Abstract
Objectives
The aim of this study was to characterize the cardiovascular disease (CVD) profile and describe the incidence and characteristics of cardiovascular (CV) events in patients with esophageal cancer (EC) following chemoradiation and surgery.
Background
Underlying CVD is a concern in patients with EC receiving curative treatment with chemoradiation and surgery.
Methods
Consecutive patients with EC referred for curative treatment were enrolled. Clinical CVD status, ongoing CVD treatment, cardiac function, and physical performance were assessed before chemoradiation. During a 90-day follow-up period, all CV events were noted and classified after in-depth medical record review. CV events were defined by major adverse CV events (transient ischemic attack, imaging-verified new stroke, unstable angina, heart failure or cardiomyopathy) or by Common Terminology Criteria for Adverse Events grade ≥3 (arrhythmia, thromboembolic events, or pericardial effusion requiring pericardiocentesis).
Results
Among 55 patients enrolled (median age 67 years; range: 50-86 years; 89% men), 22% had CVD prior to chemoradiation, and 11% with pre-existing CVD were inadequately treated according to current CV guidelines. Thirteen patients (24%) developed 15 events during follow-up. Pre-existing atrial fibrillation and a dilated left atrium were significantly associated with subsequent CV events. Left ventricular (LV) systolic dysfunction was frequently noted; 51% had impaired LV global longitudinal strain (<18%), and 16% had LV ejection fraction <50%.
Conclusions
A systematic cardiac evaluation prior to chemoradiation in patients with EC revealed a high prevalence of undetected CVD, inadequately treated pre-existing CVD, and a high incidence of CV events after chemoradiation. These findings highlight the need for a systematic baseline cardiac examination in patients with EC to optimize CVD treatment. (Impact of Cancer Therapy on Myocardial Function in Patients With Esophagus Cancer [Heartcheck]; NCT03619317)
Key Words: cardiac function, cardiovascular disease, chemoradiation, esophageal cancer
Abbreviations and Acronyms: AF, atrial fibrillation; CTCAE, Common Terminology Criteria for Adverse Events; CV, cardiovascular; CVD, cardiovascular disease; EC, esophageal cancer; GLS, global longitudinal strain; LV, left ventricular; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular event(s); METs, metabolic equivalents of task; NT-proBNP, N-terminal pro–B-type natriuretic peptide; Vo2max, peak oxygen consumption
Central Illustration
Underlying cardiovascular disease (CVD) is of concern in patients with locally advanced esophageal cancer (EC) and gastroesophageal junction cancer receiving neoadjuvant chemoradiation followed by surgery or definitive chemoradiation (1). This may be secondary to potentially accelerated CVD in patients with EC without pre-existing CVD or presence of known CVD and cardiovascular (CV) risk factors (2,3). Currently, few data exist on risk factors for developing significant acute CVD during chemoradiation and thoracic surgery in patients with EC. In this context, limited data are available regarding pretreatment cardiac function, clinical performance, and how pretreatment cardiac status may influence the subsequent neoadjuvant chemoradiation or definitive chemoradiation treatment course.
In patients with lung cancer, who resemble patients with EC in terms of thoracic radiation therapy, Atkins et al (4) found that patients with pre-existing coronary heart disease had a significantly increased risk for major adverse CV events (MACE). In contrast, a retrospective study by Hayashi et al (5) showed CV events in patients with EC, without any specific association with pre-existing CV risk factors such as hypertension, diabetes mellitus, and dyslipidemia. Pretreatment cardiac status and its relation to multimodal curative treatment in patients with EC seem to be relevant concerns given the location of the heart within the irradiated field and the subsequent risk for radiation-induced cardiac toxicity (6). In addition, cardiac toxicity from concomitant chemotherapy and subsequent thoracic surgery may contribute to additional CV events. The aim of the present study was to: 1) characterize the CV profile and treatment of consecutive patients with EC receiving neoadjuvant chemoradiation and surgery or definitive chemoradiation; 2) evaluate cardiac structure and function, focusing on left ventricular (LV) systolic and diastolic function; 3) describe the incidence and characteristics of CV events occurring during neoadjuvant chemoradiation and surgery or definitive chemoradiation; and 4) study the associations between clinical and echocardiographic parameters and subsequent CV events.
Methods
Study population
From June 2018 to February 2021, a total of 59 patients with primary EC scheduled for curative therapy were invited to participate in this prospective single-center study. Four patients were excluded, 1 patient declined the invitation, and 3 patients had early disease progression and shifted from curative to palliative treatment. A Consolidated Standards of Reporting Trials diagram is presented in Supplemental Figure 1. At their first contact to the Department of Oncology at Aarhus University Hospital, patients were informed about the study, and subsequently written consent was obtained according to the principles of the Declaration of Helsinki. The inclusion criteria were age ≥18 years; World Health Organization performance status of 0, 1, or 2; histologically verified locally advanced, nonmetastatic squamous cell carcinoma or adenocarcinoma of the esophagus or gastroesophageal junction; and referral for chemotherapy and concomitant radiation therapy followed by surgery prescribed for curative intent (7,8). Before the start of definitive chemoradiation or neoadjuvant chemoradiation and surgery, patients underwent a baseline clinical cardiac examination in the Department of Cardiology at Aarhus University Hospital. The date of the cardiac examination was considered baseline and defined the starting point (time 0) of the 90-day follow-up period. Ninety days of follow-up was chosen to ensure that a 30-day postsurgery period would be included. During the 90-day follow-up period, all CV events were noted and evaluated by in-depth medical record review by one investigator (M.M.A.S.). The study was approved by the local scientific ethics committee of the Central Denmark Region and the Danish Data Protection Agency, and all patients provided written informed consent. Furthermore, the trial was registered with ClinicalTrials.gov (NCT03619317) prior to study initiation.
Cancer treatment
Cancer treatment included weekly intravenous chemotherapy with carboplatin and paclitaxel and concomitant radiation therapy. The carboplatin dose was area under the curve 2 and the paclitaxel dose 50 mg/m2. Radiation therapy was delivered as intensity-modulated RT. The neoadjuvant chemoradiation group received 41.4 Gy in 23 fractions over 5 weeks (8); the definitive chemoradiation group received 50 Gy in 25 fractions or 50.4 Gy in 28 fractions over 6 weeks. Esophageal resections were performed no earlier than 3 to 4 weeks after completion of neoadjuvant chemoradiation by open thoracic approach, minimally invasive surgery, or a combination of both (hybrid procedure).
Pre–EC treatment CV evaluation
The baseline cardiac clinical examination comprised assessment of cardiac history, medication, and dosages; physical examination, including measurement of height, weight, and biochemical testing; 12-lead electrocardiography; comprehensive transthoracic echocardiography; and a symptom-limited, semisupine cardiopulmonary exercise test with assessment of peak oxygen consumption (Vo2max). Blood pressure was assessed using an automatic blood pressure monitor over 20 minutes during quiet resting conditions.
Echocardiography
Comprehensive transthoracic echocardiography was performed by a single investigator (M.M.A.S.) according to current guidelines (9). A commercially available ultrasound system (Vivid E95, GE Healthcare) was used, equipped with a 1.4- to 4.6-MHz phased-array transducer. Images were stored digitally using custom software (EchoPAC, GE Healthcare) for offline analysis. Echocardiographic postprocessing analyses were performed by a single investigator (M.M.A.S.) blinded to all clinical data.
LV ejection fraction (LVEF) was calculated using the Simpson biplane method of discs. LVEF outflow tract velocity-time integral measurements were obtained using pulsed-wave Doppler to calculate stroke volume. Cardiac output was calculated as stroke volume multiplied by heart rate. Global longitudinal strain (GLS) using 2-dimensional speckle-tracking analysis was assessed on standard 2-dimensional images from the apical 4-, 2-, and 3-chamber views acquired at a frame rate >55 frames/s (10). Diastolic function was evaluated from: 1) the biplane method of volume of the left atrium; 2) mitral pulsed-wave Doppler inflow at the mitral leaflet tips; and 3) tissue Doppler measurements of e′ at the lateral and septal annulus according to current guidelines of the European Society of Cardiology (11). Right ventricular systolic function was evaluated using M-mode measurement of tricuspid annular plane systolic excursion. Right ventricular systolic longitudinal function (right ventricular GLS) was measured as the average of the deformation at the free wall of the right ventricle. Valvular function was also evaluated.
Cardiopulmonary exercise protocol
Patients underwent cardiopulmonary exercise testing in a semisupine position with lateral tilt assessing Vo2max. A multistage maximal symptom-limited test was conducted using the Echo Cardiac Stress Table (Lode) (12), with work load starting at 0 W and increasing stepwise by 25 W every 3 minutes. Patients were encouraged to exercise until exhaustion (Borg rating >18) with a fixed pedaling speed of 60 rounds/min. The target respiratory exchange ratio was ≥1.1, and metabolic equivalents of task (METs) were used to indicate the rate of intensity. Patients were monitored using pulse oximetry, blood pressure measurements, and continuous 12-lead electrocardiography.
Blood samples
Venous blood samples were collected to analyze N-terminal pro–B-type natriuretic peptide (NT-proBNP) (upper limit of normal <300 ng/L), troponin T (upper limit of normal <14 ng/L), and lipid profiles.
CVD treatment evaluation
Patients with prevalent CVD or CV risk factors at baseline were classified as having 1 or more of the following conditions: heart failure or cardiomyopathy, persistent or paroxysmal atrial fibrillation (AF), coronary artery disease, valve disease, hypertension, or dyslipidemia. CVD was diagnosed and evaluated in accordance with guidelines from the European Society of Cardiology (13, 14, 15). In each patient with pre-existing CVD, CV medical treatment, including medication doses, was assessed to determine if these were prescribed in accordance with recommended guidelines, and if not, treatment was adjusted accordingly. Patients with suspicion of undiagnosed CVD were referred for cardiac examinations according to institutional clinical practice.
Definition of CV events
Significant adverse CV events were defined either according to the American Heart Association and American College of Cardiology definitions of MACE (16) or as a Common Terminology Criteria for Adverse Events (CTCAE; version 4.0) grade ≥3. MACE included: 1) transient ischemic attack; 2) imaging-verified new stroke; 3) unstable angina; or 4) HF or cardiomyopathy; the latter 2 both required hospitalization or an urgent visit to a department of cardiology. Events defined by CTCAE were grouped into arrhythmia, thromboembolic events, or pericardial effusion requiring pericardiocentesis. CV events were evaluated by one investigator (M.M.A.S.) and a senior cardiologist (S.H.P.).
Statistical analysis
Continuous variables following approximately normal distributions are expressed as mean ± SD or median (interquartile range) and were compared using a 2-sample Student’s t-test or the Wilcoxon rank sum test, depending on normality. Histograms and Q-Q plots were used to check continued values for normality. Categorical variables are presented as frequencies and corresponding percentages and were compared using the Pearson chi-square or Fisher exact test. For correlation analysis, a Spearman or Pearson correlation test was used. The CV event rate was calculated using the Kaplan-Meier method with time to first event. Individuals were followed until first CV event, death, or the end of the follow-up period, whichever came first. The log-rank test was used to compare events in subgroups. For univariable analysis, a Cox proportional hazards model was used to calculate HRs to determine whether clinical variables were associated with CV events. P values <0.05 were considered to indicate statistical significance. All statistical analyses were performed using Stata/IC version 15.1 (StataCorp).
Results
Baseline clinical CVD and cancer characteristics
Table 1 shows baseline clinical and tumor characteristics of the 55 enrolled patients, all of whom were White. Smoking history was as follows: current in 12, prior in 29, and never in 14 patients. A total of 46% of patients had an estimated 10-year survival <35%, as defined by the Charlson comorbidity index. Regarding treatment, 24% of patients were referred for definitive chemoradiation and 76% for neoadjuvant chemoradiation followed by surgery. Surgery was performed according to a minimally invasive (n = 19), open thoracic (n = 1), or hybrid procedure (n = 21).
Table 1.
Baseline Characteristics (n = 55)
| Male | 49 (89) |
| Age, y | 66 (50-86) |
| Tobacco | |
| Never | 14 (25) |
| Quit >6 wk ago | 26 (47) |
| Quit <6 wk ago | 3 (5) |
| Active | 12 (22) |
| Chronic obstructive lung disease known | 5 (9) |
| Diabetes | 2 (4) |
| Systolic BP, mm Hg | 121 (20) |
| Diastolic BP, mm Hg | 79 (12) |
| Heart rate, beats/min | 74 (13) |
| Body mass index, kg/m2 | 26 (5) |
| Body surface area, m2 | 1.9 (0.2) |
| Comorbidity index | |
| Charlson comorbidity index | |
| <5 | 9 (16) |
| 5-35 | 22 (40) |
| 36-74 | 18 (33) |
| >75 | 6 (11) |
| Clinical characteristics | |
| WHO performance status | |
| 0 | 35 (64) |
| 1 | 17 (31) |
| 2 | 3 (5) |
| NYHA functional class | |
| I | 46 (84) |
| II | 6 (11) |
| III | 3 (5) |
| IV | 0 (0) |
| Clinical frailty scale | |
| Very fit | 18 (33) |
| Well | 20 (33) |
| Managing well | 13 (24) |
| Vulnerable | 3 (5) |
| Mildly frail | 1 (2) |
| Cancer diagnosis | |
| ICD-10 code | |
| Gastroesophageal junction cancer (16.0) | 40 (73) |
| Esophageal cancer (15.3-15.9) | 15 (27) |
| Clinical TNM classification | |
| T4 | 2 (4) |
| T3 | 48 (86) |
| T2 | 5 (10) |
| N0 | 25 (46) |
| N1 | 18 (33) |
| N2 | 11 (19) |
| N3 | 1 (2) |
| Histology | |
| Adenocarcinoma | 37 (67) |
| Squamous cell carcinoma | 18 (33) |
| Biomarkers | |
| NT-proBNP, ng/L | 115 (50-209) |
| Troponin T, ng/L | 9.0 (6-9) |
| Total cholesterol, mmol/L | 4.7 ± 1.1 |
| HDL, mmol/L | 1.2 (1-1.5) |
| LDL, mmol/L | 2.7 ± 0.9 |
| Triglycerides, mmol/L | 1.5 (1-1.8) |
| Creatinine, μmol/L | 75.1 ± 18.9 |
| Hemoglobin, mmol/L | 8.7 (8.2-9.3) |
Values are n (%), median (interquartile range), or mean ± SD. To convert mmol/L to mg/dL for total cholesterol, HDL, and LDL, multiply by 38.67. To convert mmol/L to mg/dL for triglycerides, multiply by 88.57.
BP = blood pressure; HDL = high-density lipoprotein; ICD-10 = International Classification of Diseases-Tenth Revision; LDL = low-density lipoprotein; NT-proBNP = N-terminal pro–B-type natriuretic peptide; NYHA = New York Heart Association; WHO = World Health Organization.
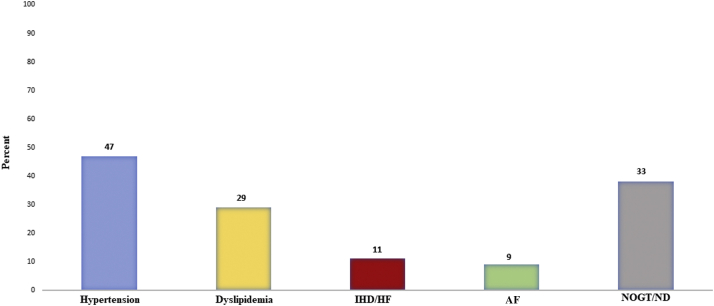
Figure 1 demonstrates prevalent CVD at the time of cancer diagnosis. At baseline assessment, among the 55 patients, 33% either had new CVD diagnoses (n = 12) or were not being treated as recommended in current guidelines (n = 6). Two patients were diagnosed with heart failure with reduced ejection fraction, 1 had ischemic heart disease, and the other had nonischemic heart failure. Twenty-six patients had arterial hypertension, 4 of whom were not being treated in accordance with guideline recommendations, and 3 had new diagnoses of hypertension. AF was present in 5 patients, and another 3 patients were diagnosed with arrhythmia at baseline (2 with AF and 1 with nonsustained ventricular tachycardia). Dyslipidemia was present in 16 patients, 2 of whom were not being treated as recommended in guidelines but were changed accordingly. Four patients were diagnosed with dyslipidemia, and relevant treatment was initiated. Nine patients (16%) had elevated NT-proBNP (>300 ng/L) at baseline, among whom 6 had pre-existing CVD. Twelve patients (22%) had elevated troponin T, 4 of whom had pre-existing CVD. No patients met the criteria of acute coronary syndrome according to the European Society of Cardiology guidelines.
Figure 1.
Pre-Existing CVD Before Beginning Esophageal Cancer Treatment
Proportion of cardiovascular disease (CVD) at the time of cancer diagnosis, as defined by atrial fibrillation (AF), ischemic heart disease (IHD) or heart failure (HF), hypertension or dyslipidemia, and a composite of these elements with either a new diagnosis (ND) or patients not treated optimally according to guidelines (NOGT).
Myocardial and cardiopulmonary exercise performance
Table 2 shows data on physical performance and echocardiographic analysis of systolic and diastolic function of the left and right ventricles. No patients had significant valvular heart disease. The average LVEF was within the normal range, although 16% of patients had an ejection fraction <50%. According to sex-specific cutpoints, 27% of men (LVEF reference <52%) and 17% of women (LVEF reference <54%) had an abnormal LVEF. GLS was generally borderline abnormally low (normal reference ≥18%). Among all 55 patients, 51% had abnormally low GLS. Abnormal diastolic function was noted in 13% and was classified as grade I diastolic dysfunction. No patients were classified as having grade II or III diastolic dysfunction. Right ventricular systolic function was found to be within the normal range. There were no elevations in pulmonary systolic pressure.
Table 2.
Echocardiographic and Cardiopulmonary Exercise Parameters
| Left ventricle | |
| LVEF, % | 55.6 ± 5.9 |
| LV cardiac output, L/min | 3.4 ± 0.9 |
| LV diastolic function | |
| LAVI, mL/m2 | 28.2 (24.8-38.1) |
| Mitral E/A ratio | 0.8 (0.7-1.0) |
| E/e′ ratio | 6.9 (5.7-7.9) |
| Right ventricle | |
| TAPSE, cm | 2.2 ± 0.4 |
| RV free wall GLS, % | 22.2 ± 5.2 |
| TGR, mm Hg | 18.5 ± 8.1 |
| Exercise | |
| METs, mL/kg/min | 5.7 (4.6-6.9) |
| Vo2max at peak exercise, mL/kg/min | 20.7 ± 5.8 |
| Vo2max at peak exercise, % of predicted | 87 ± 23 |
| Peak RER | 1.2 ± 0.1 |
| RPP | 2.2 (2.1-2.8) |
Values are mean ± SD or median (interquartile range).
GLS = global longitudinal strain; LAVI = left atrial volume index (biplane); LV = left ventricular; LVEF = left ventricular ejection fraction (Simpson biplane method); METs = metabolic equivalents of task; RER = respiratory exchange ratio; RPP = rate-pressure product; RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion; TGR = tricuspid pressure gradient of the right ventricle; Vo2max = peak oxygen consumption.
Vo2max was overall mildly reduced in this cohort of patients with EC. The median exercise capacity was 5.7 METs (interquartile range: 4.6-6.9 METs), and a mean peak respiratory exchange ratio of 1.23 ± 0.10 was reached. As reference, a respiratory exchange ratio >1.1 is considered to indicate sufficient patient effort during cardiopulmonary stress testing (12). Correlation analysis of physical performance (Vo2max) and LV function (LVEF and GLS) showed a modest but significant correlation, with r = 0.325 (P = 0.019) and r = 0.44 (P = 0.001), respectively.
CV events
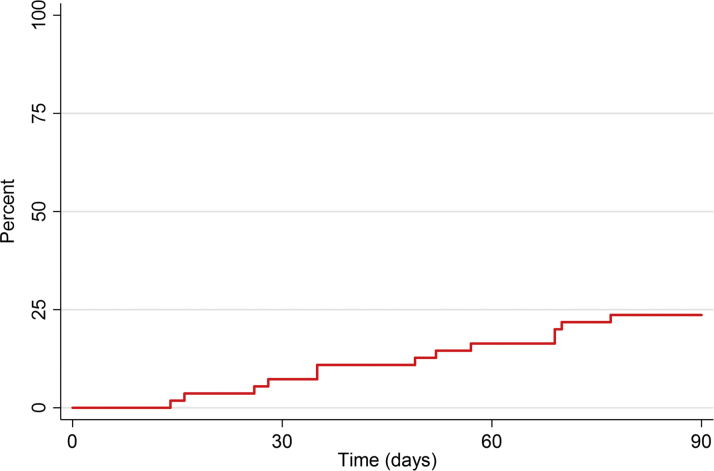
During the follow-up period of 90 days, 13 patients developed a total of 15 CV events, 4 of whom had no pre-existing CVD or elevated levels of NT-proBNP. The 90-day CV event-free rate of MACE and CTCAE grade ≥3 was 76.4% (95% CI: 62.8-85.5), as shown in Figure 2. MACE included hospitalization for unstable angina (n = 3) and stroke (n = 1), and events defined by CTCAE included onset of AF (n = 5), atrioventricular nodal re-entry tachycardia (n = 1), pericardial effusion (n = 1), and pulmonary emboli or deep venous thromboembolic events (n = 4). No patients developed heart failure requiring hospitalization. Two patients died within the 90-day follow-up period. In 1 patient, the cause of death was cancer related, and this had followed a previous CV event. The other patient died out of the hospital at day 84 of the follow-up period. The latter patient had no signs of cancer progression, no autopsy was performed, and the death was classified as of unknown cause.
Figure 2.
Overall CV Event Rate
Time to first major adverse cardiovascular (CV) event or CV event defined by Common Terminology Criteria for Adverse Events grade ≥3.
Univariable analysis of clinical, echocardiographic, and exercise characteristics in patients with CV events
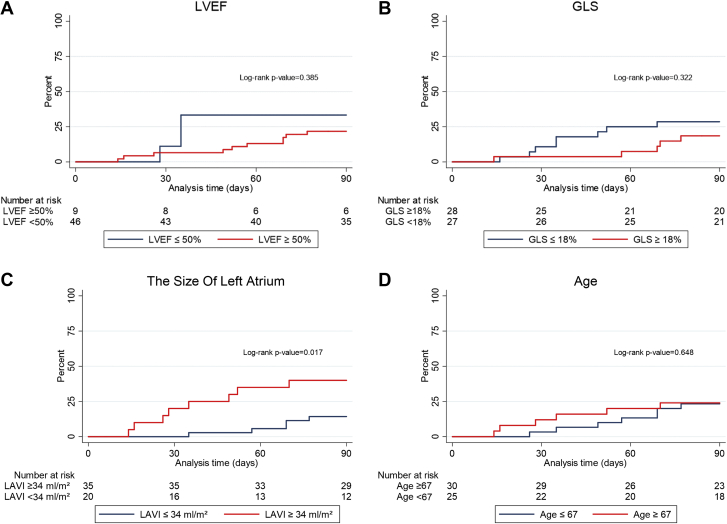
Univariable analysis was used to establish if clinical, echocardiographic, or exercise characteristics were associated with CV events (MACE plus CTCAE grade ≥3) during the 90-day follow-up period (Table 3). Using Cox proportional hazards models, left atrial volume index ≥34 mL/m2 (HR: 3.59; 95 % CI: 1.17-10.99) and preexisting AF (HR: 4.35; 95% CI: 1.18-16.06) were each associated with MACE. Of note, in patients with CV events, 61.5% had GLS <18% and 23.3% had a LVEF <50%, with HRs of 1.74 (95% CI: 0.70-5.34) and 1.76 (95% CI: 0.48-6.40), respectively. Two patients with CV events had diastolic dysfunction. No other characteristics were significantly associated with CV events. Figure 3 shows MACE plus CTCAE grade ≥3 with the CV event rate by patients according to the following categories: 1) LVEF ≥50% or <50%; 2) GLS ≥18% or <18%; 3) left atrial volume index ≥34 mL/m2 or <34 mL/m2; and 4) age ≥67 years or <67 years.
Table 3.
Univariable Associations Between Clinical Variables and Subsequent CV Events
| Patients (n = 55) | Events (n = 13) | HR | 95% CI | |
|---|---|---|---|---|
| Age | ||||
| <67 y | 30 | 7 | 1.00 | Reference |
| ≥67 y | 25 | 6 | 1.03 | 0.96-1.11 |
| Sex | ||||
| Female | 6 | 3 | 1.00 | Reference |
| Male | 49 | 10 | 2.50 | 0.69-9.11 |
| Smoking | ||||
| Never | 14 | 6 | 1.00 | Reference |
| Ever (ongoing/previous) | 41 | 7 | 0.33 | 0.11-0.98 |
| Body mass index | ||||
| ≥25 kg/m2 | 32 | 7 | 1.00 | Reference |
| <25 kg/m2 | 23 | 6 | 1.18 | 0.39-3.50 |
| WHO performance status | ||||
| 0 | 35 | 8 | 1.00 | Reference |
| 1 or 2 | 20 | 5 | 1.11 | 0.36-3.39 |
| Systolic BP | ||||
| <140 mm Hg | 46 | 10 | 1.00 | Reference |
| ≥140 mm Hg | 9 | 3 | 1.72 | 0.47-6.26 |
| Diastolic BP | ||||
| <90 mm Hg | 47 | 10 | 1.00 | Reference |
| ≥90 mm Hg | 8 | 3 | 2.04 | 0.56-7.43 |
| Heart rate | ||||
| ≥80 beats/min | 14 | 3 | 1.00 | Reference |
| <80 beats/min | 41 | 10 | 1.28 | 0.35-4.66 |
| Total cholesterol | ||||
| ≤5 mmol/L | 37 | 6 | 1.00 | Reference |
| ≥5 mmol/L | 18 | 7 | 2.68 | 0.90-7.99 |
| LDL cholesterol | ||||
| <3 mmol/L | 35 | 6 | 1.00 | Reference |
| ≥3 mmol/L | 20 | 7 | 2.22 | 0.75-6.62 |
| NT-proBNP | ||||
| ≤115 ng/L | 28 | 6 | 1.00 | Reference |
| ≥115 ng/L | 27 | 7 | 1.33 | 0.45-3.96 |
| Troponin T | ||||
| <14 ng/L | 43 | 9 | 1.00 | Reference |
| ≥14 ng/L | 12 | 4 | 1.99 | 0.61-6.48 |
| Hypertension | ||||
| No | 29 | 6 | 1.00 | Reference |
| Yes | 26 | 7 | 1.35 | 0.45-4.01 |
| Atrial fibrillation | ||||
| No | 50 | 10 | 1.00 | Reference |
| Yes | 5 | 3 | 4.35 | 1.18-16.1 |
| IHD | ||||
| No | 49 | 12 | 1.00 | Reference |
| Yes | 6 | 1 | 0.67 | 0.87-5.13 |
| LVEF | ||||
| ≥50% | 46 | 10 | 1.00 | Reference |
| <50% | 9 | 3 | 1.76 | 0.48-6.40 |
| GLS | ||||
| ≥18% | 27 | 5 | 1.00 | Reference |
| <18% | 28 | 8 | 1.74 | 0.70-5.34 |
| Diastolic dysfunction | ||||
| No | 48 | 11 | 1.00 | Reference |
| Yes | 7 | 2 | 1.28 | 0.28-5.77 |
| LAVI | ||||
| <34 mL/m2 | 35 | 5 | 1.00 | |
| ≥34 mL/m2 | 20 | 8 | 3.59 | 1.17-10.9 |
| TAPSE | ||||
| ≥18 mm | 44 | 3 | 1.00 | Reference |
| <18 mm | 11 | 10 | 1.24 | 0.34-4.50 |
| Vo2max at peak exercisea | ||||
| ≥20 mL/kg/min | 25 | 6 | 1.00 | Reference |
| <20 mL/kg/min | 27 | 7 | 1.14 | 0.38-3.40 |
| Vo2max at peak exercise, % predicteda | ||||
| ≥85% | 24 | 6 | 1.00 | Reference |
| <85% | 28 | 7 | 1.05 | 0.35-3.11 |
| Histology | ||||
| Squamous cell carcinoma | 37 | 4 | 1.00 | Reference |
| Adenocarcinoma | 18 | 9 | 1.15 | 0.35-3.73 |
| Type of treatment | ||||
| Definitive chemoradiation | 13 | 1 | 1.00 | Reference |
| Neoadjuvant chemoradiation | 42 | 12 | 4.13 | 0.54-31.8 |
| Type of surgery in neoadjuvant chemoradiation group | ||||
| Minimally invasive | 19 | 4 | 1.00 | Reference |
| Open thoracic (n = 1) or hybrid (n = 21) | 22 | 7 | 1.67 | 0.49-5.72 |
Figure 3.
CV Events: Major Adverse CV Events and CV Events Defined by Common Terminology Criteria for Adverse Events Grade ≥ 3
Cardiovascular (CV) event rate according to the following categories: (A) left ventricular ejection fraction (LVEF); (B) global longitudinal strain (GLS); (C) left atrial volume index (LAVI), and (D) median age.
Discussion
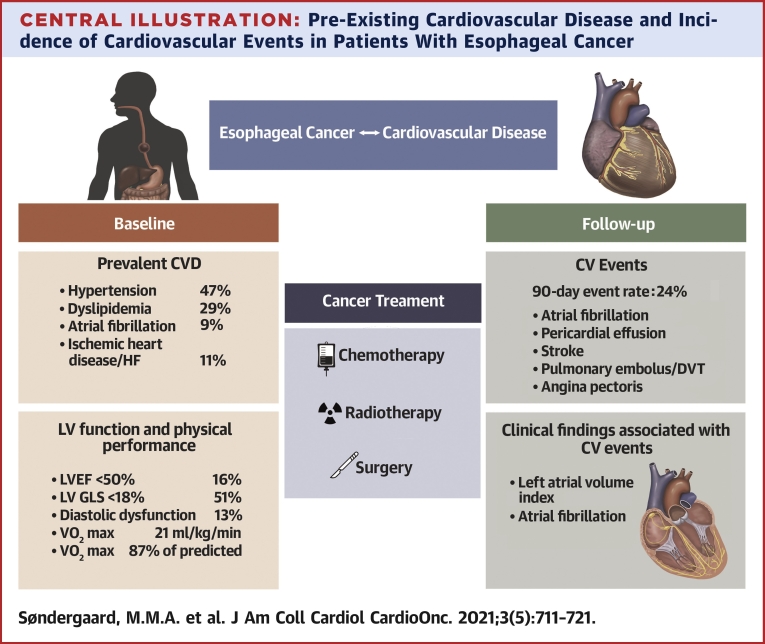
In the present study, we investigated CVD and risk factor profile, cardiac function, and cardiopulmonary fitness in patients with esophageal or gastroesophageal junction cancer before initiating cancer treatment for curative intent. CV events were registered during the treatment course and up to 90 days after baseline cardiac investigation. The main findings were as follows: 1) prior to the initiation of cancer therapy for curative intent, there was a high prevalence of CVD; 2) one third of patients who were either given a new CVD diagnosis or had previously established CVD were not being treated according to current guidelines; 3) adverse CV events were frequent and occurred in approximately one quarter of the patients within the 90-day follow-up period; and 4) left atrial dilation and prevalent AF were associated with CV events in follow-up (Central Illustration).
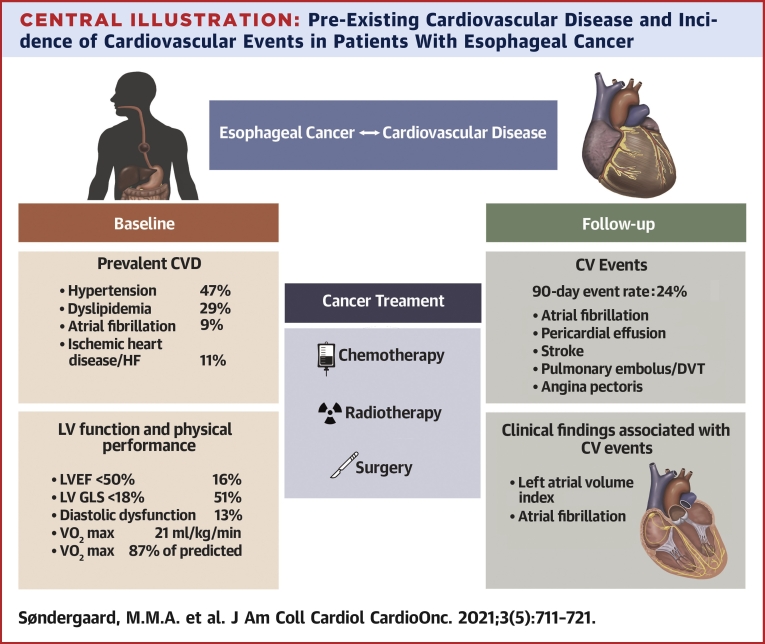
Central Illustration.
Pre-Existing Cardiovascular Disease and Incidence of Cardiovascular Events in Patients With Esophageal Cancer
A high prevalence of cardiovascular disease (CVD) was detected prior to cancer treatment with curative intent of patients with esophageal cancer. A substantial proportion of patients presented with a suboptimal medical CVD treatment and undiagnosed CVD. Left ventricular systolic function was impaired in one third of the patients and was associated with mildly reduced peak exercise capacity. A cardiovascular (CV) event rate of 24% was determined over 90 days of follow-up. Atrial fibrillation and the size of the left atrium were associated with CV events.
Prevalence of CVD prior to treatment
In this study, we found a high prevalence of CVD at baseline. Importantly, 22% of patients were diagnosed with CVD and 11% were not being treated with guideline-directed CV medical therapy. These findings suggest that in an effort to optimize patients’ CVD status, systematic cardiac evaluation should be considered prior to and during combined modality chemoradiation with or without surgery. Neoadjuvant chemoradiation followed by surgery or definitive chemoradiation carries systemic and local toxicity, and although the clinical effectiveness of routine CV evaluation prior to chemoradiation has not been tested in a clinical trial, the application of guideline-directed medical therapy in patients with prevalent CVD treated with curative intent is, generally speaking, consistent with best practice.
An important issue that remains to be clarified is whether all patients with EC should be screened for CVD by a CV specialist or if patients with EC should be referred for cardiac examination solely on the basis of specific indications (eg, pre-existing CVD, cardiac symptoms, older age, increased NT-proBNP), in an effort to promote greater cost-effectiveness and to avoid the risk of delaying necessary cancer treatment. As demonstrated previously and in the present study, established CVD and the risk profile of patients with EC seem considerable and are likely of prognostic importance (5,17). It is well established that CVD in general increases with age, especially after the sixth decade. The risk for ischemic heart disease in particular increases, with a predominance among men and smokers (2). In this cohort, 60% of patients would qualify for cardiac examination as part of diagnostic work-up prior to neoadjuvant chemoradiation or definitive chemoradiation, as they presented with either pre-existing CVD or elevated NT-proBNP levels. Furthermore, in the present study, 22% of patients were diagnosed with new CV conditions.
CV events
We report a high prevalence of CV events, with a 90-day event rate of 24%. Previous retrospective studies have reported data on CV events with an inherent significantly longer follow-up time. Among these, Hayashi et al (5) reported on cardiac events defined by CTCAE grade ≥3 (ischemic heart disease, arrhythmia, pericardial effusion, and sudden death), which occurred at a rate of 16% over a longer follow-up time (median 73 months). Witt et al (17) reported CTCAE grade ≥3 cardiac events, with a 25% CV event rate in patients treated with neoadjuvant chemoradiation and a median 70-month follow-up after surgery (17). A meta-analysis including 7 studies of postoperative short-term outcomes in patients with EC demonstrated a 12% increase in cardiopulmonary complications in patients treated with neoadjuvant chemoradiation. However, cardiopulmonary complications were not defined, nor was follow-up on cardiopulmonary complications (18). A review by Beukema et al (1) analyzed data from 13 studies and reported an overall 10.8% incidence of clinically relevant cardiotoxicity within 2 years, a considerably longer time frame than our study. All the aforementioned studies were retrospective, had inconsistent endpoints, and lacked validated definitions of cardiopulmonary complications and cardiotoxicity. To the best of our knowledge, no prior study of EC has prospectively used American Heart Association– and American College of Cardiology–defined endpoints on CV events (16). Ongoing studies by our own group are evaluating long-term CV morbidity and mortality in larger populations of treated patients with EC.
Clinical differences in patients with or without CV events
Among patients with and without CV events, we found no differences in baseline characteristics such as age, sex, body mass index, and performance status. Left atrial volume index ≥34 mL/m2 and pre-existing AF were statistically significantly associated with CV events. Patients with CV events had slightly lower LVEF and GLS, and median NT-proBNP levels were slightly higher, but none of these findings reached statistical significance. Furthermore, no differences were noted in troponin T. Hayashi et al (5) reported that smoking was an important risk factor for developing CVD but did not find that comorbidities such as diabetes mellitus, hypertension, hyperlipidemia, or other heart disease predicted the development of CV events (5). Witt et al (17) reported retrospectively on 123 patients, among whom 53 underwent neoadjuvant chemoradiation followed by surgery and 70 had surgery alone. Among these patients, pre-existing heart disease, defined as coronary artery disease or HF, was related to higher rates of cardiac events (HR: 2.56; P = 0.04). In our study, paroxysmal AF was recognized in 5 of the 15 events. All 5 patients were older than 55 years (range: 55-72 years), 4 were men, and none was receiving prophylactic antiarrhythmic drug therapy. Three of these patients with AF events had documented enlarged left atria. Hypertension and enlarged left atrium are risk factors for AF, and conversely, AF can result in an enlarged left atrium. Patients with hypertension who are older than 55 years seem to be at an increased risk for developing AF during the treatment course. These findings may potentially be used to select patients with EC for pretreatment cardiac evaluation. Patients with significantly enlarged left atria seem to carry a higher risk for events and could be evaluated for cardioprotection prophylaxis (19). These are considerations for future research.
Baseline echocardiographic and cardiopulmonary stress test findings
The echocardiographic evaluation in our cohort revealed that 27% of men and 17% of women had abnormally low LVEF and GLS. The prevalence rates of abnormal LVEF, GLS, and diastolic function are comparable with those reported in the prospective study of 40 patients with EC by Lund et al (20). GLS is a well-recognized parameter of systolic function that has been shown to be of value in detecting subclinical myocardial dysfunction in patients with normal LVEF. Furthermore, it has been suggested that GLS is superior to LVEF in detecting cardiotoxicity in patients with cancer (21). At baseline, we found that 51% of patients had GLS <18%, which is the generally accepted lower limit of normal GLS. Furthermore, we found that 16% of patients had an ejection fraction < 50% and that 35% of patients with GLS <18% had LVEF ≥ 50%.
Cardiopulmonary exercise testing with assessment of Vo2max is considered the gold standard for evaluating patients’ physical performance and in general adds prognostic information in patients with cardiomyopathy or HF (12). Prospective data on physical performance in relation to neoadjuvant chemoradiation in patients with EC are limited. Von Döbeln et al (22) showed that peak physical performance decreased after neoadjuvant chemoradiation by assessment of maximal METs. Lund et al (20) found that working capacity (maximal watts performed) decreased after neoadjuvant chemoradiation. Before chemoradiation, our patients had mildly reduced Vo2max, with a mean value of only 87% of the expected value. The lower pretreatment physical performance may be related to both cancer and cardiac performance. We noted a modest but significant correlation between maximal Vo2max, LVEF, and GLS. No correlation was observed between Vo2max and increased left atrial volume index or tricuspid annular plane systolic excursion. Low physical performance may result in worsening of CV prognosis in patients with EC undergoing treatment comprising chemoradiation and surgery. Further research on echocardiographic and cardiopulmonary fitness after treatment is needed, with longitudinal testing prior to and after chemoradiation.
Study limitations
This was a single-center study with a small sample size of 55 White patients, which limits the generalizability of our findings to patients with EC undergoing other institutional treatment practices or patients with EC with different clinical characteristics, including diverse race or ethnicity (23). Only patients undergoing EC treatment with curative intent were included. As a result, the rates of pre-existing CVD and new CV events were likely lower than in patients referred for palliative oncologic treatment. Furthermore, only short-term cardiotoxicity was examined; the long-term effects at present remain unknown and need to be addressed in future studies. Proving the beneficial effect of pretreatment cardiac examination requires a randomized controlled study, which may not be feasible. Finally, we recognize that a competing risks approach could have been considered, although there was only 1 patient death, secondary to unknown causes.
Conclusions
A systematic cardiac evaluation prior to curative treatment in patients with EC revealed a high prevalence of CVD at the time of cancer diagnosis. A substantial proportion of patients with EC presented with less optimal medical CVD treatment and undiagnosed CVD. LV systolic function was impaired in one third of the patients and was associated with mildly reduced peak exercise capacity. A high incidence of CV events was recorded within 90 days of curative treatment with chemoradiation alone or combined with subsequent thoracic surgery. These findings highlight the need for systematic baseline examination in patients with EC to optimize cardiac treatment and risk profile.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Traditional CVD risk factors and potentially accelerated CVD due to cancer treatment are of great importance for clinicians, oncologists, cardiologists, and patients. Our study suggests that there are opportunities to optimize CV status and CVD treatment among patients with esophageal cancer before initiating a treatment program with systemic toxicities.
TRANSLATIONAL OUTLOOK: Further studies are needed to validate our findings in larger populations and over an extended period of time. Robust clinical predictors of adverse CV events and the most effective CV treatment strategies to mitigate CV risk also need to be identified. Strengthening of multidisciplinary collaborations before, during, and after chemoradiation across oncology and cardiology and patients is important when considering clinical care models.
Funding Support and Author Disclosures
This research was supported by the Danish Cancer Society, the Carpenter Jorgen Holm and Wife Elisa F. Hansen’s Memorial Scholarship, and Radiumstationens Research Fund. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure, please see the online version of this paper.
Appendix
References
- 1.Beukema J.C., van Luijk P., Widder J., Langendijk J.A., Muijs C.T. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol. 2015;114:85–90. doi: 10.1016/j.radonc.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Accordino M.K., Neugut A.I., Hershman D.L. Cardiac effects of anticancer therapy in the elderly. J Clin Oncol. 2014;32:2654–2661. doi: 10.1200/JCO.2013.55.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z., Zhang H. Impact of neoadjuvant chemotherapy and chemoradiotherapy on postoperative cardiopulmonary complications in patients with esophageal cancer. Dis Esophagus. 2017;30:1–7. doi: 10.1093/dote/dox002. [DOI] [PubMed] [Google Scholar]
- 4.Atkins K.M., Rawal B., Chaunzwa T.L., et al. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J Am Coll Cardiol. 2019;73:2976–2987. doi: 10.1016/j.jacc.2019.03.500. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi Y., Iijima H., Isohashi F., et al. The heart’s exposure to radiation increases the risk of cardiac toxicity after chemoradiotherapy for superficial esophageal cancer: a retrospective cohort study. BMC Cancer. 2019;19:195. doi: 10.1186/s12885-019-5421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin S.H., Hobbs B.P., Verma V., et al. Randomized phase IIB trial of proton beam therapy versus intensity-modulated radiation therapy for locally advanced esophageal cancer. J Clin Oncol. 2020;38:1569–1579. doi: 10.1200/JCO.19.02503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiappa A., Andreoni B., Dionigi R., et al. A rationale multidisciplinary approach for treatment of esophageal and gastroesophageal junction cancer: accurate review of management and perspectives. Crit Rev Oncol Hematol. 2018;132:161–168. doi: 10.1016/j.critrevonc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro J., van Lanschot JJB, Hulshof M., et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 9.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Collier P., Phelan D., Klein A. A Test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol. 2017;69:1043–1056. doi: 10.1016/j.jacc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 12.Balady G.J., Arena R., Sietsema K., Myers J., Coke L., Fletcher G.F., et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 13.Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 15.Williams B., Mancia G., Spiering W., et al. 2018 practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2018;36:2284–2309. doi: 10.1097/HJH.0000000000001961. [DOI] [PubMed] [Google Scholar]
- 16.Hicks K.A., Tcheng J.E., Bozkurt B., et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) J Am Coll Cardiol. 2015;66:403–469. doi: 10.1016/j.jacc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Witt J.S., Jagodinsky J.C., Liu Y., et al. Cardiac toxicity in operable esophageal cancer patients treated with or without chemoradiation. Am J Clin Oncol. 2019;42:662–667. doi: 10.1097/COC.0000000000000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sathornviriyapong S., Matsuda A., Miyashita M., et al. Impact of neoadjuvant chemoradiation on short-term outcomes for esophageal squamous cell carcinoma patients: a meta-analysis. Ann Surg Oncol. 2016;23:3632–3640. doi: 10.1245/s10434-016-5298-9. [DOI] [PubMed] [Google Scholar]
- 19.Kristensen S.D., Knuuti J., Saraste A., et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on Non-Cardiac Surgery: Cardiovascular Assessment and Management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA) Eur Heart J. 2014;35:2383–2431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- 20.Lund M., Alexandersson von Döbeln G., Nilsson M., et al. Effects on heart function of neoadjuvant chemotherapy and chemoradiotherapy in patients with cancer in the esophagus or gastroesophageal junction—a prospective cohort pilot study within a randomized clinical trial. Radiat Oncol. 2015;10:16. doi: 10.1186/s13014-014-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel J., Rikhi R., Hussain M., et al. Global longitudinal strain is a better metric than left ventricular ejection fraction: lessons learned from cancer therapeutic-related cardiac dysfunction. Curr Opin Cardiol. 2020;35:170–177. doi: 10.1097/HCO.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 22.von Döbeln G.A., Nilsson M., Adell G., et al. Pulmonary function and cardiac stress test after multimodality treatment of esophageal cancer. Pract Radiat Oncol. 2016;6:e53–e59. doi: 10.1016/j.prro.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Delman A.M., Ammann A.M., Turner K.M., Vaysburg D.M., Van Haren R.M. A narrative review of socioeconomic disparities in the treatment of esophageal cancer. J Thorac Dis. 2021;13:3801–3808. doi: 10.21037/jtd-20-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.