Abstract
A 39-year-old woman with cystic bronchiectasis had repeated pulmonary infections from 1996 to 1999, and 6 of a total of 28 isolates of Escherichia coli from sputum specimens were studied. Their identical antibiotype and randomly amplified polymorphic DNA patterns indicated a single clone of E. coli, which persistently colonized the respiratory tract, causing recurrent infections.
Escherichia coli is the most abundant facultative anaerobic bacterium in the normal human intestine. Its presence is clearly associated with infections of the gastrointestinal tract, urogenital tract, and peritoneum and occasionally with infections at distant loci after bacteremia. However, it is rarely associated with pulmonary infections (2).
In March 1987, a 39-year-old woman who suffered from fever, a productive cough with copious purulent sputum, and progressive dyspnea was admitted to National Taiwan University Hospital. She had survived measles complicated with pneumonia when she was 4 years old and had had a chronic cough, purulent expectoration, and chronic dyspnea with episodic exacerbation accompanied by fever thereafter. She did not smoke cigarettes or drink alcohol. Physical examination revealed an emaciated woman with fever, respiratory distress, and cyanosis. She had engorged jugular veins, coarse crackles over bilateral lung fields, and marked clubbing of all digits. Her breath and sputum both produced a putrid odor. Chest roentgenography revealed diffuse cystic bronchiectasis of bilateral lungs with multiple air-fluid levels, pulmonary hypertension, and localized right pneumothorax. From March 1996 to February 1999, she was readmitted seven times with similar manifestations. High-resolution computed tomography of the chest done in December 1998 showed diffuse severe cystic bronchiectasis with multiple air-fluid levels and right pneumothorax. Microbiological studies (Gram and acid-fast stains and aerobic bacterial cultures) of multiple sputum specimens were performed during each hospital stay. Microscopic examinations of the sputum specimens all revealed numerous polymorphonuclear cells and predominant gram-negative bacilli. Cultures of a total of 28 sputum specimens over this 3-year period all yielded heavy growth of E. coli. Each organism was isolated either as a sole pathogen (21 occasions) or as one of mixed pathogens (with Haemophilus parainfluenzae on three occasions, Pseudomonas aeruginosa on three occasions, and Klebsiella pneumoniae on one occasion). All of these E. coli isolates had nearly identical antibiotypes, as determined by disk diffusion testing against 12 antimicrobial agents. The isolates were resistant to ampicillin, intermediately susceptible or susceptible to amoxicillin-clavulanic acid, and susceptible to cefazolin, cefotiam, cefmetazole, cefotaxime, aztreonam, gentamicin, netilmicin, amikacin, imipenem, and ciprofloxacin. Blood and urine cultures of specimens collected during this period were all negative for E. coli. In July 1996, arterial blood gas collected while the patient was breathing room air showed a pH of 7.407, pCO2 of 6.78 kPa, pO2 of 9.30 kPa, and a bicarbonate level of 31.7 mmol/liter. Intravenous antibiotic therapy including amoxicillin-clavulanic acid, cefazolin, cefmetazole, or ciprofloxacin had been given and continued for 21 to 28 days during each hospitalization. Fever usually declined within 3 to 7 days, while decreases in sputum amount and purulence took 2 to 4 weeks. Even between hospital stays her sputum remained copious and purulent. Her pulmonary function deteriorated rapidly, and arterial blood gas when the patient was on a 40%, 10-liter/min O2 mask in February 1999 showed a pH of 7.445, pCO2 of 7.45 kPa, pO2 of 9.06 kPa, and a bicarbonate level of 37.8 mmol/liter. She had remained bedridden since 1997.
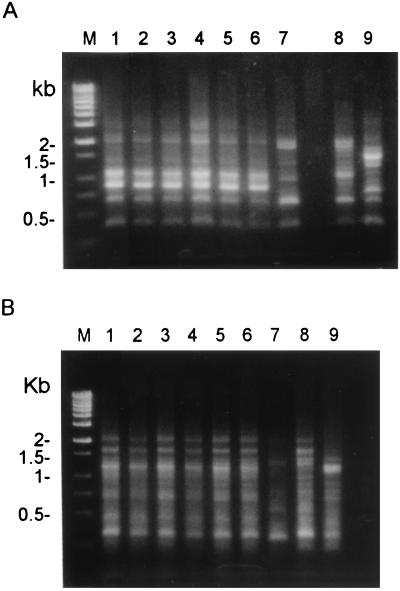
Of the 28 E. coli isolates, six were subjected to further investigations: one was isolated in 1996, two were isolated in 1997, two were isolated in 1998, and one was isolated in 1999. MICs of six antimicrobial agents were determined for these isolates using the Etest (AB Biodisk, Solna, Sweden) as described previously (6). All of the isolates had identical antibiotypes: MICs of >256 μg/ml for ampicillin, 48 μg/ml for cefazolin, 24 μg/ml for amoxicillin-clavulanic acid, 0.75 μg/ml for ciprofloxacin, 4 μg/ml for minocycline, and 32 μg/ml for trimethoprim-sulfamethoxazole. Analysis of randomly amplified polymorphic DNA (RAPD) patterns generated by arbitrarily primed PCR was performed as reported previously (7). In addition to the six isolates from our patient, three isolates (as control strains) from blood samples of three other patients were also typed. Two oligonucleotide primers were used: M13 (5′-TTATGTAAAACGACGGCCAGT-3′) and ERIC1 (5′-GTGAATCCCCAGGAGCTTACAT-3′) (Operon Technologies Inc., Alameda, Calif.). Figure 1 shows the RAPD patterns of the nine isolates obtained with the two primers. The six patient isolates had identical RAPD patterns, which were different from those of the three control strains.
FIG. 1.
RAPD patterns generated by arbitrarily primed PCR of the nine isolates of E. coli with two primers: M13 (A) and ERIC1 (B). Lanes M, molecular marker; lanes 1 to 6, six E. coli isolates from the patient with cystic bronchiectasis; lanes 7 to 9, the three control strains.
Pulmonary infection due to E. coli is rare in clinical settings. Tillotson and Lerner reported that E. coli accounted for only 0.7% of 1,882 cases of pneumonia at Detroit Receiving Hospital (15). E. coli infection usually induces bronchopneumonia with interstitial infiltration of mononuclear cells (8, 11, 15, 16). Risk factors include chronic illness, particularly diabetes mellitus, renal disease, and alcoholism. This organism seldom causes acute infection in patients with chronic bronchitis (1). Bacterial pathogens frequently associated with infections in bronchiectatic patients are P. aeruginosa, Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, and Moraxella (subgenus Branhamella) catarrhalis (1, 4, 12, 14). Only one previous report described E. coli as an isolate from sputum of patients with bronchiectasis (10). Isolation of P. aeruginosa from sputum of patients with bronchiectasis has been associated with extensive disease, more rapid deterioration of lung function, and poor prognosis (3, 4), probably because it produces toxins and enzymes which interfere with host defense as well as causing lung damage (5, 13, 17). In our patient, the cystic bronchiectatic changes were so extensive that only very scanty normal lung tissue was left. The poor lung function was a clear indication of the severity and widespread nature of the lung damage. Whether the extensive bronchiectasis was caused by the chronic infection of E. coli or whether the E. coli colonized the already damaged airways and caused additional infection is not known.
Pulmonary infection caused by E. coli may result from hematogenous dissemination from either the gastrointestinal or urinary tract and from aspiration from the pharynx (8, 11, 15, 16). In fact, 2% of the healthy population may harbor E. coli in their oropharynges, and the incidence is higher in hospitalized patients. In our patient, there was no evidence of bacteremia or bacteruria due to this microorganism. Therefore, aspiration may have been the mode of entry of E. coli into the diseased lung. However, persistent colonization of the respiratory tract in a patient by a single clone of E. coli, resulting in recurrent infection with multiple hospitalizations, has not been previously reported. In a study of bronchiectatic patients colonized by M. catarrhalis, Klingman et al. found that each patient was colonized by two to four strains and that the mean duration of colonization was 2.3 months (9). These findings suggest that the acquisition and clearance of M. catarrhalis in bronchiectatic patients are a dynamic process, which is contrary to our findings. In the present case, the E. coli clone could not be eliminated from the respiratory tract despite long-term therapy with antibiotics with in vitro activity. Even after the respiratory signs disappeared, colonization persisted, reflecting an apparent dissociation between in vitro susceptibility and in vivo response. Failure to eradicate this microorganism by treatment with antibiotics shown to have in vitro activity has been partly due to an inability to achieve sufficient drug levels in the infected cysts and inadequate drainage of sputum or infected fluid in cystic cavities.
In summary, we have described a case of persistence of a single clone of E. coli in the respiratory tract of a patient with severe cystic bronchiectasis. The potential significance of chronic persistence of this pathogen in debilitated bronchiectatic patients should be considered.
REFERENCES
- 1.Bjerkestrand G, Digranes A, Schreiner A. Bacteriological findings in transtracheal aspirates from patients with chronic bronchitis and bronchiectasis. Scand J Respir Dis. 1975;56:201–207. [PubMed] [Google Scholar]
- 2.Eisenstein B I. Enterobacteriaceae. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. Philadelphia, Pa: Churchill Livingstone; 1995. pp. 1969–1972. [Google Scholar]
- 3.Ellis D A, Thornley P E, Wightman A J, Walker M, Chalmers J, Crofton J W. Present outlook in bronchiectasis: clinical and social study and review of factors influencing prognosis. Thorax. 1981;36:659–664. doi: 10.1136/thx.36.9.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans S A, Turner S M, Bosch B J, Hardy C C, Woodhead M A. Lung function in bronchiectasis: the influence of Pseudomonas aeruginosa. Eur Respir J. 1996;9:1601–1604. doi: 10.1183/09031936.96.09081601. [DOI] [PubMed] [Google Scholar]
- 5.Hoiby N. Haemophilus influenzae, Staphylococcus aureus, Pseudomonas cepacia, and Pseudomonas aeruginosa in patients with cystic fibrosis. Chest. 1988;94:97s–102s. doi: 10.1378/chest.94.2_supplement.97s. [DOI] [PubMed] [Google Scholar]
- 6.Hsueh P R, Teng L J, Lee L N, Yang P C, Chen Y C, Ho S W, Luh K T. Indwelling device-related and recurrent infections due to Aeromonas species. Clin Infect Dis. 1998;26:651–658. doi: 10.1086/514587. [DOI] [PubMed] [Google Scholar]
- 7.Hsueh P-R, Teng L-J, Ho S-W, Hsieh W-C, Luh K-T. Clinical and microbiological characteristics of Flavobacterium indologenes infections associated with indwelling devices. J Clin Microbiol. 1996;34:1908–1913. doi: 10.1128/jcm.34.8.1908-1913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaffey P B, English II P W, Campbell G A, Rubin S A, Haque A K. Escherichia coli lobar pneumonia: fatal infection in a patient with mental retardation. South Med J. 1996;89:628–630. [PubMed] [Google Scholar]
- 9.Klingman K L, Pye A, Murphy T F, Hill S L. Dynamics of respiratory tract colonization by Branhamella catarrhalis in bronchiectasis. Am J Respir Crit Care Med. 1995;152:1072–1078. doi: 10.1164/ajrccm.152.3.7663786. [DOI] [PubMed] [Google Scholar]
- 10.Lin L J, Luh K T. Bronchiectasis: a clinical analysis of 100 cases. J Formos Med Assoc. 1982;81:1580–1585. . (In Chinese.) [PubMed] [Google Scholar]
- 11.Packham D R, Sorrell T C. Pneumonia with bacteremia due to Escherichia coli. Aust N Z J Med. 1981;11:669–672. doi: 10.1111/j.1445-5994.1981.tb03545.x. [DOI] [PubMed] [Google Scholar]
- 12.Pang J A, Cheng A, Chan H S, Poon D, French G. The bacteriology of bronchiectasis in Hong Kong investigated by protected catheter brush and bronchoalveolar lavage. Am Rev Respir Dis. 1989;139:14–17. doi: 10.1164/ajrccm/139.1.14. [DOI] [PubMed] [Google Scholar]
- 13.Pederson B K, Kharazmi A. Inhibition of human natural killer cell activity by Pseudomonas aeruginosa alkaline protease and elastase. Infect Immun. 1987;55:986–989. doi: 10.1128/iai.55.4.986-989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts D E, Cole P. Use of selective media in bacteriological investigations of patients with chronic suppurative respiratory infection. Lancet. 1980;i:796–797. doi: 10.1016/s0140-6736(80)91295-7. [DOI] [PubMed] [Google Scholar]
- 15.Tillotson J R, Lerner A M. Characteristics of pneumonias caused by Escherichia coli. N Engl J Med. 1967;277:115–122. doi: 10.1056/NEJM196707202770302. [DOI] [PubMed] [Google Scholar]
- 16.Tillotson J R, Lerner A M. Pneumonias caused by Gram negative bacilli. Medicine. 1966;45:65–76. doi: 10.1097/00005792-196601000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Wilson R, Pitt T, Taylor G, Watson D, MacDermot J, Sykes D, Roberts D, Cole P. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest. 1987;79:221–229. doi: 10.1172/JCI112787. [DOI] [PMC free article] [PubMed] [Google Scholar]