Abstract
Purpose
This study describes motor speech disorders and associated communication limitations in six variants of progressive supranuclear palsy (PSP).
Method
The presence, nature, and severity of dysarthria and apraxia of speech (AOS) were documented, along with scores on the Apraxia of Speech Rating Scale–Version 3 (ASRS-3) for 77 (40 male and 37 female) patients with PSP. Clinician-estimated and patient-estimated communication limitations were rated using the Motor Speech Disorders Severity Rating (MSDSR) Scale and the Communicative Effectiveness Survey (CES), respectively. Descriptive statistics were calculated for each of these dependent variables. One-tailed t tests were conducted to test mean differences in ASRS-3 and CES between participants with and without AOS and between participants with and without dysarthria. Spearman rank correlations were calculated between ASRS-3 scores and clinical judgments of AOS and dysarthria severity and between MSDSR and CES ratings.
Results
Nine participants (12%) had normal speech. Eighty-seven percent exhibited dysarthria; hypokinetic and mixed hypokinetic–spastic dysarthria were observed most frequently. AOS was observed in 19.5% of participants across all variants, but in only 10% exclusive of the PSP speech and language variant. Nearly half presented with AOS in which neither phonetic nor prosodic features clearly predominated. The mean ASRS-3 score for participants with AOS was significantly higher than for those without and correlated strongly with clinician judgment of AOS severity. Mean ASRS-3 was higher for participants with dysarthria than for those without but correlated weakly with dysarthria severity. Mean MSDSR and CES ratings were lower in participants with AOS compared to those without and moderately correlated with each other.
Conclusions
Motor speech disorders that negatively impact communicative effectiveness are common in PSP and occur in many variants. This is the first description of motor speech disorders across PSP variants, setting the stage for future research characterizing neuroanatomical correlates, progression of motor speech disorders, and benefits of targeted interventions.
Supplemental Material
Progressive supranuclear palsy (PSP), also known as Steele Richardson Olszewski syndrome, is characterized by parkinsonism, vertical supranuclear gaze palsy, and postural instability (see Hoglinger et al., 2017, for a review). The presenting symptoms of PSP are diverse and have led to the description of PSP variants with unique phenotypes (Ali & Josephs, 2018; Hoglinger et al., 2017) and neuroanatomical correlates (Whitwell et al., 2020). In the current diagnostic criteria, the variants, further described below, are assigned based on the combination of features in the domains of oculomotor dysfunction, postural instability, akinesia, and cognitive dysfunction and relate to the likelihood of underlying PSP pathology at autopsy (Hoglinger et al., 2017). Richardson syndrome (PSP-RS) denotes the classic presentation characterized by rigidity, akinesia, gaze palsy, and postural instability. The corticobasal syndrome variant (PSP-CBS) presents with asymmetric limb apraxia, alien limb syndrome, myoclonus, and cortical sensory loss. The Parkinsonism variant (PSP-P) is associated with asymmetric onset of tremor, moderate initial response to levodopa, and slower rate of progression. Primary complaints of freezing of gait, speech, and/or writing are characteristic of the pure akinesia gait freezing variant (denoted by PSP-PGF). Predominant postural instability is seen as PSP-PI. PSP-F denotes the frontal variant, which presents with behavioral and personality changes, and PSP-OM presents with relatively isolated oculomotor dysfunction. Finally, progressive apraxia of speech (AOS), agrammatic aphasia, or both are characteristics of the speech and language variant (PSP-SL).
Dysarthria is common in PSP (see Kim & McCann, 2015, for a review), appearing earlier with greater severity and more rapid progression compared to Parkinson's disease (Collin et al., 1995; dell'Aquila et al., 2013; Goetz et al., 2003; Kaat et al., 2007; Muller et al., 2001; Skodda et al., 2011; Tykalova et al., 2017). Dysarthria can be a presenting complaint (Collins et al., 1995; Goetz et al., 2003; Nath et al., 2003; Testa et al., 2001), and unintelligible speech is often the first major disability experienced by patients with PSP (dell'Aquila et al., 2013). The dysarthria of PSP is typically mixed (Muller et al., 2001; Rusz et al., 2015), with predominant hypokinetic (Collins et al., 1995; Metter & Hanson, 1991) or spastic features (Kluin et al., 1993; Skodda et al., 2011). Ataxic features are less common and rarely predominate (Kluin et al., 1993).
Because most of the reports detailing dysarthria in PSP predate the description of PSP variants, little is known about how dysarthria presents across variants. Skodda et al. (2011) found no difference in dysarthria displayed by participants with PSP-RS and PSP-P. In contrast, Clark et al. (2017) reported that participants with PSP-RS exhibited primarily hypokinetic features, whereas participants with PSP-SL were more likely to exhibit spastic features or experience no dysarthria at all.
Only more recently have communication limitations beyond dysarthria been described in PSP (Kim & McCann, 2015). As noted above, progressive AOS (Josephs et al., 2014, 2012) and agrammatic nonfluent aphasia (Josephs et al., 2005; Josephs & Duffy, 2008; Santos-Santos et al., 2016) are the presenting complaint of PSP-SL (Hoglinger et al., 2017; Whitwell et al., 2019). The only study examining AOS across PSP variants reported that AOS was present in all participants with PSP-SL (which is defined by the presence of AOS) but absent in participants with PSP-RS (Clark et al., 2017). The type of AOS, defined by the relative predominance of disruption to articulatory (phonetic) or prosodic features (Utianski, Duffy, Clark, Strand, Botha, et al., 2018), associated with PSP has not yet been described.
In spite of the richness of literature describing motor speech disorders in PSP, few reports have described the impact of motor speech disorders on communication participation for these individuals. One exception is dell'Aguila et al. (2013), who noted that speech became unintelligible approximately 5 years after onset of symptoms. Understanding the day-to-day impact of motor speech disorders in PSP is important for treatment planning, including the introduction of augmentative and alternative communication. Such information would also guide counseling and education to care partners, particularly when the impact of communication limitations poses unique challenges across various communication situations. The aim of the current study was to describe motor speech disorders and associated communication limitations in a large prospective cohort representing several variants of PSP.
Method
Informed consent was obtained from participants. The Mayo Clinic Institutional Review Board approved the study.
Participants
Seventy-seven (40 male and 37 female) individuals diagnosed with probable or possible PSP were recruited by the Neurodegenerative Research Group into a National Institutes of Health–funded prospective study. The diagnosis of PSP and the determination of PSP variant were established with international consensus criteria for PSP variants (Hoglinger et al., 2017). The neurological examination on which this was based was conducted by a movement disorder specialist (F. A., H. B., or K. A. J.) experienced in the differential diagnosis of Parkinsonism and related disorders. Table 1 summarizes demographic data for each of the PSP variant groups. For the purposes of this study, participants diagnosed with PSP-F or PSP-OM were grouped together as PSP-other. The use or timing of dopamine replacement or other pharmacological interventions was not recorded at the time of the speech evaluation. This cohort did not undergo formal language assessment so the presence of aphasia or nonaphasic cognitive communicative impairments is not documented.
Table 1.
Participant description.
| Participant characteristics | PSP-other n = 4 |
PSP-CBS n = 6 |
PSP-P n = 13 |
PSP-PGF n = 5 |
PSP-RS n = 41 |
PSP-SL n = 8 |
|---|---|---|---|---|---|---|
| Male:female | 0:4 | 3:3 | 9:4 | 2:3 | 22:19 | 4:4 |
| Mean age (years) | 65.3 | 66.7 | 70.6 | 74.4 | 68.3 | 73.4 |
| Mean education (years) | 15.3 | 12.7 | 15.5 | 14.4 | 15 | 15.9 |
| Mean disease duration (years) | 1.5 | 2.7 | 6.5 | 2.6 | 3.5 | 7.3 |
| Mean PSPRS (max = 100; higher score = more severe) | 28 | 35.2 | 41.5 | 37.6 | 39.8 | 43.9 |
Note. PSP = progressive supranuclear palsy; CBS = corticobasal syndrome; P = parkinsonism; PGF = pure akinesia gait freezing; RS = Richardson syndrome; SL = speech and language; PSPRS = PSP Rating Scale (Hoglinger et al., 2017).
Procedure
Each participant underwent a motor speech assessment battery administered by the first author during a single visit. Differential diagnosis and severity of motor speech disorder was determined based on perceptual ratings of speech features during conversational speech, oral reading, and repetition of words and sentences (Darley et al., 1969; Duffy, 2020). Performance on alternating and sequential motor tasks (rapid repetition of “puh,” “tuh,” “kuh,” and “puhtuhkuh”) was also considered in the assignment of differential diagnosis (Duffy, 2020). The differential diagnosis and degree of severity (mild, moderate, severe, or profound) were determined by the first author. After these subjective judgments were determined, performance on these same speech tasks was used to score the Apraxia of Speech Rating Scale–Version 3 (ASRS-3; Strand et al., 2014; Utianski, Duffy, Clark, Strand, Botha, et al., 2018), which quantifies the overall severity of speech characteristics of AOS as well as severity of phonetic and prosodic speech features. The ASRS-3 quantifies 13 speech and nonspeech features, each rated between 0 (essentially absent) 1and 4 (nearly always present and/or disruptive to intelligibility), for a maximum score of 52 reflecting greatest severity while still being able to produce speech.
Clinician-estimated communication limitation was rated using the Motor Speech Disorders Severity Rating (MSDSR) Scale based on overall intelligibility and success of communication interactions. Adapted from a scale developed to capture communication limitations related to progressive dysarthria secondary to amyotrophic lateral sclerosis (Hillel et al., 1989; Yorkston et al., 1993), the scale has been used successfully to characterize communication limitations 1accompanying progressive dysarthria and AOS associated with tauopathies (Utianski et al., 2020). Examples of ratings include the lowest rating of 1 (nonvocal), moderate rating of 5 (frequent repetition), and the highest rating of 10 (normal speech).
Reliability of clinician ratings was assessed by rescoring of 10% (n = 8) speech samples by the original examiner (H. M. C.) and a second clinician (R. L. U.), both certified speech-language pathologists experienced in the differential diagnosis of motor speech disorders and for whom reliability of perceptual judgments has been established in previous studies (Botha et al., 2018; Strand et al., 2014; Utianski, Duffy, Clark, Strand, Boland, et al., 2018; Utianski, Duffy, Clark, Strand, Botha, et al., 2018). Interrater agreement on the presence and severity of dysarthria was 100%, and on the presence and severity of AOS, it was 88%; intrarater agreement was 100% for both diagnoses, with 100% of severity estimates falling within 1 point of the original ratings, which were used in subsequent analyses. Inter- and intrarater agreement on the type of dysarthria and/or AOS were each 100%. Nonparametric Spearman rank correlation for MSDSR ratings within rater was .945 (p < .0001), and between raters, it was .984 (p = .0004).
Participant ratings of communication success were obtained using the Communicative Effectiveness Survey (CES; Donovan et al., 2008). Participants, either independently or in collaboration with a family member, rated the effectiveness of communication in eight specific situations (e.g., “conversing with a stranger over the telephone”). The CES utilizes a 4-point scale, where a rating of 1 indicates not at all effective and a rating of 4 indicates very effective, for a maximum score of 32. No test–retest reliability measures of participant ratings were obtained.
Analyses
Descriptive statistics were performed for all dependent variables (presence, nature, and severity of dysarthria and AOS, ASRS-3, MSDSR, and CES). Sample size imbalance precluded formal testing for group differences across PSP variants. Kruskal–Wallis one-way nonparametric tests were conducted to test mean differences in ASRS-3 and CES scores between participants with AOS and those without AOS and between participants with dysarthria and those without dysarthria (independent variables). Spearman rank correlations were calculated between ASRS-3 scores and clinical judgments of the severity of AOS and dysarthria, as well as between the clinician- and patient-rated measures of communication deficits.
Results
Motor Speech Disorders
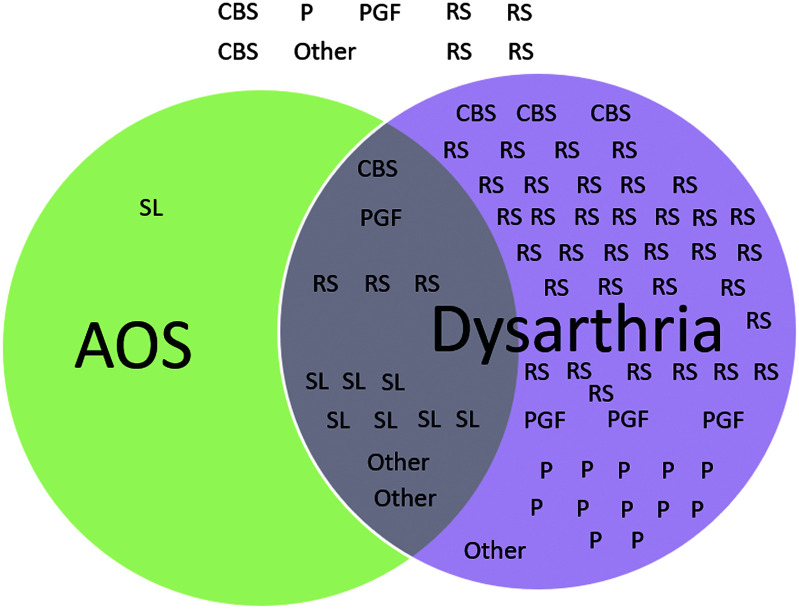
Nine participants (12% of the sample) exhibited normal speech. Eighty-seven percent of participants exhibited dysarthria (n = 67; 53 with dysarthria only), and AOS was observed in 19.5% of the total sample (n = 15; one with AOS only). Fourteen participants exhibited both dysarthria and AOS (see Figure 1).
Figure 1.
Distribution of motor speech disorders across progressive supranuclear palsy (PSP) variants. RS = Richardson syndrome; CBS = corticobasal syndrome; P = parkinsonism; PGF = pure akinesia gait freezing; SL = speech and language; AOS = apraxia of speech.
Table 2 summarizes the presence and severity of dysarthria across PSP variants. Hypokinetic dysarthria, typically characterized by tight, hoarse/breathy vocal quality, reduced loudness, monopitch and monoloudness, rapid rate and/or short rushes of speech, and imprecise articulation, was observed most frequently (52% of participants with dysarthria). Isolated spastic dysarthria, characterized by slow rate, strained–strangled vocal quality, monopitch and monloudness, and hypernaslity, was observed in 13% of participants with dysarthria. Mixed hypokinetic–spastic dysarthria was observed in 28% of participants with dysarthria; hypokinetic features predominated in 70% of these mixed dysarthrias. Other dysarthrias observed were ataxic, characterized by irregular articulatory breakdowns and telescoping of syllables (two participants), and mixed hypokinetic–spastic–ataxic, ataxic–spastic, hypokinetic–ataxic, or unilateral upper motor neuron dysarthria, characterized by mild articulatory imprecision in the setting of unilateral central facial weakness (one participant each).
Table 2.
Type and severity of dysarthria for each progressive supranuclear palsy (PSP) variant.
| Variant | Spastic | Ataxic–spastic | Ataxic | Hypokinetic–ataxic | Hypokinetic | Hypokinetic–spastic | Hypokinetic–spastic–ataxic | UUMN | |
|---|---|---|---|---|---|---|---|---|---|
| PSP-other | None (1) | ||||||||
| Mild | 1 | ||||||||
| Moderate | |||||||||
| Severe | 2 | ||||||||
| Profound | |||||||||
| PSP-CBS | None (2) | ||||||||
| Mild | 1 | 1 | 1 | ||||||
| Moderate | |||||||||
| Severe | 1 | ||||||||
| Profound | |||||||||
| PSP-P | None (1) | ||||||||
| Mild | 1 | 1 | 7 | 2 | 1 | ||||
| Moderate | |||||||||
| Severe | |||||||||
| Profound | |||||||||
| PSP-PGF | None (1) | ||||||||
| Mild | 2 | 1 | |||||||
| Moderate | |||||||||
| Severe | 1 | ||||||||
| Profound | |||||||||
| PSP-RS | None (4) | ||||||||
| Mild | 2 | 1 | 2 | 3 | 1 | ||||
| Moderate | 17 | 5 | |||||||
| Severe | 2 | 4 | |||||||
| Profound | |||||||||
| PSP-SL | None (1) | ||||||||
| Mild | |||||||||
| Moderate | |||||||||
| Severe | 2 | 2 | 2 | ||||||
| Profound | 1 |
Note. UUMN = unilateral upper motor neuron; CBS = corticobasal syndrome; P = parkinsonism; PGF = pure akinesia gait freezing; RS = Richardson syndrome; SL = speech and language.
AOS was observed in 15 participants (19.5% of the total sample; see Table 3). Nearly half of the participants with AOS exhibited the mixed subtype, in which neither phonetic nor prosodic features were judged to predominate. Next most common was phonetic subtype in five individuals and the prosodic subtype in three individuals. All of the participants in the PSP-SL group exhibited AOS, with nearly all exhibiting severe or profound AOS. AOS was observed, albeit rarely, in each of the other PSP variants represented in our sample, with the exception of PSP-P. All but one of the participants with AOS also demonstrated dysarthria. In most cases (n = 6), AOS was judged to be more severe than dysarthria. Four cases were judged to exhibit dysarthria and AOS of equal severity, and the final four exhibited dysarthria more severely than AOS.
Table 3.
Type and severity of apraxia of speech for each progressive supranuclear palsy (PSP) variant.
| Variant | Phonetic | Prosodic | Mixed | |
|---|---|---|---|---|
| PSP-other | None (2) | |||
| Mild | 2 | |||
| Moderate | ||||
| Severe | ||||
| Profound | ||||
| PSP-CBS | None (5) | |||
| Mild | ||||
| Moderate | ||||
| Severe | 1 | |||
| Profound | ||||
| PSP-P | None (13) | |||
| Mild | ||||
| Moderate | ||||
| Severe | ||||
| Profound | ||||
| PSP-PGF | None (4) | |||
| Mild | 1 | |||
| Moderate | ||||
| Severe | ||||
| Profound | ||||
| PSP-RS | None (38) | |||
| Mild | 1 | |||
| Moderate | 1 | |||
| Severe | ||||
| Profound | 1 | |||
| PSP-SL | None (0) | |||
| Mild | ||||
| Moderate | 1 | |||
| Severe | 1 | |||
| Profound | 1 | 5 |
Note. CBS = corticobasal syndrome; P = parkinsonism; PGF = pure akinesia gait freezing; RS = Richardson syndrome; SL = speech and language.
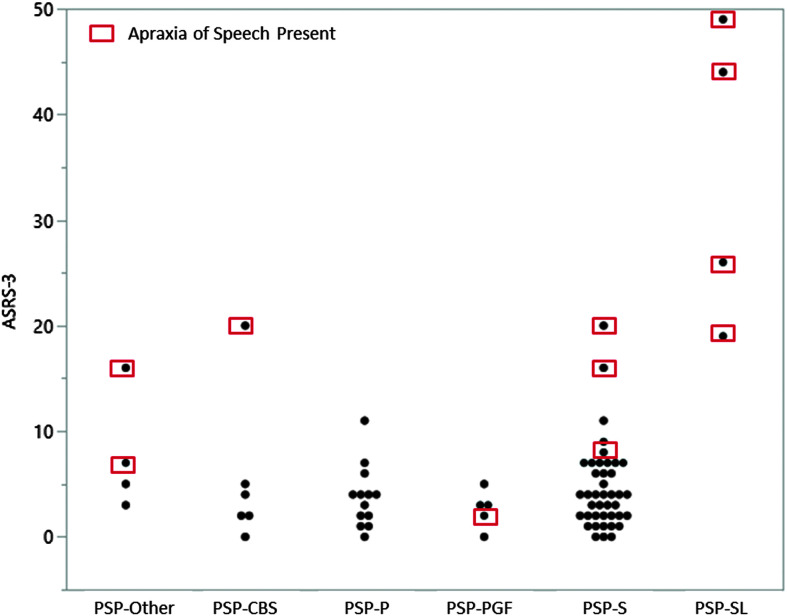
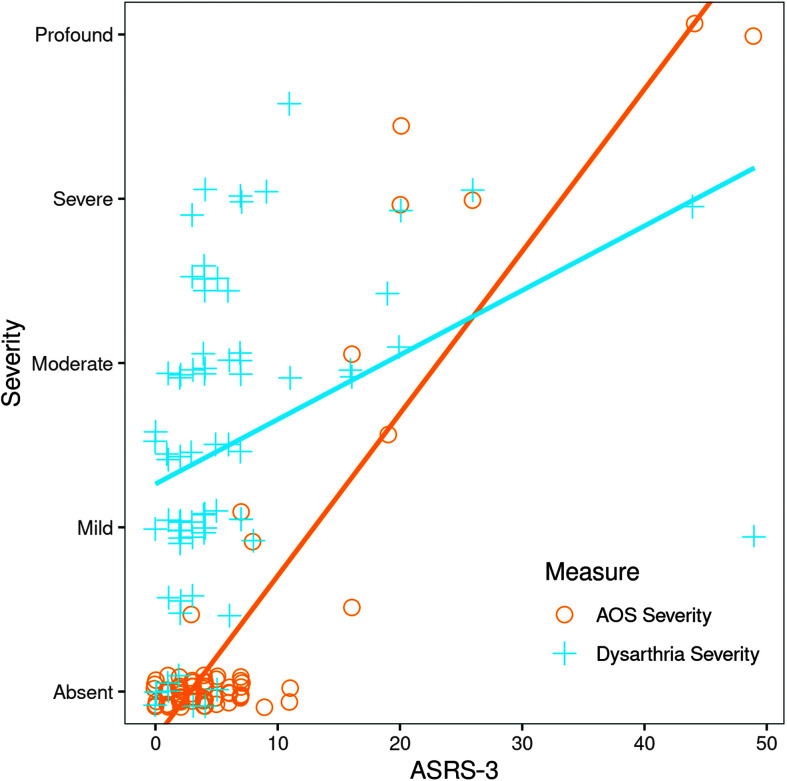
The mean ASRS-3 scores for participants with AOS are summarized in Table 4. The ASRS-3 was not scored for four of the participants with severe AOS in the PSP-SL group, as these individuals were unable to produce an adequate speech sample to rate all of the ASRS-3 items. The mean ASRS-3 score for the remaining participants with AOS (20.7) was significantly higher than the mean score for those without AOS (3.6), Kruskal–Wallis one-way test, χ2 = 21.39, p = .0001, df = 1 (see Figure 2). Mean ASRS-3 score was also significantly higher for participants with dysarthria (6.8) than those without dysarthria (1.78), Kruskal–Wallis one-way test, χ2 = 7.9, p = .0049, df = 1. The ASRS-3 score correlated strongly with clinician judgment of AOS severity (rs = .914), but not dysarthria severity (rs =.362; see Figure 3).
Table 4.
Mean Apraxia of Speech Rating Scale–Version 3 (Strand et al., 2014; Utianski, Duffy, Clark, Strand, Boland, et al., 2018) ratings (standard deviations) for participants with and without apraxia of speech (AOS).
| PSP-other n = 4 |
PSP-CBS n = 6 |
PSP-P n = 13 |
PSP-PGF n = 5 |
PSP-RS n = 41 |
PSP-SL n = 8 |
|
|---|---|---|---|---|---|---|
| ASRS-3 score when AOS is present | n = 2 | n = 1 | n = 0 | n = 1 | n = 3 | n = 8 |
| 11.5 (6.4) |
20 (NA) |
NA | 3 (NA) |
14.7 (6.1) |
34.5 (8.5) |
|
| ASRS-3 score when AOS is absent | 4 (1.41) |
2.6 (1.9) |
3.7 (2.9) |
2.5 (2.1) |
3.8 (2.7) |
NA |
Note. PSP = progressive supranuclear palsy; CBS = corticobasal syndrome; P = parkinsonism; PGF = pure akinesia gait freezing; RS = Richardson syndrome; SL = speech and language; NA = not applicable.
Figure 2.
Apraxia of Speech Rating Scale–Version 3 (ASRS-3; Strand et al., 2014; Utianski, Duffy, Clark, Strand, Boland, et al., 2018) scores for individual participants across progressive supranuclear palsy (PSP) variants. ASRS-3 was not scored for four participants in the PSP-SL group. RS = Richardson syndrome; CBS = corticobasal syndrome; P = parkinsonism; PGF = pure akinesia gait freezing; SL = speech and language.
Figure 3.
Apraxia of Speech Rating Scale–Version 3 (ASRS-3; Strand et al., 2014; Utianski, Duffy, Clark, Strand, Boland, et al., 2018) scores for individual participants across levels of dysarthria and apraxia of speech (AOS) severity. Participants who exhibited both dysarthria and AOS are represented by overlapping symbols. Scores are reported only for those participants who produced sufficient speech for the rating to be completed.
Communication Limitations
Table 5 summarizes the median and range of scores on the clinician-rated MSDSR, on which scores range from 1 to 10, with 10 reflecting no impairment. Median ratings were 8 or 7 (perceived speech changes or obvious speech abnormality, respectively) for all PSP variants, except for PSP-SL, for which the median rating was 3 (one-word responses).
Table 5.
Clinician- and patient-rated functional communication limitations.
| PSP-other | PSP-CBS | PSP-P | PSP-PGF | PSP-RS | PSP-SL | |
|---|---|---|---|---|---|---|
| Median MSDSR (range) |
8 (7–9) |
8 (3–10) |
7 (5–9) |
8 (6–9) |
7 (4–10) |
3 (1–5) |
| Mean CES (SD) |
25.25 (6.0) |
19.7 (9.9) |
18.7 (4.9) |
17.8 (6.6) |
20.0 (5.9) |
10.5 (2.1) |
Note. PSP = progressive supranuclear palsy; CBS = corticobasal syndrome; P = parkinsonism; PGF = pure akinesia gait freezing; RS = Richardson syndrome; SL = speech and language; MSDSR = Motor Speech Disorders Severity Rating Scale (Hillel et al., 1989); CES = Communicative Effective Scale (Donovan et al., 2008.
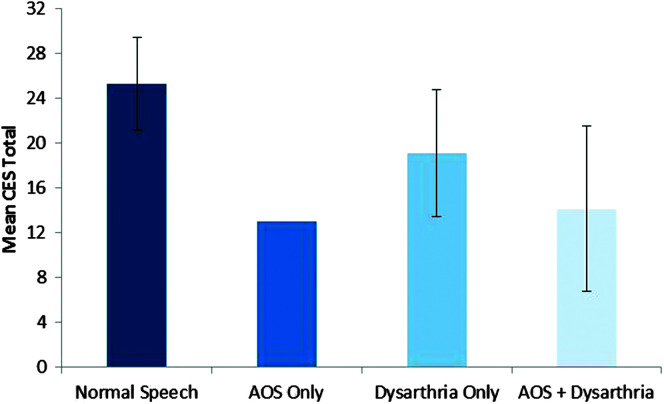
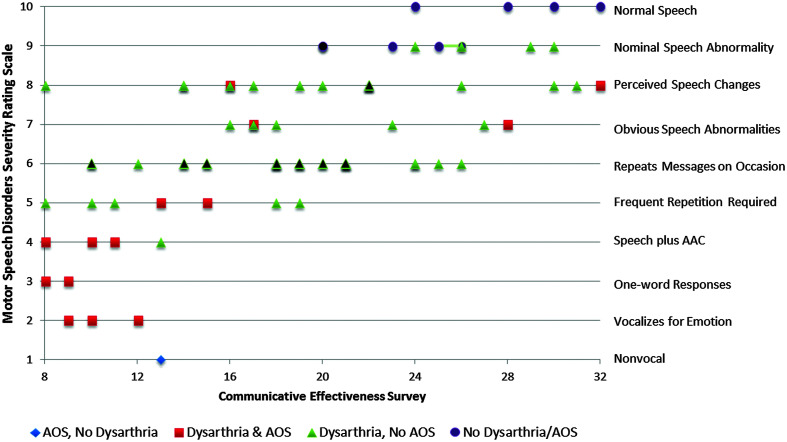
Patient-rated estimates of communication limitation were assessed using the CES, on which the possible range of scores is 8–32. Mean CES scores were quite similar for four of the PSP variants (range: 17–20), whereas PSP-SL had substantially lower mean score (10.5; greater perceived limitations) and PSP-other had substantially higher mean score (25.25; lesser perceived limitations). Figure 4 illustrates that mean CES ratings were lower in participants with AOS (14.1) compared to those without (20.0), Kruskal–Wallis one-way test, χ2 = 11.469, p = .007, df = 1, and also lower in participants with dysarthria (18.1) compared to those without dysarthria (24.1), Kruskal–Wallis one-way test, χ2 = 7.45, p = .006, df = 1. Spearman correlation between the MSDSR and CES ratings was .688 (p < .0001; see Table 6). Figure 5 plots the clinician- and patient-rated estimates of communication limitation for participants with and without AOS and/or dysarthria.
Figure 4.
Mean Communicative Effectiveness Survey (CES; Donovan et al., 2008) rating for participants with and without motor speech disorders. Error bars reflect standard deviation. AOS = apraxia of speech.
Table 6.
Correlations among severity of motor speech disorder, and clinician-rated and patient-rated measures of functional communication limitations.
| Motor Speech Disorder Severity Rating | Dysarthria severity | AOS severity | |
|---|---|---|---|
| Communication Effectiveness Survey | .688* | −.532* | −.521* |
| Motor Speech Disorder Severity Rating | −.712* | −.741* | |
| Dysarthria severity | .287 |
Note. AOS = apraxia of speech.
p = .0001.
Figure 5.
Clinician- and patient-estimated ratings of communication limitations. Black-filled markers represent data from multiple participants in the same group. AAC = augmentative and alternative communication; AOS = apraxia of speech.
Discussion
The current study explored the presence, nature, and severity of motor speech disorders in a large prospective cohort of participants with PSP. The unique contributions of this article are the inclusion of a variety of PSP phenotypes, comprehensive characterization of all motor speech disorders present, and assessment of associated communication limitations.
Motor Speech Disorders
In keeping with extant literature, dysarthria was common in this cohort. In the current cohort, hypokinetic dysarthria was the most common motor speech disorder, followed by hypokinetic-predominant mixed dysarthria. These findings are not wholly consistent with earlier reports, which generally identify mixed dysarthria as most typical of PSP (Jellinger, 2001; Muller et al., 2001; Rusz et al., 2015). Given refined diagnostic criteria, one might suspect that the participants in the current study were identified earlier in the disease process compared to previous reports, thus characterizing the speech deficits before they evolved to a more complex dysarthria. However, the mean disease duration of the entire cohort was 4.1 years, very similar to earlier reports; thus, disease duration would not seem to be a primary influencing factor. It is possible that the refined diagnostic criteria have simply led to broader recognition of PSP (Ali et al., 2019), which in turn results in a more heterogeneous sample.
Whether isolated or predominant, hypokinetic features were prominent in the majority of participants in the current sample. This differs from the accounts of Kluin et al. (1993) and Skodda et al. (2011), who reported spastic features as present in all participants and predominant when hypokinetic features were also present (95% of participants). Although Kluin et al. employed a classification scheme slightly different than the Mayo Clinic classification (Darley et al., 1969; Duffy, 2020) utilized in the current study, most of the features they describe align with those of the Mayo system. It is therefore unlikely that disparate classification schemes account for the discrepancy. Because previous reports did not differentiate among PSP variants or indicate the presence of AOS, one might speculate that the PSP-SL phenotype was overrepresented in the earlier studies. This could account for greater prevalence of spastic dysarthria (Clark et al., 2017) or perhaps characterization of the prosodic subtype of AOS (also characterized by slow rate and disrupted prosody) as a predominance of spastic features. However, because the unique presentation of this variant makes recognizing PSP more difficult (Whitwell et al., 2019), it is unlikely that this subgroup of patients would have been recruited disproportionately in early studies.
The available literature, supported by the current findings, suggests that dysarthria in PSP is heterogeneous, but not infinitely so. Clinicians performing differential diagnosis should recognize the patterns that are most consistent with PSP (i.e., isolated or mixed dysarthrias with hypokinetic, spastic, and/or ataxic features) and patterns that are not typical of PSP but more typical of other neurological disorders (e.g., the mixed flaccid–spastic dysarthria of amyotrophic lateral sclerosis).
Although observed less frequently than dysarthria, AOS was not uncommon in the current cohort. The earliest reports of progressive AOS ultimately led to the recognition of this motor speech disorder as a harbinger of PSP (Adeli et al., 2013; Duffy & Josephs, 2012; Duffy et al., 2014; Josephs et al., 2005, 2014, 2006). Furthermore, the careful characterization of this phenotype supported the inclusion of the PSP-SL variant when criteria for the diagnosis of PSP were updated (Ali et al., 2019; Hoglinger et al., 2017; Whitwell et al., 2019). In the current cohort, AOS was ubiquitous in the PSP-SL variant, consistent with diagnostic criteria for this variant.
Importantly, this is the first study to report the prevalence of AOS in variants other than PSP-SL (10%). In the current cohort, AOS was observed in each of the PSP variants represented in our sample, except for PSP-P, revealing that AOS can develop as a secondary symptom in PSP rather than only occurring as a presenting complaint, as in the PSP-SL variant. Visual inspection of the data clearly depicts the AOS in PSP-SL to be more severe than AOS in other variants. This observation is confounded, however, by the prolonged disease duration of the participants with this variant. The confound of disease duration in PSP-SL is largely unavoidable since the diagnosis of possible PSP cannot be made until the emergence of gaze palsy, which may occur 5 years or more after onset of speech or language symptoms (Hoglinger et al., 2017; Josephs et al., 2014; Whitwell et al., 2019). Indeed, each of the eight participants with PSP-SL was originally recruited for studies of progressive AOS for which a clinical diagnosis of possible and probable PSP was an exclusion criterion (Josephs et al., 2012; see Supplemental Material S1 for descriptions of the motor speech disorders evident at the initial visit for participants in the PSP-SL group).
Considered along with the extant literature describing progressive AOS, the current findings support progressive AOS as a clinical indicator of PSP. When AOS is a presenting symptom, a diagnosis of PSP-SL should be considered in the neurological differential. When appropriate, the diagnosis of primary progressive AOS and PSP-SL can co-occur to help facilitate prognostication and treatment planning. However, the presence of AOS in the context of a broader neurological syndrome should not supersede other diagnostic criteria for PSP variants or other neurodegenerative condition (e.g., corticobasal degeneration).
When AOS was present, the clinician further judged whether phonetic or prosodic abnormalities predominated (Utianski, Duffy, Clark, Strand, Boland, et al., 2018; Utianski, Duffy, Clark, Strand, Botha, et al., 2018). In the current cohort, AOS severity precluded the determination of predominant features in five participants (all in the PSP-SL group), resulting in classification of mixed AOS. Equivocal or mild AOS was also present in two PSP-other participants—in these cases, the phonetic and prosodic features were judged to be equal in prominence (i.e. mixed). The remaining participants demonstrated a predominance of phonetic (n = 5) or prosodic (n = 3) features and were distributed across PSP variants. Recent studies have demonstrated that predominant prosodic features are associated with more rapid progression to parkinsonism (Josephs et al., 2014; Whitwell et al., 2017), supporting the speculation that subtypes may “predict the nature or rate of disease evolution and the type of additional deficits that may eventually emerge” (Utianski, Duffy, Clark, Strand, Botha, et al., 2018, p. 60). This may be equally true when AOS occurs in isolation as when it occurs in the context of broader neurological disease.
The current study employed the ASRS-3 to quantify the presence and severity of speech features typical of AOS. As has been reported by our group and others, mean ASRS-3 scores were higher in participants with AOS than in those without AOS (Basilakos et al., 2015; Strand et al., 2014), and AOS judged as more severe on clinical exam was associated with higher ASRS-3 scores (Duffy et al., 2017; Strand et al., 2014; Wambaugh et al., 2019). Visual inspection of Figure 3 reveals that ASRS-3 score does not independently discriminate speakers with AOS from speakers without. Strand et al. (2014) reported a cutoff score of 8 as most sensitive for detecting AOS in a sample of speakers with aphasia and/or AOS. In the current sample of speakers with dysarthria and/or AOS, two participants with scores lower than 8 were judged by the examiner to exhibit equivocal or mild AOS. Even so, a post hoc ROC analysis of the current data set (Supplemental Material S2) once again identified an ASRS-3 cutoff score of 8 as having the highest sensitivity for detecting AOS. We observed the ASRS-3 to be well suited for quantifying the severity of AOS when it was judged clinically to be present. It may also hold promise for detecting the presence of AOS with good sensitivity, even in the presence of co-occurring dysarthria.
To our knowledge, the current study is the first to report correlations between ASRS-3 and dysarthria severity. This is a relevant observation, as even though the ASRS-3 is intended to quantify speech features consistent with AOS, many features of AOS and dysarthria overlap (Strand et al., 2014). Overlapping speech features sampled by the ASRS-3 include articulatory distortions, slow rate, and slow or distorted alternate and sequential motion rates. The weak correlation between dysarthria severity and ASRS-3 score suggests that, despite this overlap, the speech features contributing to the clinical judgment of dysarthria severity in the current cohort were distinct from those sampled by the ASRS-3 and used in the clinical judgment of AOS presence and severity. For example, dysphonia and monopitch/monoloudness are common in hypokinetic and spastic dysarthria, hypernasality is common in spastic dysarthria, and rapid rate is common in hypokinetic dysarthria. These features, characteristic of dysarthria, are not typical of AOS and are not sampled by the ASRS-3.
The motor speech disorders observed in the current sample can be considered with respect to the neuroanatomical substrates known to support speech production and/or to be disrupted in PSP syndromes. Only two reports have explored the neuropathologic correlates of dysarthria in PSP. Kluin et al. (2001) found that the severity of hypokinetic features correlated with neuronal loss and gliosis in the substantia nigra, a finding confirmed by Jellinger (2001). Jellinger also noted mild–moderate neuronal depletion and gliosis in the subthalamic nucleus that did not correlate with severity of hypokinetic dysarthria. Neither study revealed neuropathological correlates for spastic or ataxic speech features, in spite of predictions that degeneration would likely be seen in the frontal and cingulate cortices, cerebellar cortex, and/or the dentate nucleus. In contrast, in a study examining both imaging and autopsy tau burden in PSP variants, Whitwell et al. (2020) reported that the subcortical structures of the globus pallidus and striatum were involved across all variants, the dentatorubrothalamic tract involvement was observed in PSP-RS, PSP-CBS, and PSP-F, and the cortical involvement was noted in PSP-SL and PSP-CBS variants. These findings are commensurate with the preponderance of hypokinetic features across variants observed in the current study. Future analyses of the current cohort will include correlation with imaging findings to better characterize the neuroanatomical substrates of individual speech features (e.g., slow rate, strained vocal quality) and the combination of speech features that constitute a given dysarthria type (e.g., spastic dysarthria; Clark et al., 2014).
Communication Limitations
Both clinician- and patient-rated estimates of communication limitations were included in the current study. Both ratings correlated significantly with severity of the motor speech disorders, similarly for dysarthria and AOS severity, providing cross-validation for these tools. An earlier study examining communication limitations in progressive AOS (Utianski et al., 2020) was unable to discriminate the relative contributions of AOS and dysarthria to ratings of communication limitation because of the strong correlation between severity of dysarthria and severity of AOS in that sample. The current sample does not suffer that confound, so we can conclude with greater confidence that severity of each of these motor speech disorders is associated with greater communication limitations.
Clinician-rated estimates of communication correlated more strongly with dysarthria and AOS severity than did patient-rated estimates. This is not unexpected since both judgment of motor speech disorder severity and clinician-rated estimates of communication limitations were made by the same examiner. The lack of complete agreement between estimates of communication limitation made by the clinician and the patient likely reflects not only that the two estimates considered different aspects of communicative behavior but also that the clinician-rated estimates were based on a more limited sample of communicative behavior. Moreover, it is unknown the degree to which cognitive deficits may have contributed to reduced awareness of functional limitations. The correlation between clinician- and patient-rated estimates of communication limitations was slightly higher (.688) than that observed in a cohort of participants with progressive AOS with and without aphasia (.484; Utianski et al., 2020). This discrepancy is not unexpected given that, as noted by those authors, the patient-rated estimates could be influenced by both language and motor speech deficits, whereas the clinician-rated estimate emphasized motor speech deficits only. Moreover, Utianski et al.'s study employed the Communication Participation Item Bank (Baylor et al., 2013) rather than the CES. Finally, the sample reported by Utianski et al. exhibited more severe AOS relative to severity of dysarthria. The unpredictability of speech errors in AOS may lead to a different speaker experience, including greater levels of frustration, which may partially account for some of the discrepancy in the pattern of findings across studies. Additional systematic study of the unique impact of progressive AOS and combined influence of co-occurring motor speech disorders on communicative limitations and quality of life will inform the education and counseling clinicians offer to patients experiencing neurodegenerative motor speech disorders.
An important role of communication ratings is to inform clinical judgments regarding the potential benefit of interventions targeting the communicative environment, communication behaviors of the individual with PSP as well as their communication partners, and the introduction of augmentative and alternative communication options. Longitudinal studies of the progression of communication limitations in PSP have been reported (Crosson, 1985; Goetz et al., 2003; Muller et al., 2001; Testa et al., 2001), but little is known about how limitations progress or how the timing of interventions may impact such progression. Future research will examine changes in the nature and severity of motor speech disorders as well as communication limitations over the course of disease progression.
Limitations
The current sample involved only small numbers of participants in several PSP variants, which prevented statistical testing of group differences. Although our sample is commensurate with previous reports of the distribution of PSP variants (Ali et al., 2019), further study with larger sample sizes is needed to confidently establish the motor speech profile typical of each variant. The current study exclusively employed subjective judgments of motor speech function. While such description is invaluable, other levels of observation such as acoustic analysis (Duffy et al., 2017; Skodda et al., 2011) and kinematic studies would further inform our understanding of the impact of PSP on speech motor control. Future studies might also consider including formal language and cognitive-communication assessments to allow for evaluation of the impacts of the full spectrum of communication disorders experienced in this population (Burrell et al., 2018).
Conclusions
Motor speech disorders are common in PSP and negatively impact communication participation. The current study offers the first description of motor speech disorders across the PSP variants, setting the stage for future research characterizing neuroanatomical correlates, progression of motor speech disorders, and the benefits of targeted interventions.
Supplementary Material
Acknowledgments
This work was supported by funding from National Institutes of Health Grants R01-DC12519 (PI: J. L. Whitwell) and R01- NS 89757 (PI: K. A. Josephs). The authors thank Peter Martin, who contributed to the development visualization of the data for Figure 3. The authors also express their gratitude to the patients and families who generously gave their time for this research.
Funding Statement
This work was supported by funding from National Institutes of Health Grants R01-DC12519 (PI: J. L. Whitwell) and R01- NS 89757 (PI: K. A. Josephs). The authors thank Peter Martin, who contributed to the development visualization of the data for Figure 3.
References
- Adeli, A. , Whitwell, J. L. , Duffy, J. R. , Strand, E. A. , & Josephs, K. A. (2013). Ideomotor apraxia in agrammatic and logopenic variants of primary progressive aphasia. Journal of Neurology, 260(6), 1594–1600. https://doi.org/10.1007/s00415-013-6839-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, F. , & Josephs, K. (2018). The diagnosis of progressive supranuclear palsy: Current opinions and challenges. Expert Review of Neurotherapeutics, 18(7), 603–616. https://doi.org/10.1080/14737175.2018.1489241 [DOI] [PubMed] [Google Scholar]
- Ali, F. , Martin, P. R. , Botha, H. , Ahlskog, J. E. , Bower, J. H. , Masumoto, J. Y. , Maraganore, D. , Hassan, A. , Eggers, S. , Boeve, B. F. , Knopman, D. S. , Drubach, D. , Petersen, R. C. , Dunkley, E. D. , van Gerpen, J. , Uitti, R. , Whitwell, J. L. , Dickson, D. W. , & Josephs, K. A. (2019). Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Movement Disorders, 34(8), 1144–1153. https://doi.org/10.1002/mds.27619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilakos, A. , Rorden, C. , Bonilha, L. , Moser, D. , & Fridriksson, J. (2015). Patterns of poststroke brain damage that predict speech production errors in apraxia of speech and aphasia dissociate. Stroke, 46(6), 1561–1566. https://doi.org/10.1161/STROKEAHA.115.009211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor, C. , Yorkston, K. M. , Eadie, T. , Kim, J. , Chung, H. , & Amtmann, D. (2013). The communicative participation item bank (CPIB): Item bank calibration and development of a disorder-generic short form. Journal of Speech, Language, and Hearing Research, 56(4), 1190–1208. https://doi.org/10.1044/1092-4388(2012/12-0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha, H. , Utianski, R. L. , Whitwell, J. L. , Duffy, J. R. , Clark, H. M. , Strand, E. A. , Machulda, M. M. , Tosakulwong, N. , Knopman, D. S. , Petersen, R. C. , Jack, C. R., Jr. , Josephs, K. A. , & Jones, D. T. (2018). Disrupted functional connectivity in primary progressive apraxia of speech. NeuroImage: Clinical, 18, 617–629. https://doi.org/10.1016/j.nicl.2018.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell, J. R. , Ballard, K. J. , Halliday, G. M. , & Hodges, J. R. (2018). Aphasia in progressive supranuclear palsy: As severe as progressive non-fluent aphasia. Journal of Alzheimer's Disease, 61(2), 705–715. https://doi.org/10.3233/JAD-170743 [DOI] [PubMed] [Google Scholar]
- Clark, H. M. , Duffy, J. , Strand, E. , Whitwell, J. L. , & Josephs, K. (2017). Motor speech disorders accompanying RS and SL variants of progressive supanuclear palsy. Stem-, Spraak- en Taalpathologie, 22, 100. [Google Scholar]
- Clark, H. M. , Duffy, J. R. , Whitwell, J. L. , Ahlskog, J. E. , Sorenson, E. J. , & Josephs, K. A. (2014). Clinical and imaging characterization of progressive spastic dysarthria. European Journal of Neurology, 21(3), 368–376. https://doi.org/10.1111/ene.12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, S. J. , Ahlskog, J. E. , Parisi, J. E. , & Maraganore, D. M. (1995). Progressive supranuclear palsy: Neuropathologically based diagnostic clinical criteria. Journal of Neurology, Neurosurgery, & Psychiatry, 58(2), 167–173. https://doi.org/10.1136/jnnp.58.2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson, B. (1985). Subcortical functions in language: A working model. Brain and Language, 25(2), 257–292. https://doi.org/10.1016/0093-934X(85)90085-9 [DOI] [PubMed] [Google Scholar]
- Darley, F. L. , Aronson, A. E. , & Brown, J. R. (1969). Differential diagnostic patterns of dysarthria. Journal of Speech and Hearing Research, 12(2), 246–269. https://doi.org/10.1044/jshr.1202.246 [DOI] [PubMed] [Google Scholar]
- dell'Aquila, C. , Zoccolella, S. , Cardinali, V. , de Mari, M. , Iliceto, G. , Tartaglione, B. , Lamberti, P. , & Logroscino, G. (2013). Predictors of survival in a series of clinically diagnosed progressive supranuclear palsy patients. Parkinsonism & Related Disorders, 19(11), 980–985. https://doi.org/10.1016/j.parkreldis.2013.06.014 [DOI] [PubMed] [Google Scholar]
- Donovan, N. J. , Kendall, D. L. , Young, M. E. , & Rosenbek, J. C. (2008). The Communicative Effectiveness Survey: Preliminary evidence of construct validity. American Journal of Speech-Language Pathology, 17(4), 335–347. https://doi.org/10.1044/1058-0360(2008/07-0010) [DOI] [PubMed] [Google Scholar]
- Duffy, J. R. (2020). Motor speech disorders: Substrates, differential diagnosis, and management (4th ed.). Elsevier. [Google Scholar]
- Duffy, J. R. , Hanley, H. , Utianski, R. L. , Clark, H. M. , Strand, E. A. , Josephs, K. A. , & Whitwell, J. L. (2017). Temporal acoustic measures distinguish primary progressive apraxia of speech from primary progressive aphasia. Brain and Language, 168, 84–94. https://doi.org/10.1016/j.bandl.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, J. R. , & Josephs, K. A. (2012). The diagnosis and understanding of apraxia of speech: Why including neurodegenerative etiologies may be important. Journal of Speech, Language, and Hearing Research, 55(5), S1518–S1522. https://doi.org/10.1044/1092-4388(2012/11-0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, J. R. , Strand, E. A. , & Josephs, K. A. (2014). Motor speech disorders associated with primary progressive aphasia. Aphasiology, 28(8–9), 1004–1017. https://doi.org/10.1080/02687038.2013.869307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz, C. G. , Leurgans, S. , Lang, A. E. , & Litvan, I. (2003). Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology, 60(6), 917–922. https://doi.org/10.1212/01.WNL.0000052686.97625.27 [DOI] [PubMed] [Google Scholar]
- Hillel, A. D. , Miller, R. M. , Yorkston, K. , McDonald, E. , Norris, F. H. , & Konikow, N. (1989). Amyotrophic lateral sclerosis severity scale. Neuroepidemiology, 8(3), 142–150. https://doi.org/10.1159/000110176 [DOI] [PubMed] [Google Scholar]
- Hoglinger, G. U. , Respondek, G. , Stamelou, M. , Kurz, C. , Josephs, K. A. , Lang, A. E. , Mollenhauer, B. , Müller, U. , Nilsson, C. , Whitwell, J. L. , Arzberger, T. , Englund, E. , Gelpi, E. , Giese, A. , Irwin, D. J. , Meissner, W. G. , Pantelyat, A. , Rajput, A. , van Swieten, J. C. , … Movement Disorder Society-Endorsed PSP Study Group. (2017). Clinical diagnosis of progressive supranuclear palsy: The Movement Disorder Society criteria. Movement Disorders, 32(6), 853–864. https://doi.org/10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger, K. (2001). Neuropathologic correlates of dysarthria in progressive supranuclear palsy. Archives of Neurology, 58(9), 1499–1500. https://doi.org/10.1001/archneur.58.9.1499 [DOI] [PubMed] [Google Scholar]
- Josephs, K. A. , Boeve, B. F. , Duffy, J. R. , Smith, G. E. , Knopman, D. S. , Parisi, J. E. , Petersen, R. C. , & Dickson, D. W. (2005). Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase, 11(4), 283–296. https://doi.org/10.1080/13554790590963004 [DOI] [PubMed] [Google Scholar]
- Josephs, K. A. , & Duffy, J. R. (2008). Apraxia of speech and nonfluent aphasia: A new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Current Opinion in Neurology, 21(6), 688–692. https://doi.org/10.1097/WCO.0b013e3283168ddd [DOI] [PubMed] [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Strand, E. A. , Machulda, M. M. , Senjem, M. L. , Gunter, J. L. , Schwarz, C. G. , Reid, R. I. , Spychalla, A. J. , Lowe, V. J. , Jack, C. R., Jr. , & Whitwell, J. L. (2014). The evolution of primary progressive apraxia of speech. Brain, 137(Pt. 10), 2783–2795. https://doi.org/10.1093/brain/awu223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Strand, E. A. , Machulda, M. M. , Senjem, M. L. , Master, A. V. , Lowe, V. J. , Jack, C. R., Jr. , & Whitwell, J. L. (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain, 135(Pt. 5), 1522–1536. https://doi.org/10.1093/brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Strand, E. A. , Whitwell, J. L. , Layton, K. F. , Parisi, J. E. , Hauser, M. F. , Witte, R. J. , Boeve, B. F. , Knopman, D. S. , Dickson, D. W. , Jack, C. R., Jr. , & Petersen, R. C. (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129(Pt. 6), 1385–1398. https://doi.org/10.1093/brain/awl078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaat, L. D. , Boon, A. J. W. , Kamphorst, W. , Ravid, R. , Duivenvoorden, H. J. , & van Swieten, J. C. (2007). Frontal presentation in progressive supranuclear palsy. Neurology, 69(8), 723–729. https://doi.org/10.1212/01.wnl.0000267643.24870.26 [DOI] [PubMed] [Google Scholar]
- Kim, J.-H. , & McCann, C. M. (2015). Communication impairments in people with progressive supranuclear palsy: A tutorial. Journal of Communication Disorders, 56, 76–87. https://doi.org/10.1016/j.jcomdis.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Kluin, K. J. , Foster, N. L. , Berent, S. , & Gilman, S. (1993). Perceptual analysis of speech disorders in progressive supranuclear palsy. Neurology, 43(3, Pt. 1), 563–566. https://doi.org/10.1212/wnl.43.3_part_1.563 [DOI] [PubMed] [Google Scholar]
- Kluin, K. J. , Gilman, S. , Foster, N. , Sima, A. , D'Amato, C. , Bruch, L. , Bluemlein, L. , Little, R. , & Johanns, J. (2001). Neuropathological correlates of dysarthria in progressive supranuclear palsy. Archives of Neurology, 58(2), 265–269. https://doi.org/10.1001/archneur.58.2.265 [DOI] [PubMed] [Google Scholar]
- Metter, E. J. , & Hanson, W. R. (1991). Dysarthria in progressive supranuclear palsy. In Moore C., Yorkston K., & Beukelman D. (Eds.), Dysarthria and apraxia of speech: Perspectives on management (pp. 127–136). Brookes. [Google Scholar]
- Muller, J. , Wenning, G. K. , Verny, M. , McKee, A. , Chaudhuri, K. R. , Jellinger, K. , Poewe, W. , & Litvan, I. (2001). Progression of dysarthria and dysphagia in postmortem-confirmed parkinsonian disorders. Archives of Neurology, 58(2), 259–264. https://doi.org/10.1001/archneur.58.2.259 [DOI] [PubMed] [Google Scholar]
- Nath, U. , Ben-Shlomo, Y. , Thomson, R. G. , Lees, A. J. , & Burn, D. J. (2003). Clinical features and natural history of progressive supranuclear palsy: A clinical cohort study. Neurology, 60(6), 910–916. https://doi.org/10.1212/01.Wnl.0000052991.70149.68 [DOI] [PubMed] [Google Scholar]
- Rusz, J. , Bonnet, C. , Klempíř, J. , Tykalová, T. , Baborová, E. , Novotný, M. , Rulseh, A. , & Růžička, E. (2015). Speech disorders reflect differing pathophysiology in Parkinson's disease, progressive supranuclear palsy and multiple system atrophy. Journal of Neurology, 262(4), 992–1001. https://doi.org/10.1007/s00415-015-7671-1 [DOI] [PubMed] [Google Scholar]
- Santos-Santos, M. A. , Mandelli, M. L. , Binney, R. J. , Ogar, J. , Wilson, S. M. , Henry, M. L. , Hubbard, H. I. , Meese, M. , Attygalle, S. , Rosenberg, L. , Pakvasa, M. , Trojanowski, J. Q. , Grinberg, L. T. , Rosen, H. , Boxer, A. L. , Miller, B. L. , Seeley, W. W. , & Gorno-Tempini, M. L. (2016). Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurology, 73(6), 733–742. https://doi.org/10.1001/jamaneurol.2016.0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodda, S. , Visser, W. , & Schlegel, U. (2011). Acoustical analysis of speech in progressive supranuclear palsy. Journal of Voice, 25(6), 725–731. https://doi.org/10.1016/j.jvoice.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Strand, E. A. , Duffy, J. R. , Clark, H. M. , & Josephs, K. A. (2014). The Apraxia of Speech Rating Scale: A tool for diagnosis and description of apraxia of speech. Journal of Communication Disorders, 51, 43–50. https://doi.org/10.1016/j.jcomdis.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa, D. , Monza, D. , Ferrarini, M. , Soliveri, P. , Girotti, F. , & Filippini, G. (2001). Comparison of natural histories of progressive supranuclear palsy and multiple system atrophy. Neurological Sciences, 22(3), 247–251. https://doi.org/10.1007/s100720100021 [DOI] [PubMed] [Google Scholar]
- Tykalova, T. , Rusz, J. , Klempir, J. , Cmejla, R. , & Ruzicka, E. (2017). Distinct patterns of imprecise consonant articulation among Parkinson's disease, progressive supranuclear palsy and multiple system atrophy. Brain and Language, 165, 1–9. https://doi.org/10.1016/j.bandl.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Utianski, R. L. , Clark, H. M. , Duffy, J. R. , Botha, H. , Whitwell, J. L. , & Josephs, K. A. (2020). Communication limitations in patients with progressive apraxia of speech and aphasia. American Journal of Speech-Language Pathology, 29(4), 1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski, R. L. , Duffy, J. R. , Clark, H. M. , Strand, E. A. , Boland, S. M. , Machulda, M. M. , Whitwell, J. L. , & Josephs, K. A. (2018). Clinical progression in four cases of primary progressive apraxia of speech. American Journal of Speech Language Pathology, 27(4), 1303–1318. https://doi.org/10.1044/2018_AJSLP-17-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski, R. L. , Duffy, J. R. , Clark, H. M. , Strand, E. A. , Botha, H. , Schwarz, C. G. , Machulda, M. M. , Senjem, M. L. , Spychalla, A. J. , Jack, C. R., Jr. , Petersen, R. C. , Lowe, V. J. , Whitwell, J. L. , & Josephs, K. A. (2018). Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain and Language, 184, 54–65. https://doi.org/10.1016/j.bandl.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh, J. L. , Bailey, D. J. , Mauszycki, S. C. , & Bunker, L. D. (2019). Interrater reliability and concurrent validity for the Apraxia of Speech Rating Scale 3.0: Application with persons with acquired apraxia of speech and aphasia. American Journal of Speech-Language Pathology, 28(2S), 895–904. https://doi.org/10.1044/2018_AJSLP-MSC18-18-0099 [DOI] [PubMed] [Google Scholar]
- Whitwell, J. L. , Stevens, C. A. , Duffy, J. R. , Clark, H. M. , Machulda, M. M. , Strand, E. A. , Martin, P. R. , Utiansky, R. L. , Botha, H. , Spychalla, A. J. , Senjem, M. L. , Schwarz, C. G. , Jack, C. R., Jr. , Ali, F. , Hassan, A. , & Josephs, K. A. (2019). An evaluation of the progressive supranuclear palsy speech/language variant. Movement Disorders Clinical Practice, 6(6), 452–461. https://doi.org/10.1002/mdc3.12796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell, J. L. , Tosakulwong, N. , Botha, H. , Ali, F. , Clark, H. M. , Duffy, J. R. , Utianski, R. L. , Stevens, C. A. , Weigand, S. D. , Schwarza, C. G. , Senjem, M. L. , Jack, C. R., Jr. , Lowe, V. J. , Ahlskog, E. , Dickson, D. W. , & Josephs, K. A. (2020). Brain volume and flortaucipir analysis of progressive supranuclear palsy clinical variants. NeuroImage: Clinical, 25, 102152. https://doi.org/10.1016/j.nicl.2019.102152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell, J. L. , Weigand, S. D. , Duffy, J. R. , Clark, H. M. , Strand, E. A. , Machulda, M. M. , Spychalla, A. J. , Senjem, M. L. , Jack, C. R., Jr. , & Josephs, K. A. (2017). Predicting clinical decline in progressive agrammatic aphasia and apraxia of speech. Neurology, 89(22), 2271–2279. https://doi.org/10.1212/WNL.0000000000004685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston, K. M. , Strand, E. , Miller, R. , Hillel, A. D. , & Smith, K. (1993). Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. Journal of Medical Speech-Language Pathology, 1, 35–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.