Abstract
Cardiovascular disease and cancer are the 2 leading causes of death worldwide. Emerging evidence suggests common mechanisms between cancer and cardiovascular disease, including atrial fibrillation and atherosclerosis. With advances in cancer therapies, screening, and diagnostics, cancer-specific survival and outcomes have improved. This increase in survival has led to the coincidence of cardiovascular disease, including atrial fibrillation and atherosclerosis, as patients with cancer live longer. Additionally, cancer and cardiovascular disease share several risk factors and underlying pathophysiologic mechanisms, including inflammation, cancer-related factors including treatment effects, and alterations in platelet function. Patients with cancer are at increased risk for bleeding and thrombosis compared with the general population. Although optimal antithrombotic therapy, including agent choice and duration, has been extensively studied in the general population, this area remains understudied in patients with cancer despite their altered thrombotic and bleeding risk. Future investigation, including incorporation of cancer-specific characteristics to traditional thrombotic and bleeding risk scores, clinical trials in the cancer population, and the development of novel antithrombotic and anti-inflammatory strategies on the basis of shared pathophysiologic mechanisms, is warranted to improve outcomes in this patient population.
Key Words: arrhythmia, risk factor, thrombosis
Abbreviations and Acronyms: AF, atrial fibrillation; CAD, coronary artery disease; CHIP, clonal hematopoiesis of indeterminate potential; CI, confidence interval; CLEC-2, C-type lectin-like receptor 2; HR, hazard ratio; IL, interleukin; MI, myocardial infarction; PCI, percutaneous coronary intervention; ROS, reactive oxygen species; TKI, tyrosine kinase inhibitor; VTE, venous thromboembolism
Central Illustration
Highlights
-
•
Cancer and cardiovascular disease increasingly coexist, and patients with cancer are often undertreated.
-
•
Cancer and cardiovascular disease share common pathophysiology, including inflammation.
-
•
Thrombosis and bleeding risk scores often underperform in patients with cancer and cardiovascular disease.
-
•
Inclusion of cancer status in cardiovascular trials and risk scores may improve cardiovascular outcomes.
Cardiovascular disease and cancer are the leading causes of death in the developed world (1). Advances in cancer therapeutics and diagnostics have led to improved survival and cancer-specific outcomes (2,3). As patients with cancer live longer, their risk for atherosclerosis, atrial fibrillation (AF), and cardiovascular disease likewise increases. Additionally, recent evidence suggests common pathophysiological linkages between cancer and cardiovascular disease. One study demonstrated an association between 10-year atherosclerotic risk score and the risk for incident cancer (4). In part, this is due to several shared risk factors, such as smoking, obesity, and diabetes mellitus. As new data emerge, the lines between cardiology, oncology, and cardio-oncology continue to blur (5).
The management of coronary artery disease (CAD) and AF often involve balancing thrombotic and bleeding risks associated with antiplatelet and anticoagulation therapy. Cancer-specific and therapy-related risk factors have been associated with an increased risk for thrombosis and adverse cardiovascular events in this patient population (6, 7, 8). However, patients with cancer also have an increased risk for bleeding compared with those without cancer. Therefore, management of CAD and AF in the cancer population adds an extra layer of nuance and complexity not seen in the general population. Numerous trials have been conducted attempting to find the optimal type and duration of therapy in a variety of prothrombotic cardiovascular conditions, though despite their increased thrombotic and bleeding risk, patients with cancer are often excluded from these trials (9, 10, 11). Thus, there is a relative paucity of evidence to guide antithrombotic therapy in patients with cancer and cardiovascular disease but an ample amount of opportunity to explore these nuances.
An Inflammatory Common Link Among Atherosclerosis, AF, and Cancer
Recent evidence suggests that inflammation is the mutual pathophysiologic link among atherothrombosis, AF, and cancer (12,13). Additionally, atherosclerosis and cancer may influence the progression of each other. Elevated inflammatory markers, including high-sensitivity C-reactive protein and interleukin (IL)–6, have been shown to be a risk factor in the development of atherothrombosis and AF (14, 15, 16, 17). In AF, elevated C-reactive protein levels before ablation have been associated with increased likelihood of recurrent AF postablation (18). A causal role for inflammation in AF has been suggested in studies showing increased NLRP3 inflammasome activation in AF (19). Additionally, inflammatory markers have been shown to be reduced after successful ablation of AF (20).
Elevations in C-reactive protein and IL-6 have been associated with increased risk for cardiovascular events independent of cholesterol level (16,17,21). Additionally, NLRP3 inflammasome activation and IL-1β have been shown to promote atherogenesis and arterial thrombosis in preclinical animal models (22,23). This connection between inflammation and cardiovascular events has spurred trials investigating anti-inflammatory therapies in cardiovascular disease. In patients with rheumatoid arthritis, a population known to have high levels of inflammation and at elevated risk for cancer and cardiovascular disease, the ORAL Surveillance (Safety Study of Tofacitinib Versus Tumor Necrosis Factor [TNF] Inhibitor in Subjects With Rheumatoid Arthritis) study is examining the risk for major adverse cardiovascular events in patients with cancer receiving tofacitinib, a JAK1/3 inhibitor, compared with a tumor necrosis factor inhibitor (NCT02092467) (24,25). However, in the general population, anti-inflammatory therapies have shown promise in reducing cardiovascular events and cancer risk. The CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Studies) trial randomized more than 10,000 patients with previous myocardial infarction (MI) and high-sensitivity C-reactive protein levels of 2 mg/L or higher to receive the IL-1β inhibitor canakinumab (50, 150, or 300 mg every 3 months) or placebo. The results of CANTOS showed significant reductions in nonfatal MI, nonfatal stroke, and cardiovascular death in patients treated with canakinumab (26,27). Colchicine is another anti-inflammatory drug studied in patients with chronic CAD and in post-MI populations (28,29). In patients with chronic CAD, low-dose colchicine significantly reduced MI and ischemia-driven revascularization compared with placebo (28). Additionally, there was a reduction in new onset or first recurrence of AF, though it did not reach statistical significance (28). In patients with recent MI, treatment with colchicine resulted in a significant reduction in the composite primary endpoint of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina leading to revascularization, with reductions in the latter 2 endpoints driving the observed effect (29).
Inflammation also plays a role in the development and progression of cancer (30,31). IL-1 has been shown to mediate tumor angiogenesis, metastasis, and tumor immune evasion (32,33). IL-1 initiates and amplifies inflammation and can be produced by both immune (myeloid cells, macrophages, etc) and nonimmune cells in response to danger-associated molecular patterns and pathogen-associated molecular patterns (34). Additionally, IL-1 can induce the release of other proinflammatory and carcinogenic cytokines and factors, including IL-6, reactive oxygen species (ROS), and vascular endothelial growth factor (35,36). IL-6 and IL-1 cause activation of STAT3, which can induce proliferation of cancer cells and migration via increased expression of matrix metalloproteinases (37). Additionally, epithelial-to-mesenchymal transition, important in cancer cell invasiveness, metastatic potential, and cell detachment, is facilitated via IL-1, IL-6, and STAT3 signaling in chronic inflammation (37,38). The importance of IL-1 and IL-6 in the progression of cancer is highlighted in clinical trials of IL-1 and IL-6 antagonists on cancer survival. In an analysis of CANTOS, patients receiving canakinumab had a lower incidence of new lung cancer diagnosis and lower lung cancer mortality compared with those who received placebo (30). In 2 small trials (n = 47) in patients with smoldering multiple myeloma, the addition of the IL-1 receptor antagonist anakinra to dexamethasone was associated with improved progression-free and overall survival (39,40). Blockade of IL-6 with siltuximab inhibited progression of cholangiocarcinoma in a mouse model (41). Additionally, one retrospective study suggested lower incidences of cancer in patients with gout treated with colchicine, though it is unclear if this result is attributed to colchicine’s anti-inflammatory action or microtubule formation inhibition (42). The role of inflammation in the pathogenesis of cardiovascular disease and malignancy is an evolving field but one that is a common thread among these disorders (Figure 1).
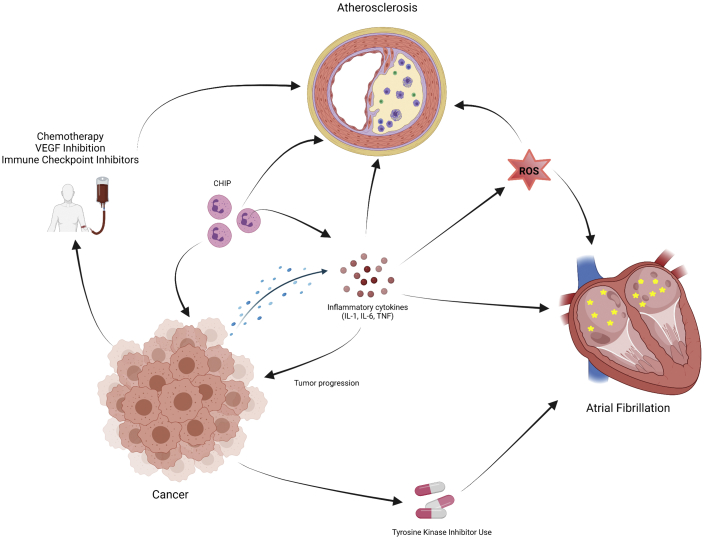
Figure 1.
Inflammation and Cancer Treatment in Atrial Fibrillation and Atherosclerosis
Arrows indicate relationships: inflammation arising from malignancy or other source as well as cancer-related treatment and clonal hematopoiesis of indeterminate potential (CHIP) can lead to progression of atherosclerosis and development of atrial fibrillation. IL = interleukin; ROS = reactive oxygen species; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
Inflammation may lead to the development of AF, atherosclerosis, and cancer via the production of ROS. ROS are by-products of cellular metabolism and oxygen use and have been associated with increased risk for the development of cancer via DNA damage and genetic destabilization (43,44). In AF, production of ROS via leukocyte-derived myeloperoxidase may lead to atrial fibrosis and adverse extracellular matrix remodeling via matrix metalloproteinases (45,46). In atherosclerosis, increased oxidative stress is associated with the severity of CAD and MI (47,48). Additionally, ROS produced by monocytes can convert oxidized low-density lipoprotein to highly oxidized low-density lipoprotein, which is phagocytosed by macrophages to form foam cells (49).
In summary:
-
•
Inflammation plays a role in the development and progression of AF, atherosclerosis, and cancer.
-
•
IL-1 and the NLRP3 inflammasome are of particular clinical interest, as pharmacologic inhibition of IL-1 was shown in the CANTOS trial to reduce cardiovascular risk, with some signal of decreased cancer mortality as well.
-
•
Inflammation’s role in the development of AF, atherosclerosis, and cancer may be mediated in part by the production of ROS.
-
•
Further studies are needed to delineate the role of anti-inflammatory therapies in cancer prevention or treatment and cardiovascular disease.
Common Mechanistic Links Between Cancer and AF
AF is the most common atrial arrhythmia, affecting approximately 2% of the general population and with increasing prevalence with age, with 18% of those 85 years and older having AF (50,51). Cancer and AF share similar risk factors, including diabetes mellitus, obesity, and cardiometabolic disease (52, 53, 54). However, recent emerging data suggest that patients with cancer have a higher prevalence of AF compared with the general population (55). One large, Danish nationwide study showed that patients with cancer had an incidence of AF of 17.4 per 1,000 person-years compared with 3.7 per 1,000 person-years in patients without cancer (56). Risk for developing AF after cancer diagnosis was highest in the first 90 days. Another nationwide study of patients with breast cancer showed an increased risk for AF among patients with breast cancer who were <60 years of age compared with the general population (57). Conversely, there is some evidence that AF itself may portend an increased risk for cancer diagnosis (58,59). However, whether this is due to detection bias and not a causal relationship is still up for debate (60). Regardless, AF and cancer share common pathophysiology and risk factors.
Treatment-related factors have been associated with the risk for the development of AF in patients with cancer (61). Postsurgical AF is common in patients with cancer, particularly in those with lung cancer (62, 63, 64). Cancer treatment (chemotherapy, radiation, etc) can also predispose patients to infectious complications, including sepsis, which can promote the development of AF (65). Some traditional cytotoxic chemotherapeutic agents have been associated with the development of AF, including platinum-based chemotherapy, paclitaxel, ifosfamide, corticosteroids, and immunotherapy (66,67). The advent of targeted therapy, including tyrosine kinase inhibitors (TKIs), in cancer treatment has recently changed the way many different types of malignancies are treated. However, these therapies have been implicated in the development of AF in patients with cancer. Ibrutinib is a Bruton TKI that is used for treatment for a variety of B-cell malignancies and is the TKI most associated with an increased risk for AF, with up to 16% of patients diagnosed with AF after initiation of therapy (68, 69, 70). The mechanism behind the development of AF in patients may be due to off-target inhibition of other tyrosine kinases in cardiac myocardial cells (71). For example, ibrutinib has been found to inhibit C-terminal Src kinase. The absence of C-terminal Src kinase in a knockout mouse model was found to induce left atrial enlargement, fibrosis, and inflammation, leading to increased AF (72). Additionally, ibrutinib may also induce AF via production of ROS (73). Immune checkpoint inhibitors are also commonly used in the treatment of certain cancers and have been known to cause cardiotoxicity, myocarditis, and AF driven by dysregulated inflammation (74).
In summary:
-
•
AF is common in patients with cancer, and both diseases share several risk factors, including diabetes and cardiometabolic diseases.
-
•
Treatment-related factors, including traditional chemotherapy and TKI use, can predispose patients with cancer to AF.
-
•
Potential therapeutic strategies to mitigate risk for AF in patients with cancer are yet to be explored.
Common Links Between Cancer and Atherosclerotic Heart Disease
Cancer and CAD share overlapping risk factors, including but not limited to obesity, smoking, diabetes, and age. Additionally, CAD and cancer may influence the progression of each other. MI has been shown to accelerate breast cancer growth and increase cancer-related mortality (75,76). A nationwide study in South Korea showed an increased risk for new malignancy after percutaneous coronary intervention (PCI) compared with age- and sex-matched control subjects who had not undergone PCI (77). Interestingly, the study showed that the risk for lung and hematologic cancers was higher than that for other types of cancer. Obesity, defined as a body mass index of 30 kg/m2 or greater, is a recognized independent risk factor for CAD and atherosclerosis (78). Obesity has also been shown to be a risk factor in the development of cancer, particularly gastrointestinal adenocarcinomas, breast, ovarian, uterine, renal, and liver cancers and multiple myeloma (79). Bariatric surgery for morbid obesity has shown associations with lower rates of both cardiovascular mortality and cancer-related mortality as well as decreased risk for incident cancer (80,81). Diabetes is another classic risk factor for CAD that is also associated with an increased risk for malignancy, especially colorectal cancer. The risk for developing colorectal cancer was increased by 20% to 38% in patients with diabetes compared with those without (82).
Age is a significant risk factor for both cancer and CAD and is also associated with increased somatic mutations in hematopoietic progenitors, leading to clonal hematopoiesis of indeterminate potential (CHIP). These age-related mutations found in genes such as DNMT3A, TET2, JAK2, and ASXL1, although they are also found in myeloid neoplasms including myelodysplastic syndromes, acute myeloid leukemia, and myeloproliferative neoplasms, in CHIP, they do not cause alterations in peripheral blood counts (83). CHIP is present in 10% of patients older than 70 years and confers a 10-fold increased risk for developing hematologic malignancy (83). The role of CHIP in cardiovascular disease is an active area of research, with compelling evidence showing an increased risk of cardiovascular death, CAD, and heart failure compared with patients without CHIP (84, 85, 86, 87). The increased risk for CAD and cardiovascular disease in CHIP is mediated by increased inflammation through increased IL-1β and IL-6 signaling, particularly in TET2-mutated CHIP (84,88,89). Mutations in JAK2 are also seen in myeloproliferative neoplasms, which have also been associated with increased rates of arterial thrombotic events, including MI (90, 91, 92). JAK2 encodes for a protein important for signal transduction for several inflammatory cytokines and hematopoietic growth factors. In a mouse model of atherosclerosis, mice that received bone marrow transplants from JAK2-mutated mice had accelerated atherosclerosis and larger plaques with increased necrotic cores (93). Additionally, JAK2 mutations lead to a thrombophilic state via alterations of platelet and endothelial adhesion molecule activation and increased formation of neutrophil extracellular traps (94,95). Pharmacologic inhibition of JAK2 in another mouse model of atherosclerosis led to decreased atherosclerotic burden (96). Tumor-associated macrophages and inflammatory cells are important participants in tumor growth and progression, so CHIP may also affect patients with solid malignancies (97). Indeed, mutations associated with CHIP have been shown to be present in solid tumors as well as peripheral blood (98, 99, 100). Additionally, one study showed that 4.5% of patients with solid malignancies harbored mutations associated with CHIP (101). The presence of CHIP was associated with increased age, prior radiation therapy, and tobacco use, and patients with CHIP had shorter survival compared with those without (101).
Although pre-existing factors may affect the risk for developing CAD and cancer, in patients with established cancer, the treatment for their malignancy may also have adverse effects on the genesis and progression of CAD. Radiation, which is used in the treatment of various types of cancers, has been shown to increase the risk for ischemic heart disease (102, 103, 104). In one study, patients who received thoracic radiation for lung cancer had progression in coronary artery calcium, a known predictor of CAD, compared with those who did not (103). Various classes of cytotoxic chemotherapy have been known to cause cardiotoxicity, cardiomyopathy, and vascular toxicity, which may contribute to increased atherosclerotic risk (105). Cisplatin and other platinum-based chemotherapeutics have been associated with an increased risk for MI via endothelial damage and plaque erosion (106). Inhibition of tumor angiogenesis via blockade of vascular endothelial growth factor either by anti–vascular endothelial growth factor monoclonal antibody (bevacizumab) or tyrosine kinase inhibition (sunitinib, pazopanib, and sorafenib) is associated with increased arterial thrombotic events, including MI (107). In a mouse model of atherosclerosis, vascular endothelial growth factor inhibition led to accelerated atherosclerosis and endothelial dysfunction (108). Additionally, exacerbation of hypertension and development of new hypertension are well-known adverse events of vascular endothelial growth factor inhibitors and add to their cardiovascular risk (105).
As targeted and immune-based therapies that have revolutionized cancer therapy are more routinely used, cardiovascular complications of these modalities have been observed. In chronic myeloid leukemia, the identification of BCR-ABL fusion gene, which leads to overproduction of a growth-stimulatory tyrosine kinase, led to the development of targeted TKIs with imatinib targeting BCR-ABL as the first. Although the risk for vascular events is relatively low with imatinib therapy, newer generations of TKIs, including dasatinib, nilotinib, and ponatinib, were shown to have substantially higher rates of vascular events compared with imatinib (109). Inhibition of off-target tyrosine kinases, including vascular endothelial growth factor, may explain this increased risk for vascular events (110). Interestingly, not all TKIs may have adverse effects on atherosclerosis. One preclinical study showed that treatment of a mouse model of atherosclerosis with erlotinib, an epidermal growth factor inhibitor, reduced plaque size, likely mediated by decreased T cell plaque infiltration and proliferation (111). Immune checkpoint inhibitors are novel therapeutic agents with expanding use in a variety of different types of cancers but may be associated with increased risk for vascular events (112). Recent evidence suggests that the use of immune checkpoint inhibitors is associated with a 3-fold increased risk for cardiovascular events. Additionally, imaging before and after immune checkpoint inhibitor use showed 3-fold increased progression of total aortic plaque volume (113).
In summary:
-
•
CHIP is a risk factor for both atherosclerosis and hematologic malignancies and may also be associated with worse outcomes in solid tumors.
-
•
Adverse effects of CHIP on cardiovascular outcomes and cancer may be due to inflammation.
-
•
Cancer-related treatments, including radiation, conventional cytotoxic chemotherapy, TKIs, and immunotherapy, can increase cardiovascular risk.
Role of Platelets and Antiplatelet Therapy in Cancer and Atherosclerotic Heart Disease
Platelets are small, anucleate cells with a complex transcriptome and play an important role in hemostasis, inflammation, and immune surveillance (114, 115, 116). Platelets are yet another link between atherosclerosis and cancer (Figure 2) (117, 118, 119). In atherosclerosis, activated platelets contribute to the inflammatory milieu by releasing various platelet-derived mediators of inflammation, including CD40 ligand, IL-1β, platelet-derived growth factor, transforming growth factor-β, ROS, and P-selectin (114,120,121). Platelets have been shown to oxidize low-density lipoprotein, which is a major driver of atherosclerotic plaque formation (122,123). Additionally, platelets also promote monocyte migration to atherosclerotic plaques and induce their differentiation to an inflammatory phenotype, leading to plaque growth and foam cell formation (124,125). Platelets also interact with neutrophils and eosinophils to promote atherosclerotic plaque formation, growth, and thrombosis by inducing neutrophil extracellular trap or eosinophil extracellular trap formation, respectively (126, 127, 128). Increased platelet activation, as measured by urinary levels of 11-dehydro-thromboxane B2 (a metabolite of thromboxane A2), has also been associated with excess vascular risk in patients with AF despite oral anticoagulation (129).
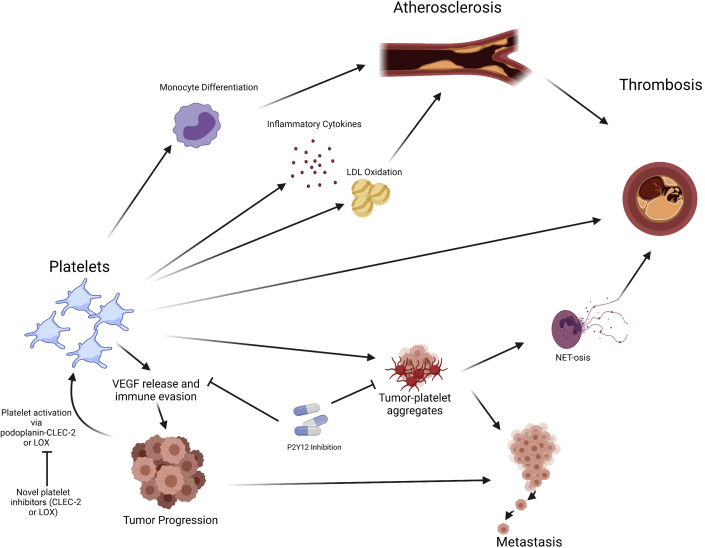
Figure 2.
Platelets Are Involved in the Progression of Atherosclerosis and Cancer
Arrows indicate relationships: platelets facilitate progression of atherosclerosis through monocyte differentiation, release of inflammatory cytokines and low-density lipoprotein (LDL) oxidation. Additionally, platelets contribute to cancer progression and metastasis through release of proangiogenic factors, immune evasion, and hematogenous spread via tumor-platelet aggregates. P2Y12 inhibition may attenuate platelet-facilitated tumor progression. CLEC-2 = C-type lectin-like type II transmembrane receptor; LOX = lysyl oxidase; NET = neutrophil extracellular trap.
Platelets have an important role in cancer development, progression, and metastasis (117,119,130,131). Thrombocytosis has been associated with worse outcomes in several types of cancer, including lung, colorectal, ovarian, and hepatocellular carcinoma (132, 133, 134, 135). Additionally, in a mouse model of metastatic lung cancer, induced thrombocytopenia led to significant improvement in survival (136). Tumors may also express proteins that bind to platelets, including podoplanin, which induces platelet aggregation through C-type lectin-like receptor 2 (CLEC-2) and facilitates hematogenous spread and thrombosis (137,138). Additionally, similar to their role in atherosclerosis, platelets facilitate the recruitment of inflammatory cells to the tumor, which contribute to tumor progression (139,140). Hematogenous tumor metastasis is facilitated by platelets by the formation of tumor-platelet thrombi via interactions with tumor cells and leukocytes through P-selectin or tissue factor (141,142). Platelets and tumor cells themselves have been shown to induce the formation of neutrophil extracellular traps, which in turn mediate some of the prothrombotic characteristics of malignancy as well as facilitate metastasis (143). Platelets also facilitate tumor immune evasion through protection from natural killer cells via the transfer of major histocompatibility complex class I from platelets to tumor and T-cell immune regulation (144,145). Platelets have also been shown to express programmed death ligand-1 and help tumors that are programmed death ligand-1 negative evade T cell-mediated immunity (146). Neoangiogenesis, important to tumor growth and metastasis, is enhanced by platelets via release of angiogenic factors, including vascular endothelial growth factor (147). Tumors can also affect platelet behavior by increasing platelet activation, release of microparticles, extracellular vesicles, and platelet granules, which may accelerate atherosclerotic plaque and increase the risk for cardiovascular events (119,148).
Although the benefits of antiplatelet therapy in primary and secondary prevention of CAD are well known (149), there is increasing evidence that antiplatelet therapy may have a benefit on cancer progression (146,150,151). Aspirin is an irreversible antagonist of cyclooxygenase-1 and -2, leading to decreased conversion of arachidonic acid to prostanoids, including thromboxane A2 (152). At low doses, aspirin exerts an antiplatelet effect via decreased thromboxane A2 production, and at high doses, it provides an anti-inflammatory effect. Aspirin may exert antitumor properties via tumor cyclooxygenase-1 and -2 inhibition and decreased platelet secretion of proangiogenic factors (131). An analysis of 5 aspirin trials showed that daily aspirin therapy was associated with reduced risk for distant metastasis, particularly in patients with adenocarcinoma (153,154). Another large cohort study involving more than 18,000 patients showed that long-term aspirin use of more than 5 years was associated with a decreased incidence of colorectal cancer and prostate cancer in men (155). In addition to reduced risk for metastatic disease, aspirin may reduce the incidence of cancer and risk for cancer-related mortality, though aspirin therapy may have to be longer than 5 years to see the benefit, as other trials with shorter follow-up times have failed to show similar decreases in risk and incidence of cancer (156, 157, 158). Other antiplatelet agents, such as P2Y12 inhibitors, have shown some preliminary evidence that they may be beneficial in certain types of malignancies, in part by preventing platelet activation–induced granule release (159,160). In mouse models of hepatocellular carcinoma associated with chronic hepatitis B virus infection and ovarian cancer, treatment with clopidogrel and ticagrelor, respectively, improved survival and tumor burden (160,161). Additionally, P2Y12 inhibition appeared to reverse some of the platelet-mediated immune evasion by tumor cells and disrupt tumor cell–induced platelet aggregates, which may be important for hematogenous metastasis (145,151).
Despite the possible antitumor effects of platelet inhibition via aspirin or P2Y12 inhibitors, the increased risk for bleeding using these products may limit their clinical usefulness in patients with cancer, thus necessitating the development of antiplatelet agents that do not produce the same bleeding risk. Alternative mechanisms of platelet inhibition that may benefit cancer survival include inhibition of CLEC-2, a platelet receptor that causes platelet activation and binds to podoplanin on cancer cells. In animal models, inhibition of CLEC-2 reduced podoplanin-induced aggregation and hematogenous metastases (137,162). Additionally, inhibition of CLEC-2 did not result in increased bleeding time in mice treated with a podoplanin-CLEC-2 inhibitor (162). Given that podoplanin has also been shown to be expressed in advanced atherosclerotic lesions, and increased levels of soluble CLEC-2 may be associated with increased risk for CAD and may predict death and vascular events in patients with stroke, inhibition of CLEC-2 and podoplanin may be a novel therapy for cardiovascular disease that has yet to be investigated in clinical trials (163, 164, 165). Inhibition of lysyl oxidase, an enzyme more commonly known for crosslink formation of collagen and elastin, provides another novel method of platelet inhibition that may be beneficial in both atherosclerotic disease and cancer. Lysyl oxidase has been shown to be elevated in patients with certain malignancies, including myeloproliferative neoplasms, which are associated with increased thrombotic risk (166). Lysyl oxidase is also a proangiogenic factor that promotes neoangiogenesis in some solid cancers by inducing vascular smooth muscle migration and proliferation (167). In atherosclerotic heart disease, lysyl oxidase has been found to be present in atherosclerotic lesions and to be associated with arterial restenosis after arterial balloon angioplasty (168, 169, 170). In myeloproliferative neoplasms, platelets have been shown to express lysyl oxidase, and increased lysyl oxidase activity is associated with increased platelet adhesion to collagen and increased arterial thrombus formation in a mouse model (166,171). In preclinical studies, inhibition of lysyl oxidase has been shown to attenuate progression of myelofibrosis, a myeloproliferative neoplasm, and triple-negative breast cancer and thus is a promising novel therapy (172, 173, 174).
In summary:
-
•
Platelets are important in the development of both atherosclerosis and cancer via release of proinflammatory and angiogenic factors and facilitating hematogenous spread of cancer cells.
-
•
Platelet inhibition can be beneficial in both atherosclerosis and cancer, but higher bleeding risk may limit the use of traditional platelet inhibitors in patients with cancer.
-
•
The development of novel platelet inhibitors that do not increase bleeding risk, such as inhibition of CLEC-2 or lysyl oxidase, is needed.
Thrombotic and Bleeding Risk in Patients With Cancer
Patients with cancer are at an increased risk for thrombotic events, both arterial and venous. Tumor-, patient-, and treatment-related factors all play a role in the thrombophilic state of malignancy (6). Venous thromboembolism (VTE) is a well-known and common complication of cancer and cancer therapy, occurring in 20% to 25% of all patients with cancer (175,176). The occurrence of VTE in patients with cancer also portends a worse prognosis, with patients who have had VTE having a 4-fold increase in risk for death compared with patients without VTE (177). Several patient-, treatment-, and tumor-related factors affect the risk for VTE in cancer (6). Additionally, interruption of anticoagulation for procedures or chemotherapy-induced thrombocytopenia has been described to increase thrombotic complications in patients with cancer (178, 179, 180). New risk factors for VTE, including tumor genetics and immune checkpoint inhibitor therapy, are being described and may improve the risk/benefit ratio of pharmacologic thromboprophylaxis (181,182).
The risk for thrombosis in patients with cancer with AF has been studied in retrospective studies. One study of elderly patients with AF found an increased risk for thromboembolic events in patients with lung cancer (183). However, in a Swedish retrospective cohort study of patients with AF off anticoagulation with and without cancer, patients with cancer did not have an increased risk for ischemic stroke after multivariable analysis (adjusted hazard ratio [HR]: 0.91; 95% confidence interval [CI]: 0.88-0.96), though they did have a significantly increased risk for intracranial (adjusted HR: 1.16; 95% CI: 1.05-1.30) and gastrointestinal (adjusted HR: 1.30; 95% CI: 1.22-1.39) hemorrhage (184). The results on gastrointestinal hemorrhage were heterogeneous, with some cancers (colorectal, prostate, myeloma, ovarian, pancreatic) showing significant risk, while others (breast, lung, biliary) did not (184). In another study of patients with cancer undergoing chemotherapy, advanced cancer stage was associated with increased risk for stroke but not chemotherapy itself after adjustment for cancer status (HR: 1.26; 95% CI: 0.78-2.03) (185). Another large retrospective cohort study of more than 2 million adults in France who had been hospitalized with AF investigated the effect of cancer on overall and cardiovascular mortality, ischemic stroke, and bleeding (186). Of the 2,435,541 patients, 399,344 had cancer, and those with cancer had increased all-cause mortality (HR: 2.00; 95% CI: 1.99-2.01) and major bleeding (HR: 1.27; 95% CI: 1.26-1.28) but not ischemic stroke. The risk for bleeding increased with higher HAS-BLED score (C index >0.70). However, the effect of cancer on ischemic stroke was heterogeneous among different cancer types, being elevated in patients with pancreatic, breast, and uterine cancer compared with those without cancer (186). Although the CHA2DS2-VASc score has been validated in estimating thrombotic risk associated with AF in the general population, it does not include cancer status and may underperform in the cancer population (187). Although the CHA2DS2-VASc score may underperform in the cancer population, it did predict ischemic stroke in this population, whereas the Khorana score, a VTE risk prediction score for cancer-associated VTE, did not (188). Given the differences in thrombotic and bleeding risks between the cancer and noncancer populations, more comprehensive risk scores need to be developed to adequately predict thrombohemorrhagic complications in patients with cancer.
Cancer is not a single entity, and tumor behavior can differ enormously among cancer types and with different treatment modalities, so it is expected that cancer type and tumor-specific factors can alter thrombotic risk in patients with cancer. Extrapolating from cancer-associated VTE risk research, tumors originating from stomach, pancreas, lung, lymphoma, gynecologic, bladder, and testicular cancer have the highest risk for thrombosis (189). Additionally, tumor genetics likely influence thrombosis risk (181). Patients with anaplastic lymphoma kinase and ROS1 rearranged non-small-cell lung cancer have a 2- to 5-fold increase in thrombosis risk compared with patients without those rearrangements (181,190). The heterogeneity of thrombosis risk among patients with cancer limits the conclusions that can be reached in analyses of randomized controlled trials that enrolled multiple tumor types, although some subanalyses and post hoc analyses that group similar types can yield actionable results (191).
Patients with malignancy may also be at an increased risk for complications following PCI for acute coronary syndrome and other CAD complications compared with the general population, though the data are mixed for thrombotic complications. In a nationwide database of patients who recently underwent PCI, patients with cancer had an increased risk for readmission for acute MI compared with those without cancer (192). The risk for readmission for acute MI was highest among lung and colon cancer (12.1% and 10.8%, respectively) compared with 5.6% for those without cancer. In another large nationwide database study of patients admitted for acute MI, patients with active cancer had a 2-fold increased risk for all-cause death, cardiac complications, and bleeding. Patients with lung cancer had the worst mortality (odds ratio: 2.71; 95% CI: 2.62-2.80) and cardiac complications (odds ratio: 2.38; 95% CI: 2.31-2.45), while patients with colon cancer had the highest rates of bleeding (odds ratio: 2.82; 95% CI: 2.68-2.98) (193). Cancer patients also had increased risk for recurrent acute coronary syndrome or death (15.2% vs 5.3%; P < 0.001) in a registry study of patients who underwent PCI for acute coronary syndrome (194). Another registry study from Japan involving 3,499 patients with acute MI treated with PCI showed increased all-cause death in patients with cancer (adjusted HR: 2.43; 95% CI: 1.73-3.42) but no increased incidences of cardiovascular events including acute MI (adjusted HR: 0.71; 95% CI: 0.35-1.42) and stroke (adjusted HR: 1.41; 95% CI: 0.67-2.97) (195). Despite the increased thrombotic risk in patients with cancer, PCI has still shown the benefit of decreased major adverse cardiovascular events in patients with cancer who had acute coronary syndrome if performed within 72 hours of admission and may be underused in this patient population (196, 197, 198). Patients with malignancy are less likely to undergo stenting, receive drug-eluting stents, receive newer generation P2Y12 inhibitors (ticagrelor or prasugrel), and receive standard of care medications (beta-blockers, statins, or angiotensin-converting enzyme inhibitors) compared with those without cancer (193,194).
In summary:
-
•
Patients with cancer and AF are at high risk for bleeding.
-
•
Risk for thrombosis in patients with cancer and AF is more heterogeneous, but certain cancer types (pancreatic, breast, uterine) and somatic mutations (anaplastic lymphoma kinase rearrangements in lung cancer, etc) may have an increased risk compared with patients without cancer.
-
•
Risk scores for AF do not include cancer status and may underperform in this patient population, necessitating the development of cancer-specific risk scores in AF.
-
•
Patients with cancer and CAD have increased adverse outcomes and are less likely to receive standard of care compared with those without cancer, presenting an opportunity for physicians to improve post-MI care in this patient population.
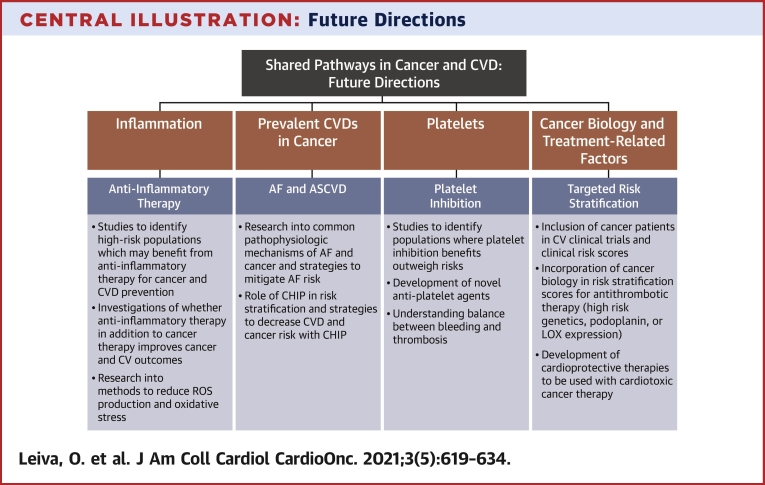
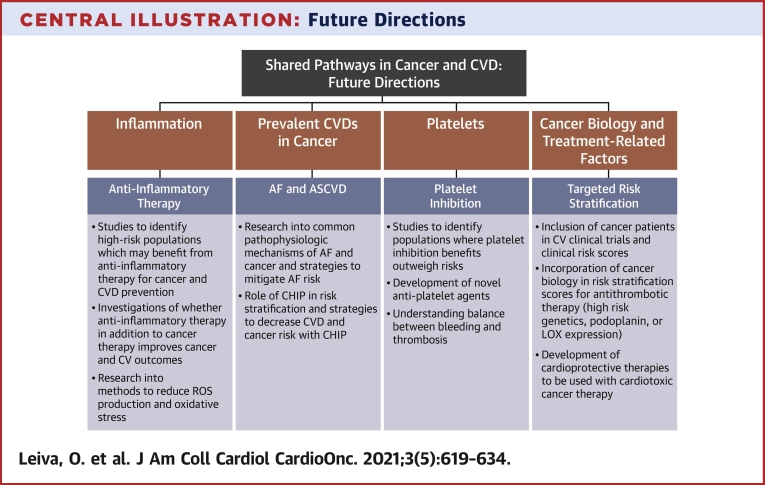
Conclusions and Future Directions
Cancer, AF, and CAD share common mechanistic links and risk factors. Inflammation is a major contributor to the progression of malignancy and cardiovascular disease. Although anti-inflammatory therapies show potential in the treatment and prevention of CAD and may reduce the risk for development of certain cancers, more studies are needed to ascertain the risks and benefits in patients with active malignancy. As cancer treatments advance and prognosis improves, patients with cancer will live longer and be at risk for developing AF and CAD. Compared with those without cancer, patients with cancer and AF or CAD have worse outcomes, including increased thrombotic and bleeding risks. Furthermore, cancer-specific factors including tumor biology and genetics may affect thrombotic risk and more studies are needed to identify high-risk patients. Traditional risk scores underperform in assessing risk for thrombosis and bleeding in patients with cancer, and development of cancer-specific risk scores or inclusion of cancer status in current risk scores may help clinicians navigate these nuanced decisions. Despite the increased risk for recurrent acute coronary syndrome, patients with cancer are less likely to be adequately treated or undergo stenting compared with patients without cancer. The duration of dual-antiplatelet therapy and choice of P2Y12 inhibitors in patients are unmet clinical needs and in need of further investigation. Additionally, inhibition of platelets may be beneficial in cancer, as it is in CAD, though bleeding risk limits the routine use of current antiplatelet agents. The development of novel pathways to inhibit platelet activation may prove to be beneficial in this patient population. Further investigation of the common underlying pathophysiology of cancer and cardiovascular disease is warranted and may lead to novel therapeutic and diagnostic strategies to improve the care of this growing patient population (Central Illustration).
Central Illustration.
Future Directions
Cancer and cardiovascular disease share several common pathophysiologic pathways. Further investigation of underlying mechanisms and development of novel therapeutics and diagnostic strategies may improve the care of this patient population.
ASCVD = atherosclerotic cardiovascular disease; CHIP = clonal hematopoiesis of indeterminate potential; CV = cardiovascular; CVD = cardiovascular disease; LOX = lysyl oxidase; ROS reactive oxygen species.
Funding Support and Author Disclosures
Dr Bhatt is an advisory board member for Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, and Regado Biosciences; is on the boards of directors of the Boston VA Research Institute, the Society of Cardiovascular Patient Care, and TobeSoft; is chair of the American Heart Association Quality Oversight Committee; is a member of data monitoring committees for the Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), the Cleveland Clinic (including for the ExCEED trial, funded by Edwards Lifesciences), Contego Medical (chair, PERFORMANCE 2), the Duke Clinical Research Institute, the Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), and the Population Health Research Institute; has received honoraria from the American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org; vice chair, ACC Accreditation Committee), the Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (editor-in-chief, Harvard Heart Letter), the Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), the Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (editor-in-chief, Journal of Invasive Cardiology), the Journal of the American College of Cardiology (guest editor, associate editor), K2P (cochair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (continuing medical education steering committees), MJH Life Sciences, the Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and US national coleader, funded by Bayer), Slack Publications (chief medical editor, Cardiology Today’s Intervention), the Society of Cardiovascular Patient Care (secretary/treasurer), and WebMD (continuing medical education steering committees); is deputy editor of Clinical Cardiology; is chair of the NCDR-ACTION Registry Steering Committee and the VA CART Research and Publications Committee; has received research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, and The Medicines Company; has received royalties from Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); is a site coinvestigator for Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), and Svelte; is a trustee of the American College of Cardiology; and has conducted unfunded research for FlowCo, Merck, and Takeda. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
Figures 1 and 2 were created using BioRender.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Dagenais G.R., Leong D.P., Rangarajan S., et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2020;395:785–794. doi: 10.1016/S0140-6736(19)32007-0. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N., Forjaz G., Mooradian M.J., et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 4.Lau E.S., Paniagua S.M., Liu E., et al. Cardiovascular risk factors are associated with future cancer. J Am Coll Cardiol CardioOnc. 2021;3:48–58. doi: 10.1016/j.jaccao.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt D.L. Birth and maturation of cardio-oncology. J Am Coll Cardiol CardioOnc. 2019;1:114–116. doi: 10.1016/j.jaccao.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leiva O., Newcomb R., Connors J.M., Al-Samkari H. Cancer and thrombosis: new insights to an old problem. J Med Vasc. 2020;45:6S8–6S16. doi: 10.1016/S2542-4513(20)30514-9. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y.F., Liu C.J., Chang P.M., et al. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol. 2013;165:355–357. doi: 10.1016/j.ijcard.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Navi B.B., Reiner A.S., Kamel H., et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 10.Levine G.N., Bates E.R., Bittl J.A., et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082–1115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 11.Howard C.E., Nambi V., Jneid H., Khalid U. Extended duration of dual-antiplatelet therapy after percutaneous coronary intervention: how long is too long? J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Y.F., Chen Y.J., Lin Y.J., Chen S.A. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 14.Schnabel R.B., Larson M.G., Yamamoto J.F., et al. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol. 2009;104:92–96. doi: 10.1016/j.amjcard.2009.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conen D., Ridker P.M., Everett B.M., et al. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. 2010;31:1730–1736. doi: 10.1093/eurheartj/ehq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridker P.M., Cushman M., Stampfer M.J., Tracy R.P., Hennekens C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 17.Ridker P.M., Hennekens C.H., Buring J.E., Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 18.Meyre P.B., Sticherling C., Spies F., et al. C-reactive protein for prediction of atrial fibrillation recurrence after catheter ablation. BMC Cardiovasc Disord. 2020;20:427. doi: 10.1186/s12872-020-01711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao C., Veleva T., Scott L., Jr., et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. doi: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotter M., Jais P., Vergnes M.C., et al. Decline in C-reactive protein after successful ablation of long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2006;47:1231–1233. doi: 10.1016/j.jacc.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 21.Liuzzo G., Biasucci L.M., Gallimore J.R., et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 22.Westerterp M., Fotakis P., Ouimet M., et al. Cholesterol efflux pathways suppress inflammasome activation, NETosis, and atherogenesis. Circulation. 2018;138:898–912. doi: 10.1161/CIRCULATIONAHA.117.032636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liberale L., Holy E.W., Akhmedov A., et al. Interleukin-1β mediates arterial thrombus formation via NET-associated tissue factor. J Clin Med. 2019;8:2072. doi: 10.3390/jcm8122072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantel A., Holmqvist M., Andersson D.C., Lund L.H., Askling J. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275–1285. doi: 10.1016/j.jacc.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Simon T.A., Thompson A., Gandhi K.K., Hochberg M.C., Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212. doi: 10.1186/s13075-015-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridker P.M., Everett B.M., Thuren T., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 27.Everett B.M., MacFadyen J.G., Thuren T., Libby P., Glynn R.J., Ridker P.M. Inhibition of interleukin-1β and reduction in atherothrombotic cardiovascular events in the CANTOS trial. J Am Coll Cardiol. 2020;76:1660–1670. doi: 10.1016/j.jacc.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Nidorf S.M., Fiolet A.T.L., Mosterd A., et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 29.Tardif J.C., Kouz S., Waters D.D., et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 30.Ridker P.M., MacFadyen J.G., Thuren T., et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 31.Bruni D., Angell H.K., Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20:662–680. doi: 10.1038/s41568-020-0285-7. [DOI] [PubMed] [Google Scholar]
- 32.Tengesdal I.W., Menon D.R., Osborne D.G., et al. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2000915118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottschlich A., Endres S., Kobold S. Therapeutic strategies for targeting IL-1 in cancer. Cancers (Basel) 2021;13:477. doi: 10.3390/cancers13030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briukhovetska D., Dorr J., Endres S., Libby P., Dinarello C.A., Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. 2021;21:481–499. doi: 10.1038/s41568-021-00363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu S., Bhagat G., Cui G., et al. Overexpression of interleukin-1β induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantovani A., Dinarello C.A., Molgora M., Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019;50:778–795. doi: 10.1016/j.immuni.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huynh J., Chand A., Gough D., Ernst M. Therapeutically exploiting STAT3 activity in cancer—using tissue repair as a road map. Nat Rev Cancer. 2019;19:82–96. doi: 10.1038/s41568-018-0090-8. [DOI] [PubMed] [Google Scholar]
- 38.Perusina Lanfranca M., Zhang Y., Girgis A., et al. Interleukin 22 signaling regulates acinar cell plasticity to promote pancreatic tumor development in mice. Gastroenterology. 2020;158:1417–1432.e11. doi: 10.1053/j.gastro.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lust J.A., Lacy M.Q., Zeldenrust S.R., et al. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol. 2016;91:571–574. doi: 10.1002/ajh.24352. [DOI] [PubMed] [Google Scholar]
- 40.Lust J.A., Lacy M.Q., Zeldenrust S.R., et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1β-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84:114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen M.L.T., Bui K.C., Scholta T., et al. Targeting interleukin 6 signaling by monoclonal antibody siltuximab on cholangiocarcinoma. J Gastroenterol Hepatol. 2021;36:1334–1345. doi: 10.1111/jgh.15307. [DOI] [PubMed] [Google Scholar]
- 42.Kuo M.C., Chang S.J., Hsieh M.C. Colchicine significantly reduces incident cancer in gout male patients: a 12-year cohort study. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moloney J.N., Cotter T.G. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Stanicka J., Russell E.G., Woolley J.F., Cotter T.G. NADPH oxidase-generated hydrogen peroxide induces DNA damage in mutant FLT3-expressing leukemia cells. J Biol Chem. 2015;290:9348–9361. doi: 10.1074/jbc.M113.510495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudolph V., Andrie R.P., Rudolph T.K., et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med. 2010;16:470–474. doi: 10.1038/nm.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedrichs K., Baldus S., Klinke A. Fibrosis in atrial fibrillation—role of reactive species and MPO. Front Physiol. 2012;3:214. doi: 10.3389/fphys.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vassalle C., Petrozzi L., Botto N., Andreassi M.G., Zucchelli G.C. Oxidative stress and its association with coronary artery disease and different atherogenic risk factors. J Intern Med. 2004;256:308–315. doi: 10.1111/j.1365-2796.2004.01373.x. [DOI] [PubMed] [Google Scholar]
- 48.Elesber A.A., Best P.J., Lennon R.J., et al. Plasma 8-iso-prostaglandin F2α, a marker of oxidative stress, is increased in patients with acute myocardial infarction. Free Radic Res. 2006;40:385–391. doi: 10.1080/10715760500539154. [DOI] [PubMed] [Google Scholar]
- 49.Madamanchi N.R., Vendrov A., Runge M.S. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 50.Camm A.J., Kirchhof P., Lip G.Y., et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 51.Heeringa J., van der Kuip D.A., Hofman A., et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 52.Freisling H., Viallon V., Lennon H., et al. Lifestyle factors and risk of multimorbidity of cancer and cardiometabolic diseases: a multinational cohort study. BMC Med. 2020;18:5. doi: 10.1186/s12916-019-1474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolin K.Y., Carson K., Colditz G.A. Obesity and cancer. Oncologist. 2010;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mechanick J.I., Farkouh M.E., Newman J.D., Garvey W.T. Cardiometabolic-based chronic disease, adiposity and dysglycemia drivers: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:525–538. doi: 10.1016/j.jacc.2019.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kattelus H., Kesaniemi Y.A., Huikuri H., Ukkola O. Cancer increases the risk of atrial fibrillation during long-term follow-up (OPERA study) PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0205454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jakobsen C.B., Lamberts M., Carlson N., et al. Incidence of atrial fibrillation in different major cancer subtypes: a nationwide population-based 12 year follow up study. BMC Cancer. 2019;19:1105. doi: 10.1186/s12885-019-6314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Souza M., Smedegaard L., Madelaire C., et al. Incidence of atrial fibrillation in conjunction with breast cancer. Heart Rhythm. 2019;16:343–348. doi: 10.1016/j.hrthm.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Conen D., Wong J.A., Sandhu R.K., et al. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 2016;1:389–396. doi: 10.1001/jamacardio.2016.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ostenfeld E.B., Erichsen R., Pedersen L., Farkas D.K., Weiss N.S., Sorensen H.T. Atrial fibrillation as a marker of occult cancer. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saliba W., Rennert H.S., Gronich N., Gruber S.B., Rennert G. Association of atrial fibrillation and cancer: Analysis from two large population-based case-control studies. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0190324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng W.L., Kao Y.H., Chen S.A., Chen Y.J. Pathophysiology of cancer therapy-provoked atrial fibrillation. Int J Cardiol. 2016;219:186–194. doi: 10.1016/j.ijcard.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Siu C.W., Tung H.M., Chu K.W., Jim M.H., Lau C.P., Tse H.F. Prevalence and predictors of new-onset atrial fibrillation after elective surgery for colorectal cancer. Pacing Clin Electrophysiol. 2005;28(suppl 1):S120–S123. doi: 10.1111/j.1540-8159.2005.00024.x. [DOI] [PubMed] [Google Scholar]
- 63.Higuchi S., Kabeya Y., Matsushita K., et al. Incidence and complications of perioperative atrial fibrillation after non-cardiac surgery for malignancy. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0216239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cardinale D., Sandri M.T., Colombo A., et al. Prevention of atrial fibrillation in high-risk patients undergoing lung cancer surgery: the PRESAGE trial. Ann Surg. 2016;264:244–251. doi: 10.1097/SLA.0000000000001626. [DOI] [PubMed] [Google Scholar]
- 65.Walkey A.J., Greiner M.A., Heckbert S.R., et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165:949–955.e3. doi: 10.1016/j.ahj.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Hooft C.S., Heeringa J., van Herpen G., Kors J.A., Kingma J.H., Stricker B.H. Drug-induced atrial fibrillation. J Am Coll Cardiol. 2004;44:2117–2124. doi: 10.1016/j.jacc.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 67.Khouri M.G., Douglas P.S., Mackey J.R., et al. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. 2012;126:2749–2763. doi: 10.1161/CIRCULATIONAHA.112.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ganatra S., Sharma A., Shah S., et al. Ibrutinib-associated atrial fibrillation. J Am Coll Cardiol EP. 2018;4:1491–1500. doi: 10.1016/j.jacep.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 69.Caldeira D., Alves D., Costa J., Ferreira J.J., Pinto F.J. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0211228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown J.R., Moslehi J., O’Brien S., et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102:1796–1805. doi: 10.3324/haematol.2017.171041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang T., Yang P., Roden D.M., Darbar D. Novel KCNA5 mutation implicates tyrosine kinase signaling in human atrial fibrillation. Heart Rhythm. 2010;7:1246–1252. doi: 10.1016/j.hrthm.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao L., Salem J.E., Clauss S., et al. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal Src kinase. Circulation. 2020;142:2443–2455. doi: 10.1161/CIRCULATIONAHA.120.049210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang X., An N., Zhong C., et al. Enhanced cardiomyocyte reactive oxygen species signaling promotes ibrutinib-induced atrial fibrillation. Redox Biol. 2020;30:101432. doi: 10.1016/j.redox.2020.101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ball S., Ghosh R.K., Wongsaengsak S., et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol. 2019;74:1714–1727. doi: 10.1016/j.jacc.2019.07.079. [DOI] [PubMed] [Google Scholar]
- 75.Koelwyn G.J., Newman A.A.C., Afonso M.S., et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat Med. 2020;26:1452–1458. doi: 10.1038/s41591-020-0964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hershman D.L., Till C., Shen S., et al. Association of cardiovascular risk factors with cardiac events and survival outcomes among patients with breast cancer enrolled in SWOG clinical trials. J Clin Oncol. 2018;36:2710–2717. doi: 10.1200/JCO.2017.77.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwak S., Choi Y.J., Kwon S., et al. De novo malignancy risk in patients undergoing the first percutaneous coronary intervention: a nationwide population-based cohort study. Int J Cardiol. 2020;313:25–31. doi: 10.1016/j.ijcard.2020.04.085. [DOI] [PubMed] [Google Scholar]
- 78.Wilson P.W., Bozeman S.R., Burton T.M., Hoaglin D.C., Ben-Joseph R., Pashos C.L. Prediction of first events of coronary heart disease and stroke with consideration of adiposity. Circulation. 2008;118:124–130. doi: 10.1161/CIRCULATIONAHA.108.772962. [DOI] [PubMed] [Google Scholar]
- 79.Lauby-Secretan B., Scoccianti C., Loomis D., et al. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adams T.D., Gress R.E., Smith S.C., et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 81.Schauer D.P., Feigelson H.S., Koebnick C., et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95–101. doi: 10.1097/SLA.0000000000002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuhara H., Steinmaus C., Cohen S.E., Corley D.A., Tei Y., Buffler P.A. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. 2011;106:1911–1921. doi: 10.1038/ajg.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jaiswal S., Fontanillas P., Flannick J., et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaiswal S., Natarajan P., Silver A.J., et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calvillo-Arguelles O., Jaiswal S., Shlush L.I., et al. Connections between clonal hematopoiesis, cardiovascular disease, and cancer: a review. JAMA Cardiol. 2019;4:380–387. doi: 10.1001/jamacardio.2019.0302. [DOI] [PubMed] [Google Scholar]
- 86.Khetarpal S.A., Qamar A., Bick A.G., et al. Clonal hematopoiesis of indeterminate potential reshapes age-related CVD: JACC review topic of the week. J Am Coll Cardiol. 2019;74:578–586. doi: 10.1016/j.jacc.2019.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sano S., Wang Y., Yura Y., et al. JAK2 (V617F)–mediated clonal hematopoiesis accelerates pathological remodeling in murine heart failure. J Am Coll Cardiol Basic Trans Science. 2019;4:684–697. doi: 10.1016/j.jacbts.2019.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fuster J.J., MacLauchlan S., Zuriaga M.A., et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bick A.G., Pirruccello J.P., Griffin G.K., et al. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation. 2020;141:124–131. doi: 10.1161/CIRCULATIONAHA.119.044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malak S., Labopin M., Saint-Martin C., Bellanne-Chantelot C., Najman A. for the French Group of Familial Myeloproliferative Disorders. Long term follow up of 93 families with myeloproliferative neoplasms: life expectancy and implications of JAK2V617F in the occurrence of complications. Blood Cells Mol Dis. 2012;49:170–176. doi: 10.1016/j.bcmd.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 91.Rossi C., Randi M.L., Zerbinati P., Rinaldi V., Girolami A. Acute coronary disease in essential thrombocythemia and polycythemia vera. J Intern Med. 1998;244:49–53. doi: 10.1046/j.1365-2796.1998.00314.x. [DOI] [PubMed] [Google Scholar]
- 92.Barbui T., Carobbio A., Cervantes F., et al. Thrombosis in primary myelofibrosis: incidence and risk factors. Blood. 2010;115:778–782. doi: 10.1182/blood-2009-08-238956. [DOI] [PubMed] [Google Scholar]
- 93.Wang W., Liu W., Fidler T., et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 (V617F) mice. Circ Res. 2018;123:e35–e47. doi: 10.1161/CIRCRESAHA.118.313283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wolach O., Sellar R.S., Martinod K., et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Edelmann B., Gupta N., Schnoeder T.M., et al. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J Clin Invest. 2018;128:4359–4371. doi: 10.1172/JCI90312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang Y., Liu W., Wang W., et al. Inhibition of JAK2 suppresses myelopoiesis and atherosclerosis in Apoe(-/-) mice. Cardiovasc Drugs Ther. 2020;34:145–152. doi: 10.1007/s10557-020-06943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Severson E.A., Riedlinger G.M., Connelly C.F., et al. Detection of clonal hematopoiesis of indeterminate potential in clinical sequencing of solid tumor specimens. Blood. 2018;131:2501–2505. doi: 10.1182/blood-2018-03-840629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coombs C.C., Gillis N.K., Tan X., et al. Identification of clonal hematopoiesis mutations in solid tumor patients undergoing unpaired next-generation sequencing assays. Clin Cancer Res. 2018;24:5918–5924. doi: 10.1158/1078-0432.CCR-18-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Z., Huang W., Yin J.C., et al. Comprehensive next-generation profiling of clonal hematopoiesis in cancer patients using paired tumor-blood sequencing for guiding personalized therapies. Clin Transl Med. 2020;10:e222. doi: 10.1002/ctm2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coombs C.C., Zehir A., Devlin S.M., et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21:374–382.e4. doi: 10.1016/j.stem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Darby S.C., Ewertz M., McGale P., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 103.Yakupovich A., Davison M.A., Kharouta M.Z., et al. Heart dose and coronary artery calcification in patients receiving thoracic irradiation for lung cancer. J Thorac Dis. 2020;12:223–231. doi: 10.21037/jtd.2020.01.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lipshultz S.E., Adams M.J., Colan S.D., et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 105.Herrmann J., Yang E.H., Iliescu C.A., et al. Vascular toxicities of cancer therapies: the old and the new—an evolving avenue. Circulation. 2016;133:1272–1289. doi: 10.1161/CIRCULATIONAHA.115.018347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ito D., Shiraishi J., Nakamura T., et al. Primary percutaneous coronary intervention and intravascular ultrasound imaging for coronary thrombosis after cisplatin-based chemotherapy. Heart Vessels. 2012;27:634–638. doi: 10.1007/s00380-011-0222-5. [DOI] [PubMed] [Google Scholar]
- 107.Ranpura V., Hapani S., Chuang J., Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. 2010;49:287–297. doi: 10.3109/02841860903524396. [DOI] [PubMed] [Google Scholar]
- 108.Winnik S., Lohmann C., Siciliani G., et al. Systemic VEGF inhibition accelerates experimental atherosclerosis and disrupts endothelial homeostasis—implications for cardiovascular safety. Int J Cardiol. 2013;168:2453–2461. doi: 10.1016/j.ijcard.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 109.Douxfils J., Haguet H., Mullier F., Chatelain C., Graux C., Dogne J.M. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta-analysis. JAMA Oncol. 2016;2:625–632. doi: 10.1001/jamaoncol.2015.5932. [DOI] [PubMed] [Google Scholar]
- 110.Mukai M., Komori K., Oka T. Mechanism and management of cancer chemotherapy-induced atherosclerosis. J Atheroscler Thromb. 2018;25:994–1002. doi: 10.5551/jat.RV17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeboudj L., Maitre M., Guyonnet L., et al. Selective EGF-receptor inhibition in CD4(+) T cells induces anergy and limits atherosclerosis. J Am Coll Cardiol. 2018;71:160–172. doi: 10.1016/j.jacc.2017.10.084. [DOI] [PubMed] [Google Scholar]
- 112.Bar J., Markel G., Gottfried T., et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122–131. doi: 10.1016/j.ejca.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 113.Drobni Z.D., Alvi R.M., Taron J., et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smyth S.S., McEver R.P., Weyrich A.S., et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 115.Jackson S.P. The growing complexity of platelet aggregation. Blood. 2007;109:5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 116.Coppinger J.A., Cagney G., Toomey S., et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 117.Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davi G., Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 119.Xu X.R., Yousef G.M., Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood. 2018;131:1777–1789. doi: 10.1182/blood-2017-05-743187. [DOI] [PubMed] [Google Scholar]
- 120.Henn V., Slupsky J.R., Grafe M., et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 121.Ghasemzadeh M., Hosseini E. Platelet granule release is associated with reactive oxygen species generation during platelet storage: a direct link between platelet pro-inflammatory and oxidation states. Thromb Res. 2017;156:101–104. doi: 10.1016/j.thromres.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 122.Siegel-Axel D., Daub K., Seizer P., Lindemann S., Gawaz M. Platelet lipoprotein interplay: trigger of foam cell formation and driver of atherosclerosis. Cardiovasc Res. 2008;78:8–17. doi: 10.1093/cvr/cvn015. [DOI] [PubMed] [Google Scholar]
- 123.Chatterjee M., Rath D., Schlotterbeck J., et al. Regulation of oxidized platelet lipidome: implications for coronary artery disease. Eur Heart J. 2017;38:1993–2005. doi: 10.1093/eurheartj/ehx146. [DOI] [PubMed] [Google Scholar]
- 124.Barrett T.J., Schlegel M., Zhou F., et al. Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aax0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Passacquale G., Vamadevan P., Pereira L., Hamid C., Corrigall V., Ferro A. Monocyte-platelet interaction induces a pro-inflammatory phenotype in circulating monocytes. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0025595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marx C., Novotny J., Salbeck D., et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood. 2019;134:1859–1872. doi: 10.1182/blood.2019000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pircher J., Engelmann B., Massberg S., Schulz C. Platelet-neutrophil crosstalk in atherothrombosis. Thromb Haemost. 2019;119:1274–1282. doi: 10.1055/s-0039-1692983. [DOI] [PubMed] [Google Scholar]
- 128.Liang X., Xiu C., Liu M., et al. Platelet-neutrophil interaction aggravates vascular inflammation and promotes the progression of atherosclerosis by activating the TLR4/NF-κB pathway. J Cell Biochem. 2019;120:5612–5619. doi: 10.1002/jcb.27844. [DOI] [PubMed] [Google Scholar]
- 129.Pastori D., Pignatelli P., Farcomeni A., et al. Urinary 11-dehydro-thromboxane B2 is associated with cardiovascular events and mortality in patients with atrial fibrillation. Am Heart J. 2015;170:490–497.e1. doi: 10.1016/j.ahj.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 130.Gay L.J., Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tao D.L., Tassi Yunga S., Williams C.D., McCarty O.J.T. Aspirin and antiplatelet treatments in cancer. Blood. 2021;137:3201–3211. doi: 10.1182/blood.2019003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ma Y., Li G., Yu M., et al. Prognostic significance of thrombocytosis in lung cancer: a systematic review and meta-analysis. Platelets. 2021;32:919–927. doi: 10.1080/09537104.2020.1810653. [DOI] [PubMed] [Google Scholar]
- 133.Zhao J.M., Wang Y.H., Yao N., et al. Poor prognosis significance of pretreatment thrombocytosis in patients with colorectal cancer: a meta-analysis. Asian Pac J Cancer Prev. 2016;17:4295–4300. [PubMed] [Google Scholar]
- 134.Hufnagel D.H., Cozzi G.D., Crispens M.A., Beeghly-Fadiel A. Platelets, Thrombocytosis, and ovarian cancer prognosis: surveying the landscape of the literature. Int J Mol Sci. 2020;21:8169. doi: 10.3390/ijms21218169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu P.H., Hsu C.Y., Su C.W., et al. Thrombocytosis is associated with worse survival in patients with hepatocellular carcinoma. Liver Int. 2020;40:2522–2534. doi: 10.1111/liv.14560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hyslop S.R., Alexander M., Thai A.A., et al. Targeting platelets for improved outcome in KRAS-driven lung adenocarcinoma. Oncogene. 2020;39:5177–5186. doi: 10.1038/s41388-020-1357-6. [DOI] [PubMed] [Google Scholar]
- 137.Shirai T., Inoue O., Tamura S., et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J Thromb Haemost. 2017;15:513–525. doi: 10.1111/jth.13604. [DOI] [PubMed] [Google Scholar]
- 138.Tawil N., Bassawon R., Meehan B., et al. Glioblastoma cell populations with distinct oncogenic programs release podoplanin as procoagulant extracellular vesicles. Blood Adv. 2021;5:1682–1694. doi: 10.1182/bloodadvances.2020002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Popivanova B.K., Kostadinova F.I., Furuichi K., et al. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69:7884–7892. doi: 10.1158/0008-5472.CAN-09-1451. [DOI] [PubMed] [Google Scholar]
- 140.Olsson A.K., Cedervall J. The pro-inflammatory role of platelets in cancer. Platelets. 2018;29:569–573. doi: 10.1080/09537104.2018.1453059. [DOI] [PubMed] [Google Scholar]
- 141.Gil-Bernabe A.M., Ferjancic S., Tlalka M., et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119:3164–3175. doi: 10.1182/blood-2011-08-376426. [DOI] [PubMed] [Google Scholar]
- 142.Kim Y.J., Borsig L., Varki N.M., Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang L., Liu Q., Zhang X., et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:133–138. doi: 10.1038/s41586-020-2394-6. [DOI] [PubMed] [Google Scholar]
- 144.Placke T., Orgel M., Schaller M., et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72:440–448. doi: 10.1158/0008-5472.CAN-11-1872. [DOI] [PubMed] [Google Scholar]
- 145.Rachidi S., Metelli A., Riesenberg B., et al. Platelets subvert T cell immunity against cancer via GARP-TGFβ axis. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aai7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zaslavsky A.B., Adams M.P., Cao X., et al. Platelet PD-L1 suppresses anti-cancer immune cell activity in PD-L1 negative tumors. Sci Rep. 2020;10:19296. doi: 10.1038/s41598-020-76351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.De Palma M., Biziato D., Petrova T.V. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 148.Zaldivia M.T.K., McFadyen J.D., Lim B., Wang X., Peter K. Platelet-derived microvesicles in cardiovascular diseases. Front Cardiovasc Med. 2017;4:74. doi: 10.3389/fcvm.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Khan S.U., Singh M., Valavoor S., et al. Dual antiplatelet therapy after percutaneous coronary intervention and drug-eluting stents: a systematic review and network meta-analysis. Circulation. 2020;142:1425–1436. doi: 10.1161/CIRCULATIONAHA.120.046308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hayashi T., Shibata M., Oe S., Miyagawa K., Honma Y., Harada M. Antiplatelet therapy improves the prognosis of patients with hepatocellular carcinoma. Cancers (Basel) 2020;12:3215. doi: 10.3390/cancers12113215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wright J.R., Chauhan M., Shah C., et al. The TICONC (Ticagrelor-Oncology) study. J Am Coll Cardiol CardioOnc. 2020;2:236–250. doi: 10.1016/j.jaccao.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ornelas A., Zacharias-Millward N., Menter D.G., et al. Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017;36:289–303. doi: 10.1007/s10555-017-9675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rothwell P.M., Wilson M., Price J.F., Belch J.F., Meade T.W., Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 154.Algra A.M., Rothwell P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 155.Jacobs E.J., Thun M.J., Bain E.B., Rodriguez C., Henley S.J., Calle E.E. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007;99:608–615. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 156.Rothwell P.M., Price J.F., Fowkes F.G., et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 157.Haykal T., Barbarawi M., Zayed Y., et al. Safety and efficacy of aspirin for primary prevention of cancer: a meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol. 2019;145:1795–1809. doi: 10.1007/s00432-019-02932-0. [DOI] [PubMed] [Google Scholar]
- 158.McNeil J.J., Gibbs P., Orchard S.G., et al. Effect of aspirin on cancer incidence and mortality in older adults. J Natl Cancer Inst. 2021;113(3):258–265. doi: 10.1093/jnci/djaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Graff J., Harder S., Wahl O., Scheuermann E.H., Gossmann J. Anti-inflammatory effects of clopidogrel intake in renal transplant patients: effects on platelet-leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers. Clin Pharmacol Ther. 2005;78:468–476. doi: 10.1016/j.clpt.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 160.Sitia G., Aiolfi R., Di Lucia P., et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A. 2012;109:E2165–E2172. doi: 10.1073/pnas.1209182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cho M.S., Noh K., Haemmerle M., et al. Role of ADP receptors on platelets in the growth of ovarian cancer. Blood. 2017;130:1235–1242. doi: 10.1182/blood-2017-02-769893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chang Y.W., Hsieh P.W., Chang Y.T., et al. Identification of a novel platelet antagonist that binds to CLEC-2 and suppresses podoplanin-induced platelet aggregation and cancer metastasis. Oncotarget. 2015;6:42733–42748. doi: 10.18632/oncotarget.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hatakeyama K., Kaneko M.K., Kato Y., et al. Podoplanin expression in advanced atherosclerotic lesions of human aortas. Thromb Res. 2012;129:e70–e76. doi: 10.1016/j.thromres.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 164.Fei M., Xiang L., Chai X., et al. Plasma soluble C-type lectin-like receptor-2 is associated with the risk of coronary artery disease. Front Med. 2020;14:81–90. doi: 10.1007/s11684-019-0692-x. [DOI] [PubMed] [Google Scholar]
- 165.Wu X., Zhang W., Li H., et al. Plasma C-type lectin-like receptor 2 as a predictor of death and vascular events in patients with acute ischemic stroke. Eur J Neurol. 2019;26:1334–1340. doi: 10.1111/ene.13984. [DOI] [PubMed] [Google Scholar]
- 166.Abbonante V., Chitalia V., Rosti V., et al. Upregulation of lysyl oxidase and adhesion to collagen of human megakaryocytes and platelets in primary myelofibrosis. Blood. 2017;130:829–831. doi: 10.1182/blood-2017-04-777417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Setargew Y.F.I., Wyllie K., Grant R.D., Chitty J.L., Cox T.R. Targeting lysyl oxidase family meditated matrix cross-linking as an anti-stromal therapy in solid tumours. Cancers (Basel) 2021;13:491. doi: 10.3390/cancers13030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rodriguez C., Martinez-Gonzalez J., Raposo B., Alcudia J.F., Guadall A., Badimon L. Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008;79:7–13. doi: 10.1093/cvr/cvn102. [DOI] [PubMed] [Google Scholar]
- 169.Kagan H.M., Raghavan J., Hollander W. Changes in aortic lysyl oxidase activity in diet-induced atherosclerosis in the rabbit. Arteriosclerosis. 1981;1:287–291. doi: 10.1161/01.atv.1.4.287. [DOI] [PubMed] [Google Scholar]
- 170.Nuthakki V.K., Fleser P.S., Malinzak L.E., et al. Lysyl oxidase expression in a rat model of arterial balloon injury. J Vasc Surg. 2004;40:123–129. doi: 10.1016/j.jvs.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 171.Matsuura S., Mi R., Koupenova M., et al. Lysyl oxidase is associated with increased thrombosis and platelet reactivity. Blood. 2016;127:1493–1501. doi: 10.1182/blood-2015-02-629667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.De Vita A., Liverani C., Molinaro R., et al. Lysyl oxidase engineered lipid nanovesicles for the treatment of triple negative breast cancer. Sci Rep. 2021;11:5107. doi: 10.1038/s41598-021-84492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saatci O., Kaymak A., Raza U., et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat Commun. 2020;11:2416. doi: 10.1038/s41467-020-16199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Leiva O., Ng S.K., Matsuura S., et al. Novel lysyl oxidase inhibitors attenuate hallmarks of primary myelofibrosis in mice. Int J Hematol. 2019;110:699–708. doi: 10.1007/s12185-019-02751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Timp J.F., Braekkan S.K., Versteeg H.H., Cannegieter S.C. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–1723. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 176.Blom J.W., Doggen C.J., Osanto S., Rosendaal F.R. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 177.Sorensen H.T., Mellemkjaer L., Olsen J.H., Baron J.A. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 178.Prandoni P., Lensing A.W., Piccioli A., et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 179.Shaw J.R., Douketis J., Le Gal G., Carrier M. Periprocedural interruption of anticoagulation in patients with cancer-associated venous thromboembolism: an analysis of thrombotic and bleeding outcomes. J Thromb Haemost. 2019;17:1171–1178. doi: 10.1111/jth.14468. [DOI] [PubMed] [Google Scholar]
- 180.Saccullo G., Malato A., Raso S., et al. Cancer patients requiring interruption of long-term warfarin because of surgery or chemotherapy induced thrombocytopenia: the use of fixed sub-therapeutic doses of low-molecular weight heparin. Am J Hematol. 2012;87:388–391. doi: 10.1002/ajh.23122. [DOI] [PubMed] [Google Scholar]
- 181.Leiva O., Connors J.M., Al-Samkari H. Impact of tumor genomic mutations on thrombotic risk in cancer patients. Cancers (Basel) 2020;12:1958. doi: 10.3390/cancers12071958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Icht O., Darzi N., Shimony S., et al. Venous thromboembolism incidence and risk assessment in lung cancer patients treated with immune checkpoint inhibitors. J Thromb Haemost. 2021;19(5):1250–1258. doi: 10.1111/jth.15272. [DOI] [PubMed] [Google Scholar]
- 183.Pastori D., Menichelli D., Bucci T., Violi F., Pignatelli P., for the ATHERO-AF Study Group Cancer-specific ischemic complications in elderly patients with atrial fibrillation: data from the prospective ATHERO-AF study. Int J Cancer. 2020;147:3424–3430. doi: 10.1002/ijc.33179. [DOI] [PubMed] [Google Scholar]
- 184.Aspberg S., Yu L., Gigante B., Smedby K.E., Singer D.E. Risk of ischemic stroke and major bleeding in patients with atrial fibrillation and cancer. J Stroke Cerebrovasc Dis. 2020;29:104560. doi: 10.1016/j.jstrokecerebrovasdis.2019.104560. [DOI] [PubMed] [Google Scholar]
- 185.Kitano T., Sasaki T., Gon Y., et al. The effect of chemotherapy on stroke risk in cancer patients. Thromb Haemost. 2020;120:714–723. doi: 10.1055/s-0040-1708484. [DOI] [PubMed] [Google Scholar]
- 186.Pastori D., Marang A., Bisson A., et al. Thromboembolism, mortality, and bleeding in 2,435,541 atrial fibrillation patients with and without cancer: a nationwide cohort study. Cancer. 2021;127(12):2122–2129. doi: 10.1002/cncr.33470. [DOI] [PubMed] [Google Scholar]
- 187.D’Souza M., Carlson N., Fosbol E., et al. CHA2DS2-VASc score and risk of thromboembolism and bleeding in patients with atrial fibrillation and recent cancer. Eur J Prev Cardiol. 2018;25:651–658. doi: 10.1177/2047487318759858. [DOI] [PubMed] [Google Scholar]
- 188.Gutierrez A., Patell R., Rybicki L., Khorana A.A. Predicting outcomes in patients with cancer and atrial fibrillation. Ther Adv Cardiovasc Dis. 2019;13 doi: 10.1177/1753944719860676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Khorana A.A., Francis C.W. Risk prediction of cancer-associated thrombosis: Appraising the first decade and developing the future. Thromb Res. 2018;164(suppl 1):S70–S76. doi: 10.1016/j.thromres.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 190.Al-Samkari H., Leiva O., Dagogo-Jack I., et al. Impact of ALK rearrangement on venous and arterial thrombotic risk in NSCLC. J Thorac Oncol. 2020;15:1497–1506. doi: 10.1016/j.jtho.2020.04.033. [DOI] [PubMed] [Google Scholar]
- 191.Kraaijpoel N., Di Nisio M., Mulder F.I., et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: results from the Hokusai VTE Cancer Study. Thromb Haemost. 2018;118:1439–1449. doi: 10.1055/s-0038-1667001. [DOI] [PubMed] [Google Scholar]
- 192.Kwok C.S., Wong C.W., Kontopantelis E., et al. Percutaneous coronary intervention in patients with cancer and readmissions within 90 days for acute myocardial infarction and bleeding in the USA. Eur Heart J. 2021;42:1019–1034. doi: 10.1093/eurheartj/ehaa1032. [DOI] [PubMed] [Google Scholar]
- 193.Bharadwaj A., Potts J., Mohamed M.O., et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J. 2020;41:2183–2193. doi: 10.1093/eurheartj/ehz851. [DOI] [PubMed] [Google Scholar]
- 194.Iannaccone M., D’Ascenzo F., Vadala P., et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: a BleeMACS substudy. Eur Heart J Acute Cardiovasc Care. 2018;7:631–638. doi: 10.1177/2048872617706501. [DOI] [PubMed] [Google Scholar]
- 195.Takeuchi T., Hikoso S., Hattori S., et al. The effect of a cancer history on patients with acute myocardial infarction after percutaneous coronary intervention. Int Heart J. 2021;62(2):238–245. doi: 10.1536/ihj.20-452. [DOI] [PubMed] [Google Scholar]
- 196.Mohamed M.O., Van Spall H.G.C., Kontopantelis E., et al. Effect of primary percutaneous coronary intervention on in-hospital outcomes among active cancer patients presenting with ST-elevation myocardial infarction: a propensity score matching analysis. Eur Heart J Acute Cardiovasc Care. 2021;10(8):829–839. doi: 10.1093/ehjacc/zuaa032. [DOI] [PubMed] [Google Scholar]
- 197.Nardi Agmon I., Perl L., Bental T., et al. Temporal trends in short and long-term outcomes after percutaneous coronary interventions among cancer patients. Heart Vessels. 2021;36(9):1283–1289. doi: 10.1007/s00380-021-01817-y. [DOI] [PubMed] [Google Scholar]
- 198.Balanescu D.V., Donisan T., Deswal A., et al. Acute myocardial infarction in a high-risk cancer population: outcomes following conservative versus invasive management. Int J Cardiol. 2020;313:1–8. doi: 10.1016/j.ijcard.2020.04.050. [DOI] [PubMed] [Google Scholar]