Abstract
Cancer patients and survivors have elevated cardiovascular risk when compared with noncancer patients. Cardio-oncology has emerged as a new subspecialty to comanage and address cardiovascular complications in cancer patients such as heart failure, atherosclerotic cardiovascular disease (ASCVD), valvular heart disease, pericardial disease, and arrhythmias. Cardiac computed tomography (CT) can be helpful in identifying both clinical and subclinical ASCVD in cancer patients and survivors. Radiation therapy treatment planning CT scans and cancer staging/re-staging imaging studies can quantify calcium scores which can identify pre-existing subclinical ASCVD. Cardiac CT can be helpful in the evaluation of cardiac tumors and pericardial diseases, especially in patients who cannot tolerate or have a contraindication to cardiac magnetic resonance. In this review, we describe the optimal utilization of cardiac CT in cancer patients, including risk assessment for ASCVD and identification of cancer treatment-related cardiovascular toxicity.
Key Words: atherosclerotic cardiovascular disease, calcium score cardio-oncology, carcinoid syndrome, cardiotoxicity, cardiovascular computed tomography, radiation therapy
Abbreviations and Acronyms: ASCVD, atherosclerotic cardiovascular disease; CT, computed tomography; PET, positron emission tomography; RT, radiation therapy; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography
Central Illustration
Highlights
-
•
Cardiac CT can assess for subclinical atherosclerosis, allowing for better risk stratification.
-
•
Cardiac CT has high accuracy to evaluate for obstructive coronary artery disease.
-
•
CAC scanning may be useful after radiation therapy to assess the extent of vascular calcifications.
-
•
Cardiac CT can accurately depict cardiac tumors, pericardial effusions, and cardiac function.
Cardiac computed tomography (CT) is a technique that allows acquisition of noncontrast and contrast images of the heart and vascular structures in a CT scanner, accounting for heart motion, with submillimetric (∼0.5 millimeter) spatial resolution (1). CT scanner technology has evolved to provide sharper and detailed images of coronary vessels and other heart structures, allowing cardiac CT to have broad indications, from chest pain evaluation to structural procedure assessment (2,3). Cardiac CT has an established role in contemporary practice in excluding coronary artery disease in intermediate-risk patients with symptoms (4). One strength that cardiac CT has in comparison to other modalities is the ability to characterize coronary lesions not only into obstructive or nonobstructive, but atherosclerotic plaque characteristics such as calcified, noncalcified, and high risk (5). The high-risk atherosclerotic plaque characteristics may predict future coronary events and have been defined as presence of positive remodeling, low-attenuation plaque, spotty calcification, and presence of “napkin-ring” sign (6,7). Moreover, with recent data from the SCOT-HEART (Scottish Computed Tomography of the Heart) (8) and ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (9) trials, cardiac CT may be incorporated into future U.S. guidelines as an initial test for chest pain evaluation, as is currently established in the NICE guidelines in the United Kingdom (10) and European Society of Cardiology guidelines (11).
Cardiac CT is used in the diagnosis and treatment of numerous cardiac conditions; however, utilization has led to increased exposure to ionizing radiation (12). Cancer is certainly the most concerning late (10-20 years) complication of diagnostic ionizing radiation, and the lifetime attributable risk of having cancer is significantly higher with childhood radiation exposure (13). Current guidelines advocate for a principle of “as low as reasonably achievable” while maintaining the diagnostic image quality (12). Current doses of coronary artery calcium (CAC) scoring have fallen significantly, and are now well below 1 millisievert (mSv), in the range of diagnostic mammography or abdominal x-rays (14). Similarly, radiation exposure with computed tomography angiography (CTA) has been reduced 10-fold over the last decade caused by better acquisition techniques, use of CT scanners with more detectors (ie, 256- or 320-slice), and new radiation reduction software, where mean doses are below 2 mSv (similar to annual background radiation) (15).
Cancer patients and survivors have elevated cardiovascular risk when compared with noncancer patients (16). Certain cancer therapeutics, such as vascular endothelial growth factor receptor inhibitors, several chemotherapies (17,18), immune checkpoint inhibitors (19), and radiation therapy (RT), are associated with increased risk of atherosclerotic cardiovascular disease (ASCVD). Shared risk factors, such as obesity, diabetes, tobacco use, hypertension, and physical inactivity, are associated with increased risk of developing both ASCVD and cancer (20). Early identification of atherosclerosis, aggressive risk factor modification, and aggressive preventive strategies for cancer patients and survivors are crucial to prevent morbidity and mortality from cardiovascular disease (CVD) in these populations (21). Cardio-oncology has emerged to comanage and address cardiovascular complications in cancer patients such as heart failure, ASCVD, valvular heart disease, and arrhythmias. In this primer, we review the current and emerging role of cardiac CT in cardio-oncology, including risk assessment for ASCVD and identification of cancer treatment related cardiovascular toxicity.
Role of Cardiac CT in the Assessment of Coronary Artery Disease in Cancer Patients and Survivors
Key points
-
•
Cardiac CT has an established role in contemporary practice in excluding coronary artery disease in intermediate-risk patients with symptoms.
-
•
Cardiac CT may identify subclinical ASCVD in cancer patients and survivors.
-
•
Quantifying CAC from a noncontrast CT from the RT treatment plans or cancer staging imaging studies can help identify pre-existing subclinical ASCVD
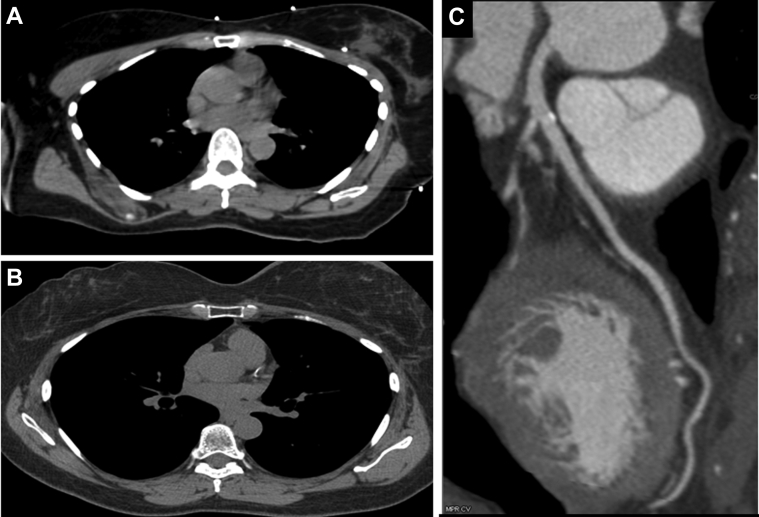
Case 1: 52-year-old woman who underwent radiation therapy (RT) for left breast cancer After 1-year post RT, patient underwent a Cardiac CT because of an episode of chest pain (Figure 1)
Figure 1.
Coronary Calcifications After Radiation Therapy
A 52-year-old woman who underwent radiation therapy (RT) for left breast cancer. In a nongated noncontrast computed tomography (CT) for RT planning, no coronary calcifications were noted (A). After 1 year post-RT, patient underwent a cardiac CT because of an episode of chest pain. Patient had a coronary calcium score of 47 (B), and nonobstructive calcified plaque was seen involving the proximal left anterior descending coronary artery and first diagonal in postcontrast images (C).
ASCVD risk and coronary calcification in cancer patients and survivors
To attenuate potentially accelerated risks of ASCVD in these patients, cardio-oncologists must have a robust and current understanding of preventive cardiology. CAC is a quantifiable imaging biomarker that describes the extent and burden of coronary calcification caused by atherosclerosis derived from a noncontrast cardiac CT (22). The presence of CAC is an independent risk factor for CV events, which can help prognosticate 10-year CV risk in addition to traditional ASCVD risk factors in the general population (23,24). In a MESA (Multi-Ethnic Study of Atherosclerosis) substudy, the incidence of new CAC was independently associated with cancer history, with an increased relative risk of about 1.3 for men and women (25).
Cardiac CT may identify subclinical ASCVD in cancer patients and survivors. There are solid data supporting the prognostic value and potential role of CAC score assessment in nongated CT studies (26). Implementing baseline CAC screening in cancer patients by utilizing nongated CT from cancer imaging, such as staging chest CTs or the CT component of positron emission tomography (PET) scans, may help in risk stratification and prompt diagnosis of ASCVD (27). Cancer patients with favorable prognoses and survivors with at least 1 risk factor for ASCVD should be appropriately screened for dyslipidemia, hypertension, and obesity for recommendations regarding lifestyle modifications as part of initial cardiovascular risk assessment (21). Discussion of initiation of aspirin and statins should include input from oncologists so as to avoid drug–drug interactions and increased bleeding complications in those at risk (Central Illustration). There may be an important role for cardiac CT in cancer patients with thrombocytopenia and anemia in low-intermediate acute coronary syndrome presentations, given that there is a higher risk of vascular complications and bleeding in some cancer patients from invasive procedures (28,29).
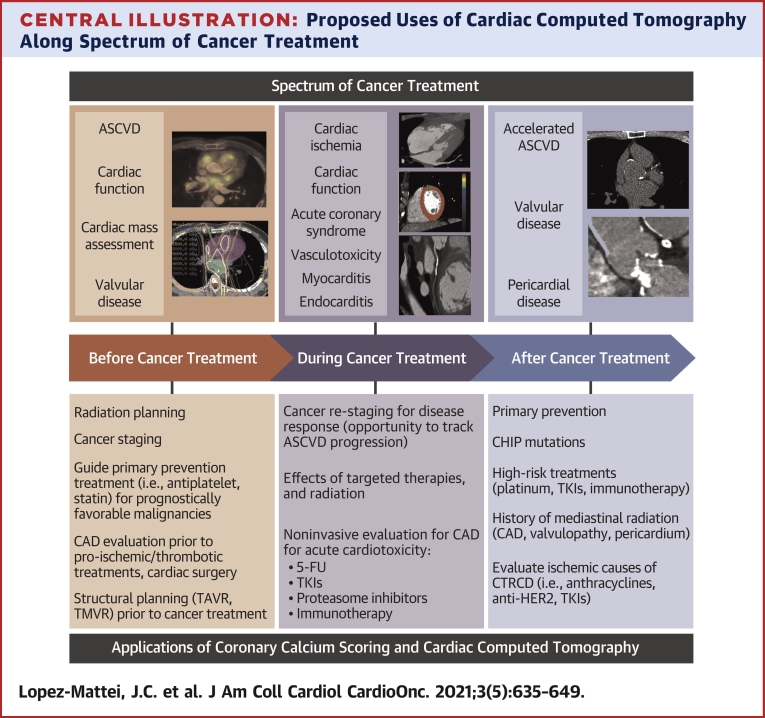
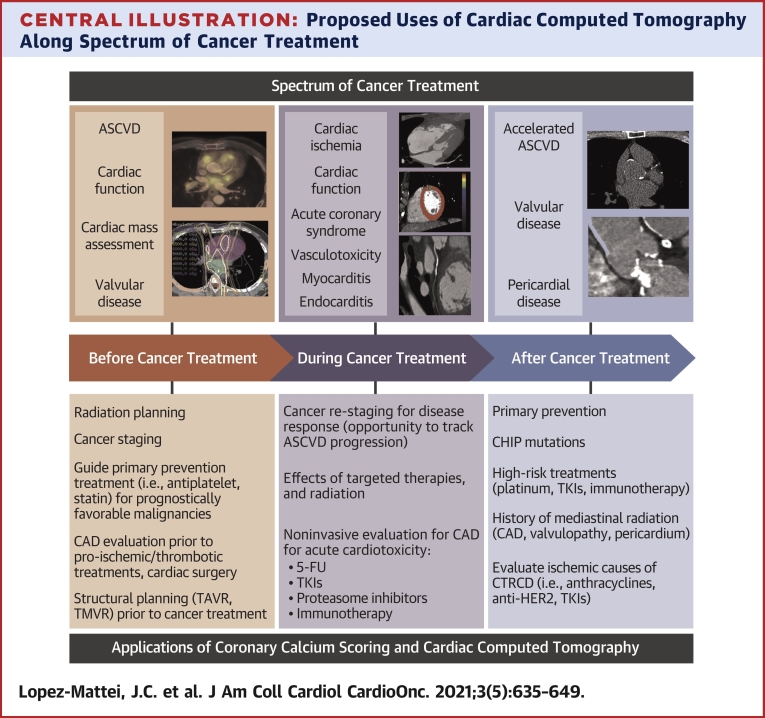
Central Illustration.
Proposed Uses of Cardiac Computed Tomography Along Spectrum of Cancer Treatment
Applications of cardiac CT across the spectrum of cancer treatment in the cancer patient, with cardiovascular disease states that can be pre-existing and/or acquired from cancer treatments. FU = fluorouracil; ASCVD = atherosclerotic cardiovascular disease; CAD = coronary artery disease; CAR-T = chimeric antigen receptor T-cell; CTRCD = cancer treatment-related cardiac dysfunction; TAVR = transcatheter aortic valve replacement; TKI = tyrosine kinase inhibitor; TMVR = transcatheter mitral valve repair.
As clinical outcomes for cancer patients continue to improve with more effective therapies, the population of cancer survivors will continue to grow. In 2019, there were an estimated 2 million new cancer diagnoses and 600,000 cancer deaths, along with millions of survivors (30). Because CVD is the second leading cause of death among cancer survivors (after cancer recurrence), screening for ASCVD should become part of survivorship care (31). Given the large overlap of ASCVD and cancer risk, dual screening for cancer and CVD has been recommended (32). Assessment of CAC on nongated noncontrast chest CTs allows for dual detection of lung cancer and CVD (33). Furthermore, emerging data suggest that breast arterial calcification on mammography correlates with CAC, allowing for identification of atherosclerosis and subsequent ASCVD risk (34). Recently, the BRAGATSTON clinical trial evaluated if automated quantification of CAC on RT planning chest CTs was useful for CVD risk prediction (35,36). The investigators found that automated CAC scoring results might be used as a fast and low-cost tool to identify patients with breast cancer at increased CVD risk (36).
Given the high CVD risk and overlap of risk factors, it is prudent to consider cardiovascular risk assessment while screening for or managing patients during and following cancer treatment. In 2019, the ACC/AHA prevention guidelines (37) recommended CAC testing in the general population to help with shared decision-making for use of preventive therapies (eg, statins, aspirin, blood pressure targets). The ability to identify subclinical atherosclerosis and redirect therapies in cancer survivors could reduce the burden of CVD risk in this population. In cancer survivorship, the focus should not solely be on the early detection of cancer recurrence, but also the assessment of ASCVD risk and use of CVD preventive therapies (lifestyle modifications and medications) to improve CVD outcomes. Identification of individuals with CAC >100 or even higher (ie, >400) in cancer patients would lead to recommendations of preventive therapies, including aspirin, statins, and more aggressive blood pressure targets, among others. Higher CAC scores are also associated with increased adherence to these therapies, including “heart smart” diet, exercise, and weight reduction (38). Incorporating CAC into pooled cohort equations or routinely incorporating CAC assessment in individuals with a history of adult or childhood cancer should be considered part of routine care in preventive cardio-oncology.
Role of cardiac CT in understanding cardiotoxicity with radiation therapy
RT is an important treatment modality for several cancers; however, there are several consequences of radiation when the heart is in the treatment field, such as ASCVD, valvular heart disease, pericardial diseases, and myocardial fibrosis (39). It is well known that radiation has direct toxic effects on the vascular endothelium (40). RT has been directly associated with the development of accelerated atherosclerosis and development of future coronary events, dependent on the heart dose received (41). Quantifying CAC from a noncontrast CT from the RT treatment plans or cancer staging imaging studies can help identify pre-existing subclinical ASCVD (27,42). Coronary artery dose volume parameters are strongly correlated with subsequent segmental coronary calcification, and may occur soon after RT and in individuals with diabetes and other conventional risk factors for ASCVD (43) (Figure 1). RT dose to the heart is currently measured as mean heart dose without considering which specific cardiac anatomical structures received the highest radiation doses. Because anatomical information is very important in ASCVD progression, monitoring doses to specific coronary territories and vascular beds might be of importance. There are important mitigation strategies to lower radiation dose to the heart in contemporary practice, such as deep inspiration breath holding and intensity modulation RT (44); these should be considered in every patient receiving RT in which the heart might be exposed in the treatment field. Several small single-center studies suggest that baseline CAC might help identify subclinical ASCVD and predict CV events in these patients (45, 46, 47). Although there are limited data, there may be a role for CAC surveillance in these patients as it may demonstrate ASCVD disease progression related to RT (43,48) (Figure 2). Cardiac CT has the ability to characterize atherosclerotic plaques and show features related to vulnerability; however, there are no data yet regarding the relationship of baseline plaque vulnerability, RT exposure, and outcomes. Currently, it is not known how efficacious preventive measures for ASCVD, such as statin and aspirin, are in patients with a history of RT. Identification of ASCVD and early treatment might improve outcomes, but this is an evolving area of research that needs further exploration.
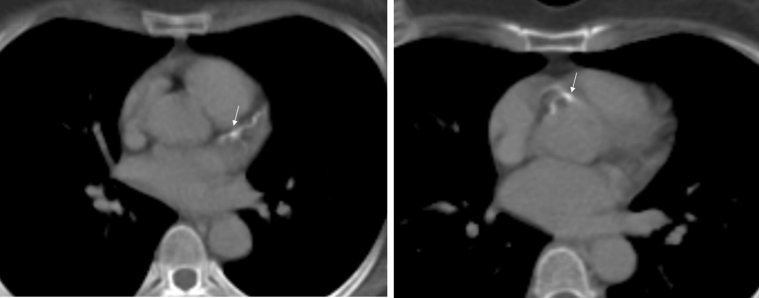
Figure 2.
Atherosclerosis From Radiation Therapy
A 46-year-old woman with history of RT for non-Hodgkin lymphoma in 1991. She had incidental finding of atherosclerosis in left anterior descending coronary artery and right coronary artery (arrows) in a nongated chest CT for an evaluation of a new malignancy. She did not have traditional risk factors for atherosclerotic cardiovascular disease other than her prior history of RT. Abbreviations as in Figure 1.
Functional vs anatomic imaging in patients treated with RT
The debate between functional vs anatomical imaging might not be settled anytime soon. However, it is clear that in patients who have received RT, ASCVD occurs in the coronary distribution that was affected by the radiation dose administered and the risk of coronary event is dose-dependent (41). Functional imaging with single-photon emission CT has been used to evaluate CV risk in patients undergoing radiation therapy. In a single-center study that enrolled 114 patients following RT to undergo rest single-photon emission CT before and after RT, it was found that 40% of patients developed new perfusion abnormalities (49). There have been several single-center studies noting a higher likelihood of abnormal myocardial perfusion imaging findings in these patients, but these findings do not predict clinical outcomes (50,51). It is important to note, however, that these studies did not correlate perfusion defects with coronary anatomy assessment, whether by invasive angiography or cardiac CT. The American Society of Echocardiography Expert Consensus for Multi-Modality Imaging Evaluation of Cardiovascular Complications of Radiotherapy in Adults recommends functional stress imaging 5-10 years after RT exposure in high risk patients (52).
Cardiac CT now has capabilities to assess ischemic burden as well by estimating fractional flow reserve (FFRct). FFRct has emerged as a powerful tool to provide important prognostic information and further inform treatment strategies (anatomic plus hemodynamic significance of stenosis) (Figure 3). FFRct affords significant improved discrimination of ischemia beyond other noninvasive tests, including myocardial perfusion imaging and PET (53). This new technique allows for evaluation of functional significance of intermediate lesions from cardiac CT images by computer modelling, which may have not been otherwise evaluable in patients with intermediate stenosis plaque or calcified plaque. This technique may avoid additional downstream testing in some cases.
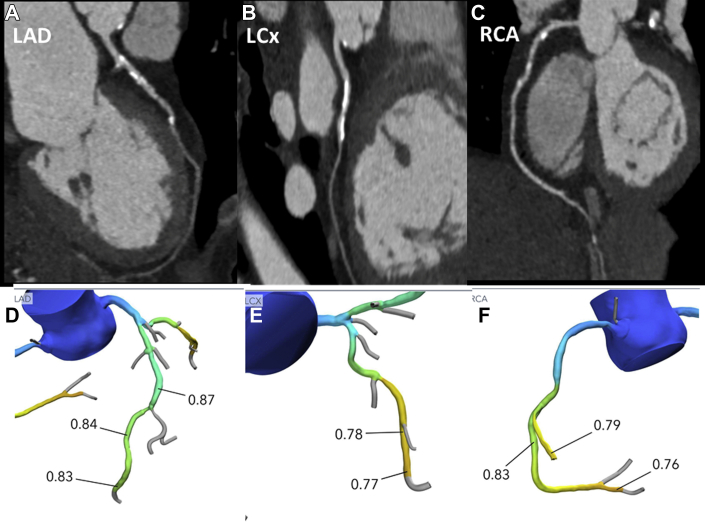
Figure 3.
CHIP and Cardiac CT Findings
A 63-year-old Hispanic woman with hypertension, dyslipidemia, and myelodysplastic syndrome post–bone marrow transplantation with persistent DNMT3A and TP53 CHIP mutations. cardiac CT was performed for a presentation of exertional chest pain, demonstrating heavily calcified, at least moderate stenoses (>50%) of the LAD (A) and LCX (B), and mild stenosis in RCA (C) with a coronary artery calcium score of 642 (99th percentile for age, sex, and race). FFRCT noninvasive analysis (Heartflow) of each vessel was also performed, demonstrating FFR values of >0.8 throughout the LAD (D), but showing borderline significant FFR values <0.8 in the mid to distal segments of the LCx (E) and RCA (F). The patient had Canadian Cardiovascular Society Class II symptoms which were stable, and improved on medical therapy. The decision was made to treat medically with referral to cardiac rehabilitation. CHIP= clonal hematopoiesis of indeterminate potential; CT = computed tomography; FFR = fractional flow reserve; LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; RCA = right coronary artery.
Role of cardiac CT in understanding cardiotoxicity and vascular toxicity from chemotherapies and targeted therapies
Cardiotoxicity is an important and concerning complication of systemic cancer therapies. It may manifest clinically as arrhythmias, hypertension, coronary vasospasm, myocardial infarction, or ventricular dysfunction. Cancer treatment-related cardiac dysfunction is defined by the American Society of Echocardiography as a decrease in left ventricular ejection fraction of >10% to a value <53% (54, 55, 56). Vascular toxicities have emerged as the second most common group of toxicities associated with anticancer therapies (57). Arterial toxicities can present as acute vasospasm, acute thrombosis, and accelerated atherosclerosis.
Cardiac CT, specifically coronary CTA, provides accurate assessment of coronary arteries with excellent sensitivity and specificity (58, 59, 60, 61, 62). In particular, the negative predictive value for the exclusion of significant coronary stenosis is very high. Coronary CTA can identify and characterize coronary atherosclerotic plaque (63, 64, 65, 66). The most common causes of cardiovascular toxicities and the role of cardiac CT in their evaluation are summarized in Table 1.
Table 1.
Proposed Uses of Cardiac CT Along Spectrum of Cancer Treatment
| Chemotherapy/Targeted Therapy Type | Examples of Specific Medications | Cardiovascular Side Effects | Role of Cardiac CT |
|---|---|---|---|
| Fluoropyrimidines | 5-FU Capecitabine |
Anginal chest pain (incidence up to 18%) (55,56,105, 106, 107, 108) more common with infusion of 5-FU Coronary vasospasm Myocardial infarction |
|
| Taxanes | Paclitaxel Docetaxel |
Myocardial ischemia (55,56) Coronary vasospasm (109) |
|
| Alkylating agents | Cyclophosphamide | Hemorrhagic myopericarditis |
|
| Immune checkpoint inhibitors | Pembrolizumab Nivolumab Ipilimumab Atezolizumab |
Myocarditis (incidence 1%-2%) (84,110, 111, 112, 113, 114, 115, 116) Increased risk of coronary atherosclerosis (117) |
|
| Vascular endothelial growth factor inhibitors | Bevacizumab Sunitinib Sorafenib Pozapanib |
Arterial hypertension Acute thromboembolic events (118, 119, 120, 121, 122) |
|
| Anthracyclines | Doxorubicin Daunorubicin Idarubicin Mitoxantrone |
Cardiomyopathy Arrhythmia Pericardial effusion |
|
| HER2/neu receptor inhibitors | Trastuzumab | Cardiomyopathy |
|
ACS = acute coronary syndrome; ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium scoring; CT = computed tomography; CTA = computed tomography angiography; FU = fluorouracil.
Role of Cardiac CT in the Evaluation of Cardiac Tumors
Key points
-
•
Cardiac CT can play an important role in identification of the location of a cardiac tumor, including its relationship to and potential involvement of surrounding cardiac structures as well as further tissue characterization.
-
•
Cardiac CT may also help differentiate intracavitary tumor from thrombus, and benign from malignant cardiac tumors in some instances with the addition of fluorodeoxyglucose PET.
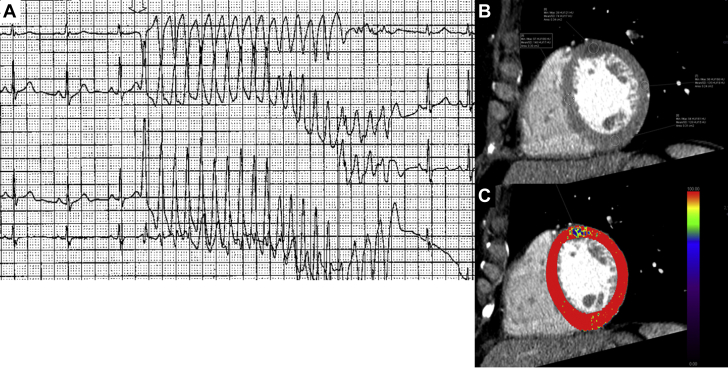
Case 2: 41-year-old woman with melanoma and an episode of ventricular tachycardia Cardiac CT was done to assess for coronary anomalies and evaluate for obstructive coronary disease caused by an episode of ventricular tachycardia (seeFigure 4)
Figure 4.
Cardiac Metastatic Disease
A 41-year-old woman with melanoma and an episode of unexplained ventricular tachycardia. Cardiac CT was done to assess for coronary anomalies and evaluate for obstructive coronary disease caused by an episode of ventricular tachycardia (A). Cardiac CT showed an area of attenuation at the mid anterior segment of the left ventricle (B), which can be seen hypoperfused in perfusion analysis (C). There was no evidence of obstructive coronary artery disease, and this area of attenuation represented intramyocardial metastasis from melanoma and the culprit for ventricular tachycardia. CT = computed tomography.
Tumor vs Thrombus
Cardiac tumors may be benign or malignant, and the latter may be primary or, more commonly, secondary (ie, metastases). Cardiac tumors may be found incidentally on imaging studies or may present symptomatically, such as with constitutional symptoms, mass effect, or embolic phenomenon. Only 5%-6% of primary tumors are malignant, most commonly sarcomas, lymphomas, and mesotheliomas (67). Cardiac metastases are up to 40 times more common than primary cardiac tumors (68). Upon autopsy, early data showed that about 12% of cancer patients had cardiac metastases, whereas more recent data suggest this occurs in 7% of cases (69). The most commonly reported metastases are from melanoma, breast, lung, and esophageal cancers (70,71) (Figure 4).
Multimodality cardiac imaging plays a significant role in the characterization of cardiac tumors, including precise location of the tumor in relation to cardiac structures (72). Due to its wide availability, transthoracic echocardiography (TTE) may often be the first imaging modality used for evaluation of a tumor, and because of its high temporal resolution, it can be especially ideal for evaluation of small mobile masses as well as valvular masses. Transesophageal echocardiography (TEE) can further evaluate valvular masses that are not well seen on TTE. Cardiac magnetic resonance (CMR) imaging is often the initial modality for further assessment of nonvalvular cardiac tumors, because of its excellent tissue characterization and multiplanar evaluation of cardiac structure. However, because of its longer acquisition time, for which some patients cannot hold their breath, and contraindication in patients with claustrophobia and certain implanted devices, CMR may not be an option in some patients. In such scenarios, because of its short acquisition time and high spatial resolution, cardiac CT can also provide further information about the tumor as well as information about extracardiac structures. Cardiac CT can play an important role in identification of the location of a cardiac tumor, including its relationship to and potential involvement of surrounding cardiac structures as well as further tissue characterization. Cardiac CT may also help differentiate intracavitary tumor from thrombus (73) and benign from malignant cardiac tumors in some instances with the addition of fluorodeoxyglucose PET (74,75). Specifically, for the detection of left atrial or left atrial appendage thrombus, the sensitivity and specificity of cardiac CT with delayed images postcontrast have been shown to be as high as 100% and 99%, respectively (76). For identification of LV thrombus, cardiac CT has been shown to have superior detection compared with 2-dimensional TTE (77), with LV thrombus demonstrating significantly lower attenuation than adjacent myocardium (73). Cardiac CT is also the optimal imaging modality for calcified masses, which are not well tissue characterized by CMR.
Cardiac CT in surgical planning for cardiac tumor resection
Resection of a cardiac tumor is usually not indicated; however, surgery may be pursued when the tumor is causing hemodynamic compromise or in the case of a primary cardiac tumor with good prognosis (78). Multiplanar imaging of a 3-dimensional acquisition data set with cardiac CT nicely allows for reconstruction of images into dedicated views for presurgical planning. Additionally, coronary CTA can be simultaneously obtained for a noninvasive evaluation of atherosclerotic coronary disease before surgery. This can provide a less-invasive, low-risk assessment for a patient with a low pretest probability of coronary artery disease, because patients—particularly with left sided masses—may be at risk for embolic events with invasive coronary angiography.
Pericardial Diseases
Key points
-
•
Imaging with cardiac CT, as well as with CMR, allows for full imaging of the pericardium.
-
•
Cardiac CT also allows for better visualization of pericardial calcification, which can be seen with constriction.
-
•
CT attenuation measurements can characterize the pericardial fluid, with values close to water likely representative of simple fluid and those above water possibly representing malignant or hemorrhagic fluid.
Case 3: 63-year-old woman with history including stage IIIA Hodgkin lymphoma, status postchemotherapy, and mantle radiation therapy completed 10 years before evaluation presents with dyspnea on exertion
Cancer may directly affect the pericardium via metastases, direct external invasion, or, less commonly, in the setting of a primary cardiac or pericardial tumor. Malignant effusions can occur as well as subsequent pericarditis, tamponade, and constrictive physiology. Additionally, many cancer therapies are associated with cardiotoxic effects on the pericardium, such as RT, chemotherapy, and immunotherapy. Radiation-associated pericarditis is more likely to occur with left-sided breast tumors vs right-sided (79) when there is greater involvement of the heart in the treatment field, and with increasing doses of radiation (80). One study revealed that the 2-year event rates for cardiotoxicity were 4%, 7%, and 21% in nonsmall cell lung cancer patients receiving a heart mean dose of <10, 10-20, and 20 Gy, respectively (81). Subsequent sequela can include pericardial fibrosis and constrictive cardiomyopathy. Certain chemotherapy agents (eg, cyclophosphamide) may lead to pericardial effusion, often necessitating pericardiocentesis or pericardial window, and 5%-15% of cancer patients have been reported to have a pericardial effusion (82). Immunotherapy, such as immune checkpoint inhibitors, may lead to a pericarditis or myopericarditis (83), which, given the significant associated morbidity and mortality with this disease state (84), requires prompt and accurate diagnosis with cardiac imaging techniques.
TTE is often the initial modality used in the assessment of pericardial disease, because it allows for evaluation of hemodynamics in addition to structure and function. However, limitations in acoustic windows do not allow for imaging of the entire pericardium, and imaging of loculated effusions (85) and detection of pericardial thickening can be challenging with this modality. Pericardial thickness by TEE has been shown to correlate well with CT (86), but this modality is limited by acoustic windows and more invasive approach. Imaging with cardiac CT, as well as with CMR, allows for full imaging of the pericardium. Cardiac CT holds the advantage of a more rapid test compared with CMR, which may be better tolerated in some patients with difficulties performing the repeated breath holds and prolonged supine positioning required with CMR. Cardiac CT also allows for better visualization of pericardial calcification, which can be seen with constriction. Thickened pericardium, when associated with heart failure symptoms, has been shown to correlate with constrictive pericarditis, and enhancement of the pericardium on contrast-enhanced CT may signal inflammation (87). Additionally, contemporary cardiac CT acquisitions provide 3-dimensional imaging that allows for complete visualization of the entire pericardium, and its multiplanar reformatted images can also be utilized to assist with presurgical planning, should pericardiectomy be pursued. For isolated or associated pericardial effusions, CT attenuation measurements can characterize pericardial fluid, with values close to water likely representative of simple fluid and those above water possibly representing malignant or hemorrhagic fluid (87).
Cardiac CT and Cancer-Related Valvular Heart Disease
Key points
-
•
In regard to assessment of valvular disease, cardiac CT can provide anatomic planning for either potential percutaneous or surgical approaches for valve replacement/repair if clinically indicated.
Case 4: 57-year-old man with a neuroendocrine tumor in small intestine and carcinoid syndrome, with severe tricuspid insufficiency caused by carcinoid valve disease
He underwent a cardiac CT for valve replacement planning (Figure 5).
Figure 5.
Carcinoid Valve Disease
A 57-year-old man with a neuroendocrine tumor in small intestine and carcinoid syndrome underwent a cardiac CT for valve replacement planning. Cardiac CT showed immobile tricuspid valve leaflets widely open in ventricular systole (A). Pulmonic valve was evaluated as well and showed mild malcoaptation in diastole and mild thickening (B). CT = computed tomography.
Radiation-induced valve disease and structural imaging with cardiac CT
As noted in the previous text, radiation induced cardiovascular disease remains a well-known sequalae of thoracic radiation that can manifest as both short- and long-term cardiovascular sequelae. Depending on dosing and radiation techniques, a whole spectrum of anatomic abnormalities may be present, including valvular disease, coronary lesions, myocardial/conduction disease, and extracardiac disease. In regard to assessment of valvular disease, cardiac CT can provide precise imaging in evaluating valvular disease and concomitant coronary artery disease, and providing anatomic planning for either potential percutaneous or surgical approaches for valve replacement/repair if clinically indicated.
Although many modifications have been made to modern radiation techniques, survivors of high-risk disease states such as Hodgkin’s disease or breast cancer remain, with many asymptomatic with subclinical cardiac disease. RT was used in up to 40% of breast cancer patients as adjuvant treatment in the Surveillance, Epidemiology, and End Results registry (88). A retrospective analysis of over 2,500 Dutch Hodgkin’s survivors who underwent mediastinal RT between 1965 and 1995 reported a cumulative incidence of cardiovascular disease of approximately 50% at 40 years, with a majority of patients experiencing multiple cardiovascular events (89). Another retrospective single institution study of 415 cancer survivors treated with RT from 1962 to 1998 demonstrated that among other sequelae, 6.2% developed clinically significant valvular dysfunction at a median of 22 years, with the most common lesion being aortic stenosis (90). More modern era studies have demonstrated associated severe cardiovascular events in patients who have received RT, with newer techniques (intensity modulated RT, proton beam therapy) (91).
Cardiac CT is already indicated in the structural intervention arena, particularly in transcatheter aortic valve replacement for aortic annulus sizing, measuring coronary artery heights, and peripheral vascular measurements (92). In cancer patients with prior radiation treatments, CT imaging can visualize the whole spectrum of sequelae from radiation treatment, with minimal modifications of imaging protocols. These findings, potentially unique to the cancer population with a history of RT, include radiation induced aortic, coronary artery, myocardial, and pericardial disease, along with pulmonary fibrosis and/or peripheral vascular disease. For patients with prohibitive anatomy for transfemoral approaches, providing anatomic information for transapical/aortic/carotid approaches for minimally invasive valve repair/replacement techniques by cardiac CT can also provide invaluable noninvasive information to weigh risks and benefits of each approach.
Although transcatheter techniques for mitral valve replacement are still being developed and investigated, multiphase electrocardiography (ECG)-gated cardiac CTA can also provide anatomic assessment of mitral annular and leaflet dimensions, and prediction of LV outflow tract outflow tract obstruction post–mitral valve replacement deployment (93,94). Primary mitral valve disease can inherently develop from RT or from functional/secondary causes from cardiomyopathic states from chemoradiation treatments.
Carcinoid heart disease
Neuroendocrine tumors (NETs) arising from the midgut or bronchial system can secrete vasoactive substances such as serotonin. Metastatic disease of the liver can lead to cardiac involvement, which is the initial presentation of up to 20% of patients with carcinoid syndrome, and can affect over one-half of those with carcinoid syndrome (95). Fibrous deposition from vasoactive substances can occur predominantly in the right-sided valves (ie, tricuspid and pulmonic) leading to right-sided failure. Although these substances are typically metabolized in the pulmonary circulation, left-sided involvement can occur if there is intracardiac shunting present (ie, patent foramen ovale, atrial septal defect, bronchial location of carcinoid, or significant carcinoid tumor burden).
Cardiac CT can provide preoperative planning for patients requiring valvular surgery including coronary artery assessment, right ventricular dimensions, as well as for myocardial (carcinoid metastases) and valvular involvement (carcinoid syndrome) (Figure 5). Even though such patients can undergo surgical replacement to restore cardiac function, prosthetic valve function can potentially deteriorate with persistent carcinoid disease; cardiac CT can be used to assess mechanisms of valve dysfunction (thrombosis, carcinoid deposits) in addition to planning for percutaneous valve-in-valve approaches. Finally, somatostatin analogues labelled with radioactive substances such as Gallium-68 are avidly taken up by NETs; thus, cardiac metastases from NET can be identified by PET (96), which can be used in combination with cardiac CT.
Cardiac CT for endocarditis assessment in cancer patients (infectious and nonbacterial thrombotic)
Endocarditis can be a complication of cancer and cancer-related treatments. These patients may be more prone to nosocomial infective endocarditis (IE) (97), and a diagnosis of IE has been noted at a higher incidence in colorectal cancer patients compared with those with lung, breast, and prostate cancers, and is associated with shorter survival (98). In addition, there is a nonbacterial, marantic thrombotic phenotype of endocarditis thought to originate from the hypercoagulable state of certain malignancies; it can be associated with embolic events including cerebrovascular strokes and can contribute significantly to morbidity (99). Although TEE is considered the standard noninvasive diagnostic imaging modality, there is increasing evidence that cardiac CTA can provide complementary imaging information—aside from coronary anatomy assessment that may be required before surgery (100). In comparing the 2 imaging modalities, TEE provides higher sensitivity in detecting valvular vegetations—particularly those of smaller size—valve perforation, and intracardiac fistula compared with cardiac CTA. However, cardiac CTA may detect pseudoaneurysm and perivalvular lesions/abscesses with higher accuracy (101,102). The ESC 2015 Guidelines for the Management of Infective Endocarditis have integrated cardiac CTA into their modified criteria for the diagnosis of IE, including cardiac CTA detection of definite paravalvular lesions as a major imaging criteria for IE (103). In addition, cardiac CTA findings of pseudoaneurysm or abscess in patients undergoing surgery for IE may be independent predictors of increased mortality in long-term follow-up (104).
In summary, cardiac CT, even in its current applications in assessment of cardiac function and use in preplanning for percutaneous structural approaches, can yield important anatomic information of the cardiac sequalae of cancer treatment, NET-associated valvular disease, or visualizing complex cardiac infections; in addition to this, it can visualize both cardiac and extracardiac-related findings that can help determine a patient’s suitability for either surgical or percutaneous approaches. Future avenues to investigate our understanding of radiation-induced valvular disease could involve evaluating RT techniques in the modern era with serial imaging combined with ECG gated protocols—either for radiation treatment or for cancer staging/surveillance purposes—to evaluate the progression of valvular remodeling from radiation treatments and carcinoid disease to better understand of the historical time course of such effects.
Conclusions
Key points
-
•
Cardiac CT has diverse roles and many potential applications for the clinical care of cancer patients and survivors.
-
•
Evaluating for presence of CAC in nongated chest CT imaging from cancer staging may allow us to better risk stratify these patients.
Cardiac CT has diverse roles and many potential applications for the clinical care of cancer patients and survivors. Evaluating for presence of CAC in nongated chest CT imaging from cancer staging may allow us to better risk stratify this growing population of patients. This is a low-cost strategy that can identify patients with ASCVD, which is an important risk factor that can affect clinical outcomes in cancer patients. Many cardiovascular toxicities from cancer therapies can be differentiated from ACS by using cardiac CT. It also can be helpful in the evaluation of cardiac tumors and pericardial diseases in patients that cannot tolerate or have a contraindication to CMR. For radiation-induced valvular heart disease, cardiac CT aids in planning of structural interventions. As highlighted in this primer, there are many opportunities where cardiac CT can be used to enhance the care of cancer patients (Central Illustration). Unlike other cardiac imaging modalities, it has the unique potential to be integrated into standard serial oncology imaging. For instance, CT imaging is already frequently performed in the cancer population for staging purposes in a variety of malignancies. Refined hybrid protocols (ie, integration of ECG gating, contrast timing to involve cardiovascular specific areas of interest) may provide an unprecedented view into the cardiovascular toxicities associated with cancer biology and its treatments over time. In cancer patients with favorable prognoses and/or those requiring long-term treatments, serial imaging could be used to improve our understanding of progression of ASCVD in specific cancer populations, allowing for potentially more aggressive cardioprotective and preventative strategies to reduce short- and long-term cardiovascular events.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Machida H., Tanaka I., Fukui R., et al. Current and novel imaging techniques in coronary CT. Radiographics. 2015;35:991–1010. doi: 10.1148/rg.2015140181. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam E.A., Wenger N.K., Brindis R.G., et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Taylor A.J., Cerqueira M., Hodgson J.M., et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–1894. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Cury R.C., Abbara S., Achenbach S., et al. CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2016;10:269–281. doi: 10.1016/j.jcct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Puchner S.B., Liu T., Mayrhofer T., et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64:684–692. doi: 10.1016/j.jacc.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motoyama S., Sarai M., Harigaya H., et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 8.Newby D.E., Adamson P.D., Berry C., et al. for the SCOT-HEART Investigators Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379:924–933. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 9.Maron D.J., Hochman J.S., Reynolds H.R., et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–1407. doi: 10.1056/NEJMoa1915922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moss A.J., Williams M.C., Newby D.E., Nicol E.D. The updated NICE guidelines: cardiac CT as the first-line test for coronary artery disease. Curr Cardiovasc Imaging Rep. 2017;10:15. doi: 10.1007/s12410-017-9412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knuuti J., Wijns W., Saraste A., et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the Diagnosis and Management of Chronic Coronary Syndromes of the European Society of Cardiology (ESC) Eur Heart J. 2019;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 12.Dahal S., Budoff M.J. Low-dose ionizing radiation and cancer risk: not so easy to tell. Quant Imaging Med Surg. 2019;9:2023–2026. doi: 10.21037/qims.2019.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Einstein A.J., Henzlova M.J., Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 14.Patel A.A., Fine J., Naghavi M., Budoff M.J. Radiation exposure and coronary artery calcium scans in the society for heart attack prevention and eradication cohort. Int J Cardiovasc Imaging. 2019;35:179–183. doi: 10.1007/s10554-018-1431-0. [DOI] [PubMed] [Google Scholar]
- 15.Madaj P., Li D., Nakanishi R., et al. Lower radiation dosing in cardiac computed tomographic angiography: the CONVERGE Registry. J Nucl Med Technol. 2019;48(1):58–62. doi: 10.2967/jnmt.119.229500. [DOI] [PubMed] [Google Scholar]
- 16.Sturgeon K.M., Deng L., Bluethmann S.M., et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889–3897. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarifa A., Albittar A., Kim P.Y., et al. Cardiac toxicities of anticancer treatments: chemotherapy, targeted therapy and immunotherapy. Curr Opin Cardiol. 2019;34:441–450. doi: 10.1097/HCO.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 18.Hassan S.A., Palaskas N., Kim P., et al. Chemotherapeutic agents and the risk of ischemia and arterial thrombosis. Curr Atheroscler Rep. 2018;20:10. doi: 10.1007/s11883-018-0702-5. [DOI] [PubMed] [Google Scholar]
- 19.Drobni Z.D., Alvi R.M., Taron J., et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142(24):2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bravo-Jaimes K., Marcellon R., Varanitskaya L., et al. Opportunities for improved cardiovascular disease prevention in oncology patients. Curr Opin Cardiol. 2020;35:531–537. doi: 10.1097/HCO.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 22.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 23.McClelland R.L., Chung H., Detrano R., Post W., Kronmal R.A. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 24.McClelland R.L., Jorgensen N.W., Budoff M., et al. 10-Year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study) J Am Coll Cardiol. 2015;66:1643–1653. doi: 10.1016/j.jacc.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitlock M.C., Yeboah J., Burke G.L., Chen H., Klepin H.D., Hundley W.G. Cancer and its association with the development of coronary artery calcification: an assessment from the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie X., Zhao Y., de Bock G.H., et al. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: systematic review and meta-analysis. Circ Cardiovasc Imaging. 2013;6:514–521. doi: 10.1161/CIRCIMAGING.113.000092. [DOI] [PubMed] [Google Scholar]
- 27.Cuddy S., Payne D.L., Murphy D.J., et al. Incidental coronary artery calcification in cancer imaging. J Am Coll Cardiol CardioOnc. 2019;1:135–137. doi: 10.1016/j.jaccao.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bharadwaj A., Potts J., Mohamed M.O., et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J. 2020;41:2183–2193. doi: 10.1093/eurheartj/ehz851. [DOI] [PubMed] [Google Scholar]
- 29.Potts J.E., Iliescu C.A., Lopez Mattei J.C., et al. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. Eur Heart J. 2019;40:1790–1800. doi: 10.1093/eurheartj/ehy769. [DOI] [PubMed] [Google Scholar]
- 30.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 31.Mehta L.S., Watson K.E., Barac A., et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30–e66. doi: 10.1161/CIR.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handy C.E., Quispe R., Pinto X., et al. Synergistic opportunities in the interplay between cancer screening and cardiovascular disease risk assessment: together we are stronger. Circulation. 2018;138:727–734. doi: 10.1161/CIRCULATIONAHA.118.035516. [DOI] [PubMed] [Google Scholar]
- 33.Budoff M.J., Lutz S.M., Kinney G.L., et al. Coronary artery calcium on noncontrast thoracic computerized tomography scans and all-cause mortality. Circulation. 2018;138:2437–2438. doi: 10.1161/CIRCULATIONAHA.118.036835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bui Q.M., Daniels L.B. A review of the role of breast arterial calcification for cardiovascular risk stratification in women. Circulation. 2019;139:1094–1101. doi: 10.1161/CIRCULATIONAHA.118.038092. [DOI] [PubMed] [Google Scholar]
- 35.Emaus M.J., Išgum I., van Velzen S.G.M., et al. Bragatston study protocol: a multicentre cohort study on automated quantification of cardiovascular calcifications on radiotherapy planning CT scans for cardiovascular risk prediction in patients with breast cancer. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-028752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gal R., van Velzen S.G.M., Hooning M.J., et al. Identification of risk of cardiovascular disease by automatic quantification of coronary artery calcifications on radiotherapy planning CT scans in patients with breast cancer. JAMA Oncology. 2021;7:1024–1032. doi: 10.1001/jamaoncol.2021.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):e177–e232. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamudu H.M., Paul T.K., Veeranki S.P., Budoff M. The effects of coronary artery calcium screening on behavioral modification, risk perception, and medication adherence among asymptomatic adults: a systematic review. Atherosclerosis. 2014;236:338–350. doi: 10.1016/j.atherosclerosis.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Desai M.Y., Jellis C.L., Kotecha R., Johnston D.R., Griffin B.P. Radiation-associated cardiac disease. J Am Coll Cardiol Img. 2018;11:1132–1149. doi: 10.1016/j.jcmg.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 40.Kirkpatrick J.B. Pathogenesis of foam cell lesions in irradiated arteries. Am J Pathol. 1967;50:291–309. [PMC free article] [PubMed] [Google Scholar]
- 41.Darby S.C., Ewertz M., McGale P., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 42.Roos C.T.G., van den Bogaard V.A.B., Greuter M.J.W., et al. Is the coronary artery calcium score associated with acute coronary events in breast cancer patients treated with radiotherapy? Radiother Oncol. 2018;126:170–176. doi: 10.1016/j.radonc.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Milgrom S.A., Varghese B., Gladish G.W., et al. Coronary artery dose-volume parameters predict risk of calcification after radiation therapy. J Cardiovasc Imaging. 2019;27:268–279. doi: 10.4250/jcvi.2019.27.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armenian S.H., Lacchetti C., Barac A., et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 45.Phillips W.J., Johnson C., Law A., et al. Reporting of coronary artery calcification on chest CT studies in breast cancer patients at high risk of cancer therapy related cardiac events. Int J Cardiol Heart Vasc. 2018;18:12–16. doi: 10.1016/j.ijcha.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gernaat S.A., Išgum I., de Vos B.D., et al. Automatic coronary artery calcium scoring on radiotherapy planning CT scans of breast cancer patients: reproducibility and association with traditional cardiovascular risk factors. PloS One. 2016;11 doi: 10.1371/journal.pone.0167925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mast M.E., Heijenbrok M.W., Petoukhova A.L., Scholten A.N., Schreur J.H., Struikmans H. Preradiotherapy calcium scores of the coronary arteries in a cohort of women with early-stage breast cancer: a comparison with a cohort of healthy women. Int J Radiat Oncol Biol Phys. 2012;83:853–858. doi: 10.1016/j.ijrobp.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Yakupovich A., Davison M.A., Kharouta M.Z., et al. Heart dose and coronary artery calcification in patients receiving thoracic irradiation for lung cancer. J Thorac Dis. 2020;12:223–231. doi: 10.21037/jtd.2020.01.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marks L.B., Yu X., Prosnitz R.G., et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 50.Sioka C., Exarchopoulos T., Tasiou I., et al. Myocardial perfusion imaging with 99 mTc - tetrofosmin SPECT in breast cancer patients that received postoperative radiotherapy: a case-control study. Radiat Oncol. 2011;6:151. doi: 10.1186/1748-717X-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gayed I., Gohar S., Liao Z., McAleer M., Bassett R., Yusuf S.W. The clinical implications of myocardial perfusion abnormalities in patients with esophageal or lung cancer after chemoradiation therapy. Int J Cardiovasc Imaging. 2009;25:487–495. doi: 10.1007/s10554-009-9440-7. [DOI] [PubMed] [Google Scholar]
- 52.Lancellotti P., Nkomo V.T., Badano L.P., et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:721–740. doi: 10.1093/ehjci/jet123. [DOI] [PubMed] [Google Scholar]
- 53.Driessen R.S., Danad I., Stuijfzand W.J., et al. Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol. 2019;73:161–173. doi: 10.1016/j.jacc.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 54.Plana J.C., Galderisi M., Barac A., et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 55.Bloom M.W., Hamo C.E., Cardinale D., et al. Cancer therapy-related cardiac dysfunction and heart failure: part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D., et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: TheTask Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology ( ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 57.Herrmann J. Vascular toxic effects of cancer therapies. Nat Rev Cardiol. 2020;82:566. doi: 10.1038/s41569-020-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Budoff M.J., Dowe D., Jollis J.G., et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 59.Knuuti J., Ballo H., Juarez Orozco L.E., et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J. 2018;55:2816. doi: 10.1093/eurheartj/ehy267. [DOI] [PubMed] [Google Scholar]
- 60.Meijboom W.B., Meijs M.F.L., Schuijf J.D., et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–2144. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 61.Miller J.M., Rochitte C.E., Dewey M., et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 62.Schuetz G.M., Schlattmann P., Dewey M. Use of 3x2 tables with an intention to diagnose approach to assess clinical performance of diagnostic tests: meta-analytical evaluation of coronary CT angiography studies. BMJ. 2012;345 doi: 10.1136/bmj.e6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdelrahman K.M., Chen M.Y., Dey A.K., et al. Coronary computed tomography angiography from clinical uses to emerging technologies: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:1226–1243. doi: 10.1016/j.jacc.2020.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Achenbach S., Moselewski F., Ropers D., et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–17. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 65.Ahmadi A., Argulian E., Leipsic J., Newby D.E., Narula J. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1608–1617. doi: 10.1016/j.jacc.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Arbab-Zadeh A., Fuster V. From detecting the vulnerable plaque to managing the vulnerable patient: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1582–1593. doi: 10.1016/j.jacc.2019.07.062. [DOI] [PubMed] [Google Scholar]
- 67.Cresti A., Chiavarelli M., Glauber M., et al. Incidence rate of primary cardiac tumors: a 14-year population study. J Cardiovasc Med (Hagerstown) 2016;17:37–43. doi: 10.2459/JCM.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 68.Butany J., Nair V., Naseemuddin A., Nair G.M., Catton C., Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6:219–228. doi: 10.1016/S1470-2045(05)70093-0. [DOI] [PubMed] [Google Scholar]
- 69.Klatt E.C., Heitz D.R. Cardiac metastases. Cancer. 1990;65:1456–1459. doi: 10.1002/1097-0142(19900315)65:6<1456::aid-cncr2820650634>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 70.Patel J.K., Didolkar M.S., Pickren J.W., Moore R.H. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 71.Goldberg A.D., Blankstein R., Padera R.F. Tumors metastatic to the heart. Circulation. 2013;128:1790–1794. doi: 10.1161/CIRCULATIONAHA.112.000790. [DOI] [PubMed] [Google Scholar]
- 72.Tyebally S., Chen D., Bhattacharyya S., et al. Cardiac tumors. J Am Coll Cardiol CardioOnc. 2020;2:293–311. doi: 10.1016/j.jaccao.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bittencourt M.S., Achenbach S., Marwan M., et al. Left ventricular thrombus attenuation characterization in computed tomography angiography. J Cardiovasc Comput Tomogr. 2012;6:121–126. doi: 10.1016/j.jcct.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 74.D’Angelo E.C., Paolisso P., Vitale G., et al. Diagnostic accuracy of cardiac computed tomography and 18-F fluorodeoxyglucose positron emission tomography in cardiac masses. J Am Coll Cardiol Img. 2020;13:2400–2411. doi: 10.1016/j.jcmg.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 75.Lopez-Mattei J.C., Lu Y. Multimodality imaging in cardiac masses: to standardize recommendations, the time is now! J Am Coll Cardiol Img. 2020;13:2412–2414. doi: 10.1016/j.jcmg.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 76.Romero J., Husain S.A., Kelesidis I., Sanz J., Medina H.M., Garcia M.J. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging. 2013;6:185–194. doi: 10.1161/CIRCIMAGING.112.000153. [DOI] [PubMed] [Google Scholar]
- 77.Goldstein J.A., Schiller N.B., Lipton M.J., Ports T.A., Brundage B.H. Evaluation of left ventricular thrombi by contrast-enhanced computed tomography and two-dimensional echocardiography. Am J Cardiol. 1986;57:757–760. doi: 10.1016/0002-9149(86)90608-9. [DOI] [PubMed] [Google Scholar]
- 78.Palaskas N., Thompson K., Gladish G., et al. Evaluation and management of cardiac tumors. Curr Treat Options Cardiovasc Med. 2018;20:29. doi: 10.1007/s11936-018-0625-z. [DOI] [PubMed] [Google Scholar]
- 79.McGale P., Darby S.C., Hall P., et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100:167–175. doi: 10.1016/j.radonc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 80.Gagliardi G., Lax I., Rutqvist L.E. Partial irradiation of the heart. Semin Radiat Oncol. 2001;11:224–233. doi: 10.1053/srao.2001.23483. [DOI] [PubMed] [Google Scholar]
- 81.Wang K., Eblan M.J., Deal A.M., et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35:1387–1394. doi: 10.1200/JCO.2016.70.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghosh A.K., Crake T., Manisty C., Westwood M. Pericardial disease in cancer patients. Curr Treat Options Cardiovasc Med. 2018;20:60. doi: 10.1007/s11936-018-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Altan M., Toki M.I., Gettinger S.N., et al. Immune checkpoint inhibitor-associated pericarditis. J Thorac Oncol. 2019;14:1102–1108. doi: 10.1016/j.jtho.2019.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mahmood S.S., Fradley M.G., Cohen J.V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yousem D., Traill T.T., Wheeler P.S., Fishman E.K. Illustrative cases in pericardial effusion misdetection: correlation of echocardiography and CT. Cardiovasc Intervent Radiol. 1987;10:162–167. doi: 10.1007/BF02577994. [DOI] [PubMed] [Google Scholar]
- 86.Ling L.H., Oh J.K., Tei C., et al. Pericardial thickness measured with transesophageal echocardiography: feasibility and potential clinical usefulness. J Am Coll Cardiol. 1997;29:1317–1323. doi: 10.1016/s0735-1097(97)82756-8. [DOI] [PubMed] [Google Scholar]
- 87.Wang Z.J., Reddy G.P., Gotway M.B., Yeh B.M., Hetts S.W., Higgins C.B. CT and MR imaging of pericardial disease. Radiographics. 2003;23:S167–S180. doi: 10.1148/rg.23si035504. [DOI] [PubMed] [Google Scholar]
- 88.Darby S.C., McGale P., Taylor C.W., Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 89.van Nimwegen F.A., Schaapveld M., Janus C.P., et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 90.Hull M.C., Morris C.G., Pepine C.J., Mendenhall N.P. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 91.Wang X., Palaskas N.L., Yusuf S.W., et al. Incidence and onset of severe cardiac events after radiotherapy for esophageal cancer. J Thorac Oncol. 2020;15:1682–1690. doi: 10.1016/j.jtho.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Achenbach S., Delgado V., Hausleiter J., Schoenhagen P., Min J.K., Leipsic J.A. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve replacement (TAVR)/transcatheter aortic valve replacement (TAVR) J Cardiovasc Comput Tomogr. 2012;6:366–380. doi: 10.1016/j.jcct.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 93.Blanke P., Dvir D., Cheung A., et al. A simplified D-shaped model of the mitral annulus to facilitate CT-based sizing before transcatheter mitral valve implantation. J Cardiovasc Comput Tomogr. 2014;8:459–467. doi: 10.1016/j.jcct.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Banks T., Razeghi O., Ntalas I., et al. Automated quantification of mitral valve geometry on multi-slice computed tomography in patients with dilated cardiomyopathy - Implications for transcatheter mitral valve replacement. J Cardiovasc Comput Tomogr. 2018;12:329–337. doi: 10.1016/j.jcct.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 95.Hassan S.A., Palaskas N.L., Agha A.M., et al. Carcinoid heart disease: a comprehensive review. Curr Cardiol Rep. 2019;21:140. doi: 10.1007/s11886-019-1207-8. [DOI] [PubMed] [Google Scholar]
- 96.Agha A.M., Lopez-Mattei J., Donisan T., et al. Multimodality imaging in carcinoid heart disease. Open Heart. 2019;6 doi: 10.1136/openhrt-2019-001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim K., Kim D., Lee S.E., et al. Infective endocarditis in cancer patients- causative organisms, predisposing procedures, and prognosis differ from infective endocarditis in non-cancer patients. Circ J. 2019;83:452–460. doi: 10.1253/circj.CJ-18-0609. [DOI] [PubMed] [Google Scholar]
- 98.García-Albéniz X., Hsu J., Lipsitch M., Logan R.W., Hernández-Díaz S., Hernán M.A. Infective endocarditis and cancer in the elderly. Eur J Epidemiol. 2016;31:41–49. doi: 10.1007/s10654-015-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.el-Shami K., Griffiths E., Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007;12:518–523. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 100.Khalique O.K., Veillet-Chowdhury M., Choi A.D., Feuchtner G., Lopez-Mattei J. Cardiac computed tomography in the contemporary evaluation of infective endocarditis. J Cardiovasc Comput Tomogr. 2021;15(4):304–312. doi: 10.1016/j.jcct.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 101.Kim I.C., Chang S., Hong G.R., et al. Comparison of cardiac computed tomography with transesophageal echocardiography for identifying vegetation and intracardiac complications in patients with infective endocarditis in the era of 3-dimensional images. Circ Cardiovasc Imaging. 2018;11 doi: 10.1161/CIRCIMAGING.117.006986. [DOI] [PubMed] [Google Scholar]
- 102.Sifaoui I., Oliver L., Tacher V., et al. Diagnostic performance of transesophageal echocardiography and cardiac computed tomography in infective endocarditis. J Am Soc Echocardiogr. 2020;33(12):1442–1453. doi: 10.1016/j.echo.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 103.Habib G., Lancellotti P., Antunes M.J., et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 104.Wang T.K.M., Saeedan M.B., Chan N., et al. Complementary diagnostic and prognostic contributions of cardiac computed tomography for infective endocarditis surgery. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.120.011126. [DOI] [PubMed] [Google Scholar]
- 105.Lestuzzi C., Tratuferi L., Viel E., Buonadonna A., Vaccher E., Berretta M. Fluoropyrimidine-associated cardiotoxicity: probably not so rare as it seems. Oncologist. 2020;25(8) doi: 10.1634/theoncologist.2020-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Polk A., Vaage-Nilsen M., Vistisen K., Nielsen D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev. 2013;39:974–984. doi: 10.1016/j.ctrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 107.Raber I., Warack S., Kanduri J., et al. Fluoropyrimidine-associated cardiotoxicity: a retrospective case-control study. Oncologist. 2020;25:e606–e609. doi: 10.1634/theoncologist.2019-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Virani S.A., Dent S., Brezden-Masley C., et al. Canadian Cardiovascular Society guidelines for evaluation and management of cardiovascular complications of cancer therapy. The. Can J Cardiol. 2016:831–841. doi: 10.1016/j.cjca.2016.02.078. [DOI] [PubMed] [Google Scholar]
- 109.Gemici G., Cinçin A., Değertekin M., Oktay A. Paclitaxel-induced ST-segment elevations. Clin Cardiol. 2009;32:E94–E96. doi: 10.1002/clc.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bonaca M.P., Olenchock B.A., Salem J.-E., et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnson D.B., Balko J.M., Compton M.L., et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kociol R.D., Cooper L.T., Fang J.C., et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141(6):e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 113.Rikhi R., Karnuta J., Hussain M., et al. Immune checkpoint inhibitors mediated lymphocytic and giant cell myocarditis: uncovering etiological mechanisms. Front Cardiovasc Med. 2021;8:721333. doi: 10.3389/fcvm.2021.721333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 115.Moslehi J.J., Salem J.-E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang L., Zlotoff D.A., Awadalla M., et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141:2031–2034. doi: 10.1161/CIRCULATIONAHA.119.044703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Drobni Z.D., Alvi R.M., Taron J., et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;29:84. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen X.-L., Lei Y.-H., Liu C.-F., et al. Angiogenesis inhibitor bevacizumab increases the risk of ischemic heart disease associated with chemotherapy: a meta-analysis. PLOS ONE. 2013;8 doi: 10.1371/journal.pone.0066721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Choueiri T.K., Schutz F.A.B., Je Y., Rosenberg J.E., Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–2285. doi: 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- 120.Qi W.-X., Shen Z., Tang L.-N., Yao Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: an up-to-date meta-analysis. Crit Rev Oncol Hematol. 2014;92:71–82. doi: 10.1016/j.critrevonc.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 121.Ranpura V., Hapani S., Chuang J., Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncologica (Stockholm, Sweden) 2010;49:287–297. doi: 10.3109/02841860903524396. [DOI] [PubMed] [Google Scholar]
- 122.Schutz F.A.B., Je Y., Azzi G.R., Nguyen P.L., Choueiri T.K. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22:1404–1412. doi: 10.1093/annonc/mdq587. [DOI] [PubMed] [Google Scholar]