Abstract
In people with HIV (PWH) on antiretroviral therapy (ART), immune dysfunction persists, including elevated expression of immune checkpoint (IC) proteins on total and HIV-specific T-cells. Reversing immune exhaustion is one strategy to enhance the elimination of HIV-infected cells that persist in PWH on ART. We aimed to evaluate whether blocking cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), T cell immunoglobulin domain and mucin domain 3 (TIM-3), T cell immunoglobulin and ITIM domain (TIGIT) and lymphocyte activation gene-3 (LAG-3) alone or in combination would enhance HIV-specific CD4+ and CD8+ T cell function ex vivo. Intracellular cytokine staining was performed using human peripheral blood mononuclear cells (PBMCs) from PWH on ART (n=11) and expression of CD107a, IFNγ, TNFα and IL-2 quantified with HIV peptides and antibodies to IC. We found that i) IC blockade enhanced the induction of CD107a and IL-2, but not IFNγ and TNFα, in response to Gag and Nef peptides, ii) the induction of CD107a and IL-2 was greatest with multiple combinations of two antibodies, and iii) antibodies to LAG-3, CTLA-4 and TIGIT in combinations showed synergistic induction of IL-2 in HIV-specific CD8+ and CD107a and IL-2 production in HIV-specific CD4+ and CD8+ T cells. These results demonstrate that the combination of antibodies to LAG-3, CTLA-4 or TIGIT can increase the frequency of cells expressing CD107a and IL-2 that associated with cytotoxicity and survival of HIV-specific CD4+ and CD8+ T cells in PWH on ART. These combinations should be further explored for an HIV cure.
INTRODUCTION
Antiretroviral therapy (ART) controls HIV replication efficiently allowing for immune recovery and a normal life expectancy in people with HIV (PWH), however virus persists indefinitely in a latent form, meaning that treatment is required lifelong (1, 2). Immune dysfunctions can also persist in PWH on ART, with evidence of increased microbial translocation, innate immune and T cell activation as well as T cell exhaustion [reviewed in (1, 2)] characterised by the upregulation of inhibitory immune checkpoint (IC) markers (3–5). To eliminate long-lived latently infected cells in PWH on ART, multiple approaches are being evaluated including strategies to enhance HIV-specific T cell function to increase clearance of infected cells, reduce the size of the reservoir as well as maintain long term control of virus replication (1). Here, we evaluated the ability of antibodies to multiple IC used either alone or in combination, to reinvigorate HIV-specific CD4+ and CD8+ T cell function.
Multiple IC markers, including cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), T cell immunoglobulin domain and mucin domain 3 (TIM-3), T cell immunoglobulin and ITIM domain (TIGIT) and lymphocyte activation gene-3 (LAG-3), remain elevated on the surface of total and HIV-specific CD4+ and CD8+ T cells in PWH on ART, compared to uninfected individuals (4–11). Blockade of each of these IC with antibodies has been previously investigated, using different ways to quantify recovery of immune function. For example, in HIV-specific CD4+ T cells, there was improved production of IFNγ and IL-2 as well as T cell proliferation following administration of antibodies to either PD-1 or CTLA-4 ex vivo (6, 12). HIV-specific CD8+ T cells also showed increased proliferation and higher expression of IFNγ and TNF⍺ ex vivo following administration of antibodies to PD-1 or PD-L1 ex vivo (13, 14). Similarly, recombinant LAG-3-Fc that competes with the binding of native membrane-bound LAG-3 to MHC-II has been shown to enhance the production of TNF⍺ and IL-2 in HIV-specific CD4+ and CD8+ T cells ex vivo (11). The relationship of these ex vivo findings to effects in vivo remain to be determined.
There is far more limited information available of the effects of IC blockade in PWH on ART in vivo. One small prospective dose finding clinical trial of anti-PD-L1 in PWH on ART without cancer using an ELISPOT assay to demonstrate enhancement of HIV-specific T cell function in two of six anti-PD-L1 recipients (15). Other small case series or case reports in PWH on ART with concomitant malignancy have similarly shown that an increase in HIV-specific CD4+ and CD8+ T cells in some but not all participants following anti-PD-1 treatment (16, 17). There are currently seven clinical trials of anti-PD1 enrolling PWH on ART, including three studies enrolling people without cancer (clinicaltrials.gov), and one study evaluating the impact of anti-PD1 and anti-CTLA-4 (18).
Beyond the blockade of single IC markers, there is increasing evidence that the clinical impact of IC blockade can be enhanced when used in combination. The best evidence has been in individuals with metastatic malignant melanoma, where there was enhanced efficacy and long-term survival with the use of anti-PD-1 together with anti-CTLA-4 compared to either antibody alone, however this has also been associated with an increased risk of toxicity (19, 20). Combinations of other antibodies to IC are in pre-clinical and clinical development (21) with clinical trials investigating anti-PD-1 in combination with anti-TIGIT or anti-LAG-3 currently underway.
In HIV infection, combination IC blockade could also potentially have an enhanced effect on restoring HIV-specific T cell function as multiple inhibitory IC are expressed on HIV-specific CD4+ (6, 11, 22, 23) and CD8+ (6, 10, 11, 13, 22, 23) T cells. Ex vivo, some combinations of IC antibodies compared to either antibody alone have shown enhanced proliferation and cytokine production of HIV-specific CD8+ T cells, including the combination of anti-PD-L1 with anti-TIGIT (13), or anti-PD-1 with anti-TIM-3, anti-BTLA or anti-CD160 (14). Whether there are additive or synergistic effects on HIV-specific T cells function following the inhibition of multiple other IC in blood from PWH on ART has not been systematically explored.
We hypothesised that combination blockade of multiple ICs compared to blockade with a single antibody would lead to an increase in cytokine-producing in HIV-specific T cells in both CD4+ and CD8+ T cells obtained from PWH on ART. To address this, we quantified the effects of monoclonal antibodies to six ICs (CTLA-4, PD-1, PD-L1, TIM-3, TIGIT and LAG-3) alone or in combination to determine the effect on the frequency of HIV-specific T cells that express CD107a, IFNγ, TNFα and IL-2 in response to HIV peptide stimulation. Similar to previous reports (24, 25), we found that in PWH on ART, there was a robust expression of IFNγ and TNFα in HIV-specific CD4+ and CD8+ T cells in the absence of antibodies to IC. However, we only observed modest enhanced expression of IFNγ and TNFα following anti-PD-1 blockade alone, with no additive effects when multiple antibodies were used in combination. In contrast, the production of IL-2 and the expression of surface CD107a in both CD4+ and CD8+ HIV-specific T cells was infrequent in the absence of IC blockade upon antigenic stimulation but was markedly enhanced with specific combinations of antibodies. These data demonstrate that significant functional impairment in IL-2 production and CD107a expression in PWH persists on ART and this can be overcome with combination IC blockade.
METHODS
Study population
Eleven PWH on combination ART were enrolled at the Alfred hospital, Melbourne, Australia with the inclusion criteria of plasma HIV RNA less than 50 copies/mL for at least three years. Clinical details are summarised in Table I. The participants were all male with suppressed viral load for at least 3 years and at the time of sample collection had plasma HIV RNA < 20 copies/mL. Leukapheresis samples were collected from HIV-1 infected individuals at the Alfred Hospital with informed consent and under institutional guidelines. The study was approved by Human Research Ethics Committees at The Alfred and Avenue Hospitals in Melbourne and the University of Melbourne Ethics Committee.
Table I:
Clinical characteristics of the study population.
| ID | Age (years) | Gender | Ethnicity | CD4+ (cells/uL) | CD4+ (%) | CD8+ (cells/uL) | CD8+ (%) | CD4:CD8 ratio | Nadir CD4+ (cells/uL) | ART regimen | Peak Viral Load (copies/mL) | Duration Viral Load <50 (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRA001 | 64 | Male | Caucasian | 403 | 24 | 1061 | 63 | 0.380 | 10 | ATV, TDF/FTC | 148,430 | 14.1 |
| PRA002 | 48 | Male | Caucasian | 1460 | 47 | 793 | 26 | 1.841 | 698 | ABC/3TC, EFV | N/A | N/A |
| PRA003 | 49 | Male | Caucasian | 833 | 31 | 767 | 29 | 1.086 | 218 | TDF/FTC, DRV, RTV | 78,300 | 11.5 |
| PRA004 | 55 | Male | Caucasian | 1036 | 40 | 1069 | 42 | 0.969 | 266 | TAF/FTC, DTG | 100,000 | 11.1 |
| PRA005 | 49 | Male | Caucasian | 388 | 28 | 717 | 51 | 0.541 | 168 | TAF/FTC, MVC | 147,000 | 12.0 |
| PRA006 | 48 | Male | Caucasian | 864 | 38 | 864 | 39 | 1.000 | 538 | EVG/TAF/FTC/COBI | 118,800 | 6.1 |
| PRA007 | 47 | Male | Caucasian | 705 | 32 | 1034 | 47 | 0.682 | 122 | DRV/COBI, TAF/FTC | 548,000 | 6.5 |
| PRA008 | 38 | Male | Other (PNG) | 281 | 25 | 328 | 30 | 0.857 | 168 | EVG/TAF/FTC/COBI | 63,300 | 8.7 |
| PRA009 | 49 | Male | Caucasian | 474 | 25 | 1085 | 56 | 0.437 | 42 | EVG/TAF/FTC/COBI | 211,930 | 7.0 |
| PRA010 | 48 | Male | Caucasian | 484 | 28 | 895 | 52 | 0.541 | 411 | TAF, FTC, RPV | N/A | N/A |
| PRA011 | 53 | Male | Caucasian | 735 | 37 | 810 | 41 | 0.907 | 300 | ABC/3TC, EFV | 365,000 | 11.2 |
| Median (IQR) | 49 (48 – 51) | N/A | N/A | 705 (439 – 849) | 31 (27 – 38) | 864 (780 – 1048) | 42 (35 – 52) | 0.857 (0.541– 0.985) | 218 (145 – 356) | N/A | 147,000 (100,000 – 211,930) | 11.1 (7 – 11.5)1 |
3TC, Lamivudine; ABC, Abacavir; ATV, Atazanavir; COBI, Cobicistat; DRV, Darunavir; DTG, Dolutegravir; EFV, Efavirenz; EVG, Elvitegravir; FTC, Emtricitabine; MVC, Maraviroc; RPV, Rilpivirine; RTV, Ritonavir; TAF, Tenofovir Alafenamide; TDF, Tenofovir Disoproxil Fumarate.
Peptide preparation
Overlapping 15 mer peptides for HIV-clade B Gag (#8117) and Nef (#5189) as well as 8–11 mer peptides to Cytomegalovirus, Epstein Barr Virus and Influenza (CEF) (#9808) were obtained from the National Institute of Health AIDS Reagent Program. Dimethyl sulfoxide (DMSO) was used to reconstitute peptides to 500 μg/mL/peptide as the working concentration. The final concentration for peptide stimulation was 2 μg/mL/peptide.
Blocking and isotype antibodies
Antibodies to ICs including IgG1 antibodies (BMS-734016 CTLA-4 Ipilimumab, BMS-986207 TIGIT, BMS-986258 TIM-3) and IgG4 (BMS-936558 PD-1 Nivolumab, BMS-936559 PD-L1 or BMS-986016 LAG-3) and the relevant isotype controls (IgG1: DT1D12-g1f-N297Q; clone 1182_RAS_Ab; or IgG4 g4P-DT1D12; clone 4A09_RAS_Ab) were a kind gift from Bristol-Myers Squibb (BMS). Other isotype antibodies evaluated included anti-β-Gal (Cat # bgal-mab1 & bgal-mab114, Invivogen), tumour-antigen (kind gift from Prof. Andrew Scott, Olivia Newton-John Cancer Research Institute, Australia) and an unknown antigen from BioLegend (Clone QA16A12 & QA16A15, BioLegend). Antibodies to each IC were used as 10 μg/mL, consistent to studies using therapeutic antibodies to PD-1/PD-L1 (26, 27) and CTLA-4 (28).
Intracellular cytokine assay
Peripheral blood mononuclear cells (PBMC) were isolated by leukapheresis and cryopreserved. Upon thawing the cryopreserved PBMC in warm RF-10 (RPMI 1640 with 10% fetal bovine serum), PBMC were adjusted in RF10 to 2 × 106 cells/mL in tissue culture flasks and incubated at 37 °C and 5% CO2 overnight. Rested PBMC were washed and adjusted to 1 × 107 viable cells/mL.
Stimulation of PBMC was performed in 200 μL reactions in a 96-well plate for 6 hours at 37°C with 5% CO2. Each well contained 1 × 106 PBMC, a cytokine secretion inhibitor cocktail (5 μg/mL Brefeldin A [B7651, Sigma] and 5 μg/mL Monensin [M5273, Sigma]), anti-CD107a (clone H4A3) and antiretrovirals (18 μM azidothymidine, 10 μM efavirenz and 20 μM raltegravir) to inhibit further rounds of viral replication following stimulation ex vivo. The cells were stimulated with either 0.4 % DMSO, 2 μg/mL Gag, Nef or a mix of Cytomegalovirus, Epstein Barr Virus and Influenza (CEF) peptides (Cat #8117, #5189 and #9808, NIH AIDS Reagent Program) or 1 μg/mL staphylococcal enterotoxin B (SEB;S4881, Sigma]). Blocking antibodies to CTLA-4, TIGIT, TIM-3, PD-1, PD-L1 and LAG-3 were added at 10 μg/mL each in various combinations and the equivalent total concentration of isotype antibodies were used as a control.
After stimulation, cells were washed in wash buffer (1% FBS and 1 mM EDTA in PBS) and stained for the live/dead marker (Cat # L34957, Invitrogen) and with antibodies to the following surface markers (CD4 [Clone RPA-T4], CD14 [Clone M5E2], CD19 [Clone HIB19], CD45RA [Clone HI100] and CCR7 [Clone 3D12]) at ambient temperature in the dark for 30 minutes. Separately, anti-PD-1 PE [Clone EH12.1] was added to the same surface staining mix in a separate well to assess PD-1 expression for the given donor. After cell fixation and permeabilisation, cells were washed in perm/wash buffer (Cat # 554714, BD Bioscience). Staining with antibodies to CD3 [Cloe UCHT1], CD8 [Clone RPA-T8], IFNγ [Clone B27], TNFα [Clone MAb11] and IL-2 [Clone MQ1–17H12] was performed at ambient temperature in the dark for 30 minutes. For wells with anti-PD-1, IL-2 was excluded from the post-permeabilisation staining mix as both were conjugated with the same fluorochrome.
After washing cells with perm/wash buffer twice, cells were fixed in 100 μL of 1% formaldehyde at ambient temperature in the dark for 15 minutes. All staining antibodies were obtained from BD Bioscience unless indicated otherwise. Within 2 hours, the LSRFortessa cytometer (BD Bioscience) was used to acquire between 210,000 and 300,000 lymphocyte events. Anti-mouse and anti-rat compensation beads (Cat # 552843 & 552844, BD Bioscience) were used for compensation. The sequential gating strategy (Supplemental Fig. 1) on the cytometric data were performed and quantified in FlowJo 9.9.6.
Statistical analyses
The fold change in percentage of cells expressing a specific cytokine following incubation with one (single), two (duo) or all six (cocktail) antibodies to ICs was assessed relative to isotype control. Normal distribution of the fold change in cytokine positive cells was evaluated by the Shapiro-Wilk test. The Wilcoxon Signed-Rank test was used to compare the percentage of cells expressing a specific cytokine following incubation with IC antibodies (alone or in combination) or appropriate IgG isotype controls. The effect size for the fold change increase of ICB relative to isotype control was calculated by post-hoc analyses using a sample size of 11, 80% power and a significance level at 0.05 for two-tailed Wilcoxon signed-ranked tests in G*Power 3.1 (29). Synergism of two antibodies was evaluated by Bliss Independent models as previously described (30). Briefly, the experimentally observed effects of a given antibody (ICB) relative to maximal stimulation with SEB was calculated as (ICB - IgG isotype) / (SEB - DMSO), where all parameters denote the frequency of cytokine-producing cells in response to HIV peptide stimulation. This was repeated for all examined cellular subsets and cytokines. To determine synergism we calculated the predicted effect of multiple antibodies, e.g. blocking antibodies to IC1 (ICB1) and IC2 (ICB2), using the formula: ICB1(observed) + ICB2 (observed) - ICB1(observed) × ICB2(observed). Wilcoxon Signed-Rank test was used to formally compare the difference between the observed and predicted fractions. If the observed minus predicted effect was > 0 with statistical significance, this indicated synergism. Correlations between the frequency of cytokine-producing cells following ICB and the T cell expression of PD-1 or the CD4:CD8 ratio were assessed using Spearman correlation. RStudio (Version 1.3.1073) and R package ggplot2 (Version 3.3.1) were used for statistical analyses and graphs.
RESULTS
Optimisation of IgG isotype antibodies to reduce non-specific activation
We initially observed that two commercial IgG isotype controls (Clone QA16A12 & QA16A15 from BioLegend) induced cytokine production above DMSO treated controls (Supplemental Fig. 2). To determine whether the higher readouts by the commercial IgG isotype were specific, we titrated the commercial IgG isotype antibodies without any additional stimulus and observed dose-dependent increases in cytokine production in both CD4+ and CD8+ T cells (Supplemental Fig. 2).
To identify a suitable IgG isotype with minimal background, we then quantified the frequency of CD4+ and CD8+ T cells that produced CD107a, IFNγ, TNFα and IL-2 following incubation with three IgGs targeting irrelevant antigens (bacterial beta-galactosidase, tumour-induced and diphtheria). All three IgGs showed lower background than the commercial IgG isotype, but the IgG isotype control that targeted bacterial β-gal and tumour showed dose-dependent stimulation at a concentration of 15 μg/mL or higher in CD8+ T cells in two of 10 donors (D49 and D81). In contrast, the diphtheria-specific IgG did not show dose-dependent stimulation in any of the 10 donors (Supplemental Fig. 2) and was therefore selected as the IgG isotype control for subsequent experiments.
Dual blockade to ICs enhanced production of IL-2 and CD107a in HIV-specific CD4+ and CD8+ T cells
We first evaluated cytokine production in response to HIV peptides, CEF peptides and a maximal stimulus of SEB. Following stimulation with peptides to either Gag or Nef, compared to DMSO, we observed a significant increase in the production of IFNγ and TNFα, but no increase in IL-2 in both CD4+ and CD8+ T cells, with CD45RA- CCR7- effector CD4+ and CD8+ T cells (Tem) as the main subset showing a response (Supplemental Fig. 3). Both Gag and Nef peptides led to an increase in expression of CD107a in CD8+ Tem but not in CD4+ T cells (Supplemental Fig. 3). All responses to HIV peptides were significantly lower than responses to either SEB on both CD4+ and CD8+ T cells or CEF peptides on CD4+ T cells (Supplemental Fig. 3). Together these findings demonstrated a low frequency of HIV-specific CD4+ and CD8+ T cells on ART with a far lower frequency of HIV-specific CD4+ and CD8+ T cells that produce IL-2, compared to the expression of surface CD107a, than IFNγ or TNFα.
We next compared the effects of IC antibodies, either alone or in combination, on the function of T cells in response to either gag or nef peptide stimulation looking at both the absolute frequency of cytokine-expressing cells (Supplemental Fig. 4) and the fold change relative to isotype control (Fig. 1). Surprisingly, most antibodies when administered alone had minimal effect on the frequency of cytokine positive cells (Fig. 1) with the exception of anti-PD-1, which induced a modest but statistically significant increase in IFNγ and TNFα production in CD4+ T cells but had no effect in CD8+ T cells (Fig. 1). The cocktail of six antibodies showed increased frequency of CD107a, IFNγ and TNFα in Gag-specific CD4+ T cells and CD8+ T cells with the exception of IFNγ in CD4+ T cells (green bars, Fig. 1A). Interestingly, the cocktail of antibodies did not induce a greater magnitude fold change than the use of two antibodies (green vs red-single/blue-dual bars, Fig. 1). The most dramatic effect of IC antibodies were seen in the frequency of IL-2+ HIV-specific T cells in response to multiple combinations of antibodies, predominantly those that included either anti-CTLA-4, anti-TIGIT or anti-LAG3. These effects were observed in total CD4+ and CD8+ T cells (Fig. 1) as well as multiple T cell subsets (Fig. 2). Similar patterns of IL-2 expression were seen following incubation with either Gag (Fig. 1A) or Nef (Fig. 1B) peptides. Combinations of two IC antibodies also increased expression of CD107a in CD4+ T cells. We observed that combinations that included anti-LAG-3 and anti-CTLA-4 showed the greatest fold change in the frequency of CD4+and CD8+ T cells expressing CD107a and IL-2 (blue bars, Fig. 1). Interestingly, apart from CD107a production in CD4+ T cells, we found no increased activity with combinations that included anti-PD-1 (Fig. 1).
Figure 1: Fold change in the frequency of cytokine+ T cells in response to HIV peptides in the presence of antibodies to immune checkpoints (ICs) relative to isotype control.
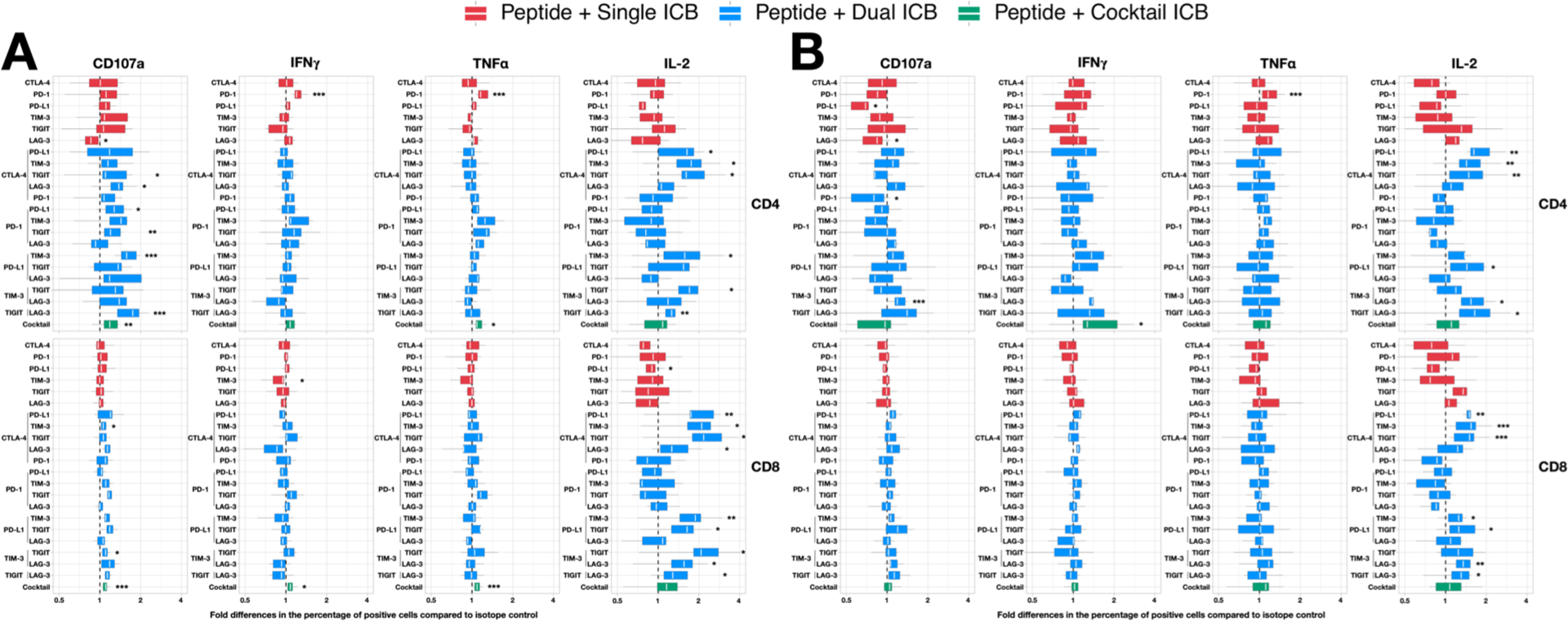
Total CD4+ and CD8+ T-cells collected from PWH on ART were incubated with antibodies to ICs either alone (red), as dual combinations (blue) or a cocktail of six antibodies (green) following incubation with overlapping peptides to either (A) gag or (B) nef and the frequency of cells expressing CD107a, IFNγ, TNFα and IL-2 quantified by flow cytometry. The fold change increase in the presence of IC antibodies relative to isotype control is shown. Data are summarised with box plots indicating the median and inter-quartile range for the 9 participants. Asterisks indicate the significant differences between the specific antibody combination and the respective IgG isotype control(s). Statistical significance was determined by Wilcoxon Signed-Rank tests. * p < 0.05, ** p < 0.01, *** p < 0.005
Figure 2: The fold change in the frequency of cytokine+ T cell subsets in response to HIV peptides in the presence of antibodies to immune checkpoints (ICs) relative to isotype control.
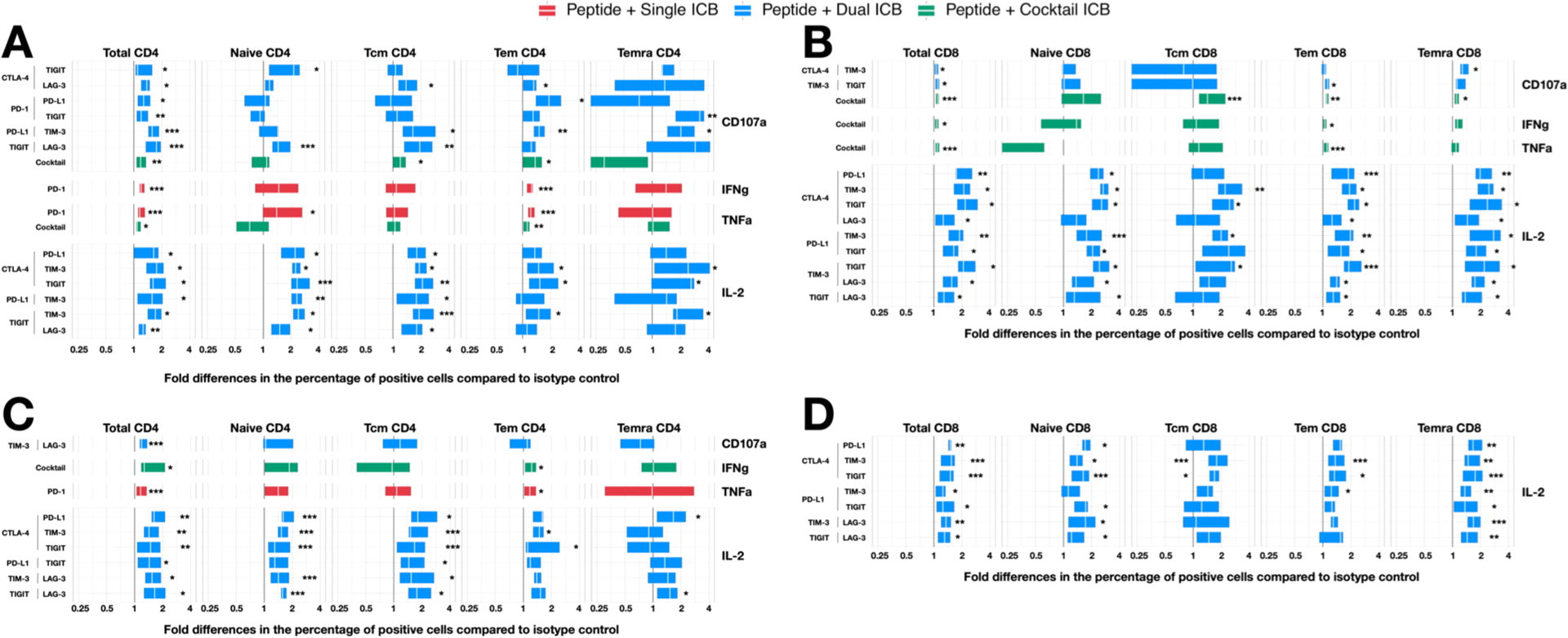
CD4+ and CD8+ T-cell subsets collected from PWH on ART were incubated with antibodies to ICs either alone (red), as dual combinations (blue) or a cocktail of six antibodies (green) following incubation with overlapping peptides to either gag or nef and the frequency of cells expressing CD107a, IFNγ, TNFα and IL-2 quantified. The fold change relative to isotype control for some combinations of IC antibodies is shown for (A) Gag-stimulated CD4+, (B) Gag-stimulated CD8+, (C) Nef-stimulated CD4+ and (D) Nef-stimulated CD8+ T-cell subsets. Only IC antibodies alone or in combination, that have a statistically significant effect on the fold change production of cytokine relative to isotype control are shown. Data is summarised with box plots indicating the median and inter-quartile range for the 9 participants. Asterisks indicate the significant differences between the specific antibody combination and the respective IgG isotype control (s). Statistical significance was determined by Wilcoxon Signed-Rank tests. * p < 0.05, ** p < 0.01, *** p < 0.005
Synergistic activity of some antibody combinations in enhancing production of CD107a and IL-2
We observed a significant increase in the frequency of HIV-specific CD4+ and CD8+ T cells that produced either IL-2 or CD107a following incubation with various combinations of two IC antibodies. To determine if the effects observed were additive or synergistic, we next used Bliss Independence modelling was used as previous described (30) to obtain the predicted readouts for every donor for each cytokine. Paired analyses were used to determine the significant differences between the experimentally observed and predicted readouts.
For expression of CD107a, we observed that antibodies to LAG-3, when combined with antibodies to either CTLA-4, TIGIT or TIM-3, resulted in a significant synergistic response in CD4+ T cells (Fig. 3A). For production of IL-2, the combination of antibody to CTLA-4 with all other antibodies (except anti-PD-1 but including anti-PD-L1) led to a significant synergistic response in both CD4+ and CD8+ T cells (Fig. 3B). Interestingly, none of the synergistic combinations of antibodies to ICs that enhanced CD107a or IL-2 responses included anti-PD-1.
Figure 3: Synergistic effects of antibodies to immune checkpoints. Intracellular cytokine staining for the frequency of T cells that produced CD107a, IFNγ, TNFα and IL-2 in response to Gag and Nef peptides in the presence of antibodies to immune checkpoints (ICs) either alone or in combination was compared to staphylococcal enterotoxin B (SEB).
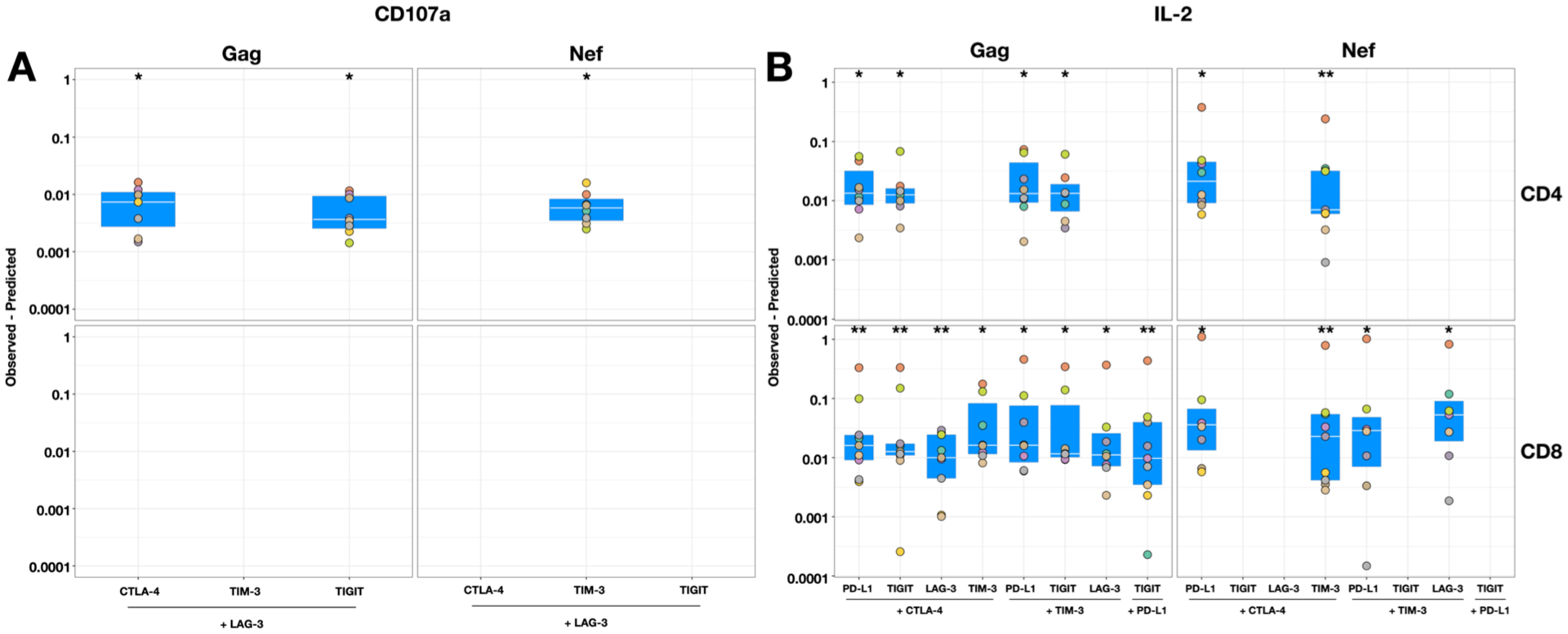
The Bliss independence model was used to calculate the difference between the predicted (response to each antibody alone) and observed fractional responses of combinations of antibodies to ICs relative to SEB. A calculated number of predicted – observed response > 0 demonstrated synergism for (A) the expression of CD107a or (B) the production of IL-2 in CD4+ (upper) and CD8+ (lower) T cells for the various combinations shown. Data are summarised with box plots indicating the median and inter-quartile range for the 9 participants. Wilcoxon Signed-Rank tests were used to determine the statistical differences between the predicted and the experimentally observed effect for a given combination. * p < 0.05, ** p < 0.005
Next, we calculated the magnitude of effect of IC antibodies relative to isotype controls with regard to CD107a and IL-2 production and the presence of synergism as determined by the Bliss Independence model (Fig. 4). This revealed that all three synergistic combinations for CD107a responses in Gag or Nef-stimulated CD4+ T cells included anti-LAG-3, of which anti-LAG-3 with anti-TIGIT showed the greatest fold change increase above IgG isotype controls (1.76-fold) (Fig. 3A). For IL-2 responses, combinations that included anti-CTLA-4 generally resulted in the greatest fold change relative to isotype controls, especially in Gag-stimulated CD8+ T cells (Fig. 3B).
Figure 4: The magnitude and synergism of antibodies to immune checkpoints (IC) used in combination to enhance the frequency of HIV-specific T-cells producing either CD107a or IL-2.
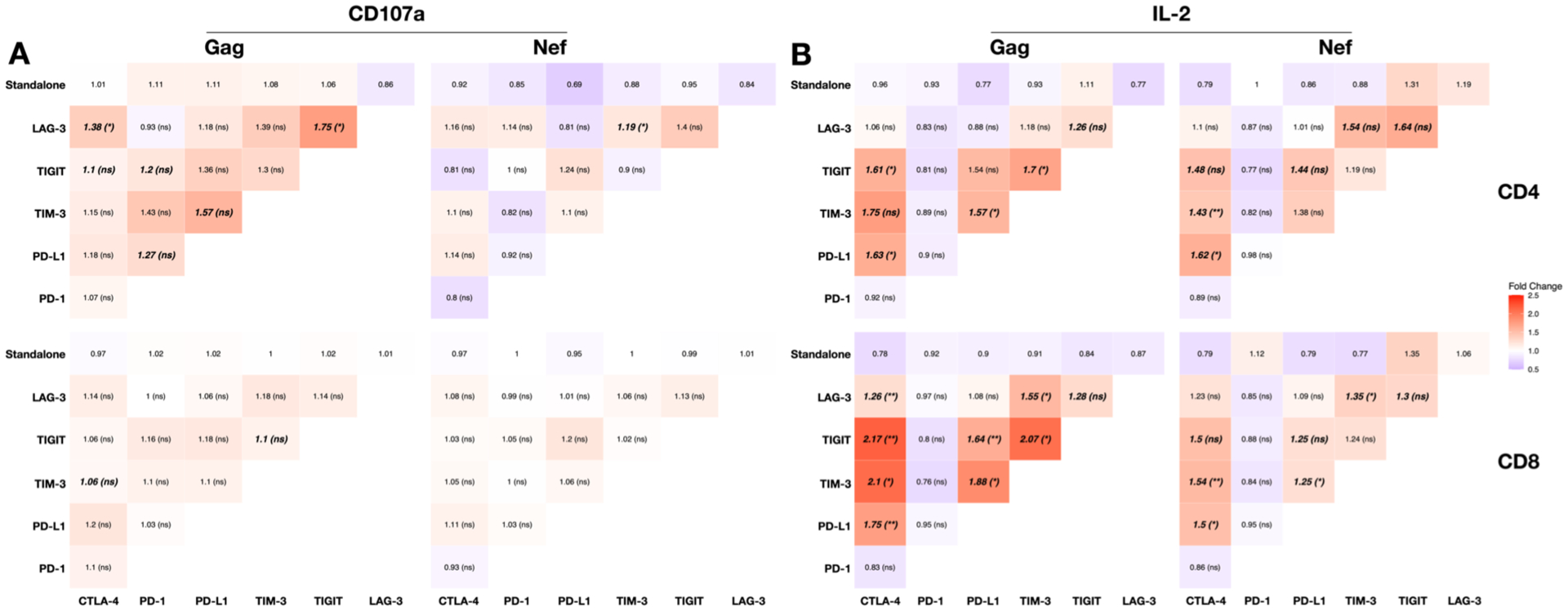
Heat maps showing the magnitude of the fold change of number of T cells expressing (A) CD107a and (B) IL-2 following stimulation with either Gag (left panels) or Nef (right panels) peptides in CD4+ (upper) and CD8+ (lower) T cells following stimulation with one or two IC antibodies relative to isotype controls. Numbers indicate the magnitude of the fold change with one antibody (top row) or two antibodies to ICs compared to IgG isotype control. The bold numbers indicate the fold changes that were significantly higher than isotype control. The asterisks represent the statistical significance for the Bliss independence tests for the specific antibody combinations. * p < 0.05, ** p < 0.005
While the combination of antibodies to CTLA-4 and either TIGIT or TIM-3 resulted in the largest fold change response, anti-CTLA-4 and anti-PD-L1 showed synergism with more breath, as indicated by the response to both Gag or Nef peptides and in CD4+ and CD8+ T cells, but at a lower magnitude fold change than anti-CTLA-4 with either anti-TIGIT or anti-TIM-3 (Fig. 3B).
Intrinsic properties of response to immune checkpoint blockade
We next hypothesised that IC antibody combinations that induced a greater fold increase in the frequency of cytokine positive cells following stimulation with either Gag or Nef peptides (Fig. 1A & B) may be due to certain clinical or cellular factors. Hence, we determined the correlation between the fold change in CD107a and IL-2 responses to combination IC blockade that were significantly higher than isotype (Fig. 1) and five clinical parameters (CD8 counts, CD4:CD8 ratio, expression of surface PD-1 and nadir and current counts of CD4).
The CD8 count was the only clinical parameter that positively correlated with the frequency of IL-2+CD8+ T cells in response to Gag (r = 0.76, p = 0.016) or Nef (r = 0.66, p = 0.04) peptide alone (Fig. 5A). Gag-stimulated CD8+ T cells subsets showed significant positive correlations between CD8 count and IL-2 production with blockade to CTLA-4 + TIM-3 (Total: r = 0.53, p = 0.148; Tcm: r = 0.81, p = 0.022; Temra: r = 0.70, p = 0.043), CTLA-4 + TIGIT (Total: r = 0.63, p = 0.076; Tcm: r = 0.90, p = 0.005; Temra: r = 0.77, p = 0.021) or TIM-3 + TIGIT (Total: r = 0.70, p = 0.043; Temra: r = 0.72, p = 0.037). Interestingly, these three combinations were identified as synergistic in previous analyses (Fig. 3B). IL-2 production in Nef-stimulated total CD8+ T cells in response to blockade to PD-L1 + TIGIT (Total: r = −0.73, p = 0.031; Temra: r = −0.73, p = 0.031) was the only combination that negatively correlated with CD8 count.
Figure 5: Heat maps showing the correlations between clinical parameters and the fold change increase in the frequency of cytokine positive cells in response to Gag and Nef peptides in the presence of antibodies to immune checkpoints.
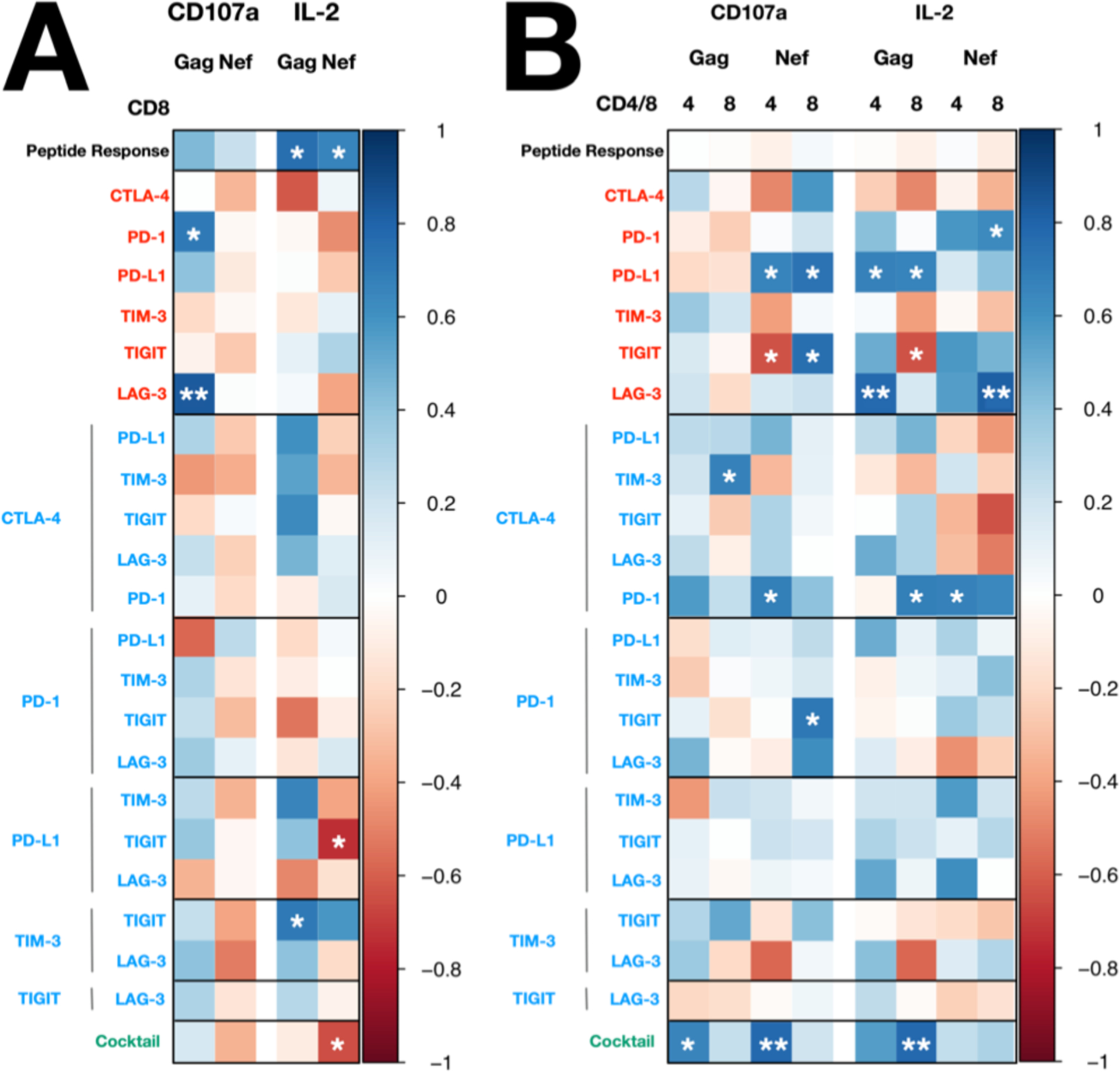
Heatmap illustrating the strength and significance of correlation coefficients between the immune checkpoint blockade response and (A) CD8 count and (B) CD4:CD8 ratio. Statistical significance was determined by Spearman’s rank correlation. Positive and negative correlations are indicated as blue and red, respectively. * p < 0.05, ** p < 0.01
The CD4:CD8 ratio also showed a positive correlation with the frequency of CD107a+ in Gag-stimulated CD4+ and CD8+ T cells with the fold change increase in cells following blockade to six IC (Total CD4+: r = 0.65, p = 0.042; Tcm CD4+: r = 0.83, p = 0.003) and CTLA-4 + TIM-3 (Total CD8+: r = 0.68, p = 0.045), respectively (Fig. 5B).
Finally, we observed no significant correlations between the responses to HIV peptides alone or with IC blockade and i) the expression of surface PD-1 for IC blockade to PD-1/PD-L1, or ii) nadir/current CD4 count in CD4 cells. Interestingly, PD-1 frequency correlated with responses to ICB that did not target PD-1 or PD-L1. While lower frequency of PD-1 showed a tendency of higher CD107a response in Gag-stimulated CD8 with blockade to CTLA-4 + TIM-3 (r = −0.72, p = 0.037), positive correlation between PD-1 frequency and the IL-2 response in Nef-stimulated CD4 with blockade to TIGIT + LAG-3 was observed (r = 0.84, p = 0.01).
DISCUSSION
Antibodies that block immune checkpoints, alone or in combination, can enhance HIV-specific T cell responses ex vivo (13, 14), but synergistic and additive effects, including which combinations provide a superior response, remain unclear. In this study, we determined the effect on HIV specific T cell function of single and combination IC blockade in PBMCs obtained from PWH on ART. While anti-PD-1 showed a modest increase in the frequency of CD4+ T cells expressing IFNγ and TNFα, antibodies to LAG-3, CTLA-4 and TIGIT in different combinations showed synergistic induction of CD107a and IL-2 production in HIV-specific T cells. Collectively, these results suggest that combination blockade involving LAG-3, CTLA-4 or TIGIT can enhance the cytokine production of HIV-specific T cells in PWH on suppressive ART.
Immune checkpoint proteins are co-expressed on different CD4+ and CD8+ T cells subsets, as we have previously shown using PBMC from healthy donors (31). Although the blockade of multiple IC, specifically anti-PD-1 and anti-CTLA-4, for the management of melanoma has shown significant clinical benefit (32), few studies have evaluated combinations of IC blockade on HIV-specific T cell function (13, 14). To systematically assess the effect of combination IC blockade, we first demonstrated that the choice of IgG isotype control could alter the interpretation of whether a given IC antibody was effective in reversing T cell function (Supplemental Fig. 2). High background levels of activation and expression of cytokines in HIV-specific cells could potentially under-estimate the effects of a specific antibody to IC. We overcame this issue using isotype controls that targeted diphtheria. Given our findings, it is important to confirm and use the isotype antibodies with minimal background for studies on the effect of IC blocking antibodies.
Prior reports have shown that in the presence of persistent antigen, as seen in chronic viral infections, exhausted antigen-specific T cells initially lose the capacity to produce IL-2 following by TNFα and IFNγ as well as in some cases degranulation (3, 33–35). Given we detected IFNγ- and TNFα- (but not IL-2 and CD107a) producing cells following stimulation with either Gag or Nef HIV peptides (Fig. 1), our findings are consistent with PWH on ART having partially (not fully) exhausted T cells.
The use of anti-PD-1 and the cocktail of six antibodies to a range of IC markers showed a modest but significant fold increase of HIV-specific T cells expressing IFNγ and TNF⍺. It is possible that the use of cytokine secretion inhibitors for the intracellular cytokine assay that we used here limited the availability of pro-inflammatory cytokines such as IL-12 (36) in the supernatant that are a prerequisite for further induction of IFNγ and TNF⍺ in CD4+ and CD8+ T cells (37–40). However, we observed clear increases in production of both IFNγ and TNF⍺ following stimulation with HIV peptides, CEF peptides and SEB using this same method (Supplemental Fig. 3). Our findings suggest that the production of IFNγ and TNFα in HIV-specific T cells from PWH on ART might already be maximal and unable to be further enhanced. In contrast, we saw minimal production of IL-2 or CD107a in response to HIV peptides in both CD4+ and CD8+ T cells (Supplemental Fig. 3) but production could be significantly enhanced with combinations of antibodies (Fig. 1). Interestingly, the frequency of cytokine positive HIV-specific CD4+ and CD8+ T cells was less following incubation with six compared to two IC antibodies, suggesting that there is a limit to the number of IC blocking antibodies that can be used together. Multiple antibodies used together could inhibit or potentially compete for Fc receptor binding sites to induce an effective antigen-specific response.
To our surprise, the largest fold increase in cytokine production (specifically IL-2 and to a lesser extent CD107a) were not seen with combinations that included anti-PD-1 but instead various combinations of antibodies to CTLA-4, LAG-3, and TIGIT. We saw remarkably similar responses for Nef- and Gag-specific IL-2 producing CD4+ and CD8+ T cells following combination IC blockade. These data suggest that the effects of combination IC blockade extend to T-cells that target either early and late viral gene products, even though the frequency of nef-specific T-cells is highly stable on ART, while gag-specific -cells decay over time (41). There may be several explanations for the additive and synergistic effects with these antibodies. First, there are distinct and different signalling pathways activated following blockade of ICs, for example, antibodies to CTLA-4 and LAG-3 inhibit calcium-independent and calcium-dependent signalling pathways respectively (42–44). Calcium influx, as a result of LAG-3 blockade (42), and protein kinase C (PKC) activation through T cell receptor ligation are required for degranulation in T cells which is indicated by expression of CD107a (45, 46). Therefore, potentially the specific combination of anti-CTLA-4 and anti-LAG-3 can also potentially enhance CD107a expression. Second, induction of IL-2 by combination IC blockade might be explained by enabling multiple transcription factors, such as Activator protein 1 (AP-1), Nuclear factor kappa B (NF-κB) and Nuclear factor of activated T cells (NFAT), all of which are required to simultaneously bind to the IL-2 promoter for IL-2 induction (47). One or more of these IL-2 specific transcription factors are inhibited following engagement of CTLA-4 (43), PD-1/PD-L1 (48), TIM-3 (49, 50), TIGIT (51) and LAG-3 (52). Consistent with our observations, treatment of PBMC from PWH on ART with antibodies to PD-L1 and TIGIT in combination, but not alone, increased IL-2 production in Gag-specific CD4+ and CD8+ T cells (13). Lastly, additive or synergistic effects of using two antibodies to ICs, could also be a result of the distribution of expression of each of the immune checkpoint markers on antigen-specific T cells. Co-expression of the ICs that we evaluated in this study on CD4+ and CD8+ T cells have been reported in PWH off ART (10, 22, 23, 53), on ART (54, 55) or both off and on ART (6, 9, 11, 13, 53).
The most striking observation was the enhanced production of IL-2 in HIV-specific CD4+ and CD8+ T cells as a result of combining antibodies to CTLA-4 with either TIGIT or TIM-3. This strategy could have several beneficial effects on the clearance of latently infected cells in PWH on ART. First, a previous study showed that the production of IL-2 from HIV-specific CD4+ T cells can enhance NK-mediated cytotoxicity (56) potentially leading to enhanced clearance of infected cells. Second, IL-2 production by HIV-specific CD8+ T cells could lead to CD4-independent proliferation and differentiation of HIV-specific CD8+ T cells (57) as seen in HIV long term non-progressors (58) as well as in PWH with a lower viral set point following primary infection (59). Third, any enhanced degranulation in CD4+ T cells from combination IC blockade might enhance the cytotoxicity of CD4+ T cells, although this is considered controversial, which in turn might assist the elimination of MHC-II-restricted HIV-infected macrophages (60). On the other hand, there could be potential counterproductive effects from increased production of IL-2 in HIV-specific CD4+ and CD8+ T cells, such as the expansion of regulatory T cells (Treg) due to their expression of CD25, a high affinity IL-2 receptor (61). Any induction of proliferation of CD4+ T cells could potentially increase the number of infected CD4+ T cells on ART, although this remains to be determined. Further work investigating the effects of the combinations of IC antibodies that enhance IL-2 production on target cell killing and number of latently infected cells is ongoing in our laboratories.
This is the first comprehensive analysis of the effects of combinations of antibodies to IC on HIV-specific T cell function, however there are several limitations in this study. First, we did not use anti-CD28 and anti-CD49d as co-stimulation during the peptide stimulation and IC blockade for the intracellular cytokine assay. Previous work has shown that the effects of IC blockers, including proliferation or the production of IFNγ and TNFα in HIV-specific cells can vary, depending on whether these co-stimulatory antibodies were included (14). We chose to not include these co-stimulatory models because anti-CD28 might enhance CTLA-4 inhibitory signalling due to less competition for CD80 (62). Second, we intentionally limited the stimulation period to 6 hours with the secretion inhibitor added at the beginning. Since other immune cells such as dendritic cells and macrophages express IC (63–66) and respond to blocking antibodies to IC, the addition of secretion inhibitors at the beginning minimised the effect of the secreted cytokines on T cells. This approach allowed us to observe the direct and immediate effects of the tested IC blockade on T cells. A longer duration of stimulation with HIV peptides and IC blockade with anti-PD-L1 (12) has previously been shown to induce a higher frequency of cells producing IFNγ, however the observed effect of IC blockade on T cells might be indirect (56, 67). Third, we only measured protein expression of cytokines using ICS. It is possible that IC blockade induced changes in mRNA but there were downstream blocks to expression of the particular cytokine. Finally, the effect size we could detect differed for each measured cytokine. Effect sizes of 0.2, 0.5 and 0.8 are considered as small, medium and large, respectively. With 11 participants, the effect size (expressed as median [IQR]) for the fold change increase of cytokine+ cells for CD107a was 0.418 [0.295 – 0.608], IFNg was 0.236 [0.095 – 0.353], TNFa was 0.186 [0.125 – 0.295] and IL-2 was 0.644 [0.295 – 0.922]. With statistical significance at 0.05 for two-tailed Wilcoxon signed-rank tests, ICB combinations with no or small biological effect would both showed as p > 0.05. This limitation, however, does not change the conclusion of the study that identified specific dual ICB combinations that significantly increased CD107a and IL-2 responses. It is important to highlight that a major obstacle to the clinical development of using IC blockade for PWH on ART as a cure strategy remains toxicity. Both anti-CTLA4 and to a lesser extent anti-PD1 have been associated with grade 3 or 4 immune related adverse events (19, 20) which would be unacceptable for PWH on ART without cancer as part of a cure strategy, as recently highlighted in work on developing a target product profile for an HIV cure (68). One strategy to reduce toxicity could be using low dose of antibody. For example anti-PD1 administered at one tenth the licensed dose in people with chronic hepatitis B, was recently shown to be safe and have prolonged and high receptor occupancy (69). Multiple early phase studies of anti-TIGIT, anti-LAG3 and other antibodies are now underway alone and in combination and these antibodies may well be associated with lower toxicity [reviewed in (70)]. Either way, significant advances will still be needed to understand, predict and ultimately reduce these toxicities to enable the use of these antibodies in PWH.
In conclusion, we identified multiple additive as well as synergistic combinations of two antibodies that blocked ICs and enhanced the frequency of cytokine producing HIV-specific T cells. The most dramatic increases were observed in the production of IL-2 in HIV-specific CD4+ and CD8+ T cells and to a lesser extent the expression of CD107a in HIV-specific CD4+ T cells. The largest fold increases in the frequency of CD107a or IL-2 positive cells were with the use of two antibodies targeting either CTLA-4, LAG-3 or TIGIT. Surprisingly, none of the antibody combinations that induced enhanced production of cytokines targeted PD-1. Further mechanistic studies are required to determine the effects of antibodies to CTLA-4 with LAG-3 or TIGIT on functional effects of HIV-specific T cells, including proliferation and killing of latently infected cells, as well as potential effects of increased IL-2 on the number of Treg and expansion of latently infected cells. Given the emerging improved safety profiles of antibodies to LAG-3 and TIGIT compared to anti-PD1 or anti-CTLA-4 (71), these antibodies could potentially be attractive for further clinical development for use in HIV cure strategies.
Supplementary Material
KEY POINTS.
IL2 and CD107a expression in HIV-specific T-cells is enhanced with IC blockade
Combinations of two antibodies to LAG-3, CTLA-4 or TIGIT showed greatest activity
CD8 count and CD4:CD8 ratio correlate with magnitude of response to IC antibodies
ACKNOWLEDGEMENT
We thank Alan Korman for the intellectual discussion and helpful suggestions throughout the design and analysis phases of the study and Andrew Scott from Olivia Cancer Research Institute for providing the tumour-specific IgG. We acknowledge the generous provision of immune checkpoint antibodies from Bristo Myers Squibb. We woud like to thank the Infectious Diseases Unit at the Alfred Hospital and the Lewin/Cameron Clinical Research Group at the Peter Doherty Institute for providing the leukapheresis samples.
This work was supported by funds from the American Foundation for AIDS Research (amfAR) Impact Grant (109226-58-RGRL) and the National Institutes of Health Delaney AIDS Research Enterprise to Find a Cure Collaboratory (Grant UM1AI126611-01). S.R.L. is an NHMRC Practitioner Fellow.
REFERENCE
- 1.Pitman MC, Lau JSY, McMahon JH, and Lewin SR. 2018. Barriers and strategies to achieve a cure for HIV. The Lancet HIV 5: e317–e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ndung’u T, McCune JM, and Deeks SG. 2019. Why and where an HIV cure is needed and how it might be achieved. Nature 576: 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ, and Kurachi M. 2015. Molecular and cellular insights into T cell exhaustion. Nature reviews. Immunology 15: 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJR, Klenerman P, Ahmed R, Freeman GJ, and Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443: 350–354. [DOI] [PubMed] [Google Scholar]
- 5.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel M-R, Delwart E, Sepulveda H, Balderas RS, Routy J-P, Haddad EK, and Sékaly R-P. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nature medicine 12: 1198–1202. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, and Walker BD. 2007. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nature immunology 8: 1246–1254. [DOI] [PubMed] [Google Scholar]
- 7.Pallikkuth S, Pahwa R, Kausalya B, Saravanan S, Pan L, Vignesh R, Iqbal S, Solomon SS, Murugavel KG, Poongulali S, Kumarasamy N, and Pahwa S. 2018. Cardiac morbidity in HIV infection is associated with checkpoint inhibitor LAG-3 on CD4 T cells. PloS one 13: e0206256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutishauser RL, Hartogensis W, Deguit CD, Krone M, Hoh R, Hecht FM, Pilcher CD, Bacchetti P, Deeks SG, Hunt PW, and McCune JM. 2017. Early and Delayed Antiretroviral Therapy Results in Comparable Reductions in CD8(+) T Cell Exhaustion Marker Expression. AIDS Research and Human Retroviruses 33: 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tauriainen J, Scharf L, Frederiksen J, Naji A, Ljunggren H-G, Sönnerborg A, Lund O, Reyes-Terán G, Hecht FM, Deeks SG, Betts MR, Buggert M, and Karlsson AC. 2017. Perturbed CD8(+) T cell TIGIT/CD226/PVR axis despite early initiation of antiretroviral treatment in HIV infected individuals. Scientific reports 7: 40354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun T-W, McCune JM, Kaul R, Rini JM, Nixon DF, and Ostrowski MA. 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. The Journal of experimental medicine 205: 2763–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian X, Zhang A, Qiu C, Wang W, Yang Y, Qiu C, Liu A, Zhu L, Yuan S, Hu H, Wang W, Wei Q, Zhang X, and Xu J. 2015. The upregulation of LAG-3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV-infected subjects. Journal of immunology (Baltimore, Md. : 1950) 194: 3873–3882. [DOI] [PubMed] [Google Scholar]
- 12.Porichis F, Kwon DS, Zupkosky J, Tighe DP, McMullen A, Brockman MA, Pavlik DF, Rodriguez-Garcia M, Pereyra F, Freeman GJ, Kavanagh DG, and Kaufmann DE. 2011. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood 118: 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, Hammond KB, Clayton KL, Ishii N, Abdel-Mohsen M, Liegler T, Mitchell BI, Hecht FM, Ostrowski M, Shikuma CM, Hansen SG, Maurer M, Korman AJ, Deeks SG, Sacha JB, and Ndhlovu LC. 2016. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS pathogens 12: e1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabmeier-Pfistershammer K, Stecher C, Zettl M, Rosskopf S, Rieger A, Zlabinger GJ, and Steinberger P. 2017. Antibodies targeting BTLA or TIM-3 enhance HIV-1 specific T cell responses in combination with PD-1 blockade. Clinical immunology (Orlando, Fla.) 183: 167–173. [DOI] [PubMed] [Google Scholar]
- 15.Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, Mason SW, Hwang CK, Grasela DM, Ray N, Cyktor JC, Coffin JM, Acosta EP, Koup RA, Mellors JW, Eron JJ, and AIDS Clinical Trials 5326 Study Team. 2017. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. The Journal of infectious diseases 215: 1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guihot A, Marcelin A-G, Massiani M-A, Samri A, Soulié C, Autran B, and Spano J-P. 2018. Drastic decrease of the HIV reservoir in a patient treated with nivolumab for lung cancer. Annals of oncology : official journal of the European Society for Medical Oncology 29: 517–518. [DOI] [PubMed] [Google Scholar]
- 17.Scully EP, Rutishauser RL, Simoneau CR, Delagrèverie H, Euler Z, Thanh C, Li JZ, Hartig H, Bakkour S, Busch M, Alter G, Marty FM, Wang CC, Deeks SG, Lorch J, and Henrich TJ. 2018. Inconsistent HIV reservoir dynamics and immune responses following anti-PD-1 therapy in cancer patients with HIV infection. Annals of oncology : official journal of the European Society for Medical Oncology 29: 2141–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen TA, Rajdev L, Rhodes A, Dantanarayana A, Tennakoon S, Chea S, Spelman T, Lensing S, Rutishauser R, Bakkour S, Busch M, Siliciano JD, Siliciano RF, Einstein MH, Dittmer DP, Chiao E, Deeks SG, Durand C, and Lewin SR. 2021. Impact of Anti–PD-1 and Anti–CTLA-4 on the Human Immunodeficiency Virus (HIV) Reservoir in People Living With HIV With Cancer on Antiretroviral Therapy: The AIDS Malignancy Consortium 095 Study. Clinical Infectious Diseases ciaa1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, and Wolchok JD. 2015. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotte A 2019. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. Journal of experimental & clinical cancer research : CR 38: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tundo GR, Sbardella D, Lacal PM, Graziani G, and Marini S. 2019. On the Horizon: Targeting Next-Generation Immune Checkpoints for Cancer Treatment. Chemotherapy 64: 62–80. [DOI] [PubMed] [Google Scholar]
- 22.Kassu A, Marcus RA, D’Souza MB, Kelly-McKnight EA, Golden-Mason L, Akkina R, Fontenot AP, Wilson CC, and Palmer BE. 2010. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. Journal of immunology (Baltimore, Md. : 1950) 185: 3007–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teigler JE, Zelinskyy G, Eller MA, Bolton DL, Marovich M, Gordon AD, Alrubayyi A, Alter G, Robb ML, Martin JN, Deeks SG, Michael NL, Dittmer U, and Streeck H. 2017. Differential Inhibitory Receptor Expression on T Cells Delineates Functional Capacities in Chronic Viral Infection. Journal of virology 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Trumble IM, Warren JA, Clutton G, Abad-Fernandez M, Kirchnerr J, Adimora AA, Deeks SG, Margolis DM, Kuruc JD, Gay CL, Archin NM, Mollan KR, Hudgens M, and Goonetilleke N. 2019. HIV-Specific T Cell Responses Are Highly Stable on Antiretroviral Therapy. Molecular therapy. Methods & clinical development 15: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appay V, Hansasuta P, Sutton J, Schrier RD, Wong JK, Furtado M, Havlir DV, Wolinsky SM, McMichael AJ, Richman DD, Rowland-Jones SL, and Spina CA. 2002. Persistent HIV-1-specific cellular responses despite prolonged therapeutic viral suppression. AIDS (London, England) 16: 161–170. [DOI] [PubMed] [Google Scholar]
- 26.Ding G, Shen T, Yan C, Zhang M, Wu Z, and Cao L. 2019. IFN-γ down-regulates the PD-1 expression and assist nivolumab in PD-1-blockade effect on CD8+ T-lymphocytes in pancreatic cancer. BMC Cancer 19: 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fromentin R, DaFonseca S, Costiniuk CT, El-Far M, Procopio FA, Hecht FM, Hoh R, Deeks SG, Hazuda DJ, Lewin SR, Routy J-P, Sékaly R-P, and Chomont N. 2019. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4+ T cells from ART-suppressed individuals. Nature communications 10: 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selby MJ, Engelhardt JJ, Johnston RJ, Lu L-S, Han M, Thudium K, Yao D, Quigley M, Valle J, Wang C, Chen B, Cardarelli PM, Blanset D, and Korman AJ. 2016. Preclinical Development of Ipilimumab and Nivolumab Combination Immunotherapy: Mouse Tumor Models, In Vitro Functional Studies, and Cynomolgus Macaque Toxicology. PloS one 11: e0161779–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faul F, Erdfelder E, Lang A-G, and Buchner A. 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 30.Laird GM, Bullen CK, Rosenbloom DIS, Martin AR, Hill AL, Durand CM, Siliciano JD, and Siliciano RF. 2015. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. The Journal of clinical investigation 125: 1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Sluis RM, Kumar NA, Pascoe RD, Zerbato JM, Evans VA, Dantanarayana AI, Anderson JL, Sékaly RP, Fromentin R, Chomont N, Cameron PU, and Lewin SR. 2020. Combination Immune Checkpoint Blockade to Reverse HIV Latency. Journal of immunology (Baltimore, Md. : 1950) 204: 1242–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Marquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, and Wolchok JD. 2019. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. The New England journal of medicine 381: 1535–1546. [DOI] [PubMed] [Google Scholar]
- 33.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, and Ahmed R. 2003. Viral Persistence Alters CD8 T-Cell Immunodominance and Tissue Distribution and Results in Distinct Stages of Functional Impairment. Journal of virology 77: 4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, and Koup RA. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107: 4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agnellini P, Wolint P, Rehr M, Cahenzli J, Karrer U, and Oxenius A. 2007. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America 104: 4565–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M, Gungabeesoon J, Freeman GJ, Warren SE, Ong S, Browning E, Twitty CG, Pierce RH, Le MH, Algazi AP, Daud AI, Pai SI, Zippelius A, Weissleder R, and Pittet MJ. 2018. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity 49: 1148–1161.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villarino AV, Tato CM, Stumhofer JS, Yao Z, Cui YK, Hennighausen L, O’Shea JJ, and Hunter CA. 2007. Helper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signals. The Journal of experimental medicine 204: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lertmemongkolchai G, Cai G, Hunter CA, and Bancroft GJ. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. The Journal of Immunology 166: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 39.Vacaflores A, Chapman NM, Harty JT, Richer MJ, and Houtman JCD. 2016. Exposure of Human CD4 T Cells to IL-12 Results in Enhanced TCR-Induced Cytokine Production, Altered TCR Signaling, and Increased Oxidative Metabolism. PloS one 11: e0157175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schurich A, Pallett LJ, Lubowiecki M, Singh HD, Gill US, Kennedy PT, Nastouli E, Tanwar S, Rosenberg W, and Maini MK. 2013. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS pathogens 9: e1003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevenson EM, Ward AR, Truong R, Thomas AS, Huang S-H, Dilling TR, Terry S, Bui JK, Mota TM, Danesh A, Lee GQ, Gramatica A, Khadka P, Alberto WDC, Gandhi RT, McMahon DK, Lalama CM, Bosch RJ, Macatangay B, Cyktor JC, Eron JJ, Mellors JW, Jones RB, and the A. A. Team.. 2020. HIV-specific T-cell responses reflect substantive in vivo interactions with infected cells despite long-term therapy. 4: 633–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsson M, Shankar EM, Che KF, Saeidi A, Elleg\aard R, Barathan M, Velu V, and Kamarulzaman A. 2013. Molecular signatures of T-cell inhibition in HIV-1 infection. Retrovirology 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraser JH, Rincón M, McCoy KD, and Le Gros G. 1999. CTLA4 ligation attenuates AP-1, NFAT and NF-kappaB activity in activated T cells. European journal of immunology 29: 838–844. [DOI] [PubMed] [Google Scholar]
- 44.Bhagwat B, Cherwinski H, Sathe M, Seghezzi W, McClanahan TK, de Waal Malefyt R, and Willingham A. 2018. Establishment of engineered cell-based assays mediating LAG3 and PD1 immune suppression enables potency measurement of blocking antibodies and assessment of signal transduction. Journal of immunological methods 456: 7–14. [DOI] [PubMed] [Google Scholar]
- 45.Lettau M, Armbrust F, Dohmen K, Drews L, Poch T, Dietz M, Kabelitz D, and Janssen O. 2018. Mechanistic peculiarities of activation-induced mobilization of cytotoxic effector proteins in human T cells. International immunology 30: 215–228. [DOI] [PubMed] [Google Scholar]
- 46.Kassahn D, Nachbur U, Conus S, Micheau O, Schneider P, Simon H-U, and Brunner T. 2009. Distinct requirements for activation-induced cell surface expression of preformed Fas/CD95 ligand and cytolytic granule markers in T cells. Cell death and differentiation 16: 115–124. [DOI] [PubMed] [Google Scholar]
- 47.Rothenberg EV, and Ward SB. 1996. A dynamic assembly of diverse transcription factors integrates activation and cell-type information for interleukin 2 gene regulation. Proceedings of the National Academy of Sciences of the United States of America 93: 9358–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jutz S, Leitner J, Schmetterer K, Doel-Perez I, Majdic O, Grabmeier-Pfistershammer K, Paster W, Huppa JB, and Steinberger P. 2016. Assessment of costimulation and coinhibition in a triple parameter T cell reporter line: Simultaneous measurement of NF-κB, NFAT and AP-1. Journal of immunological methods 430: 10–20. [DOI] [PubMed] [Google Scholar]
- 49.Tomkowicz B, Walsh E, Cotty A, Verona R, Sabins N, Kaplan F, Santulli-Marotto S, Chin C-N, Mooney J, Lingham RB, Naso M, and McCabe T. 2015. TIM-3 Suppresses Anti-CD3/CD28-Induced TCR Activation and IL-2 Expression through the NFAT Signaling Pathway. PloS one 10: e0140694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee MJ, Woo M-Y, Chwae Y-J, Kwon M-H, Kim K, and Park S. 2012. Down-regulation of interleukin-2 production by CD4(+) T cells expressing TIM-3 through suppression of NFAT dephosphorylation and AP-1 transcription. Immunobiology 217: 986–995. [DOI] [PubMed] [Google Scholar]
- 51.Anderson AC, Joller N, and Kuchroo VK. 2016. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 44: 989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romanchikova N, Ivanova V, Scheller C, Jankevics E, Jassoy C, and Serfling E. 2003. NFAT transcription factors control HIV-1 expression through a binding site downstream of TAR region. Immunobiology 208: 361–365. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto T, Price DA, Casazza JP, Ferrari G, Nason M, Chattopadhyay PK, Roederer M, Gostick E, Katsikis PD, Douek DC, Haubrich R, Petrovas C, and Koup RA. 2011. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood 117: 4805–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fromentin R, Bakeman W, Lawani MB, Khoury G, Hartogensis W, DaFonseca S, Killian M, Epling L, Hoh R, Sinclair E, Hecht FM, Bacchetti P, Deeks SG, Lewin SR, Sékaly R-P, and Chomont N. 2016. CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART. PLoS pathogens 12: e1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowler S, Chew GM, Budoff M, Chow D, Mitchell BI, D’Antoni ML, Siriwardhana C, Ndhlovu LC, and Shikuma C. 2019. PD-1+ and TIGIT+ CD4 T Cells Are Associated With Coronary Artery Calcium Progression in HIV-Infected Treated Adults. Journal of acquired immune deficiency syndromes (1999) 81: e21–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porichis F, Hart MG, Massa A, Everett HL, Morou A, Richard J, Brassard N, Veillette M, Hassan M, Ly NL, Routy J-P, Freeman GJ, Dubé M, Finzi A, and Kaufmann DE. 2018. Immune Checkpoint Blockade Restores HIV-Specific CD4 T Cell Help for NK Cells. Journal of immunology (Baltimore, Md. : 1950) 201: 971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Brien S, Thomas RM, Wertheim GB, Zhang F, Shen H, and Wells AD. 2014. Ikaros imposes a barrier to CD8+ T cell differentiation by restricting autocrine IL-2 production. Journal of immunology (Baltimore, Md. : 1950) 192: 5118–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart P-A, and Pantaleo G. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proceedings of the National Academy of Sciences of the United States of America 102: 7239–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trautmann L, Mbitikon-Kobo F-M, Goulet J-P, Peretz Y, Shi Y, Van Grevenynghe J, Procopio FA, Boulassel MR, Routy J-P, Chomont N, Haddad EK, and Sékaly R-P. 2012. Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood 120: 3466–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng N, Fujiwara M, Ueno T, Oka S, and Takiguchi M. 2009. Strong ability of Nef-specific CD4+ cytotoxic T cells to suppress human immunodeficiency virus type 1 (HIV-1) replication in HIV-1-infected CD4+ T cells and macrophages. Journal of virology 83: 7668–7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barthlott T, Moncrieffe H, Veldhoen M, Atkins CJ, Christensen J, O’Garra A, and Stockinger B. 2005. CD25+ CD4+ T cells compete with naive CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. International immunology 17: 279–288. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y, Lee CK, Lin C-H, Gassen RB, Xu X, Huang Z, Xiao C, Bonorino C, Lu L-F, Bui JD, and Hui E. 2019. PD-L1:CD80 Cis-Heterodimer Triggers the Co-stimulatory Receptor CD28 While Repressing the Inhibitory PD-1 and CTLA-4 Pathways. Immunity . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keir ME, Butte MJ, Freeman GJ, and Sharpe AH. 2008. PD-1 and its ligands in tolerance and immunity. Annual review of immunology 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia-Bates TM, Palma ML, Shen C, Gambotto A, Macatangay BJC, Ferris RL, Rinaldo CR, and Mailliard RB. 2019. Contrasting Roles of the PD-1 Signaling Pathway in Dendritic Cell-Mediated Induction and Regulation of HIV-1-Specific Effector T Cell Functions. Journal of virology 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hafler DA, and Kuchroo V. 2008. TIMs: central regulators of immune responses. The Journal of experimental medicine 205: 2699–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Graydon CG, Balasko AL, and Fowke KR. 2019. Roles, function and relevance of LAG3 in HIV infection. PLoS pathogens 15: e1007429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Porichis F, Hart MG, Zupkosky J, Barblu L, Kwon DS, McMullen A, Brennan T, Ahmed R, Freeman GJ, Kavanagh DG, and Kaufmann DE. 2014. Differential impact of PD-1 and/or interleukin-10 blockade on HIV-1-specific CD4 T cell and antigen-presenting cell functions. Journal of virology 88: 2508–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewin SR, Attoye T, Bansbach C, Doehle B, Dubé K, Dybul M, SenGupta D, Jiang A, Johnston R, Lamplough R, McCune JM, Nabel GJ, Ndung’u T, Pottage J, Ripin D, Rooney JF, Sikazwe I, Nsubuga M, Warren M, Deeks SG, and Sunnylands 2019 Working Group. 2021. Multi-stakeholder consensus on a target product profile for an HIV cure. Lancet HIV 8: e42–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, Schwabe C, and Dunbar PR. 2019. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol 71: 900–907. [DOI] [PubMed] [Google Scholar]
- 70.Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, and Lou Y. 2018. Next generation of immune checkpoint therapy in cancer: new developments and challenges. Journal of hematology & oncology 11: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin S, Xu L, Yi M, Yu S, Wu K, and Luo S. 2019. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


