ABSTRACT
Chronic kidney disease-associated pruritus (CKD-aP) is a potentially distressing condition that affects a significant proportion of patients with end-stage kidney disease undergoing dialysis. CKD-aP may lead to worsening of patients’ physical and mental health-related quality of life (HRQoL) and has also been linked with worse clinical outcomes, including increased mortality. Despite these detrimental effects, evidence from real-world studies shows that CKD-aP still remains overlooked by nephrologists and underreported by patients in clinical practice. Itch is subjective and therefore its diagnosis is often dependent on patients reporting this symptom. There is an opportunity to reduce the burden of CKD-aP on dialysis patients by increasing awareness about this condition and the availability of effective treatments. It is particularly important that nephrologists and other healthcare providers routinely ask their patients if they are experiencing itch. The differential diagnosis of CKD-aP requires a step-by-step identification and exclusion of possible alternative or concomitant causes of itch. Several simple validated self-reported assessment scales are available to evaluate the presence and severity of itch in a time-efficient manner, making them suitable for use in everyday clinical practice. The impact of CKD-aP on haemodialysis patients’ HRQoL should also be assessed on a regular basis. This review provides a comprehensive overview of the differential diagnosis of CKD-aP and the diagnostic tools that are available to identify itch and quantify its severity and impact on patient HRQoL. A suggested algorithm to guide the screening, diagnosis and assessment of CKD-aP among dialysis patients in real-world practice is provided.
Keywords: chronic pruritus, dialysis, end-stage kidney disease, itch, diagnostic algorithm, patient-reported outcomes, quality of life, uraemic pruritus, uraemic syndrome
INTRODUCTION
Chronic pruritus is defined as an itch persisting for >6 weeks [1]. It may involve the entire skin (generalized pruritus) or only particular areas (localized pruritus). Chronic pruritus is associated with a markedly reduced quality of life (QoL) and has been shown to be as debilitating as chronic pain [2]. Disturbed sleep patterns, anxiety and depression are common and may exacerbate the itching itself. One of the leading causes of chronic pruritus is chronic kidney disease-associated pruritus (CKD-aP). CKD-aP has been recognized for over a century and, before dialysis became available, was known to occur in ˂30% of patients with renal failure. In the early haemodialysis (HD) era, due to increased survival in patients with end-stage kidney disease (ESKD), the prevalence of CKD-aP was nearly 100%; thereafter, it decreased due to improved HD techniques and efficacy [3]. Recently, the prevalence of CKD-aP in HD patients has been estimated to be ∼40% [4], whereas a study of patients with CKD Stage 5 who would have otherwise been on dialysis, but were managed conservatively, reported the overall prevalence of pruritus as 74%, with 32% of these patients describing the symptom as ‘quite distressing’ or ‘very distressing’, consistent with prior findings of high prevalence of pruritus among patients with ESKD [5].
Despite the decrease in the prevalence of severe CKD-aP, itch is still a major problem affecting the QoL of dialysis patients. However, it has emphasized that until recently, studies estimating the prevalence, intensity and duration of CKD-aP were scarce, partly because nephrologists’ perception of the problem was inadequate, with itch considered a lower priority compared with the other clinical problems of patients with ESKD. Consequently, there is still no consensus about the aetiopathogenesis, differential diagnosis and treatment of itch as a symptom. The recent epidemiological study from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Phases IV–VI [4] confirmed the previous observation of increased mortality and marked deterioration in QoL in patients with CKD-aP; therefore, it is relevant to monitor and treat this condition.
The aim of this review is to provide a comprehensive overview of the chronic itch differential diagnosis, the diagnostic tools that can be used to identify patients with CKD-aP, and how to quantify its severity and impact on QoL.
DIAGNOSIS OF CKD-aP
Chronic pruritus definition and classification
Itch persisting for >6 weeks is defined as chronic pruritus, which has generally been classified into ‘dermatological’, ‘systemic’, ‘neurological’, ‘somatoform’ and ‘mixed origin’ [1].
Dermatological chronic pruritus. First, a careful examination of the skin to exclude primary lesions must be performed. In some patients (e.g. those with scabies, pemphigoid or dermatitis herpetiformis), primary lesions may be masked by secondary changes or, conversely, secondary lesions (e.g. excoriations, non-specific dermatitis, prurigo nodularis and lichen simplex chronicus) may lead to suspicion of a dermatological cause while an underlying cause should be investigated [6]. Consequently, to avoid misdiagnosis by the nephrologist, an initial assessment by the dermatologist should ideally be performed to exclude possible dermatological origins of itching.
Systemic chronic pruritus. CKD-aP is regarded as a systemic condition with a non-dermatological cause. Other systemic causes of chronic pruritus include hepato-biliary diseases (cholestatic pruritus), metabolic or endocrine diseases (such as diabetes mellitus, hyper- and hypothyroidism, iron deficiency and hyperparathyroidism), infective diseases and haematological diseases [1].
Neurological chronic pruritus. This is a peculiar condition often driven by entrapment syndromes of specific peripheral nerves, such as notalgia paraesthetica or brachioradial pruritus. Rarely, neurological chronic pruritus is the expression of central nervous system space-occupying lesions or neurodegenerative disorders such as multiple sclerosis. However, pruritus is documented in several systemic diseases associated with small-fibre neuropathy (e.g. diabetes) [7, 8].
Somatoform chronic pruritus. This is determined by delusional disorders, anxiety or depression. It is generally challenging to treat, either for a dermatologist or for a psychiatrist [9]. However, persistent and intense chronic pruritus may contribute to other psychiatric conditions like depression, insomnia and anxiety, and in some forms of intractable chronic pruritus, it may be unclear whether ‘the chicken or the egg comes first’.
Mixed chronic pruritus. Several patients with CKD-aP are better reclassified into this group. First, skin xerosis is a very frequent pattern in dialysis patients, and may contribute towards the appearance of CKD-aP [1]; moreover, it should be noted that it would be difficult to diagnose a patient with CKD-aP in the absence of other comorbid conditions that may also lead to itch, such as hyperparathyroidism, iron deficiency or Type II diabetes mellitus. Chronic pruritus is associated with small-fibre neuropathy in several systemic diseases, such as diabetes or uraemic neuropathy.
Characteristics of CKD-aP
CKD-aP has no characteristics of intensity, duration, onset or localization that would help to make a definite diagnosis [10]. Up to 50% of patients with CKD-aP complain about generalized pruritus. In the remaining patients, CKD-aP appears to predominantly affect the back, face and shunt arm. In ∼25% of patients, pruritus is reported as most severe during or immediately after dialysis. Moreover, CKD-aP often worsens at night compared with daytime [11, 12]. Once patients have developed CKD-aP, this symptom will in most cases last for months or years [13].
CKD-aP occurs in the setting of a complex metabolic environment and its causes appear to be systemic in nature. However, it seems reasonable to hypothesize that more than just one pruritogen can predispose patients to CKD-aP, and these include the concomitant presence of advanced age, diabetes mellitus, iron deficiency, anaemia, intrahepatic cholestasis, along with hepatitis B and C virus infections (which are frequent among patients on long-term HD), direct or indirect pruritic effects of uraemic toxins and metabolic derangements. This is probably why every attempt to find a single cause has failed so far [14]. However, some risk factors seem to be associated; e.g. CKD–mineral and bone disorder (CKD–MBD) is not unequivocally identified to be associated with pruritus, but Narita et al. [15] found that hypercalcaemia and hyperphosphataemia are associated with severe pruritus, and hypocalcaemia and low parathyroid hormone reduced the risk for CKD-aP. Similarly, an early analysis of the DOPPS I and II cohorts [16] confirmed 1.5-fold greater odds of pruritus in those with a calcium–phosphorus product >80 mg2/dL2. Several studies have documented an association between dialysis vintage and pruritus, but the results have been inconsistent. The study by Narita et al. [15] observed longer mean dialysis vintage among HD patients with severe pruritus versus mild pruritus (133 versus 118.8 months), while DOPPS showed a lower risk of moderate-to-severe pruritus among HD patients who were new to dialysis (ESKD ≤3 months) as well as those who had been receiving dialysis long-term (>10 years) [16].
Comorbidities such as diabetes mellitus, lung disease, smoking, hypertension, higher body mass index, elevated white cell count, lower haemoglobin and lower albumin were found to be associated with itch [16–18]. However, recent data from DOPPS IV–VI refuted both the association with CKD–MBD and the correlation with dialysis age, but identified a higher risk in patients with an older age, a greater comorbidity burden and a central venous catheter as HD venous access [4].
Step-by-step diagnosis
An attempt to standardize the diagnostic pathway towards the diagnosis of CKD-aP requires a step-by-step exclusion of all possible alternative or concomitant diagnoses.
Dermatological evaluation. It is necessary to exclude itch due to a dermatologic disease. The skin of patients with ESKD is usually atrophic and dry [19], but a correlation between xerosis and CKD-aP has not been clearly demonstrated; skin xerosis may act as a cofactor in promoting pruritus. Xerosis and hypohidrosis are often a consequence of reduced sweat secretion due to sympathetic nerve damage [14] and are therefore suggestive of dysautonomia. Nephrogenic systemic fibrosis, a rare cutaneous manifestation associated with gadolinium administration in patients with ESKD, is extremely itchy. Conversely, other specific ESKD-related skin abnormalities, such as perforating disorders, calcifying diseases (e.g. calciphylaxis), bullous dermatoses and foot ulcers, are frequent in HD patients and can assume a chronic course: these are often itchy but also frequently associated with pain.
Patient history. A history of pre-existing skin diseases in the patient history is crucial, especially if pruritus on primarily inflamed skin is assumed. For patients with CKD in particular, a possible active concomitant rheumatological disease with cutaneous involvement (e.g. systemic lupus erythematosus, scleroderma and vasculitis) must be ruled out. The presence of hepatic disease and malignancies must be considered [1]. Drug-induced pruritus without visible skin lesions accounts for ∼5% of adverse cutaneous reactions [20, 21]. Almost any drug may induce pruritus by various pathomechanisms, and therapies introduced in the past 12 months must be evaluated [1]; the more commonly prescribed drugs in patients with CKD that would be involved in chronic pruritus are some antihypertensive agents (angiotensin-converting enzyme inhibitors, clonidine and calcium antagonists), beta blockers, diuretics and allopurinol. A relevant role is played by mu-agonists opioids (e.g. morphine); they induce itch through the activation of mu-receptors. Curiously, the new drugs for CKD-aP are opioids that selectively stimulate kappa-receptors and/or inhibit mu-receptors, thus determining an itch inhibitory imbalance between the different receptors [22, 23]. A replacement of the suspected drug for a period of at least 2 weeks may be recommended to exclude a possible relationship in pruritus persistence.
Time to pruritus onset. A temporal relationship between the onset of itch and CKD or ESKD is highly significant in the diagnosis of CKD-aP. It is rare for a patient who has chronic itch prior to their diagnosis of CKD to be diagnosed with CKD-aP.
Laboratory screening. To exclude other possible aetiologies of chronic pruritus or to evaluate the potential burden of uraemic metabolic derangements, it is advisable to monitor blood cell count (particularly red blood cells and eosinophils), erythrocyte sedimentation rate; liver enzymes (transaminases, alkaline phosphatase and gamma-glutamyltransferase), thyroid-stimulating hormone, ferritin, C-reactive protein, hepatitis serology, serum cholinesterases, serum bile acids, dialysis efficiency (Kt/V) and calcium–phosphorus product.
CKD-aP: from assessment scales to QoL
Because pruritus is a subjective feeling, the objective measurement of its intensity still remains elusive. The assessment of CKD-aP is, to date, based solely on patient reports. At present, the only way to assess the presence, intensity, course and response to treatment of patients with CKD-aP remains patient-reported outcomes (PROs). PROs are defined as ‘any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else’ [24].
There is a range of PROs for pruritus evaluation, but none can be considered as standard. Moreover, most of the published literature on PROs in CKD-aP is designed for clinical studies and not for everyday use with the patient in clinical practice. Therefore, it is of primary importance to know which PROs have had a wider use in research on CKD-aP in order to apply them in clinical practice and to better describe a disease that is still underreported, and whose prognostic relevance was only recently ascertained. A list of the more commonly used and validated scales for CKD-aP is reported in Table 1.
Table 1.
More relevant PROs validated for use in the assessment of CKD-aP
| PRO | Symptoms recalling | Strengths/limitations | |
|---|---|---|---|
| Unidimensional | |||
| VAS | Worst itch in the previous 24 h | Simple and fast/highly subjective | |
| NRS | Worst itch in the previous 24 h | Simple and fast/highly subjective | |
| VRS | Worst itch in the previous 24 h | Simple and fast/highly subjective | |
| Q20 KDQOL-SF | Evaluation of the last 4 weeks | Used in large studies/not validated as a severity measure | |
| Multidimensional | |||
| 5D itch scale | 14 days | Validated to analyse the course of itch/time-consuming | |
| Skindex-10 | 7 days | Validated to evaluate CKD-aP intensity/time-consuming | |
| Dedicated to evaluating sleep | |||
| SADS | At administration | Fast/less used in studies | |
| Itch MOS | Previous week | Assesses exclusively sleep disturbance |
Subjective severity scales
Four scales are commonly used to measure itching severity: the Visual Analogue Scale (VAS), the Numeric Rating Scale (NRS), the Verbal Rating Scale (VRS) and a question from the Kidney Disease QoL-Short Form (KDQOL-SF).
The VAS is one of the most frequently used methods of pruritus severity assessment, because it provides an easy and rapid estimation of itch. The VAS, first developed in 1921 by Hayes and Patterson [25], developed originally to assess the intensity of pain, but since 1996 it has also been adopting in clinical studies for CKD-aP evaluation [26–28]; it validated for chronic pruritus in 2012.
VAS depicts a horizontal or vertical line, generally 100 mm in length, in which the extreme left represents no itching and the extreme right the worst itching imaginable. The patient is asked to draw a vertical line marking the subjective sensation of itch disturbance. The length from the left end to the vertical mark made by the patient is measured in millimetres. Separation of the scale into one-hundredths is regarded as sensitive [29].
The NRS is a similar scale, and it was originally validated for pain. It grades itching severity on a numerical scale from 0 to 10. The decimal separation is considered less sensitive but it was shown to be adequate to evaluate the efficacy of a treatment for itch [29].
The VRS includes five itching severities: no, low, moderate, severe and extremely severe [30]. A study recommended that the cut-off values for both the VAS and the NRS are converted into VRS values of 3–7–9 (i.e. >0 to <3 points represents mild pruritus, ≥3 to <7 points moderate pruritus, ≥7 to <9 severe pruritus and ≥9 points very severe pruritus) [29]. Taking into account this conversion from VAS/NRS to VRS, the scales showed good convergence, content validity and good test–retest reproducibility, as well as responsiveness to change in itch assessment.
The 24-h Worst Itching Intensity NRS (WI-NRS) is a validated version of the NRS in which patients grade the overall severity of the worst level of their itching in the previous 24 h. The European Network on Assessment of Severity and Burden of Pruritus recently has validated both the 24-h WI-VAS/NRS/VRS and the 24-h average itching intensity VAS/NRS/VRS in different languages and in different dermatoses [30] (Figure 1).
FIGURE 1:
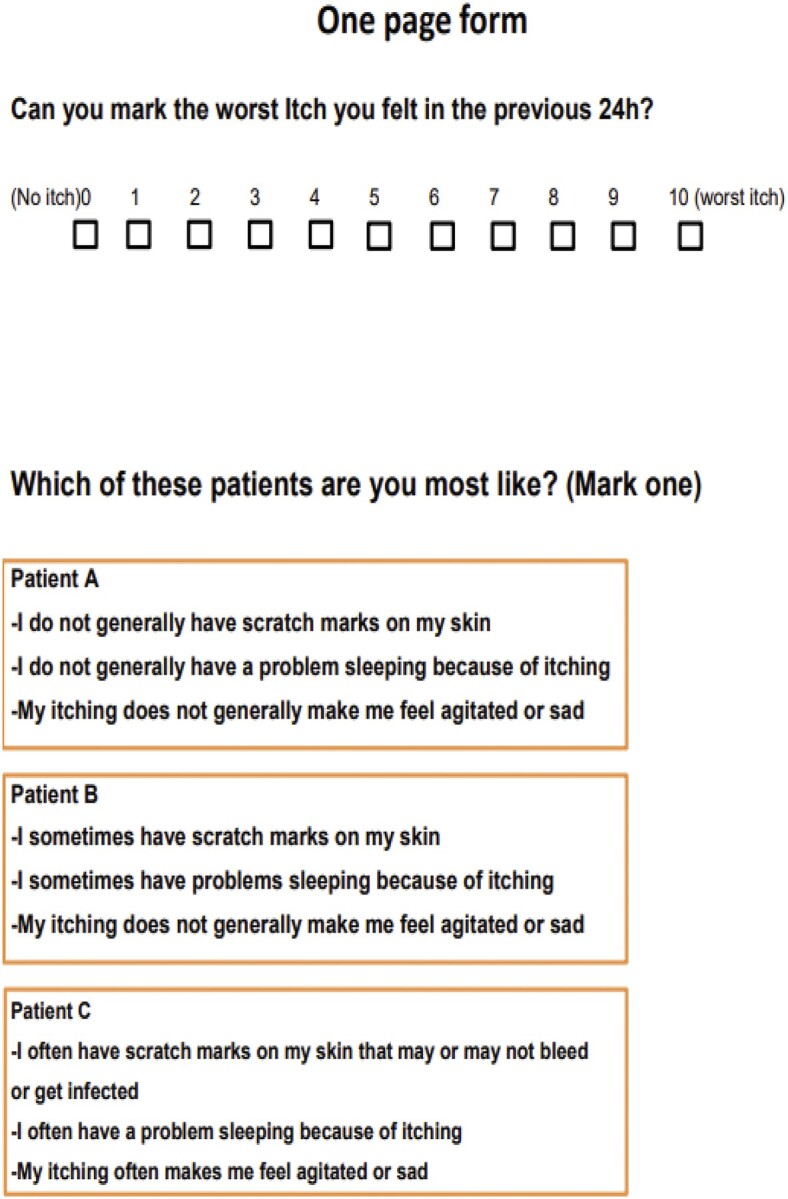
Suggested assessment scales for measuring itch severity.
Mathur et al. [13] showed that the WI-VAS and WI-NRS results correlated highly and in a reproducible manner over time in CKD-aP. Moreover, this study confirmed the impressive fluctuation and cyclicity of CKD-aP, which is the leading cause of some placebo-controlled trial failures, but pruritus rarely disappeared if the baseline WI-VAS was >40 mm.
The KDQOL-SF includes 43 kidney disease-specific questions and the 36-item short-form health survey [31]. Originally designed to test QoL in dialysis patients, this survey includes a question (question 20) that evaluates itching in the past 4 weeks: ‘During the past 4 weeks, to what extent were you bothered by: itchy skin?’ Choices include: (i) not at all bothered; (ii) somewhat bothered; (iii) moderately bothered; (iv) very much bothered; and (v) extremely bothered. This question has been the basis of the largest international studies of CKD-aP prevalence in dialysis [12, 16], which documented the prevalence of pruritus over the past 20 years.
Multidimensional scales and QoL scales
Subjective severity scales do not take into account other aspects of pruritus, such as the relative impact of pruritus on QoL. Some patients also have difficulty translating a subjective symptom, such as pruritus, into a point on a line or a number. Therefore, multidimensional itching scales try to answer to these questions. The most commonly used multidimensional itching scales are the 5D itch scale and the Itch Severity Scale (ISS) [32, 33].
The 5D itch scale was developed as a brief instrument sensitive to change over time. The scale assesses duration, degree, direction, distribution and disability associated with itching in the prior 2 weeks. The total score ranges between a minimum of 5 points (no itching) and maximum of 25 points (maximum severity). The duration, degree, direction and disability were scored from 1 to 5 points. The score for the disability dimension, with four subsections (sleep, social/leisure, housework/errands and work/school) was obtained by taking the highest score on any of the four items. The score for distribution was obtained by examining 16 body regions according to the number of affected body parts, with a maximum score of 5 points. The 5D itch scale was validated in a study involving 234 patients with chronic pruritus, of whom 36 (15%) were patients with CKD-aP [32]. The 5D itch scale score correlated strongly with the VAS score and was able to detect significant changes in pruritus over the 6-week follow-up period.
The ISS measures duration, frequency, pattern, intensity, treatment, symptoms, sensation and effect of itching on QoL. It is a valid and reliable measure of itching [33]. However, the ISS was not included among the list of important validated symptoms scales for CKD-aP [34].
The seminal study by Mathur et al. [13] compared the VAS and NRS with some novel multidimensional scales for patients with CKD-aP: the Skindex-10, the Brief Itching Inventory, the Self-Assessed Disease Severity (SADS) and an adapted sleep survey from the Medical Outcomes Study (MOS): the Itch MOS.
The Skindex-10 is a shorter version of the widely used and previously validated Skindex-16, which recalls symptoms from the previous week; the total score was the sums of disease, mood/emotional distress and social functioning domains. Each question was graded in intensity from 0 to 6.
It is important to evaluate the impact of CKD-aP on sleep disturbance. The Itch MOS was used by Mathur et al. [13] to test the frequency of various aspects of pruritus-related sleep disruption over the preceding week. This instrument includes 10 questions evaluating the effect of itching on sleep latency, disruption and daytime somnolence.
The total scores obtained from 5D itch scale, Skindex-10 and Itch MOS have recently been used in two relevant clinical studies evaluating treatment outcomes among patients with moderate to severe CKD-aP who were treated with a new drug, difelikefalin [35, 36]. The studies showed a good correlation between changes in these scales and the WI-NRS, reconfirming the observation from previous studies [13].
SADS allows patients to categorize themselves into one of three types of patient (A, B or C), depending on severity of concomitant signs and symptoms, ranging from type A (without CKD-aP) to type C (deeply bothered by itch, with scratching lesion and sleep disturbances) (Figure 1). In the study by Mathur et al. [13], SADS measurement was highly associated with all measures of health-related QoL and sleep disturbance, indicating it would be a simple way to identify patients’ perceptions. In detail, it was shown that type B of SADS correlates with a significant increase in depressive symptoms and that subjects in type C had up to 200% more antidepressant use. It was also estimated that patients in type C lost ∼2 h of sleep the night before the test [13]. Taking these results into account, and due to its ease of handling when compared with the 5D itch scale and Skindex-10, SADS would be a valid choice, in association with subjective severity scales, to monitor the multidimensional impact of CKD-aP on patient QoL and response to therapy in real-life practice.
Finally, most studies evaluating the effect of CKD-aP on QoL have used the 36-item short-form health survey, the 12-item short-form healthy survey or the previously mentioned KDQOL-SF. All the versions of the short-form are well validated and universally used; in particular, the KDQOL-SF was used to evaluate severity of self-reported itch among patients in the DOPPS studies [12, 16], which documented higher mortality (17%), worse QoL, more depression and poor sleep among patients who report pruritus.
Recently, other scales for a global assessment of pruritus have been developed and are being validated. However, few of these have included patients with CKD-aP [37].
CKD-aP assessment and routine clinical practice
As described previously, CKD-aP can be distressing and for many HD patients can lead to a significant deterioration in QoL. Although pruritus has been recognized as a consequence of uraemia, until recently, few advances have been made in characterizing its aetiopathogenetic and clinical characteristics and in the development of new treatments for this condition. Moreover, a study has recently found that ‘itching in dialysis patients’ was identified by patients as one of the top three research priorities in HD treatment and ESKD [38–41].
It was initially believed that CKD-aP was closely related to uraemia and that, consequently, improving the dialysis efficiency, altered calcium–phosphorus metabolism or anaemia would lead to symptom control. Recently, nephrologists have realized that CKD-aP had a more complex aetiopathogenesis, which cannot be managed solely with dialysis-related interventions. An analysis of the large international DOPPS cohort study (1996–2004) [16] marked a turning point in the awareness of the prognostic relevance of CKD-aP, by documenting a 17% increased mortality risk in HD patients with CKD-aP, which was attributed to the associated poor sleep quality. Subsequent DOPPS analyses (2009–18) [4, 12] confirmed these findings, with the observation that HD patients with severe pruritus had a significantly greater risk of overall mortality, compared with patients not bothered by pruritus. Furthermore, increasing pruritus severity was associated with progressive deterioration of physical and mental QoL (including depression and sleep quality) and longer post-dialysis recovery time even after adjustment for sleep quality. Furthermore, a study by Rayner et al. [12] asked the medical directors of HD facilities involved in the DOPPS about their awareness of CKD-aP; the report documented that CKD-aP was underestimated in ∼70% of the dialysis facilities and that 17% of patients did not report an itch and 18% were not receiving treatment for their itching. Additionally, medical directors still considered CKD-aP a symptom that can be resolved by increasing the dialysis dose or improving calcium–phosphorus control, reflecting the lack of awareness that these approaches are insufficient to resolve CKD-aP in 20–40% of cases [12].
Because there is a major opportunity to improve the health and wellbeing of patients on HD through increased awareness of pruritus and availability of effective treatments, it is crucial that nephrologists start enquiring routinely about itching.
The use of assessment scales and instruments to verify the persistence, severity and characteristics of CKD-aP before and after treatments are introduced should be mandatory, and in a daily clinical setting, a simple and reliable method of measuring itch intensity is highly desirable. As shown in Table 1, several PRO scales are available for the assessment of pruritus intensity including unidimensional and multidimensional measures, which also assess the impact of itch on QoL, sleep disturbance, anxiety and depression [37]. However, multidimensional scales are validated mainly for research purposes, and ‘real-life’ use is not feasible due to their length and complexity, and the amount of time required to complete them.
FUTURE DIRECTIONS
The introduction of measures to screen for the presence or absence of pruritus in patients with CKD in clinical routine is necessary to understand the real extent of the problem and to provide fundamental information to initiate a treatment and verify its effectiveness. Because nephrologists periodically check for renal anaemia, CKD–MBD indices and Kt/V, a periodic evaluation to monitor for the presence of CKD-aP and changes in itch severity over time should also be introduced in clinical practice.
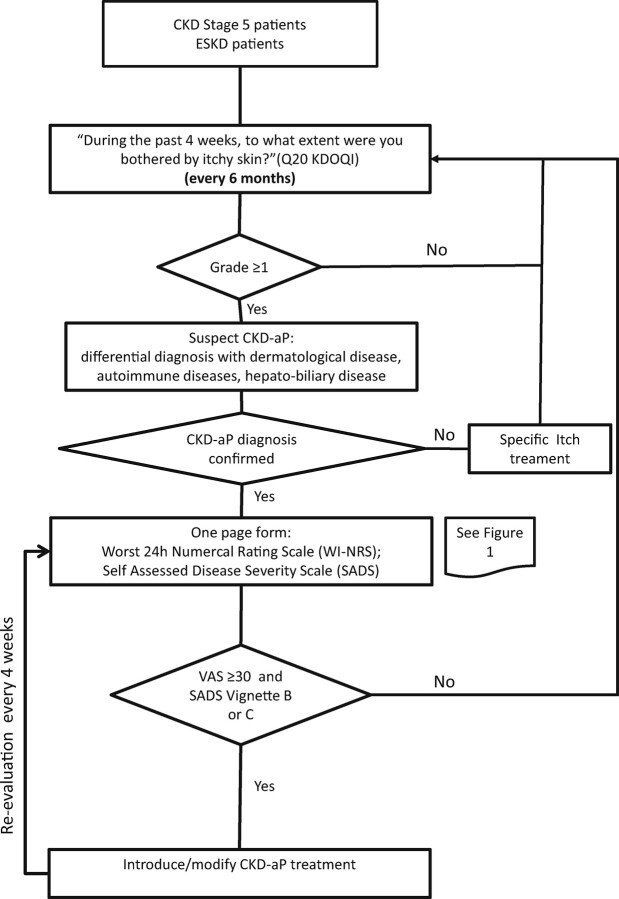
A possible way to improve the detection and assessment of CKD-aP in real-life practice is the use of a simple algorithm (Figure 2). Patients are initially screened for CKD-aP every 6 months using the single itch question from (KDQOL-SF). After CKD-aP diagnosis is confirmed, assessment of itch severity can be performed using a simple validated pruritus severity scale, such as the WI-NRS and the SADS [13], which are periodically administered every 4 weeks. These tests are simple and not time-consuming, allowing their use in a ‘real-life’ scenario. This strategy would address a series of unmet needs in CKD-aP. First, it can help overcome the communication gap between patients and the medical and paramedical staff in the HD clinic, allowing identification of patients not reporting CKD-aP or discouraged by the supposed lack of therapeutic options [12]. Secondly, the severity of pruritus would be monitored over time, helping to guide a treatment strategy based on the patient’s symptom severity.
FIGURE 2:
Suggested algorithm for screening, diagnosis and assessment of CKD-aP.
Another possible strategy to monitor the course of CKD-aP derives from the new and improved actigraphy techniques; recently, the medical community has realized the convenience and computing power of smart devices to learn more about various medical conditions, and pruritus researchers have joined this technological revolution by modernizing actigraphy into a smartwatch. This technique was validated in two proof-of-concept studies involving patients with atopic dermatitis, but further research will be required to evaluate its utility in CKD-aP [42].
CONCLUSIONS
Although the presence of CKD-aP among uraemic patients has long been recognized, this persistent and distressing symptom is frequently overlooked because of its subjective nature and the absence, until recently, of targeted therapies. The observation that CKD-aP is associated with increased morbidity and mortality in dialysis patients, together with the availability of novel targeted therapies, makes it necessary to introduce processes for correct differential diagnosis and simple subjective CKD-aP measurement tools in clinical practice. The routine use of validated PROs together with the availability of new tools to detect itching could provide long-term answers relating to the persistence and severity of CKD-aP over time and ultimately determine the effectiveness of new targeted treatments.
ACKNOWLEDGEMENTS
Editorial assistance was provided by AXON Communications (London, UK), and funded by Vifor Fresenius Medical Care Renal Pharma.
Contributor Information
Lucio Manenti, Nephrology Unit, Parma University Hospital, Parma, Italy.
Emanuela Leuci, Department of Mental Health and Pathological Addiction, Azienda USL di Parma, Parma, Italy.
FUNDING
This article is part of a supplement supported by Vifor Pharma without any influence on its content.
CONFLICT OF INTEREST STATEMENT
L.M. has received honoraria from Vifor Fresenius Medical Care Renal Pharma, Galderma Pharma and GSK Plc, participating in advisory boards. E.L. reports no conflicts of interest.
REFERENCES
- 1. Ständer S, Weisshaar E, Mettang T et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol 2007; 87: 291–294 [DOI] [PubMed] [Google Scholar]
- 2. Kini SP, DeLong LK, Veledar E et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol 2011; 147: 1153–1156 [DOI] [PubMed] [Google Scholar]
- 3. Masi CM, Cohen EP. Dialysis efficacy and itching in renal failure. Nephron 1992; 62: 257–261 [DOI] [PubMed] [Google Scholar]
- 4. Sukul N, Karaboyas A, Csomor PA et al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med 2021; 3: 42–53.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murtagh FE, Addington-Hall JM, Edmonds PM et al. Symptoms in advanced renal disease: a cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med 2007; 10: 1266–1276 [DOI] [PubMed] [Google Scholar]
- 6. Pereira MP, Steinke S, Zeidler C et al. ; EADV Task Force Pruritus Group Members. European academy of dermatology and venereology European prurigo project: expert consensus on the definition, classification and terminology of chronic prurigo. J Eur Acad Dermatol Venereol 2018; 32: 1059–1065 [DOI] [PubMed] [Google Scholar]
- 7. Brenaut E, Marcorelles P, Genestet S et al. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol 2015; 72: 328–332 [DOI] [PubMed] [Google Scholar]
- 8. Zakrzewska-Pniewska B, Jedras M. Is pruritus in chronic uremic patients related to peripheral somatic and autonomic neuropathy? Study by R-R interval variation test (RRIV) and by sympathetic skin response (SSR). Neurophysiol Clin 2001; 31: 181–193 [DOI] [PubMed] [Google Scholar]
- 9. American Psychiatric Association. DSM-IV. Diagnostic and statistical manual of mental disorders – 4th edn. Washington, DC: American Psychiatric Association, 2005 [Google Scholar]
- 10. Mettang T, Kremer AE. Uremic pruritus. Kidney Int 2015; 87: 685–691 [DOI] [PubMed] [Google Scholar]
- 11. Gilchrest BA, Stern RS, Steinman TI et al. Clinical features of pruritus among patients undergoing maintenance hemodialysis. Arch Dermatol 1982; 118: 154–156 [PubMed] [Google Scholar]
- 12. Rayner HC, Larkina M, Wang M et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol 2017; 12: 2000–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mathur VS, Lindberg J, Germain M et al. ; ITCH National Registry Investigators. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol 2010; 5: 1410–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manenti L, Tansinda P, Vaglio A. Uraemic pruritus: clinical characteristics, pathophysiology and treatment. Drugs 2009; 69: 251–263 [DOI] [PubMed] [Google Scholar]
- 15. Narita I, Alchi B, Omori K et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int 2006; 69: 1626–1632 [DOI] [PubMed] [Google Scholar]
- 16. Pisoni RL, Wikström B, Elder SJ et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2006; 21: 3495–3505 [DOI] [PubMed] [Google Scholar]
- 17. Ramakrishnan K, Bond TC, Claxton A et al. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int J Nephrol Renovasc Dis 2013; 7: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kimata N, Fuller DS, Saito A et al. Pruritus in hemodialysis patients: results from the Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS). Hemodial Int 2014; 18: 657–667 [DOI] [PubMed] [Google Scholar]
- 19. Szepietowski JC, Reich A, Schwartz RA. Uraemic xerosis. Nephrol Dial Transplant 2004; 19: 2709–2712 [DOI] [PubMed] [Google Scholar]
- 20. Reich A, Ständer S, Szepietowski JC. Drug-induced pruritus: a review. Acta Derm Venereol 2009; 89: 236–244 [DOI] [PubMed] [Google Scholar]
- 21. Weisshaar E, Szepietowski JC, Dalgard FJ et al. European S2k guideline on chronic pruritus. Acta Derm Venereol 2019; 99: 469–506 [DOI] [PubMed] [Google Scholar]
- 22. Tey HL, Yosipovitch G. Targeted treatment of pruritus: a look into the future. Br J Dermatol 2011; 165: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cowan A, Kehner GB, Inan S. Targeting itch with ligands selective for kappa opioid receptors. Handb Exp Pharmacol 2015; 226: 291–314 [DOI] [PubMed] [Google Scholar]
- 24. Food and Drug Administration. Guidance for Industry. Patient-reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims (27 May 2021, date last accessed) [DOI] [PMC free article] [PubMed]
- 25. Hayes MHS, Patterson DG. Experimental development of the graphic rating method. Psychol Bull 1921; 18: 98–99 [Google Scholar]
- 26. Morton CA, Lafferty M, Hau C et al. Pruritus and skin hydration during dialysis. Nephrol Dial Transplant 1996; 11: 2031–2036 [DOI] [PubMed] [Google Scholar]
- 27. Peer G, Kivity S, Agami O et al. Randomised crossover trial of naltrexone in uraemic pruritus. Lancet 1996; 348: 1552–1554 [DOI] [PubMed] [Google Scholar]
- 28. Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med 1969; 62: 989–993 [PMC free article] [PubMed] [Google Scholar]
- 29. Reich A, Chatzigeorkidis E, Zeidler C et al. Tailoring the cut-off values of the Visual Analogue Scale and Numeric Rating Scale in itch assessment. Acta Derm Venereol 2017; 97: 759–760 [DOI] [PubMed] [Google Scholar]
- 30. Storck M, Sandmann S, Bruland P et al. Pruritus intensity scales across Europe: a prospective validation study. J Eur Acad Dermatol Venereol 2021; 35: 1176–1185 [DOI] [PubMed] [Google Scholar]
- 31. Hays RD, Kallich JD, Mapes DL et al. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 1994; 3: 329–338 [DOI] [PubMed] [Google Scholar]
- 32. Elman S, Hynan LS, Gabriel V et al. The 5-D itch scale: a new measure of pruritus. Br J Dermatol 2010; 162: 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Majeski CJ, Johnson JA, Davison SN et al. Itch Severity Scale: a self-report instrument for the measurement of pruritus severity. Br J Dermatol 2007; 156: 667–673 [DOI] [PubMed] [Google Scholar]
- 34. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol 2015; 35: 383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fishbane S, Jamal A, Munera C et al. KALM-1 Trial Investigators. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med 2020; 382: 222–232 [DOI] [PubMed] [Google Scholar]
- 36. Fishbane S, Mathur V, Germain MJ et al. ; on behalf of the Trial Investigators. Randomized controlled trial of difelikefalin for chronic pruritus in hemodialysis patients. Kidney Int Rep 2020; 5: 600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nochaiwong S, Ruengorn C, Awiphan R et al. Development of a multidimensional assessment tool for uraemic pruritus: Uraemic Pruritus in Dialysis Patients (UP-Dial). Br J Dermatol 2017; 176: 1516–1524 [DOI] [PubMed] [Google Scholar]
- 38. Feldman R, Berman N, Reid MC et al. Improving symptom management in hemodialysis patients: identifying barriers and future directions. J Palliat Med 2013; 16: 1528–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weisbord SD, Fried LF, Mor MK et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol 2007; 2: 960–967 [DOI] [PubMed] [Google Scholar]
- 40. Weisshaar E, Matterne U, Mettang T. How do nephrologists in haemodialysis units consider the symptom of itch? Results of a survey in Germany. Nephrol Dial Transplant 2009; 24: 1328–1330 [DOI] [PubMed] [Google Scholar]
- 41. Manns B, Hemmelgarn B, Lillie E et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol 2014; 9: 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ikoma A, Ebata T, Chantalat L et al. Measurement of nocturnal scratching in patients with pruritus using a smartwatch: initial clinical studies with the Itch Tracker App. Acta Derm Venereol 2019; 99: 268–273 [DOI] [PubMed] [Google Scholar]