Central Illustration
Key Words: cancer survivorship, hormonal therapy, prostate cancer, risk factor
Abbreviations and Acronyms: ADT, androgen deprivation therapy; ARSI, androgen receptor signaling agent; CV, cardiovascular; CVD, cardiovascular disease; CVRF, cardiovascular risk factor; GnRH, gonadotropin-releasing hormone; mCSPC, metastatic castration-sensitive prostate cancer; RT, radiation therapy
Highlights
-
•
Androgen deprivation therapy is associated with metabolic derangements due to profound hypogonadism that can increase the risk of CV disease in prostate cancer survivors.
-
•
Therapeutic advances have resulted in prolonged patient exposure to androgen deprivation therapy, thereby increasing CV complications for many prostate cancer survivors.
-
•
A systematic approach to monitoring and addressing reversible CV risk factors and purposeful engagement in multidisciplinary care between oncologists, urologists, and cardiologists is critical to optimizing CV outcomes in men with prostate cancer.
Androgen deprivation therapy (ADT) is the primary systemic therapy for men with high-risk localized, recurrent, or advanced prostate cancer due to the cancer’s dependence on oncogenic androgen receptor signaling. ADT is most commonly delivered through medical castration with the use of gonadotropin-releasing hormone (GnRH) agonists or antagonists that cause profoundly low testosterone and estrogen levels and result in deep anticancer responses. ADT is used in up to 40% of men with prostate cancer at some point during the course of their treatment, with curative intent for men with localized intermediate- or high-risk disease, and with palliative intent for men with advanced/metastatic prostate cancer. Although it is associated with excellent cancer control outcomes, the hormonal shifts from ADT cause negative cardiometabolic consequences that must be recognized and managed. This is particularly critical in prostate cancer survivors with pre-existing cardiovascular disease (CVD) or cardiovascular risk factors (CVRFs), as they are at highest risk of cardiovascular complications during treatment with ADT.
Numerous studies demonstrate that prostate cancer patients have high rates of cardiovascular comorbidity, with up to 90% of men receiving ADT in a recent phase 3 randomized trial having baseline CVRFs or CVD (1). The profound hypogonadism induced by ADT is associated with a wide variety of adverse health consequences, including central adiposity, dyslipidemia (increased total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglycerides), glucose intolerance, sarcopenia, and diminished exercise tolerance, with cardiometabolic changes occurring within 12 to 24 weeks of ADT initiation (Figure 1). Although these changes are associated with the development of atherosclerosis, CV complications appear to occur more rapidly, and are more common during intermittent ADT, in which therapy exposure is cycled on and off (2). As such, additional mechanisms of cardiotoxicity beyond atherogenesis may include follicle-stimulating hormone surges that occur with initiation of ADT, abrupt transitions between hormonal states, and GnRH agonist action on vascular and myocardial cells (3). Following the recognition of such adverse effects, a joint American Heart Association/American Urologic Association committee published a scientific advisory warning of an increased risk of incident diabetes mellitus and major adverse CV events associated with ADT (4). These risks are most pronounced in men with underlying CVD or CVRFs, and in men receiving intensified or prolonged ADT exposure. Unfortunately, beyond this advisory statement, limited prospective evidence exists to guide CV monitoring and management protocols for men receiving ADT.
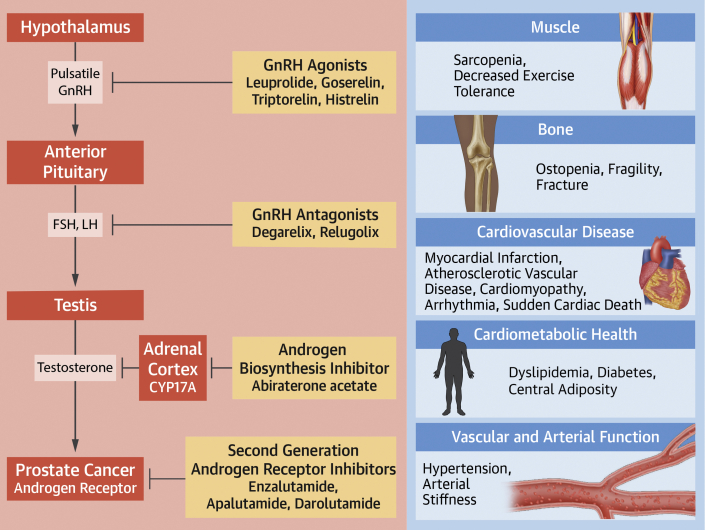
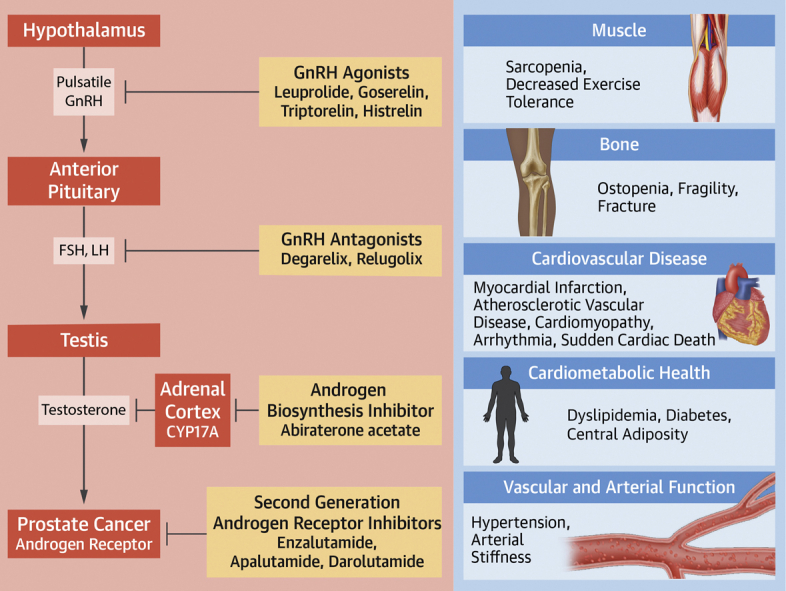
Figure 1.
Cardiometabolic Effects of Androgen Suppression Therapies in Prostate Cancer
This figure describes the mechanisms of androgen suppression for commonly used prostate cancer therapies. Adapted with permission from Hahn VS, Zhang KW, Sun L, et al. Circ Res. 2021;128:1576-1593. Cardiometabolic toxicities of androgen suppression are displayed. FSH = follicle-stimulating hormone; GnRH = gonadotropin-releasing hormone; LH = luteinizing hormone.
The optimal care for most men with prostate cancer receiving ADT includes a delicate balance between both oncologic and CV considerations, including: 1) evidence-based use, duration, and intensity of ADT; and 2) careful attention to CVRF mitigation and CVD management prior to, during, and following ADT exposure (Figure 2). This primer presents 2 clinical scenarios illustrating ADT use in contemporary oncologic practice and a recommended approach to managing CV health.
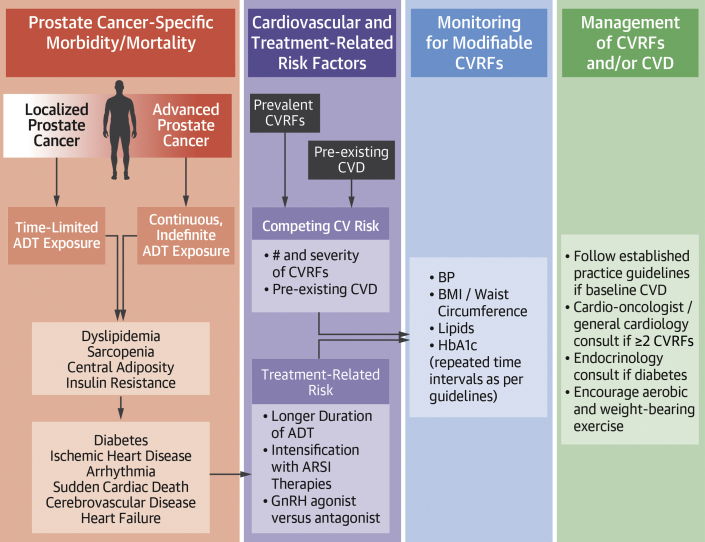
Figure 2.
Factors Influencing Cancer Control, Treatment-Related Toxicity, and Cardiovascular Outcomes
This figure describes the competing risks for morbidity and mortality in prostate cancer patients receiving systemic androgen deprivation therapy, including cancer-related, treatment-related, and cardiovascular-related risks. A recommendation is suggested for monitoring and management of cardiovascular risk factors (CVRFs) and cardiovascular disease (CVD). ADT = androgen deprivation therapy; ARSI = androgen receptor signaling agent; BMI = body mass index; BP = blood pressure; GnRH = gonadotropin-releasing hormone; HbA1c = glycosylated hemoglobin.
Case 1
A 74-year-old man presented with an elevated prostate-specific antigen of 7.2 ng/mL. Biopsy revealed Gleason 4+3=7 (grade group 3) prostate adenocarcinoma. His past medical history was significant for hypertension, previous 20 pack-year tobacco use, and coronary artery disease status post–coronary artery bypass graft 5 years ago. After consultation with both a urologist and radiation oncologist, he elected to pursue radiation therapy (RT) with concurrent 6 months of ADT with the GnRH antagonist relugolix.
Prostate cancer treatment decisions for localized disease are based on cancer risk stratification, the patient’s health status and life expectancy, and comorbid illnesses and potential complications from treatment. Because this patient has unfavorable intermediate-risk disease and a life expectancy of at least 10 years, treatment recommendations include either prostatectomy or radiation therapy with concurrent ADT, both given with curative intent (5). Neither prostatectomy nor RT has demonstrated superior cancer control outcomes in localized prostate cancer, and the differential risks of treatment, including potential treatment-associated CV complications, must be considered when choosing between the options. In this case, the risk of surgical complications, including potential CV risks related to surgery, should be weighed against the CV risk of adding ADT to RT. Even short durations (typically defined as 4-6 months) of ADT can be associated with CV complications. Indeed, the trial that demonstrated a survival benefit associated with the addition of 6 months of ADT to RT versus RT alone found that the benefit of ADT appeared to be limited to men without moderate-to-severe comorbidities (6). Because of his history of CVD and CVRFs, and concerns about surgical risk given his age and comorbidities, this patient elected to proceed with RT with ADT. However, to reduce his risk of CV complications, he opted to use the GnRH antagonist relugolix as ADT, rather than a GnRH agonist.
Relugolix is a recent Food and Drug Administration–approved oral GnRH antagonist that effectively lowered testosterone but was associated with a lower rate of major adverse CV events compared with the GnRH agonist leuprolide, especially among men with pre-existing CVD (1). Additionally, median time to testosterone recovery was more rapid with relugolix therapy, suggesting potential benefit in terms of rate of testosterone recovery for men in whom treatment cessation is the goal. However, an additional recent randomized trial comparing the relative CV toxicity of GnRH antagonist (degarelix) and GnRH agonist therapy did not find a statistical difference in major adverse CV events, and the relative CV risk profiles between agents remains unresolved (7).
Even when choosing treatments associated with lower potential CV risk, men receiving ADT should be monitored closely for the development of CVRFs or CV events. However, there are currently no standardized guidelines for CV monitoring for this population. A recent scientific statement from the American Heart Association recommends monitoring for reversible CVRFs and for development of the metabolic syndrome every 3 months (8). The ABCDE (Awareness, Blood Pressure, Cholesterol/Cigarettes, Diabetes/Diet, Exercise) algorithm can be used to systematically address reversible CVRFs and is recommended by the American Heart Association and the National Comprehensive Cancer Network for patients receiving hormonal therapies (9). We also recommend that men who have pre-existing CVD or ≥2 CVRFs consider co-management with cardio-oncology or cardiology teams whenever possible, and all patients should communicate with their primary care teams regarding the CV and metabolic risks of ADT exposure. While not specified in the American Heart Association statement, the relative risks and benefits of GnRH antagonist versus agonist therapy and the optimal duration of ADT therapy could be considered on a case-by-case basis in patients with high baseline CV risk, and treatment with ADT should be limited to situations in which there is clear cancer-control benefit. Finally, all men should be advised to regularly engage in aerobic and weight bearing exercise as tolerated to prevent or reverse weight gain and sarcopenia that can contribute to CV risk (Figure 2).
Case 2
A 68-year-old man with a history of localized prostate cancer treated with prostatectomy 5 years ago presented with a prostate-specific antigen of 15 ng/mL after being lost to follow-up for 3 years. A bone scan revealed osseous metastases in the thoracic and lumbar vertebrae and ribs. His past medical history was notable for a non–ST-segment elevation myocardial infarction 4 years prior, and ischemic cardiomyopathy (left ventricular ejection fraction of 40%), both treated with secondary risk reduction medical therapy. He had a history of type II diabetes mellitus and hypertension, both well controlled. After considering his preferences and comorbidities, the patient’s oncologist recommended systemic therapy with ADT and abiraterone acetate plus prednisone.
Approximately 40% of men will ultimately experience a prostate cancer relapse following definitive intent therapy for clinically localized disease, and 5% of incident prostate cancer diagnoses involve de novo metastatic presentations. Importantly, regardless of clinical presentation (ie, relapse following local treatment or de novo metastatic), this scenario of prostate cancer that has progressed without ongoing exposure to ADT denotes metastatic castration-sensitive prostate cancer (mCSPC). For the vast majority of such patients, palliative systemic ADT is indicated indefinitely to prolong survival, and prevent skeletal-related events (such as spinal cord compression, vertebral collapse, and pathologic fracture), cancer-related pain, and other disease-related complications.
Median survival for men with mCSPC, such as this patient, is approximately 5-6 years, with a proportion of patients demonstrating significantly longer (10+ year) survival. Over the past decade, randomized controlled trials have demonstrated that intensification of systemic therapy by adding medications to ADT improves overall survival, improves quality of life, and delays morbidity from metastatic disease, when compared with ADT alone. In the mCSPC setting, guideline-based treatments in addition to ADT include docetaxel chemotherapy, and 3 androgen receptor signaling inhibitors (ARSIs): abiraterone acetate (with prednisone), enzalutamide, and apalutamide. The choice of docetaxel versus ARSI depends on multiple patient-specific factors, including patient age and physical fitness for treatment with chemotherapy, as well as comorbidities and patient preferences.
Comorbid CVD and the presence of CVRFs are of critical importance when making decisions among the various treatment options used in combination with ADT for men with mCSPC. This patient preferred to avoid chemotherapy and opted for ARSI therapy. Importantly, ARSI therapies that are commonly used in combination with standard ADT dramatically attenuate testosterone signaling and estrogen levels even more profoundly than ADT alone, thereby contributing to increased CV risks. In particular, abiraterone acetate has been associated with an increased risk of adverse CV events in meta-analyses of randomized trials, and both abiraterone acetate and enzalutamide have been associated with an increased risk of hypertension (10). Careful monitoring using the aforementioned ABCDE strategy, co-management with a cardio-oncologist or cardiologist given his high risk status, and choosing an ARSI that does not interact with the cardiovascular medications that are currently maintaining his CV health will be of utmost importance for this patient. If his cardiac status decompensates during treatment, he and his treatment team will need to consider whether he should proceed with ADT alone (and discontinue the additional ARSI treatment), compromising cancer control but maintaining CV and overall health.
Treatment with ADT, either alone or in combination with ARSIs, can continue for years in prostate cancer survivors, resulting in prolonged treatment exposures and elevated CV risk over time. Future trials that collect complete CVRF information at baseline and define management strategies are needed to clarify optimal care. The high prevalence of baseline CVRFs and CVD and the intense androgen suppression regimens that comprise the backbone of treatment make monitoring and management of CV health for men with advanced prostate cancer of paramount importance. Strategies that employ a co-management approach including cardiologists and oncologists are likely to result in the best outcomes for these patients.
Funding Support and Author Disclosures
Dr Narayan has received research support from Bristol-Myers Squibb, Merck, TMunity Therapeutics, Pfizer, and Janssen; and consulting fees from Janssen, Pfizer, Myovant, Amgen, Regeneron, and Merck. Dr Ross has received consulting/speaker fees from Astellas, Bayer, Blue Earth, GenomeDx Biosciences, Janssen, Myovant, and Tempus. Dr Parikh has received grant support from Humana, the National Institutes of Health, the Prostate Cancer Foundation, the National Palliative Care Research Center, the Conquer Cancer Foundation, and the Veterans Administration; received personal fees and equity from GNS Healthcare and Onc.AI; received personal fees from the Cancer Study Group and Nanology; and honorarium from Flatiron and Medscape; and served on the board (unpaid) of the Coalition to Transform Advanced Care. Dr Nohria has received research support from Amgen; and served as a consultant for AstraZeneca, Bantam Pharmaceuticals, Boehringer Ingelheim, and Takeda Oncology. Dr Morgans has received research support from Astellas, Seattle Genetics, Bayer, Sanofi, and Myovant; and consulting fees from Astellas, AstraZeneca, AAA, Bayer, Clovis, Janssen, Dendreon, Myovant, Merck, Exelixis, Novartis, Pfizer, Blue Earth, Myriad, Sanofi, and Lantheus.
Footnotes
Charlotte Manisty, MD, PhD, served as the Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Shore N.D., Saad F., Cookson M.S., et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382:2187–2196. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 2.Hershman D.L., Unger J.M., Wright J.D., et al. Adverse health events following intermittent and continuous androgen deprivation in patients with metastatic prostate cancer. JAMA Oncol. 2016;2:453–461. doi: 10.1001/jamaoncol.2015.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Farrell S., Garmo H., Holmberg L., et al. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33:1243–1251. doi: 10.1200/JCO.2014.59.1792. [DOI] [PubMed] [Google Scholar]
- 4.Levine G.N., D'Amico A.V., Berger P., et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Circulation. 2010;121:833–840. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bill-Axelson A., Holmberg L., Garmo H., et al. Radical prostatectomy or watchful waiting in prostate cancer – 29-year follow-up. N Engl J Med. 2018;379:2319–2329. doi: 10.1056/NEJMoa1807801. [DOI] [PubMed] [Google Scholar]
- 6.D'Amico A.V., Chen M.-H., Renshaw A., et al. Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2015;314:1291–1293. doi: 10.1001/jama.2015.8577. [DOI] [PubMed] [Google Scholar]
- 7.Lopes R., Higano C., Slovin S., et al. Cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer: the primary results of the PRONOUNCE randomized trial. Circulation. 2021;144:1295–1307. doi: 10.1161/CIRCULATIONAHA.121.056810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okwuosa T.M., Morgans A., Rhee J.-W., et al. Impact of hormonal therapies for treatment of hormone-dependent cancers (breast and prostate) on the cardiovascular system: Effects and modifications: a scientific statement from the American Heart Association. Circ Genomic Precis Med. 2021;3 doi: 10.1161/HCG.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia N., Santos M., Jones L.W., et al. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. Circulation. 2016;133:537–541. doi: 10.1161/CIRCULATIONAHA.115.012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreira R.B., Debiasi M., Francini E., et al. Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: a meta-analysis of randomized controlled trials. Oncotarget. 2017;8:84572–84578. doi: 10.18632/oncotarget.20028. [DOI] [PMC free article] [PubMed] [Google Scholar]