Abstract
Acinetobacter baumannii is the main causative pathogen of nosocomial infections that causes severe infections in the lungs. In this study, we analyzed the histopathological characteristics of lung infection with two strains of A. baumannii (ATCC 19606 and the clinical isolate TK1090) and Pseudomonas aeruginosa PAO-1 in C3H/HeN mice to evaluate the virulence of A. baumannii. Survival was evaluated over 14 days. At 1, 2, 5, or 14 days postinfection, mice of C3H/HeN were sacrificed, and histopathological analysis of lung specimens was also performed. Histopathological changes and accumulation of neutrophils and macrophages in the lungs after infection with A. baumannii and P. aeruginosa were analyzed. Following intratracheal inoculation, the lethality of ATCC 19606- and TK1090-infected mice was lower than that of PAO-1-infected mice. However, when mice were inoculated with a sub-lethal dose of A. baumannii, the lung bacterial burden remained in the mice until 14 days post-infection. Additionally, histopathological analysis revealed that macrophages infiltrated the lung foci of ATCC 19606-, TK1090-, and PAO-1-infected mice. Although neutrophils infiltrated the lung foci of ATCC 19606- and TK1090-infected mice, they poorly infiltrated the lung foci of PAO-1-infected mice. Accumulation of these cells in the lung foci of ATCC 19606- and TK1090-infected mice, but not PAO-1-infected mice, was observed for 14 days post-infection. These results suggest that A. baumannii is not completely eliminated despite the infiltration of immune cells in the lungs and that inflammation lasts for prolonged periods in the lungs. Further studies are required to understand the mechanism of A. baumannii infection, and novel drugs and vaccines should be developed to prevent A. baumannii infection.
Keywords: Acinetobacter baumannii, neutrophils, macrophages, a mouse lung infection model
Introduction
Acinetobacter baumannii is an important opportunistic pathogen associated with nosocomial infections, including bacteremia, pneumonia, meningitis, urinary tract infections, and wound infections (Fournier and Rich et al. 2006; Munoz-Price and Weinstein 2008). Recently, multidrug-resistant A. baumannii, the main causative pathogen of nosocomial infections worldwide, has become a significant problem (Kempf and Rolain 2012; Antunes et al. 2014; Ushizawa et al. 2016). Additionally, although A. baumannii is regarded as a low-virulence pathogen (Peleg et al. 2008), it possesses several mechanisms of pathogenicity, including biofilm formation, adherence, and invasion of lung epithelial cells, host cell death, and iron acquisition (Uppalapati et al. 2020). Thus, the development of new treatments or vaccine therapies is required to manage A. baumannii infection (Gellings et al. 2020). To achieve this, the virulence of A. baumannii and the host immune response against the pathogen must be clarified (Eveillard et al. 2010).
Animal models for bacterial infection are often considered valuable tools for developing new drugs and vaccines (Byrne et al. 2020). Some mouse models of A. baumannii infection have been established, and immune responses and treatments against the pathogen have been analyzed (Crandon et al. 2009; Qiu et al. 2009a; 2009b; Jacobs et al. 2010). In particular, neutrophils and alveolar macrophages (AMs) play important roles in host resistance to respiratory infections by A. baumannii (van Faassen et al. 2007; Qiu et al. 2012; Lee et al. 2020). In addition, Toll-like receptor (TLR) 2 and 4 play key roles in eliciting innate immune responses against A. baumannii infection (Knapp et al. 2006; Kim et al. 2014). These results suggest that innate immune responses play important roles in host resistance to early A. baumannii infection. In pneumoniae models of A. baumannii infection, hypervirulent strains (LAC-4 and SJZ24) induced rapid bacterial replication, cytokine induction in the lungs, significant extrapulmonary dissemination, and severe bacteremia by 24 h postintranasal inoculation (Harris et al. 2013; Zeng et al. 2019). These studies have focused on immune responses during the early stage of A. baumannii infection because of the lethal pneumonia models of A. baumannii. Although A. baumannii is regarded as a low-virulence pathogen, it possesses several mechanisms of evading immune responses (e.g., capsule (Geisinger and Isberg 2015) and catalase expression (Sato et al. 2019), and inhibition of neutrophil extracellular traps (NETs) (Kamoshida et al. 2018). Thus, it is a possibility that A. baumannii can survive in the host for prolonged periods. However, there is relatively little literature on the immune responses against A. baumannii for long-term persistent infection and histopathological analysis of A. baumannii lung infection.
Both A. baumannii and P. aeruginosa are included among the six critical nosocomial pathogens: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter spp. (ESKAPE) that acquire multidrug resistance and virulence (Mulani et al. 2019). Additionally, A. baumannii and P. aeruginosa are opportunistic pathogens associated with nosocomial infections, including pneumonia, so that these bacteria have gained importance as a human pathogen in hospital environments. In this study, we established an A. baumannii mouse lung infection model in commonly used experimental C3H/HeN mice and analyzed whether A. baumannii showed long-term infection compared to P. aeruginosa as a major bacteria caused by pneumoniae. Additionally, A. baumannii isolated from a patient with pneumonia (TK1090 strain) was evaluated whether the clinical isolate was a high virulent strain or not. In this model, although intratracheal inoculation with a high dose of A. baumannii was shown to be lethal, in low doses of A. baumannii inoculation, temporal changes in the exudation of immune cells, such as neutrophils and macrophages, to the infected foci and changes in pulmonary lesions were observed.
Experimental
Materials and Methods
All methods were performed in accordance with relevant guidelines and regulations.
Mice. Five-week-old female C3H/HeN mice were purchased from Japan SLC (Shizuoka, Japan). All animal experiments were performed in accordance with the Institutional Animal Care and Use Committee of Teikyo University Animal Ethics Committee (Approval No. 12–079). Five animals were housed per cage and provided with sterilized food and water ad libitum.
Bacterial culture. ATCC 19606 strains of A. baumannii and P. aeruginosa PAO-1 were used as standard strains in this study. The A. baumannii TK1090 strain was isolated during an Acinetobacter outbreak in 2010 from a patient with pneumonia and preserved at Teikyo University of Medical Hospital, Tokyo, Japan. The isolates were streaked onto blood agar plates and cultivated for 24 hours to obtain monoclonal colonies and identified as A. baumannii by DNA sequencing of a partial RNA polymerase β-subunit (rpoB) gene (La Scola et al. 2006). Additionally, the isolates were confirmed as non-clonal by pulsed-field gel electrophoresis (data not shown). After identification, these isolates were stored in glycerol stocks at –80°C at the Department of Microbiology and Immunology, Teikyo University School of Medicine. Bacteria were grown until the mid-logarithmic phase at 37°C in Luria-Bertani broth (Difco, Detroit, MI) and then washed and resuspended in sterile isotonic saline (106–108 CFU/50 μl).
Infection. Mice were infected by the strains of A. baumannii and P. aeruginosa PAO-1 as described by Bernabeu-Wittel et al. (2005). Mice were then anesthetized by the intraperitoneal administration of 0.6 ml/kg of a mixed anesthetic consisting of 15 μg/ml dimorphoramine (Eisai Tokyo, Japan), 120 μg/ml xylazine (Bayer, Leverkusen, Germany), and 600 μg/ml sodium pentobarbital (Dainippon Sumitomo Pharma, Tokyo, Japan). A suspension of bacteria in a volume of 50 μl was inoculated intratracheally. A 25-gauge X 23/8 disposable needle (Top, Tokyo, Japan) was used to deliver the inoculum to the trachea. Mice fully recovered within 30 min after the procedure, and none died due to the inoculation procedure.
Survival studies. Survival of the animals (n = 7 or 8 for uninfected and infected-mice, respectively) was checked daily for 14 days post-infection. On day 1 after the infection and at the end of the experiment, the surviving mice were killed by CO2 anoxia. Their lungs and kidneys were removed and placed in 1 ml of sterile saline and homogenized. A total of 0.05 ml of each homogenate was spread onto LB agar plates, and the bacterial count (CFU) was confirmed by the growth of the bacteria on LB agar after 24 h of incubation at 37°C.
Histological examination. Lung tissues from the pneumonia mouse model were harvested under sterile conditions. All lung samples were fixed with 4% formalin, embedded in paraffin, sliced, and stained with hematoxylin and eosin (HE). The slices were observed at 100 × magnification (Luna et al. 2019). Granulocyte staining was performed as described by Gong et al. (2013). Neutrophils and macrophages were immunostained with anti-Ly-6G antibody (clone: RB6-8C5; Abcam, Tokyo, Japan) and anti-CD68 antibody (clone: FA-11; Abcam), respectively, and were detected using diaminobenzidine (DAB) method and restained with hematoxylin. The sections were observed under a light microscope (Nikon). The number of granulocytes was analyzed using HALO® image analysis software, the standard image analysis platform for quantitative tissue analysis in digital pathology (Albuquerque, NM, USA).
Statistical analysis. Bacterial density and immune cell rates were compared using the unpaired t-test. Differences were considered significant if p < 0.05.
Results
Survival rates of A. baumannii and P. aeruginosa-infected C3H/HeN mice. We examined the survival rates of C3H/HeN mice after intratracheal infection with A. baumannii and P. aeruginosa. When 1.5 × 108 CFU of ATCC 19606 and 3.2 × 108 CFU of TK1090 were inoculated, all mice died within three days post-infection (Figs. 1A and 1B). Meanwhile, when 9 × 107 of ATCC 19606 and 1.5 × 108 CFU of TK1090 were inoculated, the survival rates were 50–80% (Fig. 1A and 1B). When 3.8×107 CFU of P. aeruginosa PAO-1 was inoculated, all mice died within three days. Meanwhile, when 2.5 ×106 of P. aeruginosa PAO-1 was inoculated, the survival rate was 50% (Fig. 1C). These results indicate that although the ATCC 19606 and TK1090 strains of A. baumannii have low virulence compared with P. aeruginosa PAO-1, the inoculation of a high dose of A. baumannii to the lungs leads to the development of a lethal phenotype.
Fig. 1.
Survival rates of mice with a lung infection. Survival rates of CH3/He mice following intratracheal inoculation with ATCC 19606 (A), TK1090 (B), and PAO-1 (C) strains. Groups of mice (ATCC 19606, TK1090, and PAO-1; n = 7 or 8 for uninfected and infected mice, respectively) were intratracheally inoculated as indicated, and their clinical outcome was monitored daily for up to 14 days.
Lung bacterial burden remains in A. baumannii-infected C3H/HeN mice for the long term. To confirm the number of bacteria in the lungs of C3H/HeN mice after infection, a sub-lethal dose (107 CFU) of ATCC 19606 and TK10190 were inoculated into the lungs of the mice. On day 1 after inoculation, 8 × 106 CFU/g of ATCC19606 and 2.5 × 107 CFU/g of TK1090 were detected in the lungs. Moreover, 1.4 × 103 CFU/g of ATCC 19606 and 2.1 × 104 CFU/g of TK1090 were detected in the kidneys (Fig. 2A). Additionally, 1.4×103 CFU/g of ATCC 19606 and 2.5 × 103 CFU/g of TK1090 were detected in the lungs on day 14 after inoculation; however, neither of the pathogens was detected in the kidneys (Fig. 2B). These results indicate the long-term colonization of A. baumannii in the lungs of C3H/HeN mice.
Fig. 2.
Bacterial densities in mice infected with Acinetobacter baumannii. A. baumannii bacterial load in the lungs and kidneys of female C3H/HeN mice (groups of 3 mice for ATCC 19606- and TK1090-infected mice, respectively) intratracheally inoculated with 107 CFU of ATCC 19606 (open) and TK109 (closed) strains. Bacterial load in the respective organs was determined by quantitative bacteriology at 1 day (A) and 14 days (B) post-infection. The data are presented as the mean + standard deviation (SD). The detection limits for bacterial load were 1.3 log10 CFU/organ for the lung and kidney. Asterisks indicate statistically significant differences in the number of bacteria (*p < 0.05; ATCC 19606 vs. TK1090; unpaired t-test).
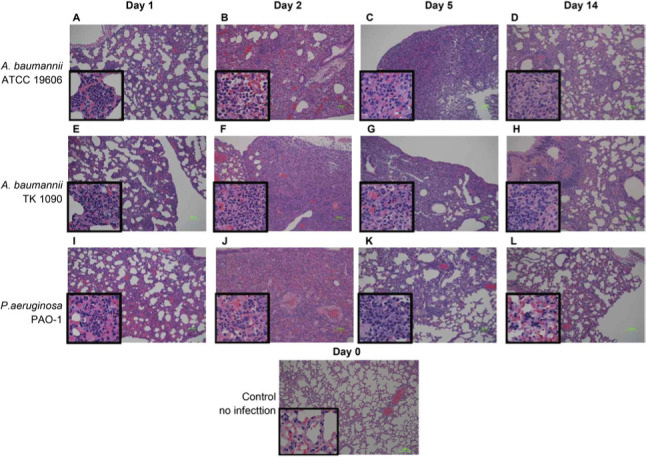
A. baumannii elicits pulmonary inflammation in C3H/HeN mice for the long term. To analyze histopathological changes in the lungs of C3H/HeN mice after infection with A. baumannii, 1×107 CFU of ATCC 19606, 1 × 107 CFU of TK1090 of A. baumannii, and 1×106 CFU of P. aeruginosa PAO-1, which were less than the lethal dose of the bacteria, were inoculated into the lungs of C3H/HeN mice. After the inoculation, the pathogen-infected lungs were resected at 0, 1, 2, 5, and 14 days post-infection, and the individual samples were stained with HE and pathologically analyzed (Fig. 3). On day 1 after inoculation, inflammation was observed in the alveoli of ATCC 19606-, TK1090-, and PAO-1-infected mice and inflammatory infiltrates were observed in the alveolar lumen of these mice, as shown in the magnified images (Fig. 3A, 3E, and 3I). On days 2 and 5 after inoculation, inflammatory infiltrates were extensively observed in the lung tissues of these mice (Fig. 3B, 3C, 3F, 3G, 3J, and 3K). However, on day 14 after inoculation, although the areas of inflammation and inflammatory cell counts were reduced in these mice, the A. baumannii-infected mice recovered from inflammation at a slower rate than the P. aeruginosa-infected mice (Fig. 3D, 3H, and 3L). These results suggest that inflammation caused by A. baumannii infection is prolonged in the lungs of mice compared with P. aeruginosa infection.
Fig. 3.
Histopathological analysis of the lung tissues from Acinetobacter baumannii- and Pseudomonas aeruginosa-infected mice. The lung tissues from C3H/HeN mice infected with ATCC 19606 (A, B, C, and D), TK1090 (E, F, G, and H), and PAO-1 (I, J, K, and L) strains and uninfected mouse (M) are shown. The lung tissues from the mice infected with ATCC 19606 at 1 day (A), 2 days (B), 5 days (C), and 14 days (D) post-infection. The lung tissues from the mice infected with TK1090 at 1 day (E), 2 days (F), 5 days (G), and 14 days (H) post-infection. The lung tissues from the mice infected with PAO-1 at 1 day (I), 2 days (J), 5 days (K), and 14 days (L) post-infection. Photomicrograph images (magnification, 100×; hematoxylin and eosin staining). Squares represent high magnification (400×).
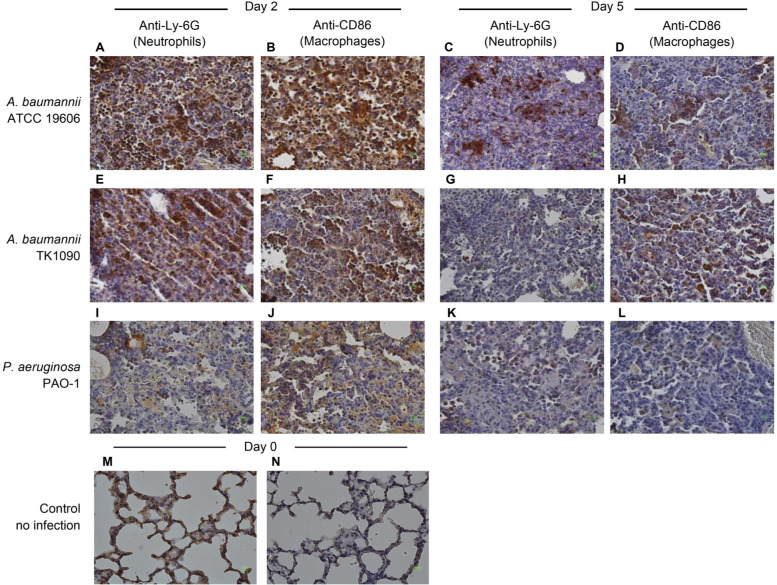
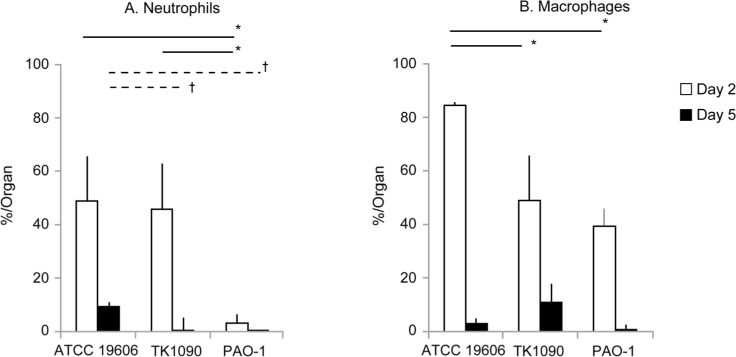
A. baumannii infection prolongs the accumulation of neutrophils and macrophages in the lung tissues. To identify the infiltrating cells in the lung tissues of A. baumannii-infected mice, lung tissues were immunostained using anti-Ly-6G and anti-CD68 antibodies to detect neutrophils and macrophages, respectively. On day 2 after inoculation with A. baumannii, infiltration of neutrophils and macrophages was observed in the lung tissues of ATCC 19606- and TK1090-infected mice (Fig. 4A, 4B, 4E, and 4F). On day 5 after inoculation, infiltration of neutrophils was mainly observed in the inflamed tissues of ATCC 19606-infected mice (Fig. 4C and 4D), whereas infiltration of macrophages was mainly observed in the inflamed tissues of TK1090-infected mice (Fig. 4G and 4H). In the case of PAO1 infection, although only the infiltration of macrophages was observed in the lung tissues of the mice at two days post-infection (Fig. 4I and 4J), neither neutrophils nor macrophages were detected in the lung tissues of the mice at five days post-infection (Fig. 4K and 4L). Moreover, we counted the number of immune cells in the inflamed tissues, as shown in Fig. 5. On day 2 after infection, 49% and 46% of neutrophils were detected in the inflamed tissue of ATCC 19606- and TK1090-infected mice (Fig. 5A). Additionally, 85% and 49% of macrophages were detected in the inflamed tissue of TK1090-infected mice (Fig. 5A). On day 5 after infection, 9.5% of neutrophils were detected in the inflamed tissue of ATCC 19606-infected mice (Fig. 5A, ATCC 19606), whereas 11% of macrophages were detected in the inflamed tissue of TK1090-infected mice (Fig. 5B, TK1090). Meanwhile, 3% of neutrophils and 39% of macrophages were detected in the inflamed tissue of PAO-1-infected mice at two days post-infection (Fig. 5A and 5B, PAO-1). These results suggest that A. baumannii induces the infiltration of inflammatory cells and prolongs the accumulation of neutrophils and macrophages in the lung tissues. Additionally, different induction patterns of immune cells were observed between ATCC 19606 and TK 1090 strains, suggesting that the virulence of A. baumannii is strain-dependent.
Fig. 4.
Infiltration of neutrophils and macrophages in the lung tissues of Acinetobacter baumannii- and Pseudomonas aeruginosa-infected mice. The lung tissues from C3H/HeN mice infected with ATCC19606 (A, B, C, and D), TK1090 (E, F, G, and H), and PAO-1 (I, J, K, and L) strains and uninfected mice (M and N) are shown. The lung tissues from the mice infected with ATCC 19606 at 1 day (A and B) and 5 days (C and D) post-infection. The lung tissues from the mice infected with TK1090 at 1 day (E and F) and 5 days (G and H) post-infection. The lung tissues from the mice infected with PAO-1 at 1 day (I and J) and 5 days (K and L) post-infection. Neutrophils and macrophages were detected by immunostaining with anti-Ly-6G antibody (A, C, E, G, I, and K) and anti-CD68 antibody (B, D, F, H, J, and L) using diaminobenzidine (DAB) method and restained with hematoxylin, respectively. Photomicrograph images (magnification, 100×).
Fig. 5.
Frequency of neutrophils and macrophages accumulated in the lung tissues of Acinetobacter baumannii- and Pseudomonas aeruginosa-infected mice. Infiltrated neutrophils and macrophages in the lung tissues of the infected mice were detected by monoclonal antibodies using diaminobenzidine (DAB) method and restained with hematoxylin, and were measured by the HALO image analysis system for counting of cell numbers. The frequencies of neutrophils (A) and macrophages (B) in the mice post-infection are shown. The frequencies of infected mice on day 2 and day 5 are shown as open and closed bars, respectively. Symbols indicate statistically significant differences (*p < 0.05, C3H/HeN mice infected with A. baumannii strains ATCC 19606 or TK1090 at 2 days post-infection vs. C3H/HeN mice infected with P. aeruginosa PAO-1 at 2 days post-infection, unpaired t-test; †p < 0.05, C3H/HeN mice infected with A. baumannii strains ATCC 19606 or TK1090 at 5 days post-infection vs. C3H/HeN mice infected with P. aeruginosa PAO-1 at 5 days post-infection, unpaired t-test).
Discussion
A. baumannii mainly infects the respiratory tract, and pneumonia is one of the main clinical manifestations of A. baumannii infection. In the present study, we established an A. baumannii pneumonia infection model in C3H/HeN mice and showed the temporal changes in the accumulation of neutrophils and macrophages, which are the main cells involved in innate immunity, in infected foci as well as temporal changes in viable bacterial cell count and lung lesions. In the lung infection model of C3H/HeN mice, the virulence of P. aeruginosa PAO-1 strain was the highest, in terms of lethal effect, followed by that of A. baumannii reference ATCC 19606 strain and clinical isolate TK1090 strain.
A previous study has reported that C57BL/6 mice were intranasally inoculated with A. baumannii ATCC 19606, and lung bacterial burden was detected in the mice at 1 day post-infection (Harris et al. 2013). Similarly, we detected lung bacterial burden in C3H/HeN mice at 1 day post-infection and subsequently detected them at 14 days post-infection. Although ATCC 19606 strain shows lower virulence than other clinical isolates, there are various researches of A. baumannii virulence using ATCC 19606 in vitro (e.g., biofilm formation, adherence, and invasion of lung epithelial cells, host cell death, and iron acquisition) (Uppalapati et al. 2020). Especially, in vitro studies suggested that A. baumannii induces inflammatory lung responses in pneumonia by adhering to lung epithelial cells (Uppalapati et al. 2020) and survives within macrophages in vitro (Sato et al. 2019). These results indicate that A. baumannii infection may prolong pneumonia. Therefore, A. baumannii ATCC 19606 is a useful strain to clarify the pathogenicity of A. baumannii infection and immune responses in pneumonia model. This study established a pneumonia model of C3H/HeN mice infected with ATCC 19606 strain. Our mouse model is expected to serve the knowledge of pneumonia caused by A. baumannii infection and becomes a valuable tool for the analysis of A. baumannii infection.
In the A. baumannii pneumonia model, inflammation was induced in the lungs, and the infiltration of neutrophils and macrophages was observed during the early phase of infection. Neutrophils play an important role in protecting against A. baumannii infection (Knapp et al. 2006; van Faassen et al. 2007; Qiu et al. 2009b). The analysis of cells collected from bronchoalveolar lavage fluid or crushed resected lung tissues by fluorescence-activated cell sorting (FACS), using a fluorescent antibody in a mouse lung infection model, has been reported in a study (Qiu et al. 2012). These methods allow the simple measurement of cells that exuded into infected lung tissues. However, analysis using tissue slices is essential to detect immune cells accumulated in the foci and elucidate the relationship between these cells and lung lesions. In the analysis of lesions caused by pneumonia, many inflammatory cells were detected by HE staining. In addition, immunostaining with specific antibodies allows the detection of neutrophils and macrophages. Accumulation of neutrophils was observed in the infected foci in an A. baumannii lung infection mouse model using histopathological analysis (Qiu et al. 2009a). Additionally, inflammatory cytokines, such as TNF-α, were detected in the lungs of mice (Qiu et al. 2009a).
When co-cultured with A. baumannii in vitro, human neutrophils strongly produce not only TNF-α but also IL-8 (Kamoshida et al. 2016). These inflammatory cytokines contribute to the exudation of macrophages (Joly-Guillou et al. 2000; Qiu et al. 2009a; 2009b). Therefore, these studies suggest that immune cells, such as neutrophils and macrophages, accumulate in A. baumannii lung infection foci and play an important role eliminating A. baumannii. Likewise, in our study, the accumulation of neutrophils and macrophages on day 2 after A. baumannii infection in the mice with pneumonia implies the involvement of inflammatory cytokines produced in the lung tissues and subsequent activation of complement components, such as C5a. Moreover, on day 5 after A. baumannii infection, although the number of neutrophils and macrophages was decreased in the lung tissues, these cells accumulated in the lung lesions. However, viable bacterial cells were detected in the lung tissues even on day 14 after infection, suggesting that this pathogen might be resistant to phagocytosis by neutrophils. Our previous study demonstrated that A. baumannii-stimulated phagocytosis by neutrophils, and ROS production was weaker than that stimulated by P. aeruginosa (Kamoshida et al. 2016). Meanwhile, the reason the mice did not die, although the pathogen survived for a long time without undergoing phagocytosis by neutrophils, might be related to the fact that the pathogen did not produce exotoxins and that its virulence was weak. In contrast, although phagocytosed by neutrophils, P. aeruginosa produces pathogenic factors, such as leucocidins, exotoxins, and pyocyanin, which can destroy immune cells, such as neutrophils (Gellatly and Hancock 2013; Kamoshida et al. 2016). The reason for the low neutrophil count on day 2 after P. aeruginosa lung infection could be attributed to the phagocytosis by neutrophils and associated neutrophil damage by the pathogenic factors produced by P. aeruginosa (Fig. 4 and 5). The extent of lung tissue damage caused by P. aeruginosa may also depend on these pathogenic factors (Usher et al. 2002; Lau et al. 2004; Allen et al. 2005; Gellatly and Hancock 2013; Yong et al. 2018).
Our study demonstrated that viable bacterial cells were detected in the kidneys 24 h after the inoculation of A. baumannii in the lungs, showing that systemic dissemination of infection occurred in the early stages. Since A. baumannii is not flagellated, it does not exhibit motility. Regarding the systemic dissemination of this pathogen, we hypothesized that although A. baumannii is attached to neutrophils, it does not undergo phagocytosis but adheres to neutrophils to migrate from infected lesions (Kamoshida et al. 2016). Thus, pulmonary inflammation and systemic dissemination (e.g., bacteremia) should be noted in A. baumannii via respiratory tract infections.
Previous studies have reported A. baumannii-infected mouse models using C57BL/6 (H2b) and Balb/c (H2d) (García-Patiño et al. 2017). When these mice were infected with a sub-lethal dose of A. baumannii, bacteria were eliminated in their mice within seven days. As we showed that A. baumannii was not eliminated in the lungs of C3H/HeN (H2k) mice for at least 14 days, these results imply the possibility that the difference of mouse MHC haplotype effects the immune responses for A. baumannii infection. The MHC haplotype affects T-cell recognition of Mycobacterium tuberculosis antigens (Kamath et al. 2004). Further studies might be required to analyze the immune responses for A. baumannii infection in a different strain of mice.
We established a mouse model of A. baumannii lung infection and analyzed the temporal and histopathological changes in lung lesions, accumulation of neutrophils and macrophages involved in innate immunity in infected foci, and viable bacterial cell counts in the foci. Although immune responses against A. baumannii for long-term infection have not been made evident in this study, we demonstrated that the pathogen survived even in the convalescent phase of infection, that is, at 14 days after infection. We believe that this mouse model will be helpful for analyzing the virulence of this pathogen, which survives by escaping from the biodefense mechanism, and for developing future treatments of infectious diseases and a methodology for vaccine efficacy studies.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) [KAKENHI 17K10032 & 20K08827] and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) [Private University Research Branding Project].
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Allen L, Dockrell DH, Pattery T, Lee DG, Cornelis P, Hellewell PG, Whyte MK. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J Immunol. 2005. Mar 15;174(6):3643–3649. 10.4049/jimmunol.174.6.3643 [DOI] [PubMed] [Google Scholar]
- Antunes LC, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014. Aug;71(3):292–301. 10.1111/2049-632X.12125 [DOI] [PubMed] [Google Scholar]
- Bernabeu-Wittel M, Pichardo C, García-Curiel A, Pachón-Ibáñez ME, Ibáñez-Martínez J, Jiménez-Mejías ME, Pachón J. Pharmacokinetic/pharmacodynamic assessment of the in vivo efficacy of imipenem alone or in combination with amikacin for the treatment of experimental multiresistant Acinetobacter baumannii pneumonia. Clin Microbiol Infect 2005;11:319–325. 10.1111/j.1469-0691.2005.01095.x [DOI] [PubMed] [Google Scholar]
- Byrne JM, Waack U, Weinstein EA, Joshi A, Shurland SM, Iarikov D, Bulitta JB, Diep BA, Guina T, Hope WW, et al. FDA Public Workshop Summary: advancing animal models for antibacterial drug development. Antimicrob Agents Chemother. 2020. Dec 16;65(1): e01983-20. 10.1128/AAC.01983-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandon JL, Kim A, Nicolau DP. Comparison of tigecycline penetration into the epithelial lining fluid of infected and uninfected murine lungs. J Antimicrob Chemother. 2009. Oct;64(4):837–839. 10.1093/jac/dkp301 [DOI] [PubMed] [Google Scholar]
- Eveillard M, Soltner C, Kempf M, Saint-André JP, Lemarié C, Randrianarivelo C, Seifert H, Wolff M, Joly-Guillou ML. The virulence variability of different Acinetobacter baumannii strains in experimental pneumonia. J Infect. 2010. Feb;60(2):154–161. 10.1016/j.jinf.2009.09.004 [DOI] [PubMed] [Google Scholar]
- Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006. Mar 1;42(5):692–699. 10.1086/500202 [DOI] [PubMed] [Google Scholar]
- García-Patiño MG, García-Contreras R, Licona-Limón P. The immune response against Acinetobacter baumannii, an emerging pathogen in nosocomial infections. Front Immunol. 2017. Apr 12;8:441. 10.3389/fimmu.2017.00441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisinger E, Isberg RR. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 2015. Feb 13;11(2):e1004691. 10.1371/journal.ppat.1004691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013. Apr;67(3): 159–173. 10.1111/2049-632X.12033 [DOI] [PubMed] [Google Scholar]
- Gellings PS, Wilkins AA, Morici LA. Recent advances in the pursuit of an effective Acinetobacter baumannii vaccine. Pathogens. 2020. Dec 19;9(12):1066. 10.3390/pathogens9121066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Cumpian AM, Caetano MS, Ochoa CE, De la Garza MM, Lapid DJ, Mirabolfathinejad SG, Dickey BF, Zhou Q, Moghaddam SJ. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol Cancer. 2013. Dec 9; 12(1):154. 10.1186/1476-4598-12-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G, Kuo Lee R, Lam CK, Kanzaki G, Patel GB, Xu HH, Chen W. A mouse model of Acinetobacter baumannii-associated pneumonia using a clinically isolated hypervirulent strain. Antimicrob Agents Chemother. 2013. Aug;57(8):3601–3613. 10.1128/AAC.00944-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AC, Hood I, Boyd KL, Olson PD, Morrison JM, Carson S, Sayood K, Iwen PC, Skaar EP, Dunman PM. Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect Immun. 2010. May;78(5):1952–1962. 10.1128/IAI.00889-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly-Guillou ML, Wolff M, Farinotti R, Bryskier A, Carbon C. In vivo activity of levofloxacin alone or in combination with imipenem or amikacin in a mouse model of Acinetobacter baumannii pneumonia. J Antimicrob Chemother. 2000. Nov;46(5):827–830. 10.1093/jac/46.5.827 [DOI] [PubMed] [Google Scholar]
- Kamath AB, Alt J, Debbabi H, Taylor C, Behar SM. The major histocompatibility complex haplotype affects T-cell recognition of mycobacterial antigens but not resistance to Mycobacterium tuberculosis in C3H mice. Infect Immun. 2004. Dec;72(12):6790–6798. 10.1128/IAI.72.12.6790-6798.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoshida G, Kikuchi-Ueda T, Nishida S, Tansho-Nagakawa S, Ubagai T, Ono Y. Pathogenic bacterium Acinetobacter baumannii inhibits the formation of neutrophil extracellular traps by suppressing neutrophil adhesion. Front Immunol. 2018. Feb 7;9:178. 10.3389/fimmu.2018.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoshida G, Tansho-Nagakawa S, Kikuchi-Ueda T, Nakano R, Hikosaka K, Nishida S, Ubagai T, Higashi S, Ono Y. A novel bacterial transport mechanism of Acinetobacter baumannii via activated human neutrophils through interleukin-8. J Leukoc Biol. 2016. Dec; 100(6):1405–1412. 10.1189/jlb.4AB0116-023RR [DOI] [PubMed] [Google Scholar]
- Kempf M, Rolain JM. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents. 2012. Feb;39(2):105–114. 10.1016/j.ijantimicag.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Kim CH, Kim DJ, Lee SJ, Jeong YJ, Kang MJ, Lee JY, Choi JA, Kwon SJ, Park JH, Park JH. Toll-like receptor 2 promotes bacterial clearance during the initial stage of pulmonary infection with Acinetobacter baumannii. Mol Med Rep. 2014. Apr;9(4):1410–1414. 10.3892/mmr.2014.1966 [DOI] [PubMed] [Google Scholar]
- Knapp S, Wieland CW, Florquin S, Pantophlet R, Dijkshoorn L, Tshimbalanga N, Akira S, van der Poll T. Differential roles of CD14 and toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am J Respir Crit Care Med. 2006. Jan 1;173(1):122–129. 10.1164/rccm.200505-730OC [DOI] [PubMed] [Google Scholar]
- La Scola B, Gundi VA, Khamis A, Raoult D. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol. 2006. Mar;44(3):827–832. 10.1128/JCM.44.3.827-832.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau GW, Ran H, Kong F, Hassett DJ, Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect Immun. 2004. Jul;72(7):4275–4278. 10.1128/IAI.72.7.4275-4278.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Aslanyan L, Vidyasagar A, Brennan MB, Tauber MS, Carrillo-Sepulveda MA, Dores MR, Rigel NW, Martinez LR. Depletion of alveolar macrophages increases pulmonary neutrophil infiltration, tissue damage, and sepsis in a murine model of Acinetobacter baumannii pneumonia. Infect Immun. 2020. Jun 22; 88(7):e00128-20. 10.1128/IAI.00128-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna BM, Yan J, Reyna Z, Moon E, Nielsen TB, Reza H, Lu P, Bonomo R, Louie A, Drusano G, et al. Natural history of Acinetobacter baumannii infection in mice. PLoS One. 2019. Jul 18;14(7): e0219824. 10.1371/journal.pone.0219824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulani MS, Kamble EE, Kumkar SN, Tawre MS, Pardesi KR. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front Microbiol. 2019. Apr 1;10:539. 10.3389/fmicb.2019.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008. Mar 20;358(12):1271–81. 10.1056/NEJMra070741 [DOI] [PubMed] [Google Scholar]
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008. Jul;21(3): 538–582. 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, KuoLee R, Harris G, Chen W. High susceptibility to respiratory Acinetobacter baumannii infection in A/J mice is associated with a delay in early pulmonary recruitment of neutrophils. Microbes Infect. 2009a. Oct;11(12):946–955. 10.1016/j.micinf.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Qiu H, Kuolee R, Harris G, Chen W. Role of NADPH phagocyte oxidase in host defense against acute respiratory Acinetobacter baumannii infection in mice. Infect Immun. 2009b. Mar;77(3):1015–1021. 10.1128/IAI.01029-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, KuoLee R, Harris G, Van Rooijen N, Patel GB, Van Rooijen N, Patel GB, Chen W. Role of macrophages in early host resistance to respiratory Acinetobacter baumannii infection. PLoS One. 2012;7(6):e40019. 10.1371/journal.pone.0040019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Unno Y, Miyazaki C, Ubagai T, Ono Y. Multidrug-resistant Acinetobacter baumannii resists reactive oxygen species and survives in macrophages. Sci Rep. 2019. Nov 25;9(1):17462. 10.1038/s41598-019-53846-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppalapati, SR., Sett A, Pathania, R. The outer membrane proteins OmpA, CarO, and OprD of Acinetobacter baumannii confer a two-pronged defense in facilitating its success as a potent human pathogen. Front Microbiol. 2020. Oct 6;11:589234. 10.3389/fmicb.2020.589234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher LR, Lawson RA, Geary I, Taylor CJ, Bingle CD, Taylor GW, Whyte MK. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J Immunol. 2002. Feb 15;168(4):1861–1868. 10.4049/jimmunol.168.4.1861 [DOI] [PubMed] [Google Scholar]
- Ushizawa H, Yahata Y, Endo T, Iwashima T, Misawa M, Sonobe M, Yamagishi T, Kamiya H, Nakashima K, Matsui T, et al. A epidemiological investigation of a nosocomial outbreak of multidrug-resistant Acinetobacter baumannii in a Critical Care Center in Japan, 2011–2012. Jpn J Infect Dis. 2016;69(2):143–148. 10.7883/yoken.JJID.2015.049 [DOI] [PubMed] [Google Scholar]
- van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun. 2007. Dec;75(12):5597–5608. 10.1128/IAI.00762-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VF, Soh MM, Jaggi TK, Mac Aogáin M, Chotirmall SH. The microbial endocrinology of Pseudomonas aeruginosa: inflammatory and immune perspectives. Arch Immunol Ther Exp. 2018. Oct;66(5):329–339. 10.1007/s00005-018-0510-1 [DOI] [PubMed] [Google Scholar]
- Zeng X, Gu H, Cheng Y, Jia KR, Liu D, Yuan Y, Chen ZF, Peng LS, Zou QM, Shi Y. A lethal pneumonia model of Acinetobacter baumannii: an investigation in immunocompetent mice. lin Microbiol Infect. 2019. Apr;25(4):516.e1-516.e4. 10.1016/j.cmi.2018.12.020 [DOI] [PubMed] [Google Scholar]