percentage of HIV-uninfected men compared to HIV-infected as high risk (14% vs 17%).
Table 1:
Baseline characteristics
| HIV− | HIV+ | p-value | ||
|---|---|---|---|---|
| N=211 | N=284 | |||
| Age | 55(50–63) | 51(47–56) | <0.001 | |
| Systolic Blood Pressure (mmHg) | 127 (117–137) | 125.00 (115–136) | 0.23 | |
| Fasting glucose (mg/dL) | 97 (88–103) | 96 (90–105) | 0.21 | |
| Total Cholesterol (mg/dL) | 194 (167–218) | 184(162–212) | 0.089 | |
| HDL Cholesterol (mg/dL) | 51 (44–62) | 46 (38–54) | <0.001 | |
| LDL Cholesterol (mg/dL) | 114 (91–139) | 104 (83–134) | 0.019 | |
| Triglycerides | 102 (72–140) | 122 (89–188) | <0.001 | |
| Body Mass Index (kg/m2) | 26.0 (23.8–29.1) | 25.5 (23.2–28.3) | 0.046 | |
| Black | 49 (23%) | 95 (33%) | 0.013 | |
| Smoking Status | Never | 47 (22%) | 83 (29%) | 0.008 |
| Former | 125 (59%) | 128 (45%) | ||
| Current | 39 (18%) | 73 (26%) | ||
| Diabetes | No | 189 (90%) | 244 (86%) | 0.22 |
| Yes | 22 (10%) | 40 (14%) | ||
| Hypertension meds | No | 147 (70%) | 202 (71%) | 0.72 |
| Yes | 64 (30%) | 82 (29%) | ||
| Cholesterol lowering meds | No | 141 (67%) | 185 (65%) | 0.70 |
| Yes | 70 (33%) | 99 (35%) | ||
| Statin use | 66 (31%) | 81 (29%) | 0.51 | |
| HCV | Negative | 203 (96%) | 245 (86%) | <0.001 |
| Positive | 6 ( 3%) | 24 ( 8%) | ||
| Cleared | 2 ( 1%) | 15 ( 5%) | ||
| Pooled Cohort Equation (PCE) | Low | 67 (32%) | 105 (37%) | 0.028 |
| Moderate | 31 (15%) | 60 (21%) | ||
| High | 113 (54%) | 119 (42%) | ||
| Framingham Risk Score | Low | 127 (60%) | 194 (68%) | 0.015 |
| Moderate | 54 (26%) | 43 (15%) | ||
| High | 30 (14%) | 47 (17%) | ||
| DAD Risk Score | Low/Moderate | - | 124 (44%) | - |
| High | - | 160 (56%) | ||
| HIV-specific factors | ||||
| Undetectable viral load (< 50 copies) | - | 232 (82%) | ||
| On HAART | - | 257 (91%) | ||
| Time on HAART (years) | - | 9.3 (6.3–12.4) | ||
| Current Indinavir use | - | 2 (0.70%) | ||
| Indinavir exposure (years) | - | 0.0 (0.0–0.5) | ||
| Current Lopinavir use | - | 24 (8.5%) | ||
| Lopinavir exposure (years) | - | 0.0 (0.0–0.1) | ||
| Current Abacavir use | - | 6 (2.1%) | ||
| CD4+ T-cell count (cells/mm3) | - | 602 (422–787) | ||
| CD4+ T-cell nadir (cells/mm3) | - | 306 (210–434) | ||
| History of Clinical AIDS | - | 27 (9.5%) |
HDL= High Density Lipoprotein; LDL= Low Density Lipoprotein, HAART-Highly active antiretroviral therapy
Fig-2.
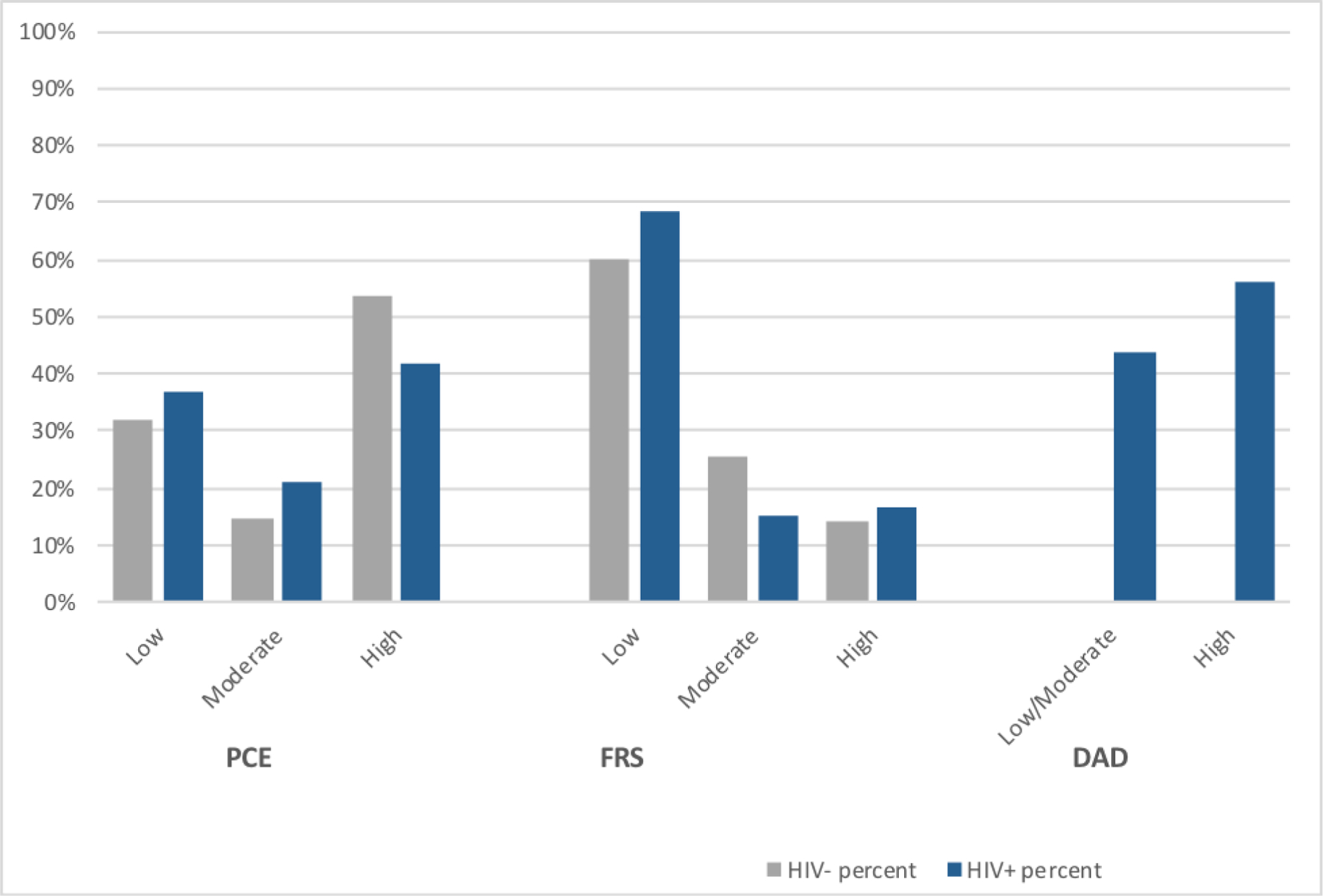
Distribution of 10-year cardiovascular disease risk by HIV status for risk scores among Multicenter AIDS Cohort Study participants. D:A:D risk score predicts 5 year risk
PCE risk categories and Plaque Progression
A. Incident CAC and CAC progression:
Among participants with no CAC at baseline, 32% of HIV-infected and 27% of HIV uninfected individuals who were categorized as low risk had developed incident CAC. Multivariable Poisson regression analysis revealed that HIV-infected men in the high risk PCE risk score group had significantly higher risk of incident CAC (aIRR 2.04, 95% CI1.03, 4.04, p<0.05) compared to HIV uninfected participants in the high risk PCE risk score group. There were no significant associations between PCE risk scores and incident CAC among HIV-uninfected men. There were no associations between CAC progression and PCE risk scores among those with CAC>0 at baseline in either HIV-uninfected or infected men when comparing the moderate and high PCE group relative to the low risk group. There was also no difference by HIV status (p-values for interaction between HIV and moderate or high risk PCE group are >0.05).
B. Total and Non-Calcified Plaque progression:
Several HIV-uninfected (17%) and HIV-infected (30%) men that were classified as low risk by PCE, had highest (3rd tertile) progression of NCP( supplementary- Table 1 A,B). Progression of TPV and NCPV in highest relative to lowest tertile of plaque was statistically significant for both HIV-uninfected and HIV-infected individuals in the high risk PCE group compared to low risk group. However, the odds of plaque progression were larger in HIV-uninfected men (Figure-3). This difference was statistically significant (p-value for HIV-interaction: 0.04). There was no significant risk of TPV and NCPV progression for men in the moderate PCE risk group relative to the low risk group, regardless of HIV status.
Figure-3:
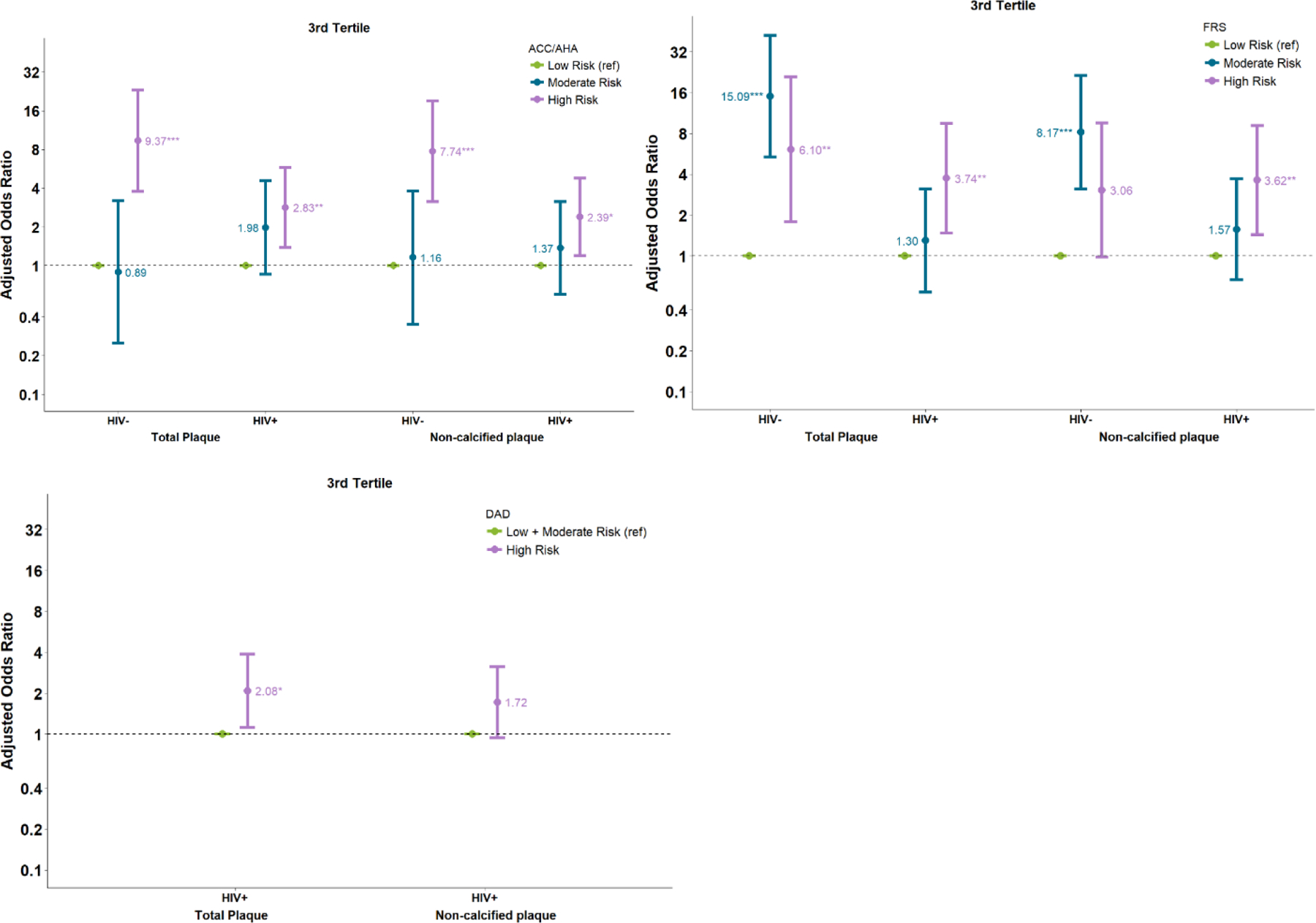
Association of risk scores and plaque progression in third tertile, which refers to group of participants with greatest plaque progression (a) PCE (b)FRS (c)DAD
FRS categories and Plaque Progression
A. Incident CAC and CAC Progression:
Among those with no CAC at baseline, HIV-infected men in the high risk FRS group had a higher risk of incident CAC (aIRR 2.48, 95% CI 1.20, 5.12, p<0.05) compared to men in the low risk FRS group. Again, no significant associations between FRS and incident CAC was seen among HIV-uninfected men. There were no associations between CAC progression and FRS risk scores among those with CAC>0 at baseline in either HIV-uninfected or infected men when comparing the high FRS group relative to the low risk group.
B. Total plaque and Noncalcified plaque Progression:
Among HIV-uninfected men there was a significant risk of plaque progression for those in the moderate and high FRS groups compared to the low risk group. For example, adjusted odds of TPV progression in highest compared to lowest tertile of plaque was 15.0 (95% CI 5.3, 42.2, p<0.001) for the moderate risk group and 6.1 (95% CI 1.7, 20.9, p<0.01) for the high risk group. However, among HIV-infected individuals these associations were smaller (Figure-3). The difference by HIV status was also only significant among men in the moderate risk group (p-values for HIV interaction: 0.016 for noncalcified plaque and <0.001 for total plaque).
D:A:D risk and Plaque progression
A. Incident CAC and CAC progression:
HIV-infected men categorized as high risk by the D:A:D risk score had no statistically higher risk of incident CAC or CAC progression compared to HIV-infected men categorized as moderate/low risk.
B. Total Plaque and Noncalcified Plaque Progression:
HIV-infected men categorized as high risk in the D:A:D risk score, were twice as likely to experience TPV progression in highest tertile of plaque (aOR 2.08, 95% CI 1.12, 3.88, p<0.05) as the men in the combined moderate and low risk group (Figure-3).
ROC curves and sensitivity analyses:
We further evaluated the discrimination of the continuous risk scores for TP, NCP, CAC progression and incident CAC (Figure 4). The cut-off values for prediction of TPV and NCPV progression, based on the lower boundary of the third tertile of progression, were at 17.1 and 12.2 mm3 plaque volume, annually, respectively . For prediction of TPV progression using the PCE, the area under the ROC curve (AUC) was marginally higher for the HIV-uninfected compared to HIV-infected men (AUC 0.69 vs. 0.60, p=0.08) (Fig-4A). AUC under the ROC curve was significantly higher for the HIV-uninfected men compared with HIV-infected men for the prediction of TPV progression using FRS (AUC 0.66 vs. 0.52, p<0.05) (Fig-4B).The AUC for TPV and NCPV progression prediction using D:A:D was 0.59 and 0.56, respectively (Fig-4E, Fig-4F) for HIV-infected. When comparing the prediction of TPV progression between PCE and FRS in HIV-infected, we observed that PCE was better at predicting plaque progression (AUC 0.60 vs. 0.520, P<0.05)
Fig-4-.
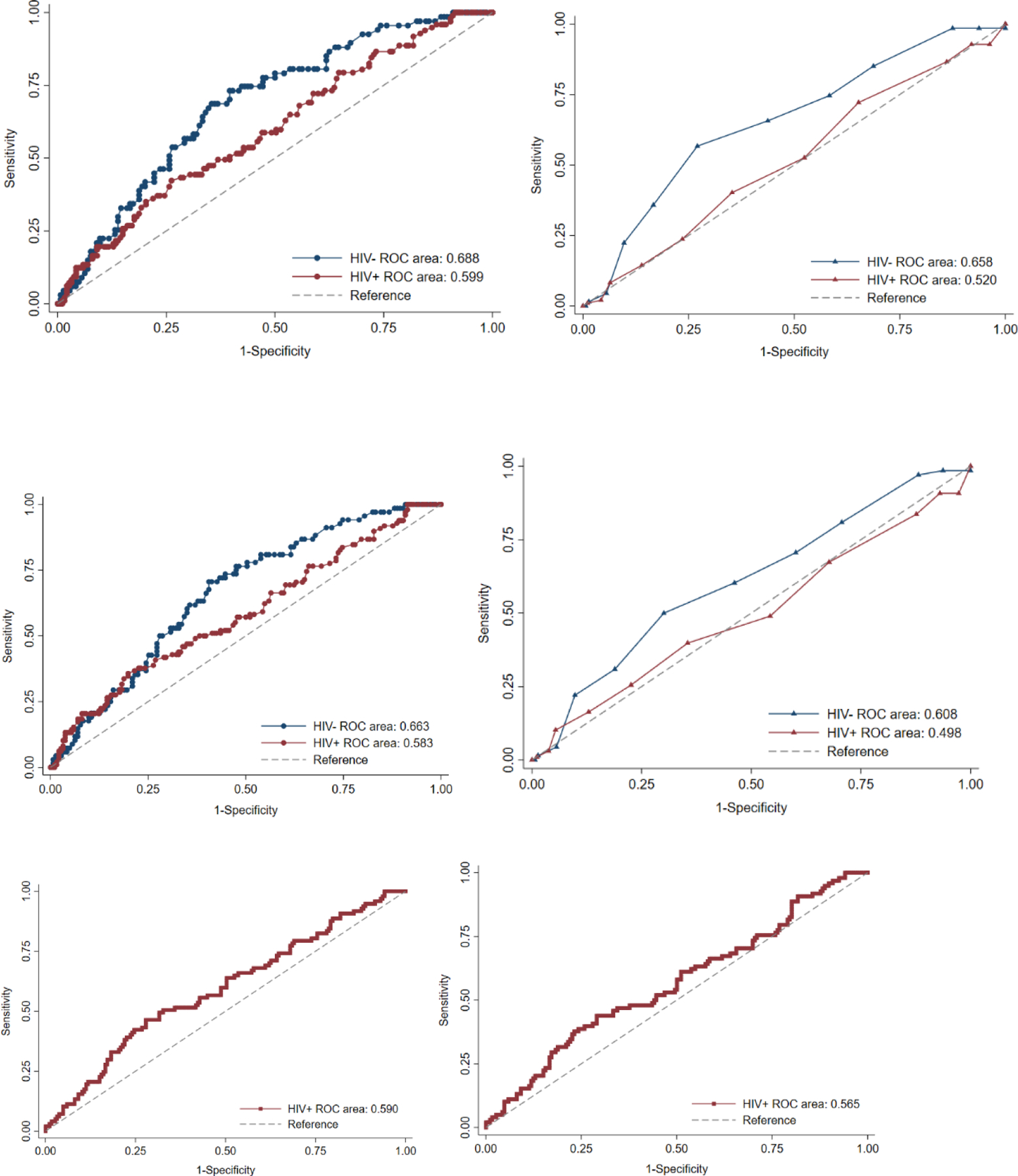
Receiver operating characteristic curves for (A) Total plaque progression (TPP) by PCE (B) TPP by FRS (C)Non-calcified plaque progression (NCPP) by PCE (D)NCPP by FRS (E)TPP by D:A:D (F)NCPP by D:A:D
The ROC curves showed no difference between HIV-uninfected and HIV-infected in the ability of PCE or FRS risk scores to predict incident CAC. Among HIV-infected, there was also no difference in the discriminatory ability for incident CAC comparing all the three risk scores The ROC’s for prediction of CAC progression (defined as >20% change in annualized CAC score) did not perform any better than chance (all curves crossed reference line)
Discussion:
In this large prospective observational study of HIV-infected and HIV-uninfected men, we evaluated the association between baseline CVD risk scores and progression of coronary artery plaque volume. High risk as per FRS and PCE risk score at baseline demonstrated stronger associations with and better discrimination of TPV and NCPV plaque progression among HIV-uninfected compared to HIV-infected men. However, all AUC values for TPV and NCPV progression were under 0.8, a commonly cited value for effective discrimination. Interestingly, the cardiovascular risk scores were not predicting better than chance alone for incident CAC and CAC progression, regardless of HIV-serostatus. Among the HIV-infected men, the D: A: D was not superior to the PCE or FRS for predicting plaque progression. In the CVD risk prediction in the HIV outpatient study, D:A:D and PCE both underestimated risk of CVD with estimated to observed ratios of 0.80 and 0.88 respectively, while FRS accurately estimated risk of CVD events with estimated to observed ratio of 1.0118. Contrary to this, another study reported that CVD risk was underestimated by FRS and PCE in HIV-infected individuals19.
Current guidelines using PCE risk score recommend statin and intensive lifestyle modification for patients in high risk category of PCE 20. Our results showed that for HIV-uninfected men, this will miss out around 25–36% of men with TPV/NCPV progression in highest tertile, and/or annualized CAC progression >20%, and around 50% of HIV-infected individuals. Our results are consistent with a previous study which showed according to PCE, 74% of HIV-infected individuals with high risk plaque features would not be recommended for statin therapy21.
D:A:D score has been developed specifically for HIV-infected population to predict 5 year CVD risk. The D:A:D adjusts for traditional risk factors with CVD outcomes in the HIV-infected population, additionally it takes into account certain ART agents that are known to increase CVD risk9. However, the discriminatory ability of D:A:D to predict plaque progression was no better than the other two CVD risk scores, with sensitivity to predict incident CAC or significant plaque progression, i.e annualized CAC>20% or TPV/NCPV progression in highest tertile around 60% in high risk group. In internal-external validation studies in D:A:D cohort reported that D:A:D equations more accurately predicted subclinical atherosclerosis measured by intimal-media thickness and CVD risk than the FRS, which overestimated9–11. Population differences in cohorts studied and percentage of participants exposed to proatherogenic antiretroviral medications may provide explanations for differences in these results18. In our cohort, small percentage of participants were taking abacavir, indinavir or lopinavir (2.1%, 0.7%, 8.5%, respectively); and therefore their inclusion in the D:A:D risk score calculation will not help to better calibrate risk for the majority of our population.
CVD risk scores are developed to predict cardiovascular events, nonetheless, total plaque, noncalcified plaque, incident CAC, and CAC progression are very strong surrogate markers for CVD events. Several prospective and retrospective studies have reported that greater progression of CAC is associated with higher CVD events. Budoff et al. in MESA reported that an increase of 5 units CAC per year in those with no prevalent CAC at baseline had 50% greater risk for CHD events, while participants with prevalent CAC at baseline, were at a 2–4 fold increased risk of CHD events with CAC increase of 100 units or more per year22. In another study, Raggi et al.23 found CAC score change of >15% per year (P<0.001) as an independent predictor of time to myocardial infarction regardless of baseline CAC score.
2018 cholesterol guidelines recommend considering delaying statins in individuals with no prevalent CAC, however it suggests that CAC scores of zero in chronic inflammatory conditions such as HIV, may still be associated with higher risk of ASCVD20. Some reports have shown that HIV-infected individuals with no prevalent CAC had higher prevalence and burden of noncalcified plaque volume (NCPV), independent of traditional risk factors8,24. NCPV is associated with CVD events and provide additional prognostic information beyond CAC. In a large single center study Hou Zu et al.25 showed cumulative probability of 3 year MACE was highest for noncalcified as opposed to calcified plaque ( 37.7% and 5.5% respectively).26 . Efficacy of statins in reducing CVD in HIV-infected population have been established27.
The strengths of our study include standardized collection of clinical and laboratory data in a diverse multiethnic population to calculate risk scores and ample time (median of 4.5 years) between baseline and follow-up scan to assess progression of subclinical atherosclerosis. Furthermore, we utilized cardiac CT to study subclinical atherosclerosis, which is very specific and provides an opportunity to assess multi-vessel quantitative atherosclerosis features, such as calcified and noncalcified plaque volume. Our study has several limitations. We present association of CVD risk scores and atherosclerotic plaque progression rather than events. The limited number of events prevent us from presenting CV event outcomes. Secondly, our study consists of only men, hence we are not able to comment on the predictive value of CVD risk scores in women living with HIV. Furthermore, some of the CI in our study were large, could be due to the wide distribution of annualized plaque change.. Nonetheless, our results are concordant with baseline cross sectional sub-study of MACS28.
In conclusion, associations between CVD risk scores with plaque progression were stronger in HIV-uninfected individuals as compared to HIV-infected individuals. The high risk D: A:D had higher sensitivity but poorer specificity as compared to PCE and FRS in HIV-infected men. No score had satisfactory discriminatory ability to predict plaque progression in HIV-infected or HIV-uninfected population. Improved CVD risk scores are needed to identify high-risk HIV-infected men for more aggressive CVD risk prevention strategies.
Supplementary Material
References
- 1.Collaboration ATC. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. The Lancet 2006;368:451–8. [DOI] [PubMed] [Google Scholar]
- 2.Wada N, Jacobson LP, Cohen M, French A, Phair J, Muñoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. American journal of epidemiology 2013;177:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Annals of internal medicine 2014;160:458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004;109:1603–8. [DOI] [PubMed] [Google Scholar]
- 5.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation 2019;140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monroe AK, Haberlen SA, Post WS, et al. Cardiovascular disease risk scores’ relationship to subclinical cardiovascular disease among HIV-infected and–uninfected men. AIDS (London, England) 2016;30:2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parra S, Coll B, Aragones G, et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV‐infected patients: relationships with serum markers of oxidation and inflammation. HIV medicine 2010;11:225–31. [DOI] [PubMed] [Google Scholar]
- 8.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS (London, England) 2010;24:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friis-Møller N, Thiebaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. European journal of cardiovascular prevention & rehabilitation 2010;17:491–501. [DOI] [PubMed] [Google Scholar]
- 10.Serrano-Villar S, Estrada V, Gomez-Garre D, et al. Diagnosis of subclinical atherosclerosis in HIV-infected patients: higher accuracy of the D:A:D risk equation over Framingham and SCORE algorithms. European journal of preventive cardiology 2014;21:739–48. [DOI] [PubMed] [Google Scholar]
- 11.Friis-Møller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D: A: D) study. European journal of preventive cardiology 2016;23:214–23. [DOI] [PubMed] [Google Scholar]
- 12.Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010;3:1229–36. [DOI] [PubMed] [Google Scholar]
- 13.Nadjiri J, Hausleiter J, Jähnichen C, et al. Incremental prognostic value of quantitative plaque assessment in coronary CT angiography during 5 years of follow up. Journal of cardiovascular computed tomography 2016;10:97–104. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi R, Post WS, Osawa K, et al. Multicenter AIDS Cohort Study Quantitative Coronary Plaque Progression Study: rationale and design. Coronary artery disease 2018;29:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 16.Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. Journal of cardiovascular computed tomography 2014;8:342–58. [DOI] [PubMed] [Google Scholar]
- 17.Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol 2004;24:1272–7. [DOI] [PubMed] [Google Scholar]
- 18.Thompson-Paul AM, Lichtenstein KA, Armon C, et al. Cardiovascular disease risk prediction in the HIV outpatient study. Clinical Infectious Diseases 2016;63:1508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regan S, Meigs JB, Massaro J, D’Agostino RB, Grinspoon SK, Triant VA. Evaluation of the ACC/AHA CVD risk prediction algorithm among HIV-infected patients. Conference on retroviruses and opportunistic infections; 2015. p. 23–6. [Google Scholar]
- 20.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology 2018:25709.
- 21.Zanni MV, Fitch KV, Feldpausch M, et al. 2013 ACC/AHA and 2004 ATP III Cholesterol Guidelines Applied to HIV-Infected Patients with/without Subclinical High Risk Coronary Plaque. AIDS (London, England) 2014;28:2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budoff MJ, Young R, Lopez VA, et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). Journal of the American College of Cardiology 2013;61:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. Journal of the American College of Cardiology 2004;43:1663–9. [DOI] [PubMed] [Google Scholar]
- 24.Metkus TS, Brown T, Budoff M, et al. HIV infection is associated with an increased prevalence of coronary noncalcified plaque among participants with a coronary artery calcium score of zero: Multicenter AIDS Cohort Study (MACS). HIV medicine 2015;16:635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou Z-h, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC: Cardiovascular Imaging 2012;5:990–9. [DOI] [PubMed] [Google Scholar]
- 26.Rumberger JA. CT defined atherosclerotic plaque type and severity: using the results of the diagnostic microscope to sharpen the clarity of peering through the prognostic telescope. JACC Cardiovascular imaging 2012;5:1000–2. [DOI] [PubMed] [Google Scholar]
- 27.Gili Sebastiano, Grosso Marra Walter, D’Ascenzo Fabrizio, Lonni Enrica, Calcagno Andrea, Cannillo Margherita, Ballocca Flavia et al. “Comparative safety and efficacy of statins for primary prevention in human immunodeficiency virus-positive patients: a systematic review and meta-analysis.” European heart journal 37, no. 48 (2016): 3600–3609. [DOI] [PubMed] [Google Scholar]
- 28.Monroe AK, Haberlen SA, Post WS, et al. Cardiovascular disease risk scores’ relationship to subclinical cardiovascular disease among HIV-infected and HIV-uninfected men. Aids 2016;30:2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


