Abstract
Objective:
Antibody blockade of the “don’t eat me” signal CD47 enhances efferocytosis and reduces lesion size and necrotic core formation in murine atherosclerosis. TNFα expression directly enhances CD47 expression, and elevated TNFα is observed in the absence of the pro-efferocytosis receptor low-density lipoprotein receptor-related protein 1 (LRP1), a regulator of atherogenesis and inflammation. Thus, we tested the hypothesis that CD47 blockade requires the presence of macrophage LRP1 to enhance efferocytosis, temper TNFα-dependent inflammation, and limit atherosclerosis.
Approach and Results:
Mice lacking systemic apoE (apoE−/−), alone or in combination with the loss of macrophage LRP1 (DKO), were fed a Western-type diet for 12-weeks while receiving anti-CD47 antibody (anti-CD47) or IgG every other day. In apoE−/− mice, treatment with anti-CD47 reduced lesion size by 25.4%, decreased necrotic core area by 34.5%, and decreased the ratio of free:macrophage-associated apoptotic bodies by 47.6% compared to IgG controls (p<0.05), confirming previous reports. DKO mice treated with anti-CD47 showed no differences in lesion size, necrotic core area or the ratio of free:macrophage-associated apoptotic bodies compared to IgG controls. In vitro efferocytosis was 30% higher when apoE−/− phagocytes were incubated with anti-CD47 compared to IgG controls (p<0.05); however, anti-CD47 had no effect on efferocytosis in DKO phagocytes. Analyses of mRNA and protein showed increased CD47 expression in anti-inflammatory IL-4 treated LRP1−/− macrophages compared to wildtype, but no differences were observed in inflammatory LPS-treated macrophages.
Conclusions:
The pro-efferocytosis receptor LRP1 in macrophages is necessary for anti-CD47 blockade to enhance efferocytosis, limit atherogenesis, and decrease necrotic core formation in the apoE−/− model of atherosclerosis.
Keywords: Cardiovascular Physiology, Coronary artery disease, phagocytosis
Subject Terms: Atherosclerosis, Inflammation, Animal Models of Human Disease, Vascular Biology
Graphical Abstract
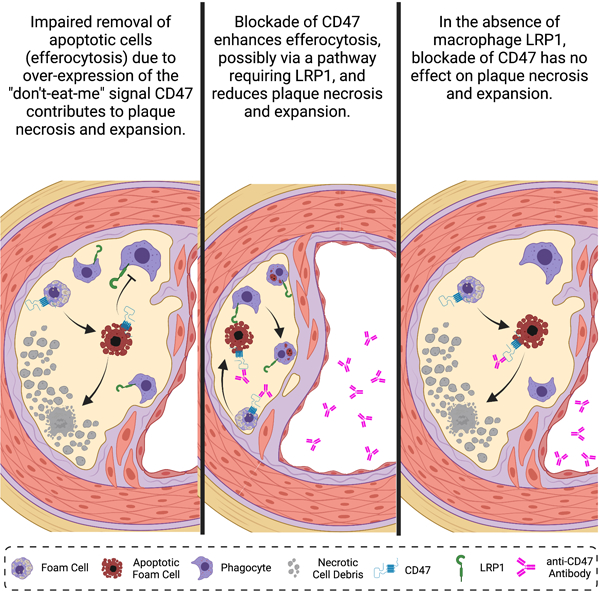
Introduction
Clinically unstable atherosclerotic plaques are characterized by the presence of a large necrotic core covered by a thin fibrous cap. Necrotic cores develop as sub-intimal apoptotic cells undergo necrosis and accumulate as a result of inefficient clearance (a process called efferocytosis) by phagocytes. Although efferocytosis is the physiological process responsible for the clearance of billions of apoptotic cell bodies every day1, it is often overwhelmed and impaired in plaques of advanced atherosclerosis2–4. Impaired efferocytosis during atherosclerosis can be attributed to either a loss of phagocytic capacity (e.g., enhanced cleavage of MERTK5) in lesion macrophages, or to increased expression of CD47 on apoptotic cells6. In either case, a steady production of apoptotic cells that are not cleared leads to secondary necrosis and the release of atherogenic intracellular content7, including matrix degrading metalloproteinases8, angiogenic cytokines9 and thrombogenic tissue factors10–12 all known to accelerate atherosclerosis and increase lesion instability.
Low-density lipoprotein (LDL) receptor-related protein 1 (LRP1) is a multi-ligand receptor that acts as a backup for LDL receptor (LDLR) in the liver13 and as an efferocytosis receptor on macrophages14, 15. We reported that loss of macrophage LRP1 reduces lipoprotein internalization and paradoxically accelerates atherosclerosis progression16. We later determined that this effect is due to enhanced inflammation with increased secretion of the cytokine TNFα14, increased accumulation of non-engulfed apoptotic debris, and accelerated necrotic core formation17. It was previously shown that LRP1, and its co-receptor calreticulin, interact with phosphatidylserine-binding complement factor C1q to engulf apoptotic cells18. In addition, LRP1 is an important mediator of efferocytosis in splenic dendritic cells15. Because of its ability to bind both phosphatidylserine and LRP1, it has been suggested that apolipoprotein E (apoE) facilitates efferocytosis, perhaps by acting as a bridging molecule. Indeed, hyperlipidemic apoE−/− mice readily develop atherosclerosis with marked accumulation of apoptotic cells, and their macrophages show impaired efferocytosis of apoptotic thymocytes in vitro19.
While LRP1 and apoE promote efferocytosis, the other side of the scale is balanced by “don’t eat me” signals, including CD47. This was originally found to be increased in a variety of cancers, rendering tumor cells immune to phagocytosis20, 21. A recent study from some of the authors of the present report (Kojima et al.6) identified the upregulation of CD47 in the necrotic core of human atherosclerotic plaques, and demonstrated that treatment of apoE−/− mice with a therapeutic anti-CD47 antibody (anti-CD47) limits plaque formation, reduces necrotic core development, and decreases the ratio of free:macrophage-associated apoptotic bodies in atherosclerotic plaques relative to a non-specific IgG control. CD47 expression correlates with that of TNFα in human atherosclerotic plaques and is modulated via NFκB6. In fact, treatment of apoptotic cancer cells and smooth muscle cells with TNFα increases CD47 expression and impairs efferocytosis in vitro6, 22. Previous work by some of the authors of the present report (Zhu et al23) demonstrated that the use of the anti-TNFα antibody adalimumab in LDLR−/− mice limits atherogenesis, decreases apoptotic cell accumulation in plaques, and reduces the size of the necrotic core compared to IgG controls. Interestingly, these effects were ameliorated in the absence of macrophage LRP1 and occurred independently of apoE. The latter result suggests that anti-TNFα therapy may limit necrosis by inhibiting CD47 expression via a mechanism requiring the presence of macrophage LRP1. Thus, we sought to determine whether CD47 blockade requires macrophage LRP1 to limit atherogenesis, reduce necrosis, and enhance efferocytosis.
In this study, we determine that the loss of macrophage LRP1 limits the anti-atherogenic effects of CD47 blockade in vivo, and show that the dual loss of LRP1 and apoE reduces efferocytosis during CD47 blockade in vitro.
Materials and Methods
Study design
Mice with floxed loxP sites flanking LRP1 were crossed with mice expressing Cre recombinase under the control of the macrophage-specific lysozyme M promoter to generate mice with macrophages deficient in LRP1 (MΦLRP1−/−) as previously described16. MΦLRP1−/− mice were then crossed with apoE−/− mice (The Jackson Laboratory) to generate apoE−/−/MΦLRP1−/− double knockout (DKO) mice. To replicate our previous work reporting the effects of CD47 blockade in male apoE−/− mice6, 16, we only used male mice in this study. 8-week-old male apoE−/− mice (N=7–8/group) and DKO mice (N=12–16/group) were fed a western-type diet (42% kcal from fat, Harlan) while receiving anti-mouse CD47 antibody (200mg/injection; BioXCell) or IgG control via intraperitoneal (i.p.) injection every other day for 12 weeks. A terminal bleed was performed after 12 weeks on Western-type diet and used for serum lipid quantification. Mice were humanely euthanized, perfused with 1X PBS and hearts frozen, unfixed, in OCT for atherosclerosis analysis. Animal care and experimental procedures were performed according to the regulation of the Institutional Animal Care and Usage Committee of Oregon Health & Science University. This study was designed in adherence to the guidelines for experimental atherosclerosis studies as recommended in the American Heart Association statement, which includes the use of our mouse models (including proper use of sample size and use of both sexes where appropriate), quantification of atherosclerosis, characterizing plasma lipids, statistical analyses, and presentation of data24.
Atherosclerosis analysis
Frozen sections of aortic sinus were stained with oil red-O. Briefly, unfixed hearts frozen in OCT were subjected to serial sectioning (5μm sections) of the aortic sinus using a Leica CM1950 Cryostat. Slides were then fixed in 4% paraformaldehyde, stained with oil red-O and hematoxylin, and images captured using Fisher Scientific light microscope (microscope name). Lesion area was quantified using ImageJ software via blinded-analysis on two slides from each mouse, each containing 5 serial sections which were averaged. Sectional lesion area is reported in μm6.
Serum Lipid Quantification
Total serum cholesterol and total serum triglyceride levels from whole blood was determined by enzymatic colorimetric assays using colorimetric kits (Pointe Scientific Cholesterol Reagent and Triglycerides Reagent kits).
In vivo quantification of apoptosis, efferocytosis and necrotic core
Serial cryosections (5μm) of the aortic root were used as described previously6, 16. Five sequential cryo-sections on the same glass slide were used for each staining to quantify macrophages using anti-rat Mac2 (0.625mg/mL; eBioscience) and apoptotic cells using TUNEL (Roche). Briefly, sections were fixed in 4% paraformaldehyde for 15 minutes, washed three times with PBS, permeabilized with 0.1% Sodium citrate and 0.1% Triton X-100 solution (pH 6.0), washed again and blocked with background buster (Innovex) at 37°C for 1 hour. TUNEL staining was performed using Roche’s In Situ Cell Death Detection Kit (Millipore Sigma) according to the manufacturer’s instructions. Again, sections were washed three times in PBS and incubated with anti-rat Mac2 antibodies at 4°C overnight. Sections were then washed with PBS three times, incubated with Alexa Fluor 594 anti-rat IgG (2.5mg/mL; Thermo Fisher) at 37°C for 1 hour. Images were captured using Nikon A1 confocal microscope and analyzed using ImageJ version 2.1.0/1.53c software. Necrotic core area was determined as described previously6. Briefly, five sequential cryo-sections on the same glass slide were stained using Mason’s trichrome stain according to the manufacturer’s protocol. Images were captured using Fisher Scientific light microscope (microscope name). Necrotic core area was quantified using ImageJ via blinded-analysis on the 5 serial sections which were averaged. Necrotic core area was defined as negative cellular area within lesions and reported in µm2.
In vitro macrophage polarization, gene expression, and protein expression
We chose to study peritoneal macrophages based on their propensity to emulate the inflammatory M1 phenotype observed in advanced murine atherosclerosis25. Peritoneal macrophages were collected from wildtype and MΦLRP1−/−(n≥6), 3 days after i.p. injection with 3% thioglycollate and seeded in 6-well plates at 3x106 cells/well in DMEM with 10% FBS. Thioglycollate-elicited macrophages were utilized in this study due to their advanced phagocytic activity compared to non-elicited resident peritoneal macrophages26. 4 hours later, floating cells were removed and attached cells were washed twice with 1X PBS. Cells were cultured in DMEM with 1% BSA overnight for macrophage synchronization. Macrophages destined for an inflammatory phenotype were first primed for 6–8 hours in DMEM with 0.1% FBS containing IFNg (50ng/mL). To generate inflammatory macrophages, naïve macrophages were treated with IFNg (50ng/mL) and lipopolysaccharide (100ng/mL) for 12 hours. To generate anti-inflammatory macrophages, naïve macrophages were treated with interleukin-4 (25ng/mL) for 12 hours. Cells were washed 3x in cold 1X PBS and collected for RNA or protein. RNA was extracted using E.Z.N.A. Total RNA Kit (Omega Bio-Tek) according to the manufacturer’s protocol. Cd47, Sirpa, Calr, Rpl32 and 18s primers were from Thermo Fisher and real-time PCR was performed using Applied Biosystems ViiA7. Protein was collected by lysing cells with RIPA buffer (Sigma-Aldrich) containing 1X Pierce protease inhibitor (Thermo Fisher). Protein lysate subjected to Western blotting by running protein lysate (30mg) onto Bolt Bis-Tris Plus Gels (4–12%) and transferred to nitrocellulose membrane. CD47 was detected with goat anti-CD47 (0.25mg/mL; R&D Systems; Cat# AF1866) with a smear containing multiple bands stretching from 40kD to 50kD resulting from the presence of 4 alternatively spliced isoforms as previously described27, 28. CD47 protein was quantified as the entire smear from 40–50kD (bracketed in Figure 2J) and normalized to beta-actin (mouse anti-beta actin; 1:5000; Millipore Sigma; Cat# A5441) as a loading control using Licor’s Image Studio Software (Version 5.2.5). Experiments were performed in triplicate and repeated three times.
In vitro efferocytosis
In vitro efferocytosis was performed using primary murine macrophages from apoE−/−, and DKO mice (N≥6), 3 days after i.p. injection with 3% thioglycollate. Phagocytes were labeled using BMQC violet cell tracker and apoptotic substrates using CFDA-SE cell tracker (green) according to the manufacturers’ protocols. BMQC violet-labled macrophages were treated with 1μM staurosporine for 4 hours to induce apoptosis as previously described6,14. BMQC violet-labeled apoptotic cells were then incubated with adherent CFDA-SE-labeled macrophages for 2 hours at a 2:1 ratio respectively in the presence of anti-CD47 antibody (10mg/mL), IgG control, or media only. Following the 2-hour incubation, wells were washed three times in 1X PBS to remove non-adherent apoptotic cells, and remaining cells were fixed in 1% paraformaldehyde prior to analysis using the BD LSRII flow cytometer. Efferocytosis is determined by dividing the number of BMQC violet+ CFDA-SE+ double positive cells by the total number of CFDA-SE+ phagocytes and reported as a percent of maximum.
Statistical Analyses
Data have been analyzed for normality using the Shapiro-Wilk test (α=0.05) and equal variance using an F-test to justify the use of parametric analyses such as Student t test. Only the ratio of spleen weight to body weight in DKO mice (Figure 1I) was found to have significantly different variance, therefore we applied the nonparametric Mann Whitney test. For images, data were collected from at least 5 sections for each mouse and averaged. Data are expressed as mean ± SEM unless otherwise stated. For comparison of 2 groups, we performed a Student t test. For efferocytosis and gene expression analyses, we used 2-way ANOVA with the Sidak multiple comparisons test. P<0.05 was considered significant. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Figure 1: Anti-CD47 does affect atherogenesis or efferocytosis in the absence of LRP1 in vivo.
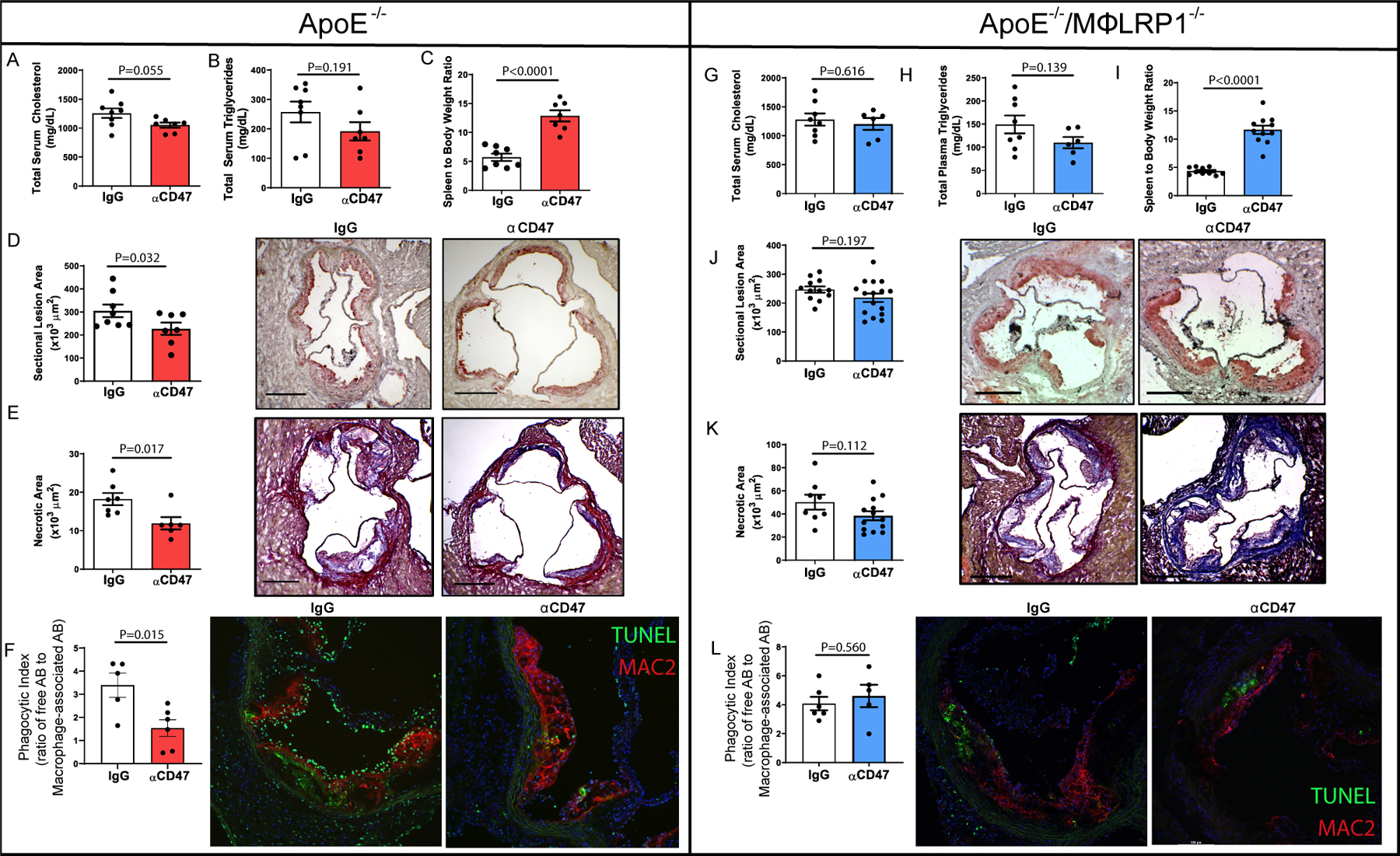
ApoE−/− mice were fed Western-type diet for 12 weeks while receiving anti-CD47 antibody (αCD47; 200μg/injection; N=7) or IgG (N=8) every other day. Mice were bled prior to euthanasia and serum was collected to determine A) Total serum cholesterol and B) total serum triglycerides. C) Spleen to body weight ratios were reported. Hearts were used to obtain frozen serial sections of aortic sinus. Frozen sinus sections were stained with D) Oil red-O to quantify lesion area (x103 μm2) One-tailed Student’s t-test was performed to determine differences between treatments. *P<0.05. E) Masson’s Trichrome stain was performed, and necrotic core area was quantified as regions of acellularity (x103 μm2). F) Immunofluorescent staining was performed using antibodies for Mac2 (red) to quantify macrophages and TUNEL (green) to label apoptotic cells. The ratio of free:macrophage-associated apoptotic bodies was determined as the ratio of the number of TUNEL+ cells not associated with Mac2+ staining to TUNEL+ cells within a region of Mac2+ area. To determine whether macrophage LRP1 is required for αCD47efficacy, apoE−/−/MΦLRP1−/− (DKO) mice were fed Western-type diet for 12 weeks while receiving anti-CD47 antibody (200μg/injection; N=16) or IgG (N=12) every other day. Mice were bled prior to euthanasia and serum was used to determine G) Total serum cholesterol and H) total serum triglycerides. I) Spleen to body weight ratios were reported and two-tailed Mann Whitney test was used to determine differences between treatment groups. Hearts were perfused with O.C.T. and used to obtain frozen serial sections of aortic sinus. Frozen sinus sections were stained with J) Oil red-O to quantify lesion area (x103 μm2). K) Masson’s Trichrome stain was performed, and necrotic core area was quantified as regions of acellularity (x103 μm2). L) Immunofluorescent staining was performed using antibodies targeting Mac2 (red) to quantify macrophages and TUNEL (green) to label apoptotic cells. The ratio of free:macrophage-associated apoptotic bodies was determined as described above. Two-tailed students t-test was performed to determine differences between treatment groups unless otherwise stated.
Results
Anti-CD47 does not influence atherogenesis or efferocytosis in the absence of LRP1.
We first confirmed the previously published claim that anti-CD47 antibody treatment limits atherogenesis in experimental atherosclerosis. ApoE−/− mice were fed Western type diet for 12 weeks while receiving anti-CD47 or IgG control every other day for 12 weeks (N=7–8/treatment). There were no differences in serum cholesterol or triglyceride levels between mice receiving anti-CD47 or control IgG (Fig. 1A/B). In support of the expected effect of CD47 blockade, spleen to body weight ratios were 2.3-fold larger in anti-CD47-treated mice compared with IgG-treated controls (P<0.0001; Fig. 1C), due to the fact that anti-CD47 therapy enhances clearance of red blood cells in the spleen, thus enlarging the organ6. Sectional lesion area was 25.4% smaller in anti-CD47-treated mice compared with IgG-treated controls (227,200 ± 69,310µm2 and 304,600 ± 77,390µm2 respectively; P<0.05; Fig. 1D). Mason’s trichrome stain was used to determine regions of necrotic area defined as acellular areas. Necrotic core area was 34.5% smaller in anti-CD47-treated mice (18,300 ± 4,168µm2) compared with IgG-treated controls (11,940 ± 3,878µm2; P<0.05; Fig. 1E). To determine the effect of anti-CD47 antibody therapy on the phagocytic index in vivo (defined as the ratio of unengulfed apoptotic bodies to macrophage-associated apoptotic bodies in lesions), frozen sections of the aortic sinus were stained for macrophages by immunofluorescence using Mac2 and for apoptotic cells using TUNEL. Mice treated with IgG antibody demonstrated a 2.1-fold higher in vivo phagocytic index compared to those treated with anti-CD47 (3.23±1.11 and 1.54±0.88 respectively; P<0.05; Fig. 1E).
To determine whether anti-CD47 therapy enhances efferocytosis and limits atherogenesis in the absence of LRP1, ApoE−/−/MΦLPR1−/− double knockout (DKO) mice were fed Western type diet for 12 weeks while receiving anti-CD47 or IgG control every other day for 12 weeks (N=14/treatment). There were no differences in serum cholesterol or triglyceride levels between mice receiving anti-CD47 or control IgG (Fig. 1G/H). Similar to apoE−/− mice, spleen to body weight ratios were 2.7-fold larger in anti-CD47-treated mice compared with IgG-treated controls (P<0.0001; Fig. 1I). On the other hand, treatment of anti-CD47 did not significantly alter sectional lesion area in DKO mice compared with their IgG-treated controls (218,600 ± 59,570µm2 and 246,800 ± 37,050µm2 respectively; P=0.162; Fig. 1J). Similarly, anti-CD47 therapy did not reduce the necrotic core compared to IgG-treated controls in the DKO mice (38,450 ± 14,160µm2 and 50,250 ± 18,150µm2 respectively; P=0.11; Fig. 1K). DKO mice treated with IgG antibody demonstrated no differences in their in vivo phagocytic index compared to those treated with anti-CD47 (4.60 ± 1.74 and 4.08 ± 1.15 respectively; P=0.429; Fig. 1L).
The ability of anti-CD47 antibody to enhance efferocytosis is inhibited by the combined loss of LRP1 and apoE.
To determine whether anti-CD47 enhances efferocytosis in the absence of LRP1 in vitro, we pretreated BMQC (violet)-labeled apoptotic peritoneal macrophages from apoE−/− and DKO mice with anti-CD47 (10μg/mL) or IgG control for 30 minutes and then added them to tissue culture wells containing CFDA-SE (green)-labeled apoE−/− or DKO macrophages for two hours to allow for efferocytosis. After a 2-hour incubation, non-adherent apoptotic macrophages were washed away, and the remaining macrophages fixed in 1% PFA for flow cytometry-based determination of efferocytosis, defined as the percentage of violet+ green+ double positive cells over the total number of green+ macrophages (Fig. 2A–D). Blockade of CD47 increased efferocytosis by 30% in apoE−/− phagocytes (engulfing apoE−/− apoptotic substrate), compared to IgG-treated controls (P<0.05; Fig. 2F, left side). On the other hand, anti-CD47 antibody treatment had no effect on efferocytosis of DKO phagocytes engulfing DKO apoptotic substrates compared to IgG-treated controls (Fig. 2F, right side).
Figure 2: Dual loss of macrophage-LRP1 and systemic apoE abolishes the ability of anti-CD47 antibody to enhance efferocytosis in vitro.
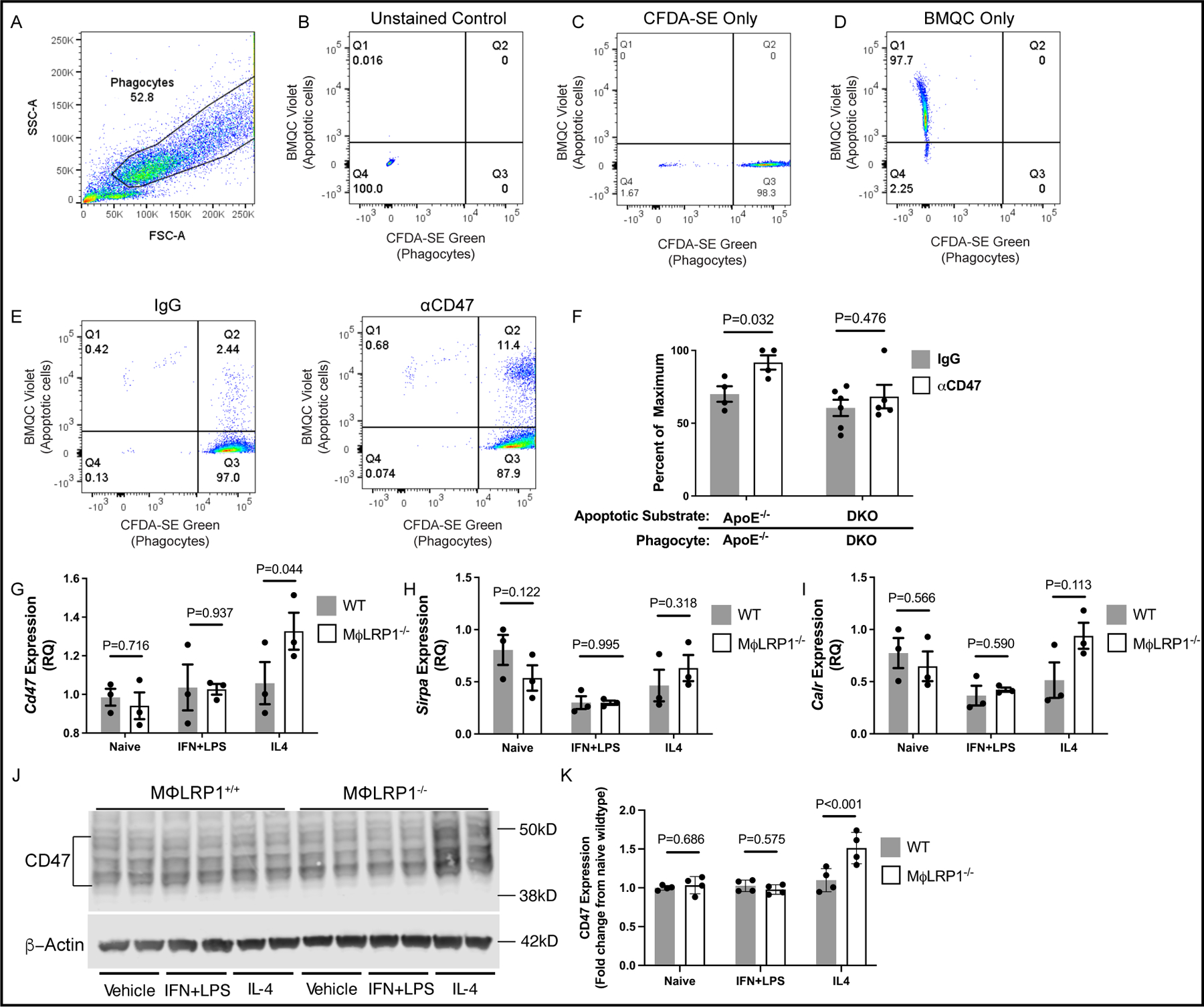
ApoE−/− and DKO mice (N=9/genotype) were injected intraperitoneally with 4% thioglycollate broth and euthanized 3 days later to isolate peritoneal macrophages. Macrophages were fluorescently labeled with CFSDA-SE (green) or BMQC (Violet) live cell dyes. Violet-labeled macrophages were treated with staurosporine (1uM) to induce apoptosis. Apoptotic macrophages were treated with anti-CD47 antibody (αCD47; 10ug/mL) or IgG for 30 minutes. Apoptotic macrophages were incubated with green-labeled macrophages (phagocytes) at a 2:1 ratio for 2 hours to allow for efferocytosis. Efferocytosis was quantified using an LSRII flow cytometer. (A) The phagocyte population was gated first, and (B) unstained, (C) green only and (D) violet only single-stained controls were used to establish fluorescent signal thresholds. Phagocytes positive for apoptotic cell uptake are found in quadrant 2 (Q2). Efferocytosis is calculated as the number of phagocytes positive for apoptotic cell uptake divided by the total number of green+ phagocytes and presented as a percent of maximum uptake. (F) Violet-labeled apoptotic apoE−/− and DKO macrophages were treated with αCD47 or IgG and incubated with phagocytes of their respective genotype. Two-way ANOVA with Sidak’s multiple comparison’s test was used to determine the effect of antibody treatment between genotypes. Peritoneal macrophages from WT and MΦLRP1−/− mice (N=9/genotype) were treated with vehicle, interferon gamma (IFNγ; 50ng/mL) and lipopolysaccharide (LPS; 100ng/mL) and interleukin-4 (IL-4; 25ng/mL) for 12 hours to differentiate macrophages to naïve, inflammatory, or anti-inflammatory subtypes respectively. RNA was used to determine gene expression of (G) Cd47, (H) Sirpa and (I) Calr using qRT-PCR. (J) Western blotting using protein lysate was used to determine the protein expression of CD47 in differentiated peritoneal macrophages using beta actin as a loading control. (K) Li-Cor Image Studio Software was used to quantify protein signal intensity. Multiple t-tests were performed using the Holm-Sidak method to determine differences in expression between genotypes.
To test whether the loss of LRP1 on macrophages disrupts efferocytosis by either upregulating CD47 or its receptor SIRPα (encoded by the gene Sirpa), or by downregulating LRP1’s ligand calreticulin (encoded by the gene Calr) we performed qRT-PCR on peritoneal macrophages from wildtype and LRP1−/− mice that were either naïve or differentiated to inflammatory or anti-inflammatory phenotypes as described in Expanded Materials and Methods. The expression of Cd47 was upregulated in anti-inflammatory LRP1−/− peritoneal macrophages compared to wildtype controls, but not in the naïve or inflammatory subsets (Fig. 2G). On the other hand, we observed no differences in expression of Sirpa or calr between wildtype and LRP1−/− macrophages, regardless of phenotype (Fig. 2H and I). Consistent with gene expression, CD47 protein was upregulated in IL-4 treated anti-inflammatory LRP1−/− macrophages compared to wildtype controls (Fig 2J and K; P<0.001). No other differences in protein expression were observed regardless of genotype or inflammatory phenotype.
Discussion
In this study we show that the atherosclerosis-limiting effect of CD47 blockade requires the presence of LRP1 on macrophages to enhance efferocytosis, reduce necrosis, and decrease plaque area in the apoE−/− model of hypercholesterolemia. While we confirm that anti-CD47 treatment enhances efferocytosis and reduces plaque area in apoE−/− mice fed Western type diet, we also prove that in mice lacking both systemic apoE and macrophage LRP1 the effects of CD47 blockade on necrotic core formation and apoptotic body phagocytosis are completely nullified. Additionally, in vitro experiments revealed that the combined loss of LRP1 and apoE completely abolishes anti-CD47’s effect on efferocytosis likely via their role as a pro-efferocytosis receptor and its ligand, respectively.
We have previously demonstrated that the “don’t eat me” anti-efferocytosis signaling protein CD47 is significantly upregulated in necrotic cores of advanced human and murine atherosclerotic plaques and is a key contributor to necrosis6. The use of anti-CD47 antibody decreased plaque growth, reduced necrotic core area and lowered the phagocytic index in the apoE−/− mouse model of atherosclerosis, including mice treated with angiotensin II infusion and mice with established lesions6. The cytokine TNFα stimulates CD47 expression via downstream activation of the classical inflammatory transcription factor NF-κB1, which also binds to the promoter region of CD47. We later determined that TNFα is capable of activating NF-κB1 binding to regions of super enhancers near the CD47 gene in several cancer cell lines, that blocking CD47 or TNFα with antibodies increases their phagocytosis by macrophages22, and that other interventions that block the CD47 axis also prevent atherosclerosis and enhance efferocytosis29.
LRP1−/− macrophages have increased expression and secretion of TNFα compared to wildtype controls14, but the expression of CD47 in atherosclerotic plaques or macrophages of LRP1−/− mice has not been investigated. Herein, we demonstrate that, in vitro, only anti-inflammatory LRP1−/− peritoneal macrophages upregulate CD47 mRNA and protein, whereas naïve and pro-inflammatory subsets show no differences among genotypes. Because CD47 inhibits phagocytosis through its interactions with SIRPα on phagocytes21, we tested whether loss of LRP1 upregulates Sirpa on macrophages but found no differences in expression compared to wildtype controls. Thus, we propose that the loss of LRP1 in macrophages impairs efferocytosis so significantly that inhibiting CD47 is no longer able to counter LRP1 effects and promote effective clearance of apoptotic cells. We describe increased CD47 expression in LRP1−/− macrophages treated with IL-4 compared to wildtype controls, but not in naïve or IFNγ/LPS treated inflammatory macrophages. Because established plaques predominantly include inflammatory macrophages during atherosclerosis progression30–32, it is unlikely our in vivo observations were a result of differences in CD47 expression in plaques.
The current findings are in apparent disagreement with our previous report that mice lacking macrophage LRP1 undergo accelerated atherogenesis and develop lesions with large necrotic cores and increased apoptotic cell burden relative to controls14, 16, 17. However, in these previous studies we had transplanted macrophage LRP1−/− bone marrow into adult apoE−/− or LDLR−/− mice, whereas in the current study macrophage LRP1 was absent during embryo development. LRP1 loss early in embryonic development likely impacts aortic macrophage function and lesion development differently than its loss later in life, perhaps because of counter-regulatory adjustments to the absence of LRP1 during embryogenesis or because resident aortic tissue macrophages have a different contribution to the progression of atherosclerotic lesions33. Further studies are required to delineate the role of LRP1 in the development of atherosclerosis with regard to its expression in resident aortic tissue macrophages compared to recruited macrophages.
LRP1 is a critical receptor on phagocytes for efferocytosis via binding to its ligand calreticulin and phosphatidylserine18. We have previously reported that LRP1−/− macrophages are less efficient phagocytes compared to their wildtype counterparts in engulfing either wildtype or LRP1−/− apoptotic macrophages14. Herein, our data clearly show that the dual loss of LRP1 and apoE on macrophages is sufficient to block the anti-CD47-dependent effects on phagocytosis leading to necrosis and exacerbated atherosclerosis. Based on our study’s design, we can only conclude that loss of macrophage LRP1 negates anti-CD47’s beneficial effects, but we cannot exclude the possibility that deletion of other pro-efferocytosis receptors would have a similar detriment to anti-CD47’s efficacy. Notably, others have demonstrated that loss/inhibition of other pro-efferocytosis receptors such as MERTK blocks efferocytosis and enhances necrotic core formation5. Thus, it is feasible that deletion of other macrophage efferocytosis receptors may limit anti-CD47 antibody’s ability to enhance efferocytosis and limit atherogenesis. Clinically, LRP1 is a known risk locus for abdominal aortic aneurysms34 and atherogenic smooth muscle cell-specific Lrp1 knockout mice develop aortic aneurysms on Western type diet. LRP1 was also identified in 2017 as a coronary artery disease risk locus35. The risk allele at rs11172113 is found in the first intron of the LRP1 gene and is associated with decreased gene expression in both atherosclerotic and nonatherosclerotic arterial tissue35. We have previously shown that LRP1 expression is elevated in progressing atherosclerotic plaques, as compared to those undergoing regression, in a murine model of atherosclerosis36. Thus, reduced LRP1 expression during clinical atherogenesis has potential to increase CAD event risk via reduced efferocytosis and enhanced plaque necrosis as has been shown in mouse models by us and others14, 16, 17, 37.
ApoE is a likely mediator of phagocytosis due to its binding affinity to both phosphatidylserine and LRP1. In fact, we and others have previously demonstrated that loss of apoE diminishes the phagocytic capacity of macrophages14, 19. However, whether apoE is acting through a receptor-ligand interaction to drive this process remains unknown. Grainger et al. have shown that the addition of exogenous human apoE3 enhances the phagocytosis of apoptotic thymocytes by primary peritoneal macrophages but does not modulate uptake of fluorescence-labeled latex beads, suggesting that its effects are specific to apoptotic bodies19. Our data show that anti-CD47 therapy does not require apoE to enhance efferocytosis in vivo and in vitro.
ApoE and LRP1 interact to promote clearance of apoptotic bodies, and with CD47 and Sirpα help ensure a homeostatic balance of efferocytosis. Our finding that LRP1 is required for the anti-atherosclerotic effect of anti-CD47 demonstrates the complexity of the efferocytosis rheostat in the atheroma, which relies upon a tightly orchestrated balance of “eat me” and “don’t eat me” signals5, 38–40, and suggests that disruption of other mediators of efferocytosis may trigger counter-effects on the regulation of efferocytosis by anti-CD47 therapy. Our data show that targeted therapies to promote efferocytosis must consider the entire milieu of pro-efferocytosis components. Finally, while the role of macrophage derived LRP1 during atherosclerosis progression is clearly related to effective clearance of apoptotic cells, we have recently reported that its loss actually promotes plaque regression, suggesting that impaired efferocytosis has no critical role in plaque regression31. On the other hand, a recent study by Yurdagul et al. suggests that disruption of efferocytosis does impair plaque regression41, and thus studies are needed to clarify the role of efferocytosis in the regressing plaque.
Supplementary Material
Highlights.
CD47 is a strong “don’t eat me” signal whose blockade increases in vivo efferocytosis and limits atherosclerosis.
Macrophage LRP1 is required for CD47 blockade to limit atherogenesis.
CD47 and LRP1 likely cooperate in the arterial plaque to control efferocytosis.
These two targets can be exploited for pharmaceutical targeting to protect against atherosclerosis development.
Acknowledgements
Graphical abstract was created with BioRender.com
Sources of Funding
The authors acknowledge the National Institutes of Health (R01 HL057986 to Sergio Fazio, R35 HL144475 to Nicholas Leeper and R01 HL132985 to Nathalie Pamir) and the NRSA for support (T32 HL094294 to Paul Mueller).
Abbreviations
- TNFα
Tumor necrosis factor alpha
- LRP1
Low-density lipoprotein receptor-related protein 1
- anti-CD47
Anti-CD47 antibody
- DKO
apoE−/−/macrophage-specific LRP1−/−
- LDL
Low-density Lipoprotein
- LDLR
LDL receptor
- ApoE
apolipoprotein E
- LPS
Lipopolysaccharide
- IFNγ
Interferon gamma
Footnotes
Disclosures
NJL is a co-founder and equity interest holder in Bitterroot Bio, Inc. SF is currently Chair for CV/Metabolism in the Scientific Council at Regeneron Pharmaceuticals. HT is currently an employee of Sanofi-Aventis, but his contributions to this manuscript were during his tenure at Oregon Health & Science University.
References
- 1.Kinchen JM and Ravichandran KS. Phagocytic signaling: you can touch, but you can’t eat. Curr Biol 2008;18:R521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayner KJ. Cell Death in the Vessel Wall: The Good, the Bad, the Ugly. Arterioscler Thromb Vasc Biol 2017;37:e75–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kojima Y, Weissman IL and Leeper NJ. The Role of Efferocytosis in Atherosclerosis. Circulation 2017;135:476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yurdagul A Jr, Doran AC, Cai B, Fredman G and Tabas IA. Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis. Front Cardiovasc Med 2017;4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, Spite M, Fredman G and Tabas I. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. The Journal of clinical investigation 2017;127:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojima Y, Volkmer JP, McKenna K, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016;536:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seimon T and Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. Journal of lipid research 2009;50 Suppl:S382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galis ZS, Sukhova GK, Lark MW and Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. The Journal of clinical investigation 1994;94:2493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle JJ, Wilson B, Bicknell R, Harrower S, Weissberg PL and Fan TP. Expression of angiogenic factor thymidine phosphorylase and angiogenesis in human atherosclerosis. The Journal of pathology. 2000;192:234–42. [DOI] [PubMed] [Google Scholar]
- 10.Kockx MM and Herman AG. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovascular research 2000;45:736–46. [DOI] [PubMed] [Google Scholar]
- 11.Mallat Z, Hugel B, Ohan J, Lesèche G, Freyssinet JM and Tedgui A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation 1999;99:348–53. [DOI] [PubMed] [Google Scholar]
- 12.Greeno EW, Bach RR and Moldow CF. Apoptosis is associated with increased cell surface tissue factor procoagulant activity. Laboratory investigation; a journal of technical methods and pathology 1996;75:281–9. [PubMed] [Google Scholar]
- 13.Lillis AP, Van Duyn LB, Murphy-Ullrich JE and Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev 2008;88:887–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yancey PG, Blakemore J, Ding L, Fan D, Overton CD, Zhang Y, Linton MF and Fazio S. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler Thromb Vasc Biol. 2010;30:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramanian M, Hayes CD, Thome JJ, Thorp E, Matsushima GK, Herz J, Farber DL, Liu K, Lakshmana M and Tabas I. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and antigen cross-presentation in vivo. The Journal of clinical investigation 2014;124:1296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overton CD, Yancey PG, Major AS, Linton MF and Fazio S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res 2007;100:670–7. [DOI] [PubMed] [Google Scholar]
- 17.Yancey PG, Ding Y, Fan D, Blakemore JL, Zhang Y, Ding L, Zhang J, Linton MF and Fazio S. Low-density lipoprotein receptor-related protein 1 prevents early atherosclerosis by limiting lesional apoptosis and inflammatory Ly-6Chigh monocytosis: evidence that the effects are not apolipoprotein E dependent. Circulation 2011;124:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly S, Roake W, Brown S, Young P, Naik H, Wordsworth P, Isenberg DA, Reid KB and Eggleton P. Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum 2006;54:1543–56. [DOI] [PubMed] [Google Scholar]
- 19.Grainger DJ, Reckless J and McKilligin E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. Journal of immunology (Baltimore, Md : 1950) 2004;173:6366–75. [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N and Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009;138:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012;109:6662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betancur PA, Abraham BJ, Yiu YY, et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun 2017;8:14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Giunzioni I, Tavori H, Covarrubias R, Ding L, Zhang Y, Ormseth M, Major AS, Stafford JM, Linton MF and Fazio S. Loss of Macrophage Low-Density Lipoprotein Receptor-Related Protein 1 Confers Resistance to the Antiatherogenic Effects of Tumor Necrosis Factor-alpha Inhibition. Arterioscler Thromb Vasc Biol 2016;36:1483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daugherty A, Tall AR, Daemen M, Falk E, Fisher EA, García-Cardeña G, Lusis AJ, Owens AP 3rd, Rosenfeld ME and Virmani R. Recommendation on Design, Execution, and Reporting of Animal Atherosclerosis Studies: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol 2017;37:e131–e157. [DOI] [PubMed] [Google Scholar]
- 25.Bisgaard LS, Mogensen CK, Rosendahl A, Cucak H, Nielsen LB, Rasmussen SE and Pedersen TX. Bone marrow-derived and peritoneal macrophages have different inflammatory response to oxLDL and M1/M2 marker expression - implications for atherosclerosis research. Scientific reports 2016;6:35234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlou S, Wang L, Xu H and Chen M. Higher phagocytic activity of thioglycollate-elicited peritoneal macrophages is related to metabolic status of the cells. J Inflamm (Lond) 2017;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed M, Luissint AC, Azcutia V, Fan S, O’Leary MN, Quiros M, Brazil J, Nusrat A and Parkos CA. Epithelial CD47 is critical for mucosal repair in the murine intestine in vivo. Nat Commun 2019;10:5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG and Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47). J Cell Sci 1995;108 ( Pt 11):3419–25. [DOI] [PubMed] [Google Scholar]
- 29.Flores AM, Hosseini-Nassab N, Jarr KU, et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nature nanotechnology 2020;15:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Libby P, Tabas I, Fredman G and Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res 2014;114:1867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A and Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PloS one 2010;5:e8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore KJ, Sheedy FJ and Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013;13:709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams JW, Zaitsev K, Kim KW, et al. Limited proliferation capacity of aortic intima resident macrophages requires monocyte recruitment for atherosclerotic plaque progression. Nat Immunol 2020;21:1194–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bown MJ, Jones GT, Harrison SC, et al. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am J Hum Genet 2011;89:619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb TR, Erdmann J, Stirrups KE, et al. Systematic Evaluation of Pleiotropy Identifies 6 Further Loci Associated With Coronary Artery Disease. Journal of the American College of Cardiology 2017;69:823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller PA, Zhu L, Tavori H, Huynh K, Giunzioni I, Stafford JM, Linton MF and Fazio S. Deletion of Macrophage Low-Density Lipoprotein Receptor-Related Protein 1 (LRP1) Accelerates Atherosclerosis Regression and Increases C-C Chemokine Receptor Type 7 (CCR7) Expression in Plaque Macrophages. Circulation 2018;138:1850–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xian X, Ding Y, Dieckmann M, Zhou L, Plattner F, Liu M, Parks JS, Hammer RE, Boucher P, Tsai S and Herz J. LRP1 integrates murine macrophage cholesterol homeostasis and inflammatory responses in atherosclerosis. eLife 2017;6:e29292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao H, Yancey PG, Babaev VR, Blakemore JL, Zhang Y, Ding L, Fazio S and Linton MF. Macrophage SR-BI mediates efferocytosis via Src/PI3K/Rac1 signaling and reduces atherosclerotic lesion necrosis. Journal of lipid research 2015;56:1449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foks AC, Engelbertsen D, Kuperwaser F, Alberts-Grill N, Gonen A, Witztum JL, Lederer J, Jarolim P, DeKruyff RH, Freeman GJ and Lichtman AH. Blockade of Tim-1 and Tim-4 Enhances Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol 2016;36:456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kojima Y, Downing K, Kundu R, Miller C, Dewey F, Lancero H, Raaz U, Perisic L, Hedin U, Schadt E, Maegdefessel L, Quertermous T and Leeper NJ. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. The Journal of clinical investigation 2014;124:1083–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yurdagul A Jr., Subramanian M, Wang X, et al. Macrophage Metabolism of Apoptotic Cell-Derived Arginine Promotes Continual Efferocytosis and Resolution of Injury. Cell metabolism 2020;31:518–533.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


