1. Introduction
Attendance is an important factor for successful treatment outcomes among individuals with substance use disorders (National Institute on Drug Abuse, 2018; Onken et al., 1997). In substance use disorder treatment, greater attendance is associated with greater substance abstinence (e.g., Connors et al., 2002; Dearing et al., 2005; Kelly et al., 2017; Sebastian et al., 2012; Simpson & Joe, 2004). However, a recent meta-analysis of 151 studies representing 26,243 participants estimated that approximately 30% of individuals with substance use disorders discontinue treatment prematurely (Lappan et al., 2020). Interventions for increasing treatment attendance are critical for enhancing abstinence, which seems integral to long-term recovery (e.g., Roos et al., 2019).
Contingency management (CM) is an evidence-based treatment for substance use disorders (National Institute on Drug Abuse, 2018) and carries substantial promise for increasing treatment attendance. CM provides tangible rewards upon demonstration of a target behavior. The target behavior is most often drug abstinence (i.e., ABS CM), which is efficacious at promoting abstinence post-treatment (Lussier et al., 2006; Prendergast et al., 2006). A common criticism of CM is that its effects may not endure after the discontinuation of rewards (Petry et al., 2017). However, a recent meta-analysis directly challenges this criticism, finding long-term benefits of CM in comparison to other substance use treatments, including cognitive behavioral therapy (Ginley et al., 2021). Research has observed larger effects when the CM protocol reinforces a target behavior immediately, at larger magnitudes, more frequently, and on escalating schedules (Griffith et al., 2000; Lussier et al., 2006; Roll et al., 1996). In addition to abstinence, CM can also be used to reinforce, and successfully improve, treatment attendance (i.e., ATT CM; Alessi et al., 2007; Petry et al., 2012b; Petry et al., 2018).
Although research demonstrates ATT CM increases attendance, less is known about ATT CM’s effect on nontargeted outcomes, such as drug abstinence. This latter point is important because substance use treatment clinics implement ATT CM to not only to improve patients’ attendance but also to ultimately improve abstinence. Between studies, the effect of ATT CM on attendance varies greatly, with studies reporting approximate effect sizes ranging from d = 0.15 to 0.64 (e.g., Alessi et al., 2007; Carroll et al., 2006; Carroll et al., 2012; Petry et al., 2018). By contrast, the overall evidence of ATT CM’s effect on abstinence is mixed, with some studies suggesting improvements in abstinence (e.g., Petry et al., 2012b; Petry et al., 2018) and other studies suggesting no effect on abstinence (e.g., Carroll et al., 2012). The variation in the effect size magnitudes for ATT CM on attendance and the discrepancies in the overall findings for ATT CM on abstinence warrant additional investigation.
A meta-analysis that quantifies the overall effect of ATT CM on attendance and abstinence is important for informing when to use ABS CM versus ATT CM. Such decisions are central for clinicians at substance use treatment clinics who may prefer to implement ATT CM because of the cost and perceived logistic challenges associated with biochemical verification of abstinence (e.g., urine drug screens) in ABS CM. Quantifying ATT CM’s effect on attendance will provide clarification of the overall impact on increasing treatment attendance; and quantifying ATT CM’s effect on abstinence will allow comparisons to ABS CM’s effect on abstinence (e.g., Ainscough et al., 2017; Benishek et al., 2014; Griffith et al., 2000; Lussier et al., 2006; Prendergast et al., 2006). Together, these estimates will allow clinicians to determine the appropriateness of ABS CM and ATT CM within their clinics based on each approach’s cost and desired outcomes.
The current study aimed to quantify ATT CM’s effect on attendance and drug abstinence relative to comparison treatments that did not reinforce attendance. This study employed meta-analysis to synthesize the results of past studies that showed ATT CM had variable effects on attendance and discrepant effects on abstinence. Given the relatively small number of studies on ATT CM, this study included studies that blended abstinence and attendance targets (i.e., ABS CM + ATT CM). The inclusion of these studies allowed comparisons of ATT CM and ABS CM + ATT CM’s effects on attendance and abstinence. The study also explored moderating effect of critical CM parameters (i.e., escalation with or without reset conditions, frequency, immediacy, and magnitude of rewards) on attendance, as these parameters have been found to be important moderators of CM effects in past meta-analyses on ABS CM (Benishek et al., 2014; Griffith et al., 2000; Lussier et al., 2006).
2. Method
2.1. Inclusion and exclusion criteria
The current study included published results on CM protocols reinforcing treatment attendance. This study defined treatment attendance as the appearance at a scheduled appointment for psychosocial treatment, psychotherapy, pharmacotherapy, or other intervention (e.g., case, management, drug court). We included studies if they: 1) published results in English; 2) evaluated the efficacy of CM treatment distributing tangible (e.g., prizes or vouchers) reinforcement for attendance at treatment for marijuana, opioids, stimulants, or polysubstance use; and 3) involved a randomized controlled trial utilizing a control condition that did not reinforce treatment attendance. This study excluded studies if they: 1) enrolled any participants under 18 years of age or 2) presented secondary data from another trial included in the meta-analysis.
The study included both studies of ABS CM + ATT CM and ATT CM in the meta-analysis. However, we included ABS CM + ATT CM protocols only if they continuously reinforced both abstinence and attendance for the entire duration of treatment and not for a limited timeframe. Thus, the study excluded CM protocols for cannabis use disorder from the meta-analysis if the protocol reinforced attendance in the early stages of the protocol and then switched to reinforcing abstinence in the latter stages of the protocol (e.g., Litt et al., 2013). These CM protocols switch from attendance reinforcement to abstinence reinforcement because participants cannot produce negative urine drug screens for cannabis during the initial treatment period (i.e., due to the long biological half-life of cannabis). Such treatments were qualitatively different from the treatments described here and cannot address the study aims.
2.2. Search strategy
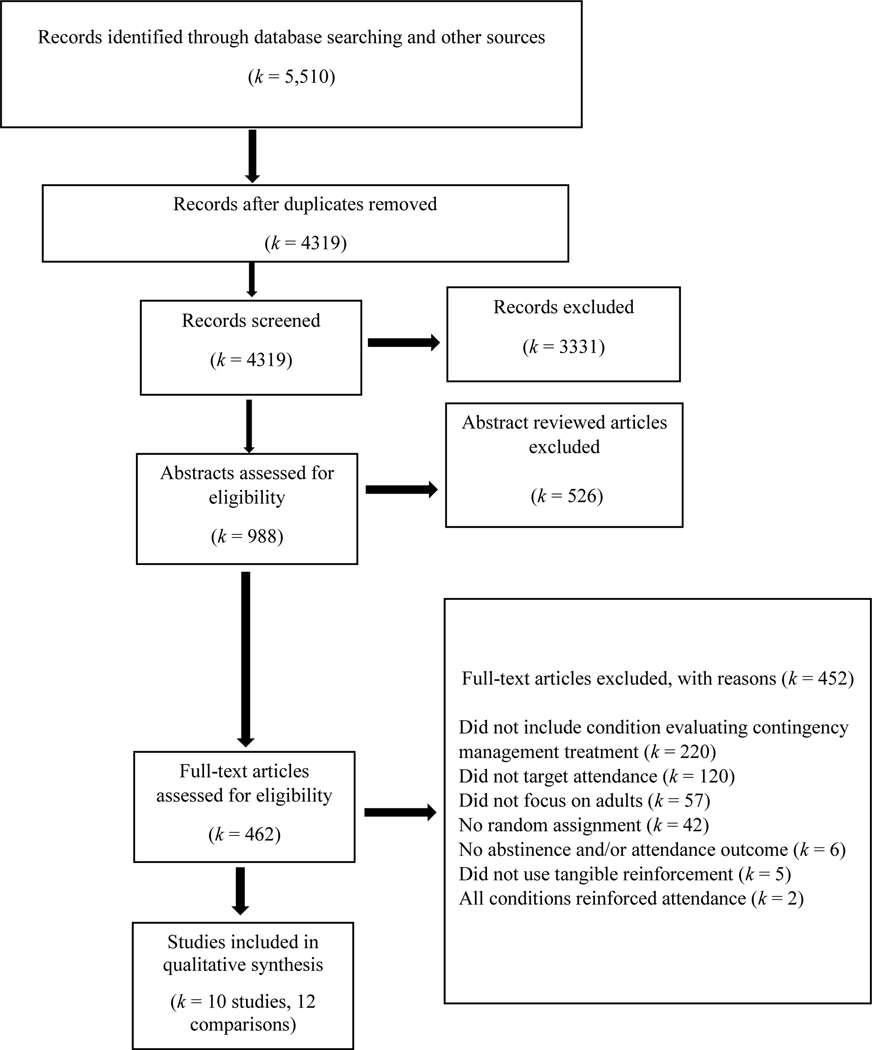
The study team reviewed studies published in any year through July 2020 based on PRISMA guidelines (Moher et al., 2009, see Figure 1). The team performed searches in PsycINFO, PubMed, and the Cochrane Database of Systematic Reviews (see Table 1 for a comprehensive description of the search terms). To ensure that we included all relevant studies, the study team searched reference lists of identified CM review articles using the search terms (k = 17) and also searched reference lists of studies that met inclusion/exclusion criteria (k = 10).
Figure 1.
Flowchart of records identified and reviewed.
Table 1.
Search terms and Boolean phrase used in the meta-analysis on contingency management for treatment attendance.
| Databases | Boolean Phrase |
|---|---|
| Cochrane Database of Systematic Reviews, PsycINFO, PubMed | [“motivational interview*” OR “motivational enhancement” OR “motivation* intervention” OR “contingency management” OR “voucher” OR “behavioral contracting” OR “token economy” OR “community reinforcement” OR “matrix model” OR “aftercare” OR “relapse prevention” OR “twelve step” OR “12 step” OR “twelve step facilitation” OR “12 step facilitation” OR “family thera*” OR “family intervention*” OR “couples thera*” OR “couples intervention” OR “seeking safety” OR “mindful*”] AND [“randomize*” OR “random” OR “randomly” OR “clinical trial” OR “control* trial”] AND [“substance*” OR “drug*” OR “cocaine” OR “crack” OR “stimulant” OR “amphetamine” OR “methamphetamine” OR “heroin” OR “opiate*” OR “opioid” OR “marijuana” OR “cannabis”]. |
2.3. Screening studies
In a multistep process, the study screened study titles and abstracts that the search strategy had captured. The first step involved two authors independently determining if articles met inclusion criteria based on study titles and abstracts. The second step involved the same two reviewers independently determining if articles met inclusion criteria at the full-text level. A codebook informed their decisions for inclusion or exclusion. The reviewers resolved all discrepancies through consensus, and, when needed, discussion with a third reviewer. Figure 1 displays the flowchart of this process. The initial search yielded 5,512 records, which led to the identification of 10 independent studies.
2.4. Data extraction
When the original publication did not report information needed to conduct meta-analysis, study staff emailed authors with requests for their data. If they received no response within two weeks, staff sent a reminder email. We requested data for five studies. However, we did not include any of these five because authors did not respond or indicated that data were destroyed due to age.
The primary outcomes extracted from each study were treatment attendance and drug abstinence. The study defined treatment attendance as the number of sessions or the number of treatment days attended. The study defined drug abstinence as the longest duration of continuous abstinence during treatment or the percentage of negative urine samples submitted during treatment. If studies reported both outcomes, we prioritized for extraction the longest duration of continuous abstinence due to the strong association between continuous abstinence and long-term outcomes (Preston et al., 1998; Stitzer et al., 2007). Five studies (50%) used the longest duration of continuous abstinence, and five studies (50%) used the percentage of negative urine samples submitted during treatment. All studies used urine toxicology to verify drug abstinence results.
This study also extracted study, participant, and treatment variables from each study. The study variable was publication year. Participant variables were the mean age and the percentage identifying as each of the following: female, Hispanic, African American/Black, and White. Treatment variables were drugs treated (marijuana only, opioids only, stimulants only, and polysubstance use) and several parameters of CM, including whether reinforcement escalated with sustained attendance (i.e., participants could earn rewards of increasing value for consecutive attendance of treatment sessions that may or may not be reset based on unexcused absences), the frequency of reinforcers (i.e., the number of times reinforcement could be earned per week), timing of reinforcement (delayed versus immediate), reinforcer magnitude in average maximum value available per participant during CM, and CM delivery method (prize versus voucher).
2.5. Risk of bias
The study used the Cochrane Risk of Bias tool to assess possible bias in the included studies (Higgins & Green, 2011). Criteria that the study assessed were: random sequence generation, allocation concealment, masking of outcome assessors, and incomplete outcome data. The study did not assess selective outcome reporting because most treatment trials are still not prospectively registered (Bradley et al., 2017). Each criterion was designated as high, low, or unclear risk of bias. Using these criteria, the study computed an overall quality indicator for each study. The study team designated as high quality studies with three or more indicators of low risk of bias, and studies with two or fewer indicators of low risk as low quality.
2.6. Analytic plan
The study team performed effect size calculations in Comprehensive Meta-Analysis v3.3070. Cohen’s d effect sizes quantified the effect of CM on attendance and abstinence outcomes. If studies contained multiple treatment conditions to one control condition, then we combined the Cohen’s d effect sizes and averaged them into one effect size. For abstinence, the study aggregated binary data for abstinent and nonabstinent outcomes from the treatment conditions and compared them to the abstinent and nonabstinent outcomes of a single control condition (Borenstein et al., 2009). This study combined and averaged effect sizes from multiple treatment conditions to prevent the overweighting of effects from control conditions. A one study removed analysis estimated the impact of each study on the overall Cohen’s d for attendance and abstinence.
Due to expected differences in the types of samples recruited, the parameters of CM, and in the various treatment settings, the study used a random effects analysis. The Q-statistic tested heterogeneity in the individual study effect sizes and determined if heterogeneity varied significantly around the summary effect size of all studies (Hedges & Olkin, 1985). The I2 statistic estimated the magnitude of heterogeneity between studies (Higgins et al., 2003).
The research team conducted multiple tests to test for possible publication bias, including the examination of a funnel plot, the Eggers regression test for asymmetry (Egger et al., 1997), and calculation of the fail-safe N (Rosenthal, 1979). An asymmetrical funnel plot indicated publication bias. The Egger test assessed the relation between error and study effect sizes using linear regression, with a significant regression indicating publication bias. A fail-safe N determined the number of nonsignificant studies not identified by a review that would be needed for a significant effect size to no longer be significant (Rosenthal, 1979).
Random effects meta-regressions tested for possible relations between the effect of CM on attendance and continuous variables. For each meta-regression, the study regressed Cohen’s d values for each treatment-control comparison on continuous moderators. Continuous moderators included participants’ mean age, the percentage of participants who identified as female, the percentage of participants who identified as African American/Black, the percentage of participants who identified as White, the study publication year, the frequency of reinforcers, the magnitude of reinforcers, and the duration of the CM protocol (see Tables 2 and 3 for a full list of moderators). The study did not conduct a meta-regression of attendance on the percentage of Hispanic participants because too few studies reported that demographic information (Higgins & Green, 2011).
Table 2.
Description of studies and study conditions included in the meta-analysis on contingency management targeting attendance.
| Study | Study Location | Total N | Treatment Conditions (n) | Comparison Condition (n) | Mean Age | % Female | % Hispanic | % African American/ Black | % White |
|---|---|---|---|---|---|---|---|---|---|
| Alessi et al. (2007) | USA | 103 | CM + IOP (46) | IOP (57) | 37 | 50 | 12 | 46 | 36 |
| Carroll et al. (2006) | USA | 136 | CM + MET, CBT (33) CM + drug counseling (34) |
MET, CBT (36) Drug counseling (33) |
21 | 10 | 13 | 60 | 23 |
| Carroll et al. (2012) | USA | 68 | CM + CBT (32) | CBT (36) | 25 | 16 | 15 | 65 | 16 |
| Chen et al. (2013) | China | 246 | CM + methadone (126) | Methadone (120) | 38 | 8 | 0 | 0 | 0 |
| Jones et al. (2001) | USA | 80 | CM + methadone (44) | Methadone (36) | 28 | 100 | nr | 76 | nr |
| Kidorf et al. (2013) | USA | 125 | CM + case management (62) | Case management (63) | 39 | 54 | nr | nr | 65 |
| Marlowe et al. (2008) | USA | 269 | CM + drug court (90) CM + drug court (89) |
Drug court (90) | 24 | 20 | 24 | 61 | 18 |
| Petry et al. (2011) | USA | 239 | CM + IOP (117) | IOP (122) | 38 | 43 | 9 | 30 | 56 |
| Petry et al. (2012) | USA | 215 | CM + IOP (107) | IOP (108) | 37 | 54 | 16 | 33 | 48 |
| Petry et al. (2018) | USA | 360 | CM + MET, CBT, TSF (271) | MET, CBT, TSF (89) | 38 | 52 | nr | 31 | 59 |
Notes. CBT = cognitive-behavioral therapy; CM = contingency management; IOP = intensive outpatient treatment; MET = motivational enhancement therapy; nr = not reported; TSF = twelve step facilitation; USA = United States of America
Table 3.
Design features of studies included in the meta-analysis and assessment of their study quality.
| Study | CM condition | Drugs Treated | Abstinence and Attendance | Prize or Voucher | Escalation (reset) | Frequencya | Reinforcer Magnitudeb | Treatment Duration (in weeks) | Overall Study Quality |
|---|---|---|---|---|---|---|---|---|---|
| Alessi et al. (2007) | CM + IOP | P | ABS + ATT | Prize | Yes (yes) | 5 | N/A | 12 | - |
| Carroll et al. (2006) | CM + MET, CBT CM + drug counseling |
M | ABS + ATT ABS + ATT |
Voucher Voucher |
Yes (yes) Yes (yes) |
1 1 |
$880 $880 |
8 8 |
- |
| Carroll et al. (2012) | CM + CBT | M | ATT | Prize | Yes (yes) | 1 | $250 | 12 | + |
| Chen et al. (2013) | CM + methadone | O | ABS + ATT | Prize | Yes (yes) | 1 | $42 | 12 | - |
| Jones et al. (2001) | CM + methadone | P | ABS + ATT | Voucher | Yes (no) | 7 | $525 | 2 | + |
| Kidorf et al. (2013) | CM + case management | P | ATT | Voucher | No | 7 | $300 | 12 | + |
| Marlowe et al. (2008) | CM + drug court CM + drug court |
P | ATT ATT |
Voucher Voucher |
Yes (no) No | 0.2 0.2 |
$390 $390 |
56 56 |
- |
| Petry et al. (2011) | CM + IOP | P | ABS + ATT | Prize | Yes (yes) | 5 | N/A | 12 | + |
| Petry et al. (2012) | CM + IOP | S | ATT | Prize | Yes (yes) | 5 | $250 | 12 | + |
| Petry et al. (2018) | CM + MET, CBT, TSF | S | ATT | Prize | Yes (yes) | 2 | $190 | 12 | + |
Notes. All studies immediately reinforced attendance. + = high study quality (defined as studies meeting three or more indicators of low bias per the Cochrane Risk of Bias tool); - = low study quality (defined as studies meeting two or fewer indicators of low bias per the Cochrane Risk of Bias tool); ABS + ATT = CM protocol reinforced abstinence and attendance simultaneously; ATT = CM protocol reinforced attendance alone; CBT = cognitive-behavioral therapy; CM = contingency management; IOP = intensive outpatient treatment; M = marijuana; MET = motivational enhancement therapy; N/A = cannot be determined; O = opioids; P = polysubstance use; S = stimulants; TSF = twelve step facilitation;
Refers to the available number of times reinforcement was earned per week
Refers to the average maximum value that participants could earn during the CM protocol
Subgroup analyses tested differences in the effect of CM on attendance among various categorical moderators. The categorical moderators included CM form (ATT CM versus ABS CM + ATT CM), drug treated (marijuana only, opioids only, polydrug use, or stimulants only), study quality (low versus high), delivery method (prizes versus vouchers), escalation of reinforcers with or without reset conditions (yes/no), and immediate reinforcers (yes/no). The study team conducted subgroup analyses with the mixed effects model, and tested significance with the fixed effects model. The study did not examine effect of moderators on abstinence because moderators of abstinence have been covered extensively elsewhere (Benishek et al., 2014; Griffith et al., 2000; Lussier et al., 2006).
3. Results
3.1. Study characteristics
Across the 10 studies, a total of 1,841 participants were allocated to 12 CM treatments. Table 2 displays each included study along with descriptions of the treatment conditions and demographic characteristics of the samples. All studies were conducted in the United States of America except for one study conducted in China. Publication dates ranged from 2001 to 2018. Sample sizes ranged from 68 to 360 (M = 184.1, SD = 95.8, median = 175.5). The mean age of participants was 32.5 (SD = 7.1, median = 37.0). Approximately 40.7% (SD = 28.2, median = 46.5) identified as female. Participants were racially diverse with 12.7% (SD = 7.3, median = 13.0) identifying as Hispanic, 44.7% (SD = 23.5, median = 46.0) identifying as African American/Black, and 35.7% (SD = 22.6, median = 36.0) identifying as White.
Table 3 presents descriptive information on the parameters of the CM protocols used in each study as well as the indicators of study quality. Of the 12 CM treatments, 50% were ABS CM + ATT CM and 50% were ATT CM. Half (50%) used prize CM and half (50%) used voucher CM. The duration of CM protocols ranged from 3 to 56 weeks. The majority of treatments utilized escalating reinforcers with or without a reset condition (83%), and all treatments delivered reinforcement immediately (100%) upon session attendance; therefore, the study dropped this moderator (immediacy) from further analyses. Reinforcement frequency ranged between once every five weeks to seven times per week. The average maximum magnitude of reinforcement available per participant per week was $58.18 (range = $3.50 to $262.50). Across entire CM protocols, the average maximum magnitude of reinforcement available per participant was $353.38 (range = $42 to $880). Per the Cochrane Risk of Bias tool, 60% of the studies were high quality and 40% were low quality.
3.2. Attendance outcomes
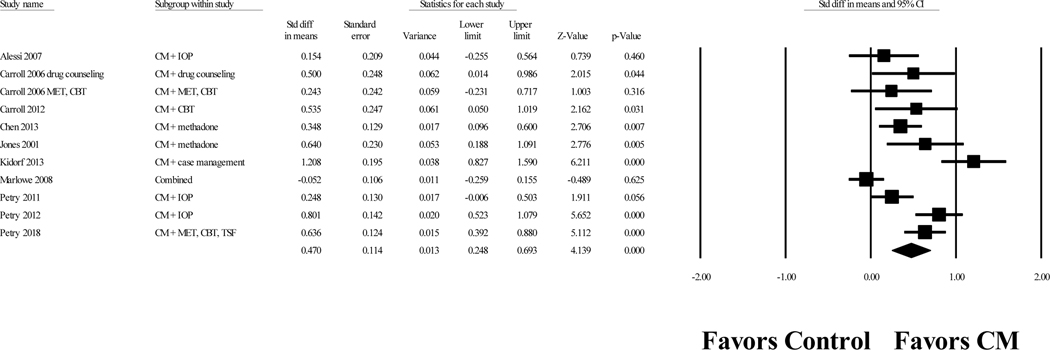
The overall Cohen’s d value for the effect of CM on attendance was 0.47, 95% confidence interval (CI) [0.25, 0.69], p < .001. Figure 2 displays a forest plot of the effect sizes for each study. The effect sizes were highly heterogeneous, Q(10) = 52.02, p < .001, I2 = 80.78. A one study removed analysis resulted in Cohen’s d values ranging from 0.40 to 0.53, indicating that the results were not highly influenced by any single study. A funnel plot of the effect sizes was symmetrical, and the Egger’s regression test did not indicate publication bias (p = 0.14). The fail-safe N indicated that 221 unpublished studies with nonsignificant results would be necessary to reduce this result to a nonsignificant level.
Figure 2.
Forrest plot of Cohen’s d values for contingency management treatment versus comparison conditions on attendance.
Notes. Values are truncated after the third decimal point. The last row in the Forrest plot represents the overall Cohen’s d value of all studies. “Combined” indicates a study with multiple treatment conditions and one control condition. These “combined” studies aggregated data from the multiple treatment conditions and compared the aggregated data to the data of the control condition to create one effect size.
CBT = cognitive-behavioral therapy; CM = contingency management; MET = motivational enhancement therapy; IOP = intensive outpatient treatment; TSF = twelve step facilitation
3.3. Moderators of attendance
Tables 4 and 5 display the results of the meta-regressions and subgroup analyses on attendance. Results of the meta-regressions indicated that there was one significant relation, which was between the effect of ATT CM on attendance and the frequency of reinforcers (i.e., number of times reinforced per week), p = 0.04. For each one unit increase in the number of times reinforced per week, the Cohen’s d value of ATT CM on attendance increased by 0.08. The subgroup analyses indicated that there was one significant moderator of the effect of CM on attendance, which was study quality. Effects of ATT CM on attendance were significantly higher for high quality studies (d = 0.67) than low quality studies (d = 0.20), p = 0.007. The study found no significant difference in attendance between ATT CM and ABS CM + ATT CM.
Table 4.
Results from meta-regressions of several possible moderators on attendance for contingency management treatment targeting attendance.
| Moderator (k) | Point estimate | 95% CI | Z-value | p-value |
|---|---|---|---|---|
| Participant age (11) | ||||
| Slope | 0.02 | −0.01, 0.05 | 1.23 | 0.22 |
| Intercept | −0.12 | −1.07, 0.84 | −0.24 | 0.81 |
| Participant gender: % female (11) | ||||
| Slope | 0.01 | −0.00, 0.01 | 1.52 | 0.13 |
| Intercept | 0.24 | −0.11, 0.59 | 1.34 | 0.18 |
| Participant race: % African American/Black (10) | ||||
| Slope | −0.00 | −0.01, 0.01 | −0.28 | 0.78 |
| Intercept | 0.45 | 0.03, 0.87 | 2.08 | 0.04 |
| Participant race: % White (10) | ||||
| Slope | 0.01 | −0.00, 0.02 | 1.87 | 0.06 |
| Intercept | 0.13 | −0.26, 0.52 | 0.64 | 0.52 |
| Publication year (11) | ||||
| Slope | 0.02 | −0.02, 0.07 | 1.02 | 0.31 |
| Intercept | −50.04 | −147.00, 46.91 | −1.01 | 0.32 |
| Frequency of reinforcers (11) | ||||
| Slope | 0.08 | 0.03, 0.15 | 2.03 | 0.04 |
| Intercept | 0.22 | −0.08, 0.52 | 1.42 | 0.16 |
| Reinforcer magnitude (9) | ||||
| Slope | 0.00 | −0.00, 0.00 | 0.15 | 0.88 |
| Intercept | 0.52 | 0.19, 0.84 | 3.13 | 0.002 |
| Treatment duration (10) | ||||
| Slope | −0.01 | −0.02, 0.00 | −1.92 | 0.05 |
| Intercept | 0.66 | 0.39, 0.93 | 4.75 | < 0.001 |
Table 5.
Results from subgroup analyses of several possible moderating variables on attendance for contingency management treatment targeting attendance.
| Moderator (k) | Cohen’s d Attendance | 95% CI | Q-value | p-value |
|---|---|---|---|---|
| Abstinence + Attendance (11) | 1.47 | 0.22 | ||
| ABS + ATT (6) | 0.33 | 0.19, 0.47 | ||
| ATT (5) | 0.62 | 0.17, 1.06 | ||
| Drugs treated (11) | 6.43 | 0.09 | ||
| Marijuana (3) | 0.42 | 0.14, 0.70 | ||
| Opioids (1) | 0.35 | 0.10, 0.60 | ||
| Polysubstance use (5) | 0.42 | 0.00, 0.84 | ||
| Stimulants (2) | 0.71 | 0.52, 0.89 | ||
| Escalating reinforcers (11) | 0.22 | 0.64 | ||
| No (2) | 0.65 | −0.42, 1.73 | ||
| Yes (9) | 0.39 | 0.19, 0.59 | ||
| Study quality (11) | 7.34 | 0.007 | ||
| High (6) | 0.67 | 0.41, 0.93 | ||
| Low (5) | 0.20 | −0.01, 0.42 | ||
| Type of reinforcer (11) | 0.02 | 0.89 | ||
| Prizes (6) | 0.46 | 0.26, 0.66 | ||
| Voucher (5) | 0.50 | −0.00, 1.00 |
Notes. ABS + ATT = CM protocol reinforced abstinence and attendance simultaneously; ATT = CM protocol reinforced attendance alone
3.4. Drug abstinence outcomes
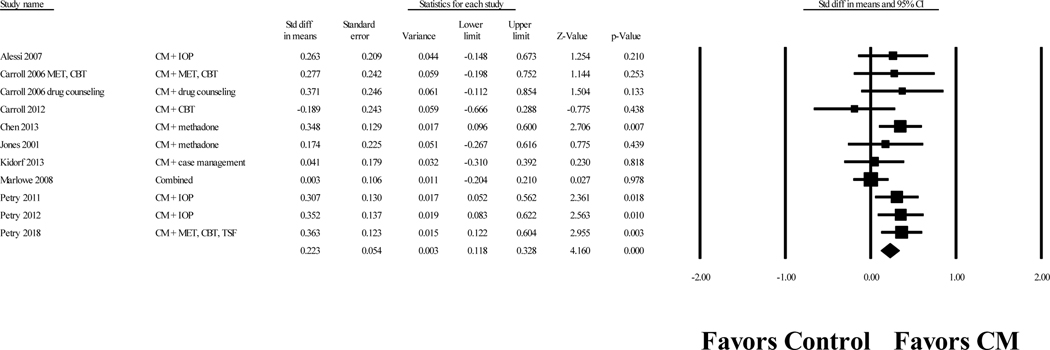
For all 12 CM treatments (both ATT CM and ABS CM + ATT CM), the weighted Cohen’s d effect size on abstinence was 0.22, 95% CI [0.12, 0.33], p < .001. Figure 3 displays a forest plot of the effect sizes for each study. The effect sizes were not significantly heterogeneous, Q(10) = 12.28, p = 0.27, I2 = 18.57. A one study removed analysis resulted in Cohen’s d values ranging from 0.20 to 0.28, indicating that the results were not highly influenced by any single study. A funnel plot of the effect sizes was symmetrical, and the Egger’s regression test did not indicate publication bias (p = 0.42). The fail-safe N indicated that 46 unpublished studies with nonsignificant results would be necessary to reduce this result to a nonsignificant level. ABS CM + ATT CM (d = 0.30) and ATT CM (d = 0.15) effect sizes did not significantly differ, p = 0.21.
Figure 3.
Forest plot of Cohen’s d values for contingency management treatment versus comparison conditions on drug abstinence at posttreatment
Notes. Values are truncated after the third decimal point. The last row in the Forrest plot represents the overall Cohen’s d value of all studies. “Combined” indicates a study with multiple treatment conditions and one control condition. These “combined” studies aggregated data from the multiple treatment conditions and compared the aggregated data to the data of the control condition to create one effect size.
CBT = cognitive-behavioral therapy; CM = contingency management; MET = motivational enhancement therapy; IOP = intensive outpatient treatment; TSF = twelve step facilitation
4. Discussion
The current meta-analysis examined the effect of ATT CM on treatment attendance and drug abstinence. This study identified a total of 12 treatments across 10 studies (6 ATT CM and 6 ABS CM + ATT CM) comprising 1,841 participants. The results indicated that ATT CM had a moderate effect on attendance and a small effect on abstinence. The moderate effect of ATT CM on attendance (d = 0.47) translated to about 68% of those receiving ATT CM evidencing greater treatment attendance compared to participants receiving the same treatment but without tangible attendance rewards. The small effect of ATT CM on abstinence (d = 0.22) translated to about a 54% greater likelihood of abstinence at post-treatment for participants receiving ATT CM compared to participants receiving the same treatment but without tangible attendance rewards.
The results of the current meta-analysis have important implications for clinical practice. The moderate effect of ATT CM on attendance suggests ATT CM is worth the associated cost if the clinician’s desired goal is to increase clients’ treatment attendance. Heterogeneity in the overall effect was large, which may be due to the variation in the frequency of attendance rewards. More frequent attendance rewards were significantly associated with larger effect sizes. Importantly, studies using ATT CM with less than one attendance reward per week appeared to produce no significant effect on attendance (Marlowe et al., 2008), suggesting rewards should occur at least once per week. This research again highlights the importance of the frequency parameter when designing a CM protocol. No other CM parameters (i.e., immediacy, magnitude, escalation of magnitude) were significantly associated with enhanced attendance outcomes. However, no other associations were likely because a lack of variability in CM parameters existed in the included studies, as all CM protocols provided immediate reinforcers and almost all provided escalated reinforcers with or without reset conditions. Consistent with research on ABS CM, ATT CM protocols should include immediate, escalated reinforcers of sufficient magnitude (Griffith et al., 2000; Lussier et al., 2006; Roll et al., 1996).
The small effect of ATT CM on abstinence suggests attendance reinforcement confers some benefits to nonreinforced targets. However, the small effect on abstinence found in this meta-analysis suggests ABS CM is the treatment of choice for promoting abstinence. The effect of ATT CM on abstinence was d = 0.22, which is substantially smaller than the effect of ABS CM on abstinence (Cohen’s d’s between 0.42 and 0.58; Ainscough et al., 2017; Benishek et al., 2014; Dutra et al., 2008; Prendergast et al., 2006).
The current meta-analysis indicated that no statistically significant differences existed in attendance or abstinence outcomes between ATT CM and ABS CM + ATT CM. However, these nonsignificant findings may be due to insufficient statistical power. For attendance, ATT CM’s effect size was d = 0.62 and ABS CM + ATT CM’s effect size was d = 0.33. For abstinence, ATT CM’s effect size was d = 0.15 and ABS CM + ATT CM’s effect size was d = 0.30. These differences (0.29 for attendance and 0.15 for abstinence) are clinically meaningful, and future studies should be conducted to experimentally compare the various conditions to demonstrate additive effects. Researchers have conducted head-to-head comparisons of ABS and ATT CM (e.g., Petry et al., 2012b), but the systematic article search did not identify any experimental manipulations that directly compared ABS CM + ATT CM to ABS CM or ATT CM.
Clinicians should consider these results in conjunction with the possible administrative burdens (i.e., money, time) associated with ABS CM, ATT CM, and ABS CM + ATT CM. ABS CM requires greater administrative burden than ATT CM due to the need to purchase rapid onsite urine test kits, administer urine tests, and review urine test results with clients. ATT CM simply requires clinicians to evaluate whether clients attended treatment, which can be streamlined by implementing well-defined clinic rules for attendance and absences. ABS CM + ATT CM requires clinicians to bear both burdens, but with benefits in both targeted behaviors.
Clinicians should also consider practical barriers, including financial costs and investment in staff time for training. CM training can be relatively circumscribed and limited in number of hours. The Veterans Affairs national roll-out of CM used a training structure of 1.5 days (Petry et al., 2014; Rash et al., 2013), with clinical leaders attending and returning to their home clinics to train additional staff. This training content could easily be restructured into multiple 2 to 4 hour sessions spread over several weeks to better accommodate clinicians’ schedules. Notably, the training duration to achieve initial proficiency in CM is shorter and easier to accommodate compared to some other empirically supported behavioral treatments (Rash et al., 2013). For example, achieving initial proficiency in motivational interviewing often requires months of spaced practice that incorporates performance feedback from experts (Hall et al., 2016). However, all empirically supported behavioral treatments, including CM, require investments for clinicians to achieve initial proficiency and to maintain it over time (Petry et al., 2010; Petry et al., 2012; Rash et al., 2020; Schwalbe et al., 2014). An important future research direction would be comparing the investments of training providers in CM relative to the investments of training providers in other empirically supported behavioral treatments (e.g., cognitive behavioral therapy, motivational interviewing, twelve-step facilitation).
Regardless of the behavioral target, providers should be trained on how to conduct CM protocols that adhere to key parameters (Rash et al., 2012; Rash et al., 2020). Such training might involve discussion of expectations related to differential outcomes based on the selected target behavior (i.e., what to reasonably expect in terms of abstinence changes when reinforcing attendance only). For those considering ATT CM, the results presented here suggest it should be implemented only when frequent opportunities for reinforcement are present (i.e., at least once per week for the duration of the treatment period and higher frequency is better). Specific to stimulant use, CM in combination with another behavioral treatment (e.g., community reinforcement approach, twelve-step facilitation, and cognitive behavioral therapy) appears especially efficacious in promoting abstinence compared to these treatments alone (De Crescenzo et al., 2018; Dutra et al., 2008). CM trainings can address how to integrate CM into common behavioral therapies. An additional consideration relates to the pharmacological treatment of opioid use disorder where studies suggest little added value of non-CM psychosocial treatment above and beyond pharmacological plus medical management treatment for these individuals (e.g., Weiss et al., 2011). Thus, little need exists for a CM program that reinforces attendance to psychosocial treatment in this context, and abstinence or medication adherence targets may be more suitable for CM programs in these settings. Discussion of these findings during trainings can inform the intentional selection of ATT CM versus ABS CM for treatment programs to maximize clients’ gains.
This study highlighted the racial and ethnic diversity of samples included in previous CM studies, which is a strength of this field of research. Many CM studies have been conducted in real-world substance use treatment centers and, thus, samples are more likely to reflect patients receiving services in such settings. In turn, the significant effects of CM, both observed in this meta-analysis and the large majority of CM studies, are likely to hold true when CM is implemented by real-world providers outside of the context of research studies. Further, the diversity of the sample observed in this study allows for the generalization of findings across racial and ethnic categories. Researchers are encouraged to continue to conduct CM studies in collaboration with community-based substance use treatment centers to ensure generalizability to diverse and historically marginalized communities, many of which have a disproportionate need for effective substance use treatments.
Several limitations should be considered when interpreting the results of this meta-analysis. First, we could not include five studies identified in the literature review due to insufficient reporting on treatment attendance and drug abstinence, which may lead to a misrepresentation of the literature. However, the research team found no evidence of publication bias based on funnel plots, the Egger’s regression test, and calculation of the fail-safe N. Second, we did not examine the effect of ATT CM on nonabstinence substance use treatment outcomes. An emerging line of research suggests reductions in substance use frequency and improvements in overall life functioning may be important for evaluating the effectiveness of treatments (e.g., Kiluk et al., 2017; Roos et al., 2019). Future studies should examine the effect of ATT CM on nonabstinence outcomes. Third, only one study included in this meta-analysis was conducted outside of the United States, and we do not know whether the results of the meta-analysis would generalize to other countries. Comparatively fewer studies have been conducted on CM treatments outside of the United States, and further studies are necessary to understand the generalizability of the results from the current study to other cultures.
With these qualifications, the current study adds to the growing literature on the efficacy of CM for treating substance use disorders and proves helpful for both researchers and clinicians. For researchers, this study provided clarification about the strength of ATT CM’s effects on attendance and abstinence. Furthermore, the systematic search indicated that one possible future direction for research is to conduct experiments that compare ABS CM + ATT CM to either ABS CM alone or ATT CM alone. These experiments will further understanding of the additive effects of ABS CM and ATT CM, which is necessary because implementing CM for both target behaviors is likely to increase costs. These increased costs would be justifiable only if the use of both approaches simultaneously and substantially benefits clients’ outcomes. This study provided clarification for clinicians about when to use ABS CM versus ATT CM. Clinicians should use ATT CM if their desired goal is to increase clients’ attendance to treatment, as long as they adopt a CM protocol with sufficient frequency. Clinicians should use ABS CM if their desired goal is abstinence.
Highlights.
Incentives for treatment attendance promote attendance and abstinence
More frequent incentives were significantly associated with greater attendance
Effects of attendance incentives on abstinence were less robust than abstinence incentives
Acknowledgments
This work was supported by the National Institutes of Health, award numbers T32AA018108, R01AA023502, P50AA027055, R01DA047183, K23DA034879, and R01MD013550. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References marked with an asterisk indicate studies included in the meta-analysis
- Ainscough TS, McNeill A, Strang J, Calder R, & Brose LS (2017). Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug and Alcohol Dependence, 178, 318–339. 10.1016/j.drugalcdep.2017.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Alessi SM, Hanson T, Wieners M, & Petry NM (2007). Low-cost contingency management in community clinics: Delivering incentives partially in group therapy. Experimental and Clinical Psychopharmacology, 15(3), 293–300. 10.1037/1064-1297.15.3.293 [DOI] [PubMed] [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, & Festinger DS (2014). Prize based contingency management for the treatment of substance abusers: A meta analysis. Addiction, 109(9), 1426–1436. 10.1111/add.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins J, & Rothstein HR (2009). Chapter 25: Multiple comparisons within a study (pp. 239–242). John Wiley & Sons, Ltd. [Google Scholar]
- Bradley HA, Rucklidge JJ, & Mulder RT (2017). A systematic review of trial registration and selective outcome reporting in psychotherapy randomized controlled trials. Acta Psychiatrica Scandinavica, 135(1), 65–77. doi: 10.1111/acps.12647 [DOI] [PubMed] [Google Scholar]
- *Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, & Rounsaville BJ (2006). The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. Journal of Consulting and Clinical Psychology, 74, 955–966. 10.1037/0022-006X.74.5.955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Carroll KM, Nich C, LaPaglia DM, Peters EN, Easton CJ, & Petry NM (2012). Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: Less can be more, more or less. Addiction, 107, 1650–1659. 10.1111/j.1360-0443.2012.03877.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Chen W, Hong Y, Zou X, McLaughlin MM, Xia Y, & Ling L. (2013). Effectiveness of prize-based contingency management in a methadone maintenance program in China. Drug and Alcohol Dependence, 133, 270–274. 10.1016/j.drugalcdep.2013.05.028 [DOI] [PubMed] [Google Scholar]
- Connors GJ, Walitzer KS, & Dermen KH (2002). Preparing clients for alcoholism treatment: Effects on treatment participation and outcomes. Journal of Consulting and Clinical Psychology, 70, 1161–1169. 10.1037/0022-006X.70.5.1161 [DOI] [PubMed] [Google Scholar]
- Dearing RL, Barrick C, Dermen KH, & Walitzer KS (2005). Indicators of client engagement: Influences on alcohol treatment satisfaction and outcomes. Psychology of Addictive Behaviors, 19, 71–78. 10.1037/0893-164X.19.1.71 [DOI] [PubMed] [Google Scholar]
- De Crescenzo F, Ciabattini M, D’Alò GL, De Giorgi R, Del Giovane C, Cassar C, Janiri L, Clark N, Ostacher MJ, & Cipriani A. (2018). Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: A systematic review and network meta-analysis. PLoS Medicine, 15, e1002715. 10.1371/journal.pmed.1002715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, & Otto MW (2008). A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry, 165(2), 179–187. 10.1176/appi.ajp.2007.06111851 [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, & Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. British Medical Journal, 315, 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginley MK, Pfund RA, Rash CJ, & Zajac K. (2021). Long-term efficacy of contingency management treatment based on objective indicators of abstinence from illicit substance use up to one-year following treatment: A meta-analysis. Journal of Consulting and Clinical Psychology, 89, 58–71. 10.1037/ccp0000552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, & Simpson DD (2000). Contingency management in outpatient methadone treatment: A meta-analysis. Drug and Alcohol Dependence, 58, 55–66. 10.1016/S0376-8716(99)00068-X [DOI] [PubMed] [Google Scholar]
- Hall K, Staiger PK, Simpson A, Best D, & Lubman DI (2016). After 30 years of dissemination, have we achieved sustained practice change in motivational interviewing? Addiction, 111, 1144–1150. 10.1111/add.13014 [DOI] [PubMed] [Google Scholar]
- Hedges LV, & Olkin I. (1985). Statistical methods for meta-analysis. Orlando, FL: Academic Press. [Google Scholar]
- Higgins JP, & Green S. (Eds.). (2011). Cochrane Handbook for Systematic Reviews of Interventions (Vol. 4). John Wiley & Sons. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ: British Medical Journal, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Jones HE, Haug N, Silverman K, Stitzer ML, & Svikis D. (2001). The effectiveness of incentives in enhancing treatment attendance and drug abstinence in methadone-maintained pregnant women. Drug and Alcohol Dependence, 61, 297–306. 10.1016/S0376-8716(00)00152-6 [DOI] [PubMed] [Google Scholar]
- Kelly JF, Kaminer Y, Kahler CW, Hoeppner B, Yeterian J, Cristello JV, & Timko C. (2017). A pilot randomized clinical trial testing integrated 12-Step facilitation (iTSF) treatment for adolescent substance use disorder. Addiction, 112, 2155–2166. 10.1111/add.13920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Kidorf M, Brooner RK, Gandotra N, Antoine D, King VL, Peirce J, & Ghazarian S. (2013). Reinforcing integrated psychiatric service attendance in an opioid-agonist program: A randomized and controlled trial. Drug and Alcohol Dependence, 133, 30–36. 10.1016/j.drugalcdep.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Babuscio TA, Nich C, & Carroll KM (2017). Initial validation of a proxy indicator of functioning as a potential tool for establishing a clinically meaningful cocaine use outcome. Drug and Alcohol Dependence, 179, 400–407. 10.1016/j.drugalcdep.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappan SN, Brown AW, & Hendricks PS (2020). Dropout rates of in person psychosocial substance use disorder treatments: A systematic review and meta analysis. Addiction, 115, 201–217. 10.1111/add.14793 [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, & Petry NM (2013). Behavioral treatment for marijuana dependence: Randomized trial of contingency management and self-efficacy enhancement. Addictive Behaviors, 38, 1764–1775. 10.1016/j.addbeh.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, & Higgins ST (2006). A meta-analysis of voucher based reinforcement therapy for substance use disorders. Addiction, 101, 192–203. 10.1111/j.1360-0443.2006.01311.x [DOI] [PubMed] [Google Scholar]
- *Marlowe DB, Festinger DS, Dugosh KL, Arabia PL, & Kirby KC (2008). An effectiveness trial of contingency management in a felony preadjudication drug court. Journal of Applied Behavior Analysis, 41, 565–577. 10.1901/jaba.2008.41-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151, 264–269. 10.1371/journal.pmed.1000097 [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. (2018). Principles of drug addiction treatment: A research-based guide (3rd ed.). Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services. [Google Scholar]
- Onken LS, Blaine JD, & Boren JJ (1997). Beyond the therapeutic alliance: Keeping the drug-dependent individual in treatment (Vol. 165). US Department of Health and Human Services, National Institutes of Health, National Institute on Drug Abuse, Division of Clinical and Services Research. [Google Scholar]
- Petry NM, Alessi SM, & Ledgerwood DM (2012a). Contingency management delivered by community therapists in outpatient settings. Drug and Alcohol Dependence, 122, 86–92. 10.1016/j.drugalcdep.2011.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Ledgerwood DM, & Sierra S. (2010). Psychometric properties of the contingency management competence scale. Drug and Alcohol Dependence, 109, 167–174. 10.1016/j.drugalcdep.2009.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Olmstead TA, Rash CJ, & Zajac K. (2017). Contingency management treatment for substance use disorders: How far has it come, and where does it need to go? Psychology of Addictive Behaviors, 31, 897–906. 10.1037/adb0000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Petry NM, Alessi SM, Rash CJ, Barry D, & Carroll KM (2018). A randomized trial of contingency management reinforcing attendance at treatment: Do duration and timing of reinforcement matter? Journal of Consulting and Clinical Psychology, 86, 799–809. 10.1037/ccp0000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Petry NM, Barry D, Alessi SM, Rounsaville BJ, & Carroll KM (2012b). A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. Journal of Consulting and Clinical Psychology, 80, 276–285. 10.1037/a0026883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, DePhilippis D, Rash CJ, Drapkin M, & McKay JR (2014). Nationwide dissemination of contingency management: The Veterans Administration initiative. The American Journal on Addictions, 23, 205–210. 10.1111/j.1521-0391.2014.12092.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Petry NM, Weinstock J, & Alessi SM (2011). A randomized trial of contingency management delivered in the context of group counseling. Journal of Consulting and Clinical Psychology, 79, 686–696. 10.1037/a0024813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, & Roll J. (2006). Contingency management for treatment of substance use disorders: A meta analysis. Addiction, 101, 1546–1560. 10.1111/j.1360-0443.2006.01581.x [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Higgens ST, Brooner RK, Montoya I, Schuster CR, & Cone EJ (1998). Cocaine use early in treatment predicts outcome in a behavioral treatment program. Journal of Consulting and Clinical Psychology, 66, 691–696. 10.1037/0022-006X.66.4.691 [DOI] [PubMed] [Google Scholar]
- Rash CJ, Alessi SM, & Zajac K. (2020). Examining implementation of contingency management in real-world settings. Psychology of Addictive Behaviors, 34, 89–98. 10.1037/adb0000496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash CJ, DePhilippis D, McKay JR, Drapkin M, & Petry NM (2013). Training workshops positively impact beliefs about contingency management in a nationwide dissemination effort. Journal of Substance Abuse Treatment, 45, 306–312. 10.1016/j.jsat.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash CJ, Petry NM, Kirby KC, Martino S, Roll J, & Stitzer ML (2012). Identifying provider beliefs related to contingency management adoption using the contingency management beliefs questionnaire. Drug and Alcohol Dependence, 121, 205–212. 10.1016/j.drugalcdep.2011.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, & Badger GJ (1996). An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis, 29, 495–504. 10.1901/jaba.1996.29-495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CR, Nich C, Mun CJ, Babuscio TA, Mendonca J, Miguel AQ, DeVito EE, Yip SW, Witkiewitz K, Carroll KM, & Kiluk BD (2019). Clinical validation of reduction in cocaine frequency level as an endpoint in clinical trials for cocaine use disorder. Drug and Alcohol Dependence, 205, 107648. 10.1016/j.drugalcdep.2019.107648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. (1979). The file drawer problem and tolerance for null results. Psychological Bulletin, 86, 638–641. 10.1037/0033-2909.86.3.638 [DOI] [Google Scholar]
- Schwalbe CS, Oh HY, & Zweben A. (2014). Sustaining motivational interviewing: A meta- analysis of training studies. Addiction, 109, 1287–1294. 10.1111/add.12558 [DOI] [PubMed] [Google Scholar]
- Sebastian F, Mushtaq S, Easow JM, & Luty J. (2012). Number needed to treat further engaged opioid-dependent clients following missed appointments. Journal of Substance Use, 17, 235–239. 10.3109/14659891.2011.565108 [DOI] [Google Scholar]
- Simpson DD, & Joe GW (2004). A longitudinal evaluation of treatment engagement and recovery stages. Journal of Substance Abuse Treatment, 27, 89–97. 10.1016/j.jsat.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Peirce J, Petry NM, Kirby K, Roll J, Krasnansky J, Cohen A, Blaine J, Vandrey R, Kolodner K, & Li R. (2007). Abstinence-based incentives in methadone maintenance: Interaction with intake stimulant test results. Experimental and Clinical Psychopharmacology, 15, 344. 10.1037/11855-022 [DOI] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fielln DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, Hasson AL, Huang Z, Jacobs P, Kosinski AS, Lindblad R, McCance-Katz EF, Provost SE, Selzer J, Somoza EC, Sonne SC, & Ling W. (2011). Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Archives of General Psychiatry, 68, 1238–1246. doi: 10.1001/archgenpsychiatry.2011.121 [DOI] [PMC free article] [PubMed] [Google Scholar]