Highlights
-
•
Rhino-orbito-cerebral mucormycosis (RCOM) is a life-threatening angioinvasive disease in patients with coronavirus disease 2019 (COVID-19).
-
•
A high index of suspicion with prompt surgical and medical management is life saving.
-
•
Efforts to maintain an optimal glycaemic index are likely to helpful in preventing ROCM.
-
•
Judicious use of steroids is needed to control collateral epidemic of ROCM in India.
-
•
Gene-sequencing studies for viral mutations of COVID-19 need evaluation.
Keywords: ROCM, COVID-19, Histopathology, Debridement, Orbital, Exenteration
Abstract
Background
Opportunistic cases of rhino-orbito-cerebral mucormycosis (ROCM) have increased in India during the coronavirus disease 2019 (COVID-19) pandemic.
Aim
To study laboratory parameters, histopathological features of sinus mucosal biopsies and exenterated orbit specimens, and clinical aspects of patients with ROCM.
Materials and methods
Retrospective analysis of nasal and sinus debridement biopsies and orbital exenteration specimens of 30 patients was undertaken, along with analysis of laboratory parameters, clinical history of predisposing conditions, and medication history during COVID-19.
Results
All patients were either in recovery following COVID-19 or had ongoing infection. Most patients were diabetic with increased glycosylated haemoglobin, and most patients received steroids and antibiotics for COVID-19. Thirty sinonasal mucosal debridement specimens from various sites, nine orbital exenteration specimens and one frontal decompression craniectomy specimen were examined. Mucor spp. were observed in necrotic tissue, and the presence of vessel and nerve invasion was documented. There were four deaths.
Conclusion
ROCM is a life-threatening disease. A high index of suspicion with prompt aggressive surgical and medical management by a multi-disciplinary team can be life saving. Efforts to maintain an optimal glycaemic index is likely to be helpful in preventing ROCM. Judicious use of steroids is mandatory to control the collateral epidemic of ROCM in India.
Introduction
Patients in the recovery period following coronavirus disease 2019 (COVID-19), especially during the second wave, have shown higher mortality and morbidity rates due to secondary infections. Cases of severe opportunistic infections in patients with severe COVID-19 have been reported worldwide. However, an exponential increase in such opportunistic infections has been observed in patients in India since March 2021. Mucormycosis is now an epidemic in India among patients with COVID-19. Globally, the prevalence of mucormycosis varied from 0.005 to 1.7 per million population in 2019–2020, and its prevalence in India was 0.14 per 1000 population (Chander et al., 2018; Prakash and Chakrabarti 2019; Skiada et al., 2020). As of 30 May 2021, 11,717 cases of mucormycosis have been reported in 18 states of India (Meghna Sen, 2021).
Mucor is a fungus of member Zygomycetes, order Mucorales (Branscomb, 2002), whose spores are normally present in the environment. It causes systemic angioinvasive disease when an individual's immunity is critically low, such as in individuals with diabetes mellitus (DM); patients receiving corticosteroids or other immunosuppressive drugs; and patients with haematological and solid organ malignancies, immunodeficiency and solid organ transplantations (Skiada et al., 2020). Corticosteroid treatment affects the function of macrophages and favours germination of fungal spores (Mishra et al., 2021).
Rhino-orbito-cerebral mucormycosis (ROCM) is the most common manifestation of the disease in large case series (Jeong et al., 2019). ROCM is frequently observed in association with uncontrolled DM (Wild et al., 2004; Singh et al., 2021). Other manifestations include pulmonary mucormycosis in patients with leukaemia and lymphoma, gastrointestinal mucormycosis in patients with malnutrition, cutaneous mucormycosis in burns patients, and disseminated mucormycosis in dialysis patients (Branscomb, 2002). ROCM is a rapidly progressive disease, and any delay in appropriate management can have a serious adverse effect on patient survival.
Aim
This study aimed to investigate laboratory parameters, histopathological features of sinus mucosal biopsies and exenterated orbit specimens, and clinical aspects of patients with ROCM admitted to AIG Hospital.
Materials and methods
The case records of 30 patients with ROCM who had undergone nasal and sinus debridement biopsies and orbital exenteration between 24 April and 24 May 2021 at AIG Hospital were reviewed retrospectively. The medical records were retrieved to examine relevant clinical details, particularly date of COVID-19 positivity on reverse transcriptase polymerase chain reaction (RT-PCR); treatment given, particularly use of antibiotics, steroids (type, dosage and duration of use), remdesivir, tocilizumab, multi-vitamin supplements and oxygen therapy; hospitalization; and ventilation history. Presence of comorbid conditions such as hypertension, DM, cancer and immunosuppression because of any chronic illness were documented with available information, followed by telephone conversations. Contrast-enhanced magnetic resonance imaging (MRI) scans were reviewed for involvement of the paranasal sinuses (PNS), orbit and brain. A complete blood picture (CBP) was documented. Most cases did not have laboratory investigations such as interleukin-6 (1L-6), C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin and D-dimer, and hence these could not be analysed. Fungal filaments on potassium hydroxide (KOH) mounts, and growth on culture media such as potato dextrose agar (PDA) and Sabouraud's dextrose agar (SDA) from samples taken during sinus and nasal debridement were also documented.
Tissue samples from the sinuses and nasal turbinates were entirely processed. All exenterated eyeballs had a sample of orbital apex tissue sent separately along with the main specimen.
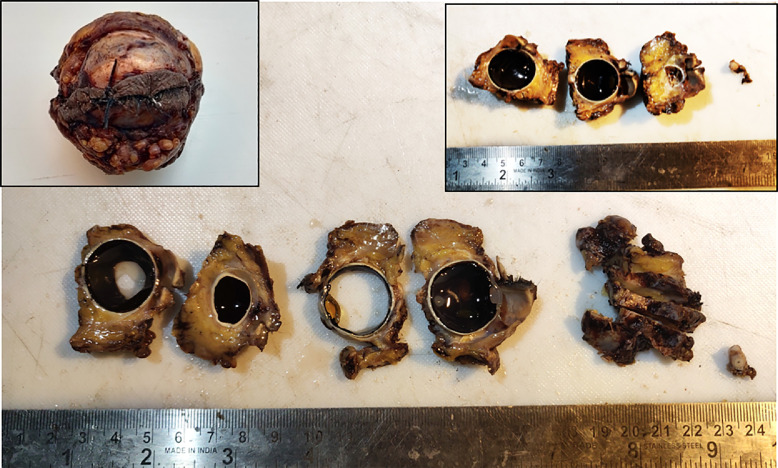
Eyeballs were grossed according to department protocol, following the protocol of the College of American Pathologists intended for grossing oncosurgical eyeball specimens. After the cut end of the orbital nerve was sampled, two vertical sections were made on either side of the cornea through the entire eyeball, and the three parts obtained were further ‘bread loafed’ and processed (Figure 1). Slides stained with haematoxylin and eosin (H&E) were prepared, and examined for the presence and type of fungi, and the density of necroinflammation. Grocott methenamine-silver (GMS) and periodic acid-Schiff (PAS) stains were used for confirmation of fungal organisms, and to demonstrate their presence in low fungal density areas. Blood vessel and neural invasion was documented wherever detected.
Figure 1.
Gross picture of ‘bread-loafed’ sections of exenterated left orbit. Left inset shows exenterated orbit. Right inset shows three vertical sections of left orbit along with left optic nerve cross-section.
Results
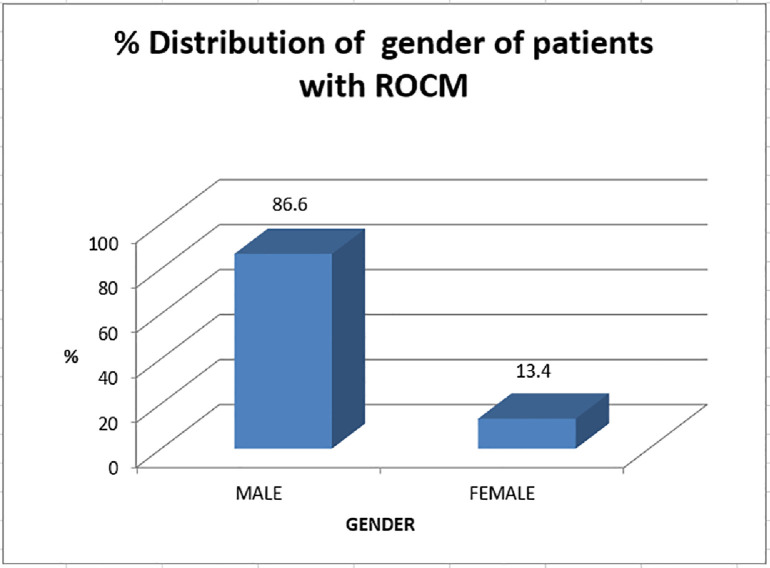
In total, 30 specimens were included in this study: 26 from male patients and four from female patients. Patients were aged 24–73 years, and five cases were aged <35 years (Figure 2a,b).
Figure 2.
Graphical representation of (a) gender distribution and (b) age distribution of patients with rhino-orbito-cerebral mucormycosis.
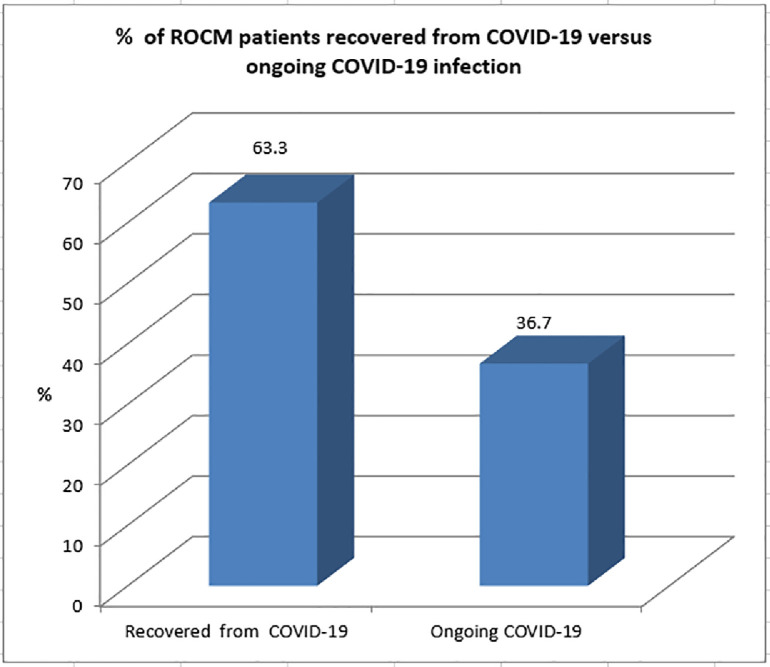
Nineteen cases were in the recovery period following COVID-19, ranging from 15 days to 2 months since symptom onet. However, 11 cases had ongoing COVID-19 (approximately 7–9 days since symptom onset) (Figure 3).
Figure 3.
Graphical representation of patients with rhino-orbito-cerebral mucormycosis during recovery after coronavirus disease 2019 (COVID-19) versus those with ongoing COVID-19.
Twenty-seven patients had a history of steroid intake (methyl prednisolone 40 mg or dexamethasone 6 mg followed by tapering doses for total of 12 days) during COVID-19. A few patients had a history of steroid intake lasting almost 3 weeks. Three patients did not have a reliable history regarding steroid intake. These three patients had high blood sugar levels. All patients had received antibiotics (doxycycline/azithromycin), three patients had received tocilizumab, and three patients had received remdesivir. All cases had received multi-vitamin supplements including zinc, vitamin C and vitamin D.
Seventeen cases had a history of DM; of these, five patients were de-novo cases of DM. In the remaining eight cases, a proper history regarding DM could not be elicited. However, 29 patients had deranged blood sugar levels on at least two occasions, and HbA1c levels in 14 patients were high (>9% in 11 cases) with values ranging from 6.8 to 15. One patient had a high blood sugar level following surgery for ROCM. Hence, all 30 patients were finally diagnosed with DM. Eight patients were hypertensive, all of whom also had DM. Of these, one patient had acute kidney injury, and another patient had previous history of pancreatitis and cerebrovascular accident. Sixteen patients had received oxygen therapy, of which eight patients required mechanical ventilation. Cycle threshold (CT) scores for RT-PCR in patients with ongoing COVID-19 ranged from 26.7 to 33.9.
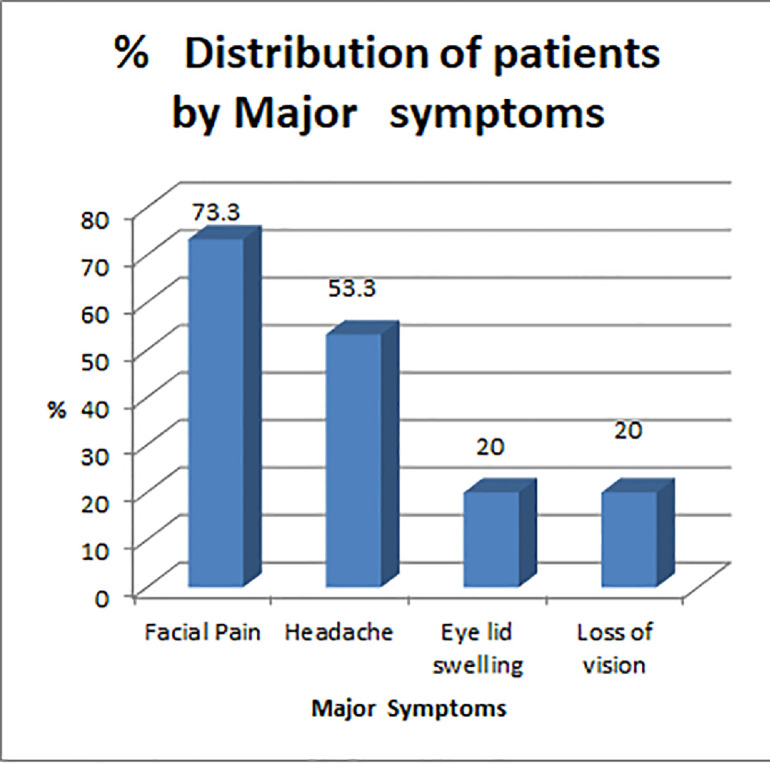
The most common symptoms were facial pain (22 cases, 73.3%), headache (16 cases, 53.3%), eyelid swelling (six cases, 20%), loss of vision (six cases, 20%), eyelid drooping (four cases, 13.3%), blocked nose (four cases, 13.3%), proptosis (four cases, 13.3%), restricted eye movements (one case, 3.3%), epistaxis (one case, 3.3%) and palatal eschar (one case, 3.3%) (Figure 4). The duration of symptoms ranged from 8 to 20 days before patients presented for medical care. The demographic and clinical features of the patients are given in Table 1.
Figure 4.
Graphical representation of common symptoms of rhino-orbito-cerebral mucormycosis.
Table 1.
Demographic and clinical features of patients with rhino-orbito-cerebral mucormycosis.
| Parameters | Values | Percentage |
|---|---|---|
| Total patients (N) | 30 | |
| Age, years (mean ± SD) | 49.06 ± 13.28 | |
| Gender (male:female) | 26:4 | |
| In recovery following COVID-19 (N) | 19 | 63.3 |
| Current COVID-19 | 11 | 36.7 |
| Steroid intake (N) | 27 | 90 |
| Antibiotic intake (N) | 30 | 100 |
| Multi-vitamin supplement (N) | 30 | 100 |
| Ongoing DM (N) | 17 | 56.7 |
| De-novo DM (N) | 5 | 16.7 |
| Deranged blood sugar levels | 8 | 26.7 |
| HbA1c (mean ± SD) | 10.6 ± 2.1 | |
| Hypertension (N) | 8 | 26.7 |
| Oxygen therapy (N) | 16 | 53.3 |
| Mechanical ventilation (N) | 8 | 26.7 |
| CT score of RT-PCR (mean ± SD) | 30.35 ± 2.30 | |
| Facial pain (N) | 22 | 73.3 |
| Headache (N) | 16 | 53.3 |
| Eyelid swelling (N) | 6 | 20 |
| Loss of vision (N) | 6 | 20 |
| Ptosis (N) | 4 | 13.3 |
| Proptosis (N) | 4 | 13.3 |
| Blocked nose (N) | 4 | 13.3 |
| Restricted ocular motility (N) | 1 | 3.3 |
| Epistaxis | 1 | 3.3 |
| Palatal eschar | 1 | 3.3 |
N, number of patients; SD, standard deviation; DM, diabetes mellitus; HbA1c, glycosylated haemoglobin; CT, cycle threshold; RT-PCR, reverse transcriptase polymerase chain reaction.
CBP showed leukocytosis in 19 cases, with the presence of neutrophilia in 22 cases and lymphopenia in seven cases. Other cases had normal haemograms. The haematological values are shown in Table 2. The neutrophil to lymphocyte ratio (NLR) was raised in most patients (25 cases). The mean prothrombin time-international normalization ratio was 1.05 (standard deviation 0.14). D-dimer was not measured in most cases.
Table 2.
Laboratory parameters, and radiological and microbiology results of patients with rhino-orbito-cerebral-mucormycosis.
| Parameters | Values | Percentage |
|---|---|---|
| WBC count (mean ± SD) | 11,469.23 ± 5489.61 | |
| Absolute neutrophil count (mean ± SD) | 10,287.97 ± 4998.11 | |
| Absolute leukocyte count (mean ± SD) | 1518.33 ± 622.04 | |
| Neutrophil:lymphocyte ratio (mean ± SD) | 8.1 ± 6.4 | |
| PT-INR (mean ± SD) | 1.05 ± 0.14 | |
| KOH mount positive for fungus (N) | 7 | 23.3 |
| Culture positive for fungus (N) | 18 | 60 |
| Rhizopus species in culture (N) | 5 | 16.7 |
| Aspergillus species in culture (N) | 1 | 3.3 |
| Lichtheimia corymbifera (N) | 1 | 3.3 |
| Mucor species in culture (N) | 10 | 33.3 |
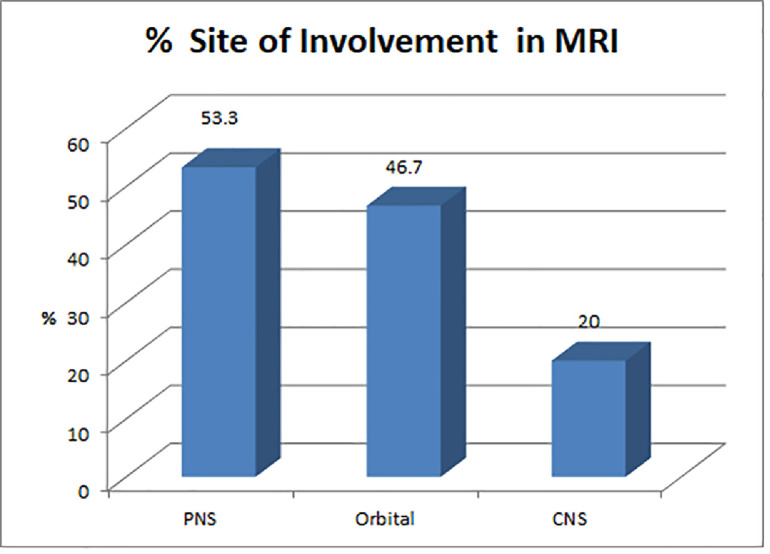
| MRI showing PNS involvement (N) | 16 | 53.3 |
| MRI showing orbital involvement (N) | 14 | 46.7 |
| MRI showing CNS involvement (N) | 6 | 20 |
WBC, white blood cell; N, number of patients; SD, standard deviation; PT-INR, prothrombin time-international normalized ratio; KOH, potassium hydroxide; MRI, magnetic resonance imaging; PNS, paranasal sinuses; CNS, central nervous system.
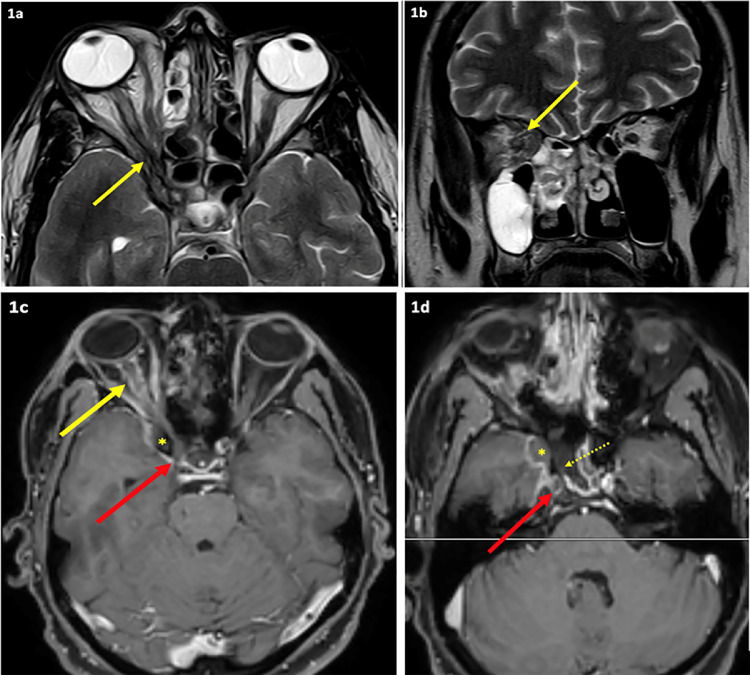
Contrast-enhanced MRI of PNS, orbits and brain (Figure 5) was performed in most cases. Orbital cellulitis, involvement of the orbital apex and involvement of the corresponding optic nerve were diagnosed in nine cases (Figure 6). Cavernous sinus involvement was seen in four cases, while one case had changes in pachymeningitis over the right frontotemporal lobe, extending along the right cavernous sinus. Six cases also had cerebral involvement. All 30 cases had involvement of at least one nasal/sinus mucosa.
Figure 5.
Graphical representation of site of involvement by Mucor spp. on magnetic resonance imaging. PNS, paranasal sinuses; CNS, central nervous system.
Figure 6.
Magnetic resonance imaging shows acute invasive fungal sinusitis involving right ethmoid, sphenoid and maxillary sinuses with right orbital apex involvement (yellow arrows in 1a and 1b), optic nerve sheath inflammation (yellow arrow in 1c), partly necrotic right sphenoid sinus walls (dotted arrow in 1d), right cavernous sinus and right internal carotid artery thrombosis (red arrows in 1c and 1d) and an extra-axial collection in the middle cranial fossa (asterisk).
All 30 cases underwent endoscopic endonasal and sinus debridement with administration of postoperative antifungals and antibiotics. Nine cases underwent orbital exenterations (three right side, six left side), and one case had central nervous system involvement and underwent bilateral frontal decompression craniectomy. Three cases received retrobulbar liposomal amphotericin B injection.
Debridement from various sites revealed fungal infection in the left maxillary (N=7), left ethmoid (N=13), left sphenoid (N=2) and left frontal (N=2) sinuses; left middle turbinate (N=9); left inferior turbinate (N=4); left pterygoid (N=1); left orbital apex and exenterated orbits (N=6); right middle turbinate (N=5); right inferior turbinate (N=6); right maxillary (N=8), right ethmoid (N=4), right sphenoid (N=2) and right frontal (N=1) sinuses; right skull base (N=1); and right orbital apex and exenterated orbits (N=3).
The left ethmoid sinus, left middle turbinate and right maxilla were the most common sino/nasal sites of involvement.
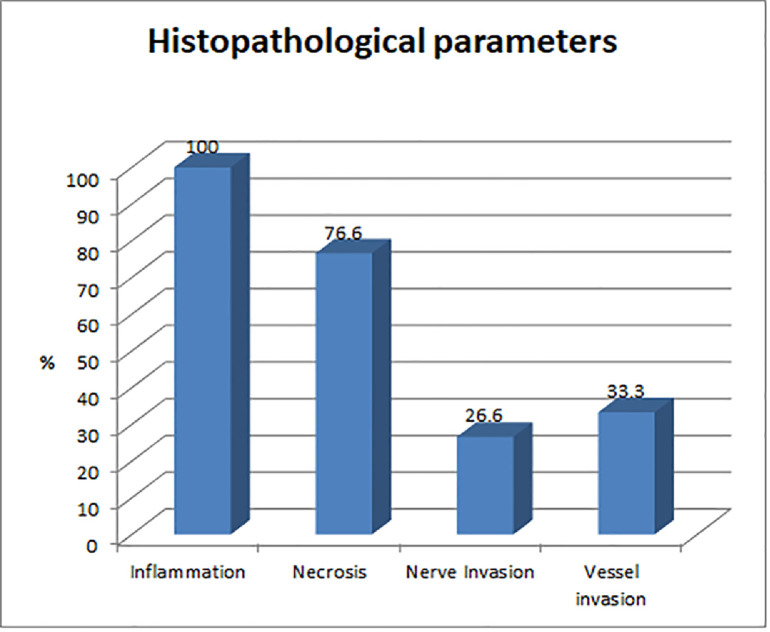
H&E sections of all nasal mucosal biopsies were evaluated for overall degree of inflammation and type of inflammatory cell predominance. The presence or absence of basement membrane thickening, subepithelial oedema (focal, pervascular, severe), hyperplastic or papillary epithelial changes, mucosal ulceration, squamous metaplasia and fibrosis was documented. The presence of Charcot–Leyden crystals, eosinophilic aggregates and fungal elements were noted. All cases with fungi were evaluated further based on morphology, presence or absence of necrosis, nerve invasion and vascular invasion (Figure 7). All 30 cases showed inflammation, predominantly lymphoplasmacytic with neutrophilic infiltrates around fungus. Twenty-three cases showed necrosis, eight cases showed perineural fungal invasion, and 10 cases showed vascular invasion by fungal elements.
Figure 7.
Graphical representation of common histopathological findings in rhino-orbito-cerebral mucormycosis.
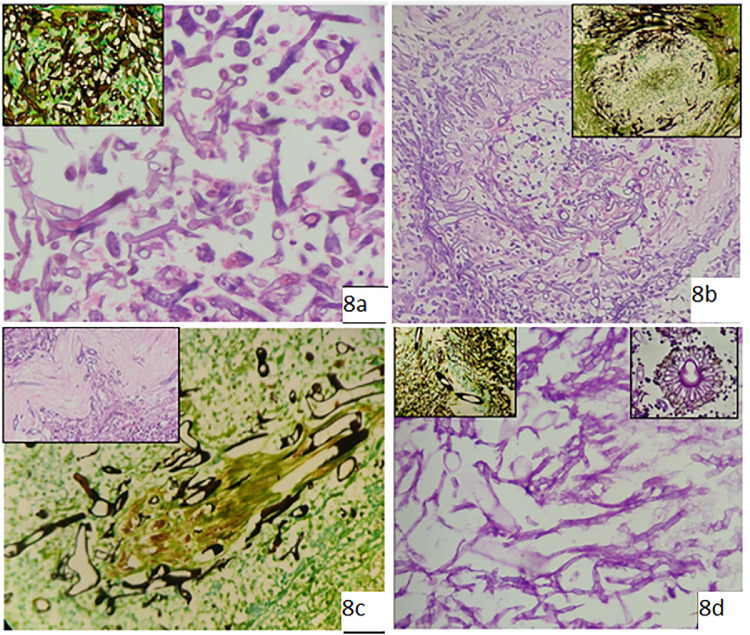
All cases had broad, pauciseptate or non-septate, wide, ribbon-like hyphae with irregular branching, mainly at 90° (Figure 8a–d). Most samples were subjected to KOH and culture studies. Culture showed fluffy, white, brown or greyish colonies on SDA/PDA. Some cases were culture and KOH negative (three cases) but fungi were detected in histopathological sections. In five cases, no fungal growth occurred on culture but KOH showed pauciseptate broad fungal hyphae. One case (Figure 8d) had co-infection with Aspergillus spp., with septate hyphae with acute angle branching and fruiting bodies (Figure 8d, inset) along with Mucor spp. The most common species isolated on culture was Rhizopus, while one case showed Lichtheimia corymbifera.
Figure 8.
Microscopic images of Mucor spp. (a) Haematoxylin and eosin (H&E)-stained section from left maxillary sinus shows many broad aseptate hyphae with predominant 90o branching (original magnification 400x). Inset shows Grocott methenamine-silver (GMS) stain. (b) H&E-stained section from right orbital tissue showing vessel wall invasion and luminal clogging by broad, aseptate hyphae (original magnification 100x). Inset shows GMS stain of the same. (c) GMS stain of a section from right orbital tissue showing neural invasion by fungi (original magnification 400x). Inset shows H&E-stained section from orbital apex tissue with neural invasion by fungi. (d) Periodic acid-Schiff-stained section from nasal mucosa demonstrating the presence of mixed fungi with broad aseptate hyphae (Mucor spp.) and thin septate hyphae with acute angle branching (Aspergillus spp.) (original magnification 400x). Inset (left) shows GMS-stained section of the same and inset (right) shows fruiting body of Aspergillus.
The radiological and microbiological results of these cases are given in Table 2, while the histopathological findings are shown in Table 3.
Table 3.
Histopathological findings in tissue samples of patients with rhino-orbito-cerebral-mucormycosis.
| Parameters | Values (N) |
|---|---|
| Total patients | 30 |
| PNS tissue sample | 30 |
| Exenterated orbital tissue | 9 |
| Skull base | 1 |
| Frontal process of maxilla | 1 |
| Inflammation | 30 |
| Necrosis | 23 |
| Perineural invasion | 8 |
| Vascular invasion | 10 |
N, number of patients; PNS, paranasal sinuses.
On follow-up at 1–3 months, there had been five deaths. Six patients had been re-admitted to hospital for systemic conditions other than ROCM or its sequelae (electrolyte imbalance, lung fibrosis, uncontrolled DM etc.) and were undergoing treatment. None of these patients had experienced recurrence of ROCM. One patient had recurrence of ROCM in the operated orbital area at 4 months and underwent repeat debridement. This patient is currently receiving posaconazole. The remaining 19 patients are doing well; antifungal therapy is ongoing with no recurrence of ROCM.
For patients with complete resolution of infection, confirmed clinically (endoscopically) and on imaging, a custom exenteration prosthesis will be fitted as part of rehabilitation. This is planned after a mean interval of 6–9 months following orbital exenteration. The prosthesis is either a stick-on adhesive type, magnetic or spectacle-mounted, based on patient preference and socket healing.
No radical maxillectomies were performed. Only partial/subtotal maxillectomies were performed, and no further reconstruction surgery was required in these patients. In cases where inferior maxillectomy was performed, the patients used an acrytic palatal obturator for the first 6 months following surgery, and then received permanent dentures after 6 months.
Discussion
COVID-19 was declared a pandemic by the World Health Organization on 11 March 2020. It has claimed millions of lives and has disrupted normal life worldwide. Due to the lack of proper understanding of the disease, its pathogenesis and effective medicines, various drugs (including steroids, antibiotics, ivermectin, remdesivir and tocilizumab), plasma therapy, oxygen therapy and ventilation have been used to reduce mortality in hypoxaemic patients.
COVID-19 is common in patients with comorbid conditions such as hypertension, DM and cardiovascular diseases. India has the dubious distinction of being the DM capital of the world (Wild et al., 2004). Patients with DM are more predisposed to COVID-19 due to increased angiotensin-converting enzyme 2 expression, impaired T-cell function, increased IL-6, increased ferritin and high HbA1c (Satish et al., 2021).
Due to pandemic challenges with lockdown, lack of adequate exercise, mental and emotional stress, and lack of medical access, increased glucose dysregulation has been noted in the majority of the population. Hyperglycaemia and acidosis impair the activity of phagocytes, which represent the main host defence mechanism against fungal infections such as mucormycosis (Waldorf, 1989). Hence, patients with DM are more susceptible to mucormycosis. In the pre-COVID-19 era, India contributed to a significant burden of mucormycosis. Studies have shown that 47% of Indians are unaware of their DM status, and only one-quarter of individuals take appropriate and timely treatment. The presence of DM was found to be an independent risk factor in a large meta-analysis of 851 cases of ROCM (Jeong et al., 2019).
As of May 2021, ROCM has been declared an epidemic in a few Indian states (i.e. Maharashtra, Gujarat, Andhra Pradesh, Madhya Pradesh and Telangana) which account for 65% of ROCM cases in India.
Mucorales are ubiquitous in nature and rarely cause disease in immunocompetent hosts (Roden et al., 2005). The fungi can thrive on organic matter such as fruits and vegetables, wet waste, and dead and decaying plants or animals. Mucorales release spores which are airborne. As a result, there is potential for most people to be exposed to these spores. The tropical climate of India is a perfect environment for them to grow. Mucormycosis is commonly acquired through inhalation of spores, or rarely through direct contact with the skin. No matter how it is acquired, the first line of defence in a healthy body eliminates the spores with the help of oxidative metabolites and cationic peptides (Green and Karras, 2012; Petrikkos et al., 2012); however, in the presence of debilitating diseases and comorbid conditions, immunosuppression and malnutrition, it is a matter of concern (Waldorf, 1989) as risk factors weaken the immune system, rendering it incompetent to fight against the fungus. In the present study, COVID-19 plus DM seems to be the likely predisposing factor for ROCM.
There was no history of patients undergoing multiple RT-PCR tests, which rules out the possibility of nasal swabs used in COVID-19 PCR tests as a predisposing factor and an entry route for Mucor spp. The test per se does not injure the nasal mucosa. In addition, PCR tests are being performed worldwide and ROCM has not been reported in other countries.
In COVID-19, viral replication promotes the inflammatory response and favours neutrophil and monocyte influx in the blood stream. There is also suppression of T-cell immunity. An imbalance between neutrophil and lymphocyte action during COVID-19 makes the patient susceptible to fungal infections (Mishra et al., 2021). In the present series, NLR was raised in 25 of 30 patients.
An increase in cytokine levels (IL-6, ferritin, LDH, CRP) increases free iron by increasing ferritin levels. Free iron is an ideal resource for mucormycosis. A low concentration of free iron in plasma is a protective factor against invasion by pathogens by increasing the activity of cyclin-dependent kinase inhibitor p21CIP1/WAF1, thus delaying the S phase of the cell cycle. Iron-binding proteins such as transferrin and lactoferrin help to maintain low levels (10−18 M) of free iron. Elevated free iron is toxic to pathogens and adds to dysfunctional chemotaxis (Symeonidis, 2009; Ibrahim, 2011; Singh et al., 2021). Low CT values of RT-PCR are associated with increased hospital admissions with severe COVID-19 and high mortality (Rajyalakshmi et al., 2021). However, in contrast to these findings, the present study found high CT values in patients with ROCM.
Administration of steroids further reduces the phagocytic activity of white blood cells and impairs the migration, ingestion and fusion functions of leukocytes; these could be contributing factors in patients with severe COVID-19 treated with steroids (Prakash and Chakrabarti, 2019). Many patients in India received steroids as the first line of medication during the first wave of COVID-19. The huge DM population and different treating physicians may be reasons for the early initiation of steroids. Unsupervised prescriptions led to irrational doses and duration of steroid intake in a few cases. Steroids in COVID-19-positive patients may have worsened glycaemic control and caused lymphopenia. Twenty-seven patients in this series had a definite history of steroid intake. In addition, it has been suggested that excess zinc intake could be the cause of fungal infection, but this was not analysed in the current study. Given that India is a heavily populated country with the majority of the population being of low socio-economic status, poor hygiene with likely repeat use of wet, dirty masks may be another factor for fungal predisposition, but large-scale studies are needed to confirm this as a causative factor.
The hyphae of pathogenic Mucorales are angioinvasive, and lead to haemorrhagic necrosis, vascular thrombosis, emboli and tissue infarction (Green and Karras, 2012). COVID-19 also causes endothelialitis with procoagulable state, which could serve as grounds for mucor angioinvasion and dissemination (Mishra et al., 2021; Singh et al., 2021).
The role of oxygen tubing and ventilator-associated ROCM is controversial as patients in home isolation have also been reported to have fungal involvement. One of the study cases was in home isolation and yet developed ROCM. In this study, 53.3% of cases had oxygen therapy and 26.7% required ventilator support.
Symptoms of ROCM can occur from the onset of COVID-19 to as late as 30–42 days post diagnosis. However, in this study, there were 11 cases of ROCM in patients with ongoing COVID-19, with patients presenting with symptoms of fungal infection on days 8–9 of COVID-19 testing. Given that the second COVID-19 wave in India was caused by the double mutant B.1.617 strain, multi-centre gene-sequencing studies are needed to confirm whether viral mutations causing exaggerated immunosuppression in DM patients could be the cause of increased predisposition to ROCM.
Headache; stuffy nose; facial pain; blood tinged, mucoid or purulent nasal discharge; blurred vision and diplopia are the common symptoms of ROCM. Clinical signs include proptosis with or without ptosis, facial swelling/discolouration, restricted ocular motility, visual loss, fixed pupils, central retinal artery occlusion, panophthalmitis, and nasal or palatal eschar.
Contrast-enhanced MRI is the preferred radiological investigation. Early signs of ROCM include nasal and paranasal mucosal thickening with irregular patchy enhancement. Black turbinates manifested as non-enhancement of turbinates is an early sentinel sign. An early sign for orbital involvement is thickening of the medial rectus. The extent of cavernous sinus involvement and ischaemic changes in the central nervous system can be determined by MRI and MR-angiography (Green and Karras, 2012). Intracranial involvement increases the mortality rate to 90% (Deutsch et al., 2019). In the present study, all 30 cases had nasal/sinus mucosal involvement, nine cases had orbital involvement, and case had cerebral involvement (Figure 1).
A recent staging of this fungal disease is as follows:
-
•
Stage 1 – involvement of the nasal mucosa;
-
•
Stage 2 – involvement of the paranasal sinuses;
-
•
Stage 3 – involvement of the orbits (3a, naolacrimal duct, medial orbit; 3b, diffuse orbital involvement; 3c, central retinal artery or ophthalmic artery occlusion, involvement of orbital apex, loss of vision; 3d, bilateral orbital involvement); and
-
•
Stage 4 – involvement of the central nervous system (Honavar, 2021).
Direct microscopy of deep or endoscopy-guided nasal swabs, and paranasal sinuses or orbital tissue using KOH mounts may be helpful for rapid diagnosis and has 90% sensitivity. Culture helps in genus and species identification, and antifungal susceptibility testing. However, only 50% of cases may show growth of organisms on culture. Molecular diagnosis (PCR) has 75% sensitivity but is not widely available commercially. Histopathological evaluation of samples from nasal mucosa, paranasal sinus mucosa and orbital, cerebral tissue including necrotic debris provides diagnostic information in 80% of samples, and identifies uncultivable fungal pathogens (Roy et al., 2017; Skiada et al., 2020). The discordance between histopathology-positive and culture-/KOH-negative reports may be because: (i) tissue samples sent to microbiology and histopathology laboratories were sampled from two different sites; (ii) KOH and culture may be negative if the amount sampled is not sufficient; (iii) Mucorales have poor growth capacity in ordinary fungal culture media; and (iv) material for culture/KOH may have been sampled from areas free of fungal elements. Histopathology, direct KOH mount and culture should be used complementary to each other for accurate diagnosis.
Mucorales have non-pigmented, wide (5–20 μm), thin-walled, ribbon-like hyphae with few septations (pauciseptate) and irregular, predominantly right-angled branching (Ribes et al., 2000). The hyphae may vary in width, appear folded or crinkled, and be sparse or fragmented. Stains that can help to highlight the fungal wall include GMS and PAS. The major morphological differentials are Aspergillus spp., other septated moulds (e.g. Fusarium and Scedosporium) and Candida spp. The presence of abundant septation and acute-angle branching should suggest the diagnosis of Aspergillus spp. or another hyaline septate mould, while yeasts with pseudohyphae should suggest Candida spp. (Guarner and Brandt, 2011). Aspergillus infection has a better outcome in terms of survival compared with mucor infection (Arndt et al., 2009).
In the present study, mucormycosis was found in all 30 cases. One case had co-infection with Aspergillus spp. (Figure 3d). Fungus was found in large numbers in necrotic and ulcerated areas rather than healthy mucosa. The posterior chamber of the eye had more fungi compared with the anterior chamber. Neural invasion and angioinvasion were demonstrated in almost all exenterated orbital specimens. The single case of cerebral involvement had presence of Mucor spp. in necrotic glial tissue.
To date, three cases of pulmonary mucormycosis have been reported in post-COVID patients in the study hospital. Compared with the high incidence of ROCM, the incidence of pulmonary mucormycosis is low. There have been no documented cases of gastrointestinal, cutaneous or renal mucormycosis at the study hospital.
Medical management includes induction with liposomal amphotericin-B 5–10 mg/kg/day for 2 weeks or intravenous posaconazole or isavuconazole. Surgical management involves early and aggressive debridement of the paranasal sinuses by turbinectomy, palatal resection or medial orbital wall resection in Stage 1, 2 and 3 disease. Orbital exenteration/neurosurgical management is used in Stage 3c disease, with occlusion of the central retinal artery or superior ophthalmic vein thrombosis, and Stage 4 disease with central nervous system involvement,
Limitations
This was a retrospective single-centre study of 30 patients. The results from laboratory investigations for cytokine storm evaluation (D-dimer, IL-6, ferritin, CRP, LDH) during COVID-19 could not be retrieved for all patients, as many were treated elsewhere and subsequently admitted to the study hospital with ROCM symptoms.
Conclusion
ROCM is a life-threatening angioinvasive disease. Most patients with COVID-19 who developed ROCM had DM or developed new-onset hyperglycaemia, and were treated with varying doses of steroids and antibiotics. Many patients developed RCOM during recovery from COVID-19. Histopathology helps with confirmation of mucor infection. Patients with ongoing COVID-19 with ROCM indicates factors such as poor hygiene, the use of wet masks for many days, and probable viral mutation leading to exaggerated immunosuppression; this needs to be confirmed by multi-centre studies and gene-sequencing analysis. Educating patients with COVID-19 to recognize the warning signs and symptoms of RCOM may help them to seek early medical care. A high index of suspicion with prompt ear/nose/throat and ophthalmology referral can be life saving. Efforts to maintain an optimal glycaemic index are helpful in the prevention of ROCM. Judicious use of steroids is mandatory to stop the collateral epidemic of mucormycosis in India amidst the COVID-19 pandemic.
Declaration of Competing Interest
None declared.
Acknowledgments
Acknowledgements
The authors wish to thank the COVID-19 treating team, AIG histopathology technicians and hospital staff for providing material for publication.
Funding
None.
Ethical approval
Not required
Author contributions
Anuradha Sekaran: conception of study, data analysis, interpretation and critical revision of manuscript.
Nayana Patil: data collection, analysis and drafting of manuscript.
Swapnali Sabhapandit: data collection, and analysis and review of manuscript.
Srinivas Kishore Sistla: data collection and review of manuscript.
Duvvur Nageshwar Reddy: conception of study and critical review of manuscript
References
- Arndt S, Aschendorff A, Echternach M, Daemmrich TD, Maier W. Rhino-orbital-cerebral mucormycosis and aspergillosis: differential diagnosis and treatment. Eur Arch Otorhinolaryngol. 2009;266:71–76. doi: 10.1007/s00405-008-0692-y. [DOI] [PubMed] [Google Scholar]
- Branscomb R. An overview of mucormycosis. Lab Med. 2002;33:453–455. [Google Scholar]
- Chander J, Kaur M, Singla N, Punia RPS, Singhal SK, Attri AK, et al. Mucormycosis: battle with the deadly enemy over a five-year period in India. J Fungi. 2018;4:46. doi: 10.3390/jof4020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch PG, Whittaker J, Prasad S. Invasive and non-invasive fungal rhinosinusitis – a review and update of the evidence. Medicina. 2019;55:1–14. doi: 10.3390/medicina55070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honavar SG. Code Mucor. Indian J Ophthalmol. 2021;69:1361–1365. doi: 10.4103/ijo.IJO_1165_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS. Host cell invasion in mucormycosis: role of iron. Curr Opin Microbiol. 2011;14:406–411. doi: 10.1016/j.mib.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JP, Karras DJ. Update on emerging infections: news from the Centers for Disease Control and Prevention. Ann Emerg Med. 2012;59:53–54. doi: 10.1016/j.annemergmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Mishra N, Mutya VSS, Thomas A, Rai G, Reddy B, Mohanan AA, et al. A case series of invasive mucormycosis in patients with COVID-19 infection. Int J Otorhinolaryngol Head Neck Surg. 2021;7:867–870. [Google Scholar]
- Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl. 1):S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5:26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajyalakshmi B, Samavedam S, Reddy PR, Aluru N. Prognostic value of ‘cycle threshold’ in confirmed COVID-19 patients. Indian J Crit Care Med. 2021;25:322–326. doi: 10.5005/jp-journals-10071-23765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- Roy P, Das S, Sharma S, Girotra V, Gupta N, Saha R, et al. Revisiting the utility of histopathological examination of biopsy: a necessity in microbiology. J Clin Diagn Res. 2017;11:DC16–DC18. doi: 10.7860/JCDR/2017/26431.9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satish D, Joy D, Ross A, Balasubramanya Mucormycosis coinfection associated with global COVID-19: a case series from India. Int J Otorhinolaryngol Head Neck Surg. 2021;7:815–820. [Google Scholar]
- Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15 doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6:265. doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symeonidis AS. The role of iron and iron chelators in zygomycosis. Clin Microbiol Infect. 2009;15(Suppl. 5):26–32. doi: 10.1111/j.1469-0691.2009.02976.x. [DOI] [PubMed] [Google Scholar]
- Waldorf AR. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol Ser. 1989;47:243–271. [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]