Abstract
Purpose
Aphasia intervention research aims to improve communication and quality of life outcomes for people with aphasia. However, few studies have evaluated the translation and implementation of evidence-based aphasia interventions to clinical practice. Treatment dosage may be difficult to translate to clinical settings, and a mismatch between dosage in research and clinical practice threatens to attenuate intervention effectiveness. The purpose of this study is to quantify a potential research–practice dosage gap in outpatient aphasia rehabilitation.
Method
This study utilized a two-part approach. First, we estimated clinical treatment dosage in an episode of care (i.e., treatment provided from outpatient assessment to discharge) via utilization in a regional provider in the United States. Second, we undertook a scoping review of aphasia interventions published from 2009 to 2019 to estimate the typical dosage used in the current aphasia literature.
Results
Outpatient clinical episodes of care included a median of 10 treatment sessions and a mean of 14.8 sessions (interquartile range: 5–20 sessions). Sessions occurred 1–2 times a week over 4–14 weeks. The median total hours of treatment was 7.5 hr (interquartile range: 3.75–15 hr). In contrast, published interventions administered a greater treatment dosage, consisting of a median of 20 hr of treatment (interquartile range: 12–30 hr) over the course of 15 sessions (interquartile range: 10–24 sessions) approximately 3 times per week.
Conclusions
Results demonstrate a meaningful research–practice dosage gap, particularly in total treatment hours and weekly treatment intensity. This gap highlights the potential for attenuation of effectiveness from research to outpatient settings. Future translational research should consider clinical dosage constraints and take steps to facilitate intervention implementation, particularly with regard to dosage. Conversely, health care advocacy and continued development of alternative delivery methods are necessary for the successful implementation of treatments with dosage that is incompatible with current clinical contexts. Pragmatic, implementation-focused trials are recommended to evaluate and optimize treatment effectiveness in outpatient clinical settings.
Supplemental Material
The fundamental goal of aphasia rehabilitation research is to improve communication and quality of life outcomes for people with aphasia. Intervention research targeting these outcomes ranges from proof of concept, feasibility studies to large effectiveness trials, primarily conducted in academic research settings. However, few studies have evaluated how well-established aphasia interventions translate into everyday clinical practice settings (Roberts et al., 2020). Substantial differences between clinical research and clinical practice settings may reduce treatment fidelity for evidence-based interventions in clinical settings and risk attenuating treatment effectiveness in clinical practice (Bauer et al., 2015). This phenomenon is described as “voltage drop” in the field of implementation science (Chambers et al., 2013). Given that the ultimate goal of aphasia rehabilitation research is to improve outcomes for people with aphasia, we need to carefully consider how well our laboratory-based treatment studies are calibrated for the clinical practice settings in which they are routinely applied. In other words, can published aphasia interventions be implemented with reasonable fidelity in routine clinical practice?
Translating treatment dosage from clinical research to clinical practice settings can be especially challenging and is one potential source of voltage drop in aphasia rehabilitation. Broadly, treatment dose refers to the amount of treatment given during an intervention and is a critical element of every aphasia intervention, regardless of setting. A framework published by Warrenet al. (2007) and extended by Baker (2012) further divides dose in behavioral interventions into subcomponents. Dose form describes the treatment task, including the therapeutic inputs, active ingredients, and client responses. Session dose is the total number of times the dose form is provided in a single treatment session. In many studies, session dose is estimated in terms of the minutes or hours of treatment provided (i.e., session duration). Session frequency describes how often treatment sessions occur (i.e., twice weekly). Together, session dose or session duration and frequency make up treatment intensity, or the amount of treatment provided in a given period. Treatment duration characterizes the total length of the treatment, typically in weeks or months. The total dose (i.e., cumulative treatment intensity) can be estimated by multiplying session dose by session frequency and treatment duration (e.g., 100 trials × 2 sessions per week × 4 weeks) or a similar combination of these parameters.
Aphasia treatment studies employ a wide range of treatment intensity and total dose. Evans et al. (2021), Kendall et al. (2019), and Conlon et al. (2020) provided approximately 60 hr of treatment across from 3, 6, or 15 weeks. Other studies have used a more modest dosage, intentionally selected to approximate local clinical practice settings. For example, a number of studies by Conroy, Carragher, and colleagues have implemented treatment 1–2 times per week for 6–8 weeks (Carragher et al., 2013; Conroy et al., 2018). The role of dosage in aphasia treatment research is unlikely to be as straightforward as “more is better.” The optimal treatment dosage for different aphasia interventions remains an area of open inquiry (e.g., Cherney, 2012; Conlon et al., 2020; Dignam et al., 2015; Mozeiko et al., 2016).
In pursuit of synthesizing dose–response relationships in the aphasia treatment literature, Harvey et al. (2020b) found significant variability in the reporting of different dose parameters and challenges with synthesizing dosage in the aggregate. Studies reviewed by Harvey et al. included between one and 100 total hours with a modal dosage of 30 hr but were not further specified.
Even less is known about the typical dosage in clinical practice settings. While clinical aphasia services are often described as limited, relatively few published studies have quantitatively described treatment dosage in clinical episodes of care (i.e., speech-language pathology services provided in a given setting from assessment to discharge). Dosage in intensive, comprehensive aphasia programs is often described in analyses of clinical outcomes (e.g., Winans-Mitrik et al., 2014). Speech-language pathology service utilization in inpatient rehabilitation settings has been previously reported for people with aphasia (Hardy et al., 2019). However, there are little empirical data on the typical dosage received in outpatient settings, defined as ambulatory care provided to individuals with aphasia after they are discharged home.
Katz et al. (2000) surveyed 175 clinicians in four countries and found that clinicians in the U.S. private sector reported providing the most outpatient sessions, between one and 20, with a mean of nine sessions. Shifts in service delivery to managed care models and health care reform may have influenced this estimate over the past 20 years. A recent study focused on access to outpatient rehabilitation services in general stroke survivors reported that Medicare beneficiaries receive an average of 8 total hours of outpatient speech-language pathology services within the first year after stroke (Skolarus et al., 2017). Given that only approximately one third of stroke survivors have aphasia (Laska et al., 2001), it is not clear how well this finding represents the treatment services received by all stroke survivors with aphasia.
Outpatient services are a crucial component of the continuum of care, facilitating the transition from inpatient rehabilitation to long-term adaptation for stroke survivors with new impairments and often-altered independence. Outpatient providers are the “last stop” in the rehabilitation medical model for people with aphasia. Additionally, outpatient clinical practice must accommodate a wide range of pragmatic barriers to service delivery. There is a great deal of variation in funding and insurance coverage for outpatient speech-language pathology services, which affects access to outpatient rehabilitation providers (Ostwald et al., 2009). Until recently, the Centers for Medicare and Medicaid Services placed clear restrictions on the amount of funding available to Medicare beneficiaries for outpatient services (Ortolan, 2017). Outpatient clinical services also require the person with aphasia to have access to consistent transportation to the clinic, which can be challenging for stroke survivors who cannot drive after their stroke (Ing et al., 2014). Furthermore, outpatient services can be constrained by clinician availability and productivity requirements (Hinckley et al., 2013; Sarno, 2004). Unlike clinical research, the amount of time available to clinicians and their clients is unlikely to be solely dedicated to a single therapy approach, which would be unlikely to address all facets of aphasia recovery.
Overall, a mismatch in treatment dosage between clinical research and outpatient clinical practice may have significant, negative consequences for outcomes in everyday clinical practice for people with aphasia. Such a mismatch is a clear threat to the external validity and effective implementation of aphasia intervention research: If an evidence-based treatment protocol provides an estimate of a treatment effect at one dose, it may not engender clinically significant changes at a lesser dose. However, neither typical dosage in clinical research nor outpatient clinical practice has been sufficiently quantified to evaluate the scope of this potential problem. Therefore, the overarching purpose of this study is to evaluate this potential research–practice dosage gap.
This study utilized a two-part approach. First, we analyzed clinical billing data from a large, regional rehabilitation provider to estimate parameters of treatment dosage for people with aphasia in outpatient clinical settings. This was intended to serve as a proxy measure for similar settings across the United States. Second, we undertook a scoping review of aphasia interventions published over a 10-year period (2009–2019) to calculate general parameters of treatment dosage across the contemporary aphasia treatment literature. We report on the findings for each of these substudies, followed by a general comparison between the two sources of data. Our research questions are as follows:
What is the typical treatment dose received by people with aphasia in an episode of care in outpatient rehabilitation clinical settings?
What is the typical treatment dose administered to people with aphasia in contemporary clinical aphasia studies?
To what extent is the dosage in contemporary aphasia treatment research aligned with current outpatient clinical practice settings?
Method
Question 1: What Is the Typical Treatment Dose Received by People With Aphasia in an Episode of Care in Outpatient Rehabilitation Clinical Settings?
To estimate treatment dosage in outpatient clinical practice settings, de-identified speech-language pathology utilization data were extracted from billing records from the Center for Rehab Services at the University of Pittsburgh Medical Center from 2009 to 2019 for records with International Classification of Diseases (ICD) diagnoses of stroke and aphasia. The Centers for Rehabilitation Services (CRS) is a major outpatient neurorehabilitation provider with more than 20 clinics across Western Pennsylvania. CRS provides comprehensive outpatient rehabilitation services across a large number of neurological and orthopedic conditions, including stroke. Data extraction was undertaken in collaboration with the Health Record Research Request Service at the University of Pittsburgh, a service in the Department of Biomedical Informatics that provisions clinical data for research purposes. While the initial goal was to extract utilization across a 10-year time span to match Study 2, data were only available from 2014 on due to the transition to a new electronic medical record vendor.
Patient records were limited to those with an existing diagnosis of International Classification of Diseases, Ninth Revision or International Classification of Diseases, Tenth Revision stroke and a diagnosis of aphasia after 2012, along with at least one billed speech-language pathology evaluation and one billed speech-language pathology treatment from an outpatient CRS speech-language pathology department. Codes used to define the study cohort were developed in collaboration with the Health Record Research Request service and are reported in the Appendix.
Evaluations were defined by Current Procedural Terminology (CPT) codes used by speech-language pathologists for the evaluation of aphasia (CPT: 92523, 92506, or 96105) within 2 years of the first dated stroke or aphasia diagnosis. Treatment sessions (i.e., each billed visit) were defined solely by the treatment code 92507 (“Treatment of speech, language, voice, communication, and or auditory processing disorder; individual”), which is billed for aphasia treatment sessions typically lasting 45 min in CRS clinics. Patient records containing CPT codes utilized for cognitive rehab were excluded to minimize the odds of including episodes of care focused on poststroke cognitive-communication deficits. These exclusionary CPT codes consisted of 97532, 97127, and G0515. This exclusion criterion eliminated < 5% of encounters; the vast majority of treatment sessions were billed under 92507.
Dosage was estimated for each episode of care, which we defined as all sessions from qualifying patient records from the initial evaluation to the last treatment session. As there is no billed code for treatment discharge, the last treatment session in an episode of care was defined as a treatment session followed by 60 days without a billed speech-language pathology session. If a new evaluation occurred within the 60-day window, the following sessions were considered to be part of a new episode of care, as evaluation codes are not typically billed for re-evaluations. Notwithstanding, additional evaluations within the 60-day window were rare in this data set.
After establishing patient-level episodes of care, we calculated summary statistics for patient demographics included in the final data set: age, sex, and race. Therapy utilization was mapped to variables of treatment dosage define here as the total number of billed treatment sessions in the episode of care, the average weekly frequency of billed treatment sessions, the total number of hours of treatment in the episode of care, the number of hours of treatment per week, and the total duration of the episode of care, in weeks. Calculations of weekly frequency excluded episodes of care with less than four sessions to minimize outstanding influence from brief episodes of care with a low frequency. Both weekly frequency and total treatment duration were calculated from the interval between the first and last treatment sessions and did not include the time between evaluation and the first treatment session. After mapping treatment sessions to dosage, summary statistics were calculated for each variable. We also calculated the percentage of people with aphasia who received more than one episode of care and the total number of sessions received per patient in the first 2 years regardless of the encounter.
Question 2: What Is the Typical Treatment Dose Administered to People With Aphasia in Contemporary Clinical Aphasia Studies?
A scoping review was used to quantify the typical treatment dose administered in aphasia intervention research. This review format was chosen as the focus was to broadly characterize aphasiologists' selection of treatment dosage, regardless of study design, quality, or outcomes. The scoping review format was particularly advantageous in methodologically examining the “extent, range, and nature” of aphasia research activity in a way that allowed us to generate summary quantitative information (Arksey & O'Malley, 2005). Arksey and O'Malley's scoping review framework was used to outline the methodology in the following five stages. The protocol was preregistered prior to the initiation of the review (https://osf.io/uyxr3).
Stage 1: Identifying Research Questions
The primary aim of this scoping review was to answer the question, “What is the typical treatment dose administered to people with aphasia in contemporary clinical aphasia studies?” We operationalized dosage following the definitions of Warren et al. (2007) into the number of treatment sessions, the number of hours per session, the number of sessions per week, the number of weeks of treatment, and the total number of hours provided in a single research treatment program, such that they mapped to statistics collected for each episode of care in the clinical data set.
Stage 2: Identifying Relevant Studies
Eligibility criteria for this study were based on several broad considerations: (a) The scoping review should focus on clinical populations similar to those included in the larger research study, which focuses on people with poststroke aphasia receiving outpatient services; (b) the time span for the scoping review should reflect current research practices and be roughly equivalent to the period of the clinical data available for Question 1 (2009–2019); and (c) the scoping review should focus on treatment studies whose primary purpose is to improve some form of communication outcomes for people with aphasia, such as a language modality, strategy use, communication effectiveness, or communication quality of life.
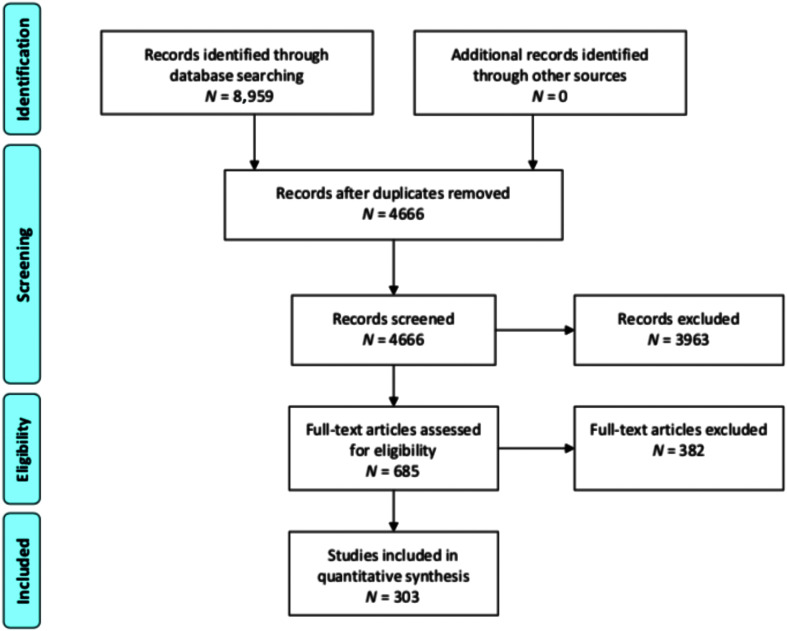
We searched Ovid Medline, Embase via Embase.com, EBSCO CINAHL, EBSCO ERIC, Ovid PsycINFO, the Cochrane Database of Systematic Reviews via Wiley, and Linguistics and Language Behavior Abstracts via ProQuest. The search strategy was developed by a health sciences librarian (the fifth author) using a combination of subject headings and keywords that described aphasia and therapy or rehabilitation. The full search strategies for all databases are reported in Supplemental Material S1. The search was limited to studies published after January 1, 2009, and results were downloaded from the databases on December 3, 2019. After the search, duplicate records were removed in EndNote.
Stage 3: Study Selection
Study screening and selection were completed by four reviewers (first, third, fourth, and sixth authors) using the review software DistillerSR (Evidence Partners). Given the criteria noted above and the large number of articles, an iterative, multilevel screening process was used. In Level 1, titles and abstracts were screened by the first author on the basis of two criteria: They (a) evaluated any sort of behavioral intervention and (b) reported that the intervention was provided to individuals with acquired aphasia (i.e., treatment studies that did not mention aphasia in the title or abstract were not included). Five percent of initial abstracts were reviewed for reliability of inclusion/exclusion by a second reviewer (third author). The percent agreement for inclusion was 96%. In Level 2, each abstract was independently screened by two reviewers to determine if (a) the study examined the effects of a behavioral intervention(s) on a communication outcome, (b) the study intervention was not specifically targeted to people with aphasia admitted to a facility (i.e., rehabilitation hospitals or skilled nursing facilities), and (c) treatment was not augmented by medication, brain stimulation, or non–speech-language interventions (e.g., acupuncture). Additional planned criteria for Level 2 were moved to Level 3 due to limited reporting of this information in study abstracts. If there was any conflict between reviewers, the article progressed to a full review in Level 3.
After Level 2, the aforementioned review team met to review and refine existing inclusion/exclusion criteria for full article review. In particular, our discussion focused on a frequently occurring challenge: how to clearly define an aphasia “treatment study” in a way that is sufficient to answer the proposed questions in the review. As the focus of this review was not on study quality and we wanted to minimize bias in our selection criteria, we elected to cast a wide net for inclusion of articles in Level 3. As a result, criteria for study inclusion in Level 3 were broader than initially planned and are specified in detail in Table 1.
Table 1.
Scoping review: Level 3 inclusion and exclusion criteria.
| Inclusion criteria |
| 1. The article reported results from an experimental/prospective study. |
| 2. The intervention could reasonably be provided by a speech-language pathologist within the scope of practice. |
| 3. The study cohort included only people with aphasia or dyads including people with aphasia (e.g., people with aphasia and a family member). |
| 4. The study evaluated at least one outcome measure related to communication within the International Classification of Functioning, Disability and Health framework. |
| Exclusion criteria |
| 1. The article described the outcomes of clinical services or clinical programs. |
| 2. The study cohort included people with other communication disorders without aphasia. |
| 3. The intervention was not specified. Typically described as providing “speech therapy” or “cognitive-linguistic therapy.” |
| 4. The intervention was intended to be provided by a provider other than a speech-language pathologist, such as a psychologist or an acupuncturist. |
| 5. The article only reported a study protocol and did not report study results. |
| 6. The intervention was specifically targeted to people with aphasia admitted to a facility (i.e., rehabilitation hospitals or skilled nursing facilities). |
| 7. The intervention was augmented by medication, brain stimulation, or non–speech-language interventions (e.g., acupuncture). |
In Level 3, the remaining articles were reviewed in full by two reviewers to ensure they met all inclusion and exclusion criteria in Table 1. Disagreement was resolved through consensus with a third reviewer. At this stage, conference proceedings and non–peer-reviewed publications were identified and removed. A number of additional duplicate articles not identified by EndNote were also removed at this stage. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; Moher et al., 2009) flow diagram reports study screening and selection for Levels 1–3 (see Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram describing identification, screening, eligibility, and inclusion.
Stage 4: Charting the Data
Key information was extracted from the articles that meet all eligibility criteria by the first author. Basic information on the article, namely, the title, authors, source, journal, year of publication, and participants, was extracted. Then for each article, we extracted parameters of treatment dosage: the total number of hours of treatment, the total number of sessions, the duration of each session, the weekly frequency of sessions, and the number of weeks of treatment. Only the parameters reported by the authors were extracted, and calculations of omitted parameters was done postextraction where possible (see below). For studies comparing two treatment conditions unrelated to dosage, we extracted the average of each parameter across conditions. Additionally, we extracted five variables pertinent to dosage to characterize studies included in this review: whether or not each study (a) was deployed using telehealth, (b) incorporated homework or home practice, (c) was implemented using specific software designed for aphasia rehabilitation, (d) reported effect sizes, and (e) included any treatment in groups or dyads. A second reviewer extracted data for 5% of included articles to assess the reliability of data extraction. Percent agreement for data extraction for sample size, home practice, effect sizes, dyads, telehealth, and group treatment was 93% (range: 82%–100%). Percent agreement for dosage extraction, calculated as the percentage of studies where both reviewers reported all dosage parameters as the same, was 92%. The small number of conflicts (2) in dosage extraction was reviewed and determined to be the result of reporting ambiguity rather than extraction error.
Stage 5: Collating, Summarizing, and Reporting the Results
As in previous studies (Cherney, 2012; Harvey et al., 2020b), we found a large variation in how often and how detailed dosage was reported across the literature. Furthermore, while we made efforts to create a flexible data-capture system, not all studies reported dosage in ways we specified. Therefore, we implemented the following postprocessing steps. (a) In studies that reported differing intensities between participants, such as studies comparing dosage between groups or single-case design studies administering treatment until a criterion was reached, each dosage parameter was averaged for the entire study, weighted by the number of participants receiving that dosage. (b) Parameters reported in a range were averaged. For instance, a study reporting that session duration lasted 45 min to 1 hr was counted as 52.5 min. (c) If a study did not report one of the six collected parameters, it was reported as n/a in the data extraction process. In postprocessing, the dosage parameter was calculated if other parameters were available. For example, if a study reported that treatment was provided 2 times per week for 4 weeks for a total of 16 hr, we then calculated that the intervention included eight sessions that were 2 hr in duration. This step was implemented with every possibility to minimize missing data.
After processing, summary statistics were calculated for each element of treatment dosage across all studies. We also calculated the percentage of studies that reported sufficient information to calculate each treatment variable and the percentage of studies that included the five additional criteria noted above. Last, while the results and discussion of this article focus on these summary statistics, we also calculated dosage parameters across studies weighted by sample size to account for potential differences in dosage between different study types and sizes, as larger studies are often considered to substantiate stronger evidence in the literature.
Question 3: To What Extent Is the Dosage in Contemporary Aphasia Treatment Research Aligned With Current Outpatient Clinical Practice Settings?
After calculating summary statistics for Studies 1 and 2, we employed robust statistical tests to compare dosage parameters. We compared each dosage parameter, with the exception of session duration, via a two-sample difference-in-medians permutation test using the R (R Core Team, 2020; 4.0.2) package infer (Bray et al., 2020). This nonparametric test evaluates whether there is a statistical difference in the medians of two distributions and is well suited to this comparison as it does not require data to be normally distributed and is robust to skewing and outliers. First, the difference in median values from each distribution is calculated. Then, a null distribution is generated via permutation by repeatedly shuffling the data from both distributions (here, 1,000 simulations). The p value is estimated by calculating the percentage of simulations from the null distribution that falls outside the difference in medians. If less than 5% of the simulated null distribution falls outside of the difference in medians, we can reject the null hypothesis that there is no difference in the two median values. Because session duration in CRS clinics is typically 45 min, we utilized a similar one-sample median permutation test to evaluate whether session duration in the scoping review was different from 45 min.
Results
Question 1: What Is the Typical Treatment Dose Received by People With Aphasia in an Episode of Care in Outpatient Rehabilitation Clinical Settings?
In total, 683 episodes of care across 24 CRS clinics from 2014 to 2019 were included in the final sample (including 602 unique patients). Of these records, 320 were for women, and 363 were for men, consistent with general findings that age-specific stroke incidence is higher for men (Reeves et al., 2008). Eighty-two percent of people with aphasia in the encounters identified as White; and 13.5%, as Black. People with aphasia identifying as Filipino, Indian, Japanese, Vietnamese, or “Other Asian” comprised less than 1% of encounters. Approximately 3% were reported as “not specified” or “declined.” Racial demographics in this sample are relatively consistent with the general racial makeup of Western Pennsylvania as published by the 2010 U.S. census, though the general demographics of stroke survivors with aphasia in Western Pennsylvania are not known. Age was widely distributed from 14 to 95 years, with a median age of 63 years. This age range is notably lower than general stroke incidence, where about two thirds of strokes occur in people 65 years or older (Hall et al., 2012). We suspect that this difference is likely due to evaluating utilization within an outpatient setting, which is associated with younger patients than inpatient or home health rehabilitation settings (Chan et al., 2009). Older adults are more likely to be discharged to skilled nursing following stroke (Nguyen et al., 2015).
Summary statistics for estimates of treatment dosage are reported in Table 2. The median number of sessions per encounter was 10 sessions, and the mean number of sessions per encounter was 14.5 sessions (interquartile range: 5–20 total sessions). Of the 683 episodes of care included in this data set, 570 consisted of more than three total sessions and were included in the weekly frequency and intensity calculations. The median number of sessions per week was 1.4 (interquartile range: 1.1–1.8). Last, the median treatment duration was 7.7 weeks (interquartile range: 4–14.6). The median number of hours of treatment was 7.5. Dosage parameters were notable for positive skewing. Additionally, 10% of patients attended more than one episode of care in the first 2 years after the first reported diagnosis. When estimating the number of sessions for 2 years following the initial evaluation collapsing across episodes of care, the mean was 16.8 and the median was 11 sessions across all encounters. Less than 2% of patients attended more than two episodes of care.
Table 2.
Outpatient dosage statistics from 2014 to 2019 for episodes of care with International Classification of Diseases (ICD) diagnosis of stroke and aphasia at the Centers for Rehabilitation Services in Western Pennsylvania.
| Variable | Mean | Median | Minimum | Q25 | Q75 | Maximum |
|---|---|---|---|---|---|---|
| Total sessions | 14.5 | 10.0 | 1.0 | 5.0 | 20.0 | 99.0 |
| Total hours | 10.9 | 7.5 | 0.8 | 3.8 | 15.0 | 74.3 |
| Hours per week | 1.1 | 1.1 | 0.3 | 0.8 | 1.4 | 2.6 |
| Sessions per week | 1.5 | 1.4 | 0.4 | 1.1 | 1.8 | 3.6 |
| Total weeks | 10.6 | 7.7 | 0.1 | 4.0 | 14.6 | 51.3 |
Note. Dosage variables are calculated across individual episodes of care. Session duration is 45 min per session for all treatment sessions. A total of 683 episodes of care were included in the study, 570 of which had more than four sessions and were included in estimates of weekly frequency.
Question 2: What Is the Typical Treatment Dose Administered to People With Aphasia in Contemporary Clinical Aphasia Studies?
The comprehensive search identified 8,959 potential articles, and a final total of 303 articles were included. A final list of articles is reported in Supplemental Material S2. Included articles provided a wide range of interventions from short-term treatment paradigms focused on identifying underlying treatment mechanisms to large-scale group interventions focused on efficacy. Articles included a range of study types from single-subject experimental designs to larger, randomized controlled trials. Studies included a total of 2,987 participants. The median age of participants with aphasia across all articles was 59 years (range: 11–92 years). 1 Furthermore, 58.4% of participants were male, and 41.6% of participants were female. Of the articles included, 78.8% reported sufficient information to calculate all dosage parameters of interest. We were able to calculate the total number of hours in 85.8% of articles, the total number of sessions in 92.1% of articles, session duration in 82.5% of articles, the weekly frequency in 87.5% of articles, and treatment duration in weeks in 92.4% of articles.
Additionally, we found that 26.8% of studies included some form of home practice, which was inconsistently included in the dosage parameters, and adherence was not consistently tracked. A percentage (20.2%) of studies utilized a specific app or software (e.g., AphasiaScripts; Lee & Cherney, 2008) for at least some aspect of the intervention, and 6.3% utilized telehealth or videoconferencing. Last, 13.1% of studies included at least some treatment in groups or dyads, and 45.4% of studies reported effect sizes. Study statistics are also reported in Table 3.
Table 3.
Percentage of studies providing sufficient information to calculate dosage parameters (top) and study characteristics of studies included in the scoping review (bottom).
| Dosage variable | Percent reported |
|---|---|
| Total sessions | 92.1 |
| Total hours | 85.8 |
| Hours per session | 82.5 |
| Hours per week | 83.8 |
| Sessions per week | 87.5 |
| Total weeks | 92.4 |
|
Study characteristic |
Percentage of studies |
| Included group or dyad | 13.1 |
| Reported effect sizes | 45.4 |
| Used specific software or app | 20.2 |
| Included home practice | 26.8 |
| Utilized telehealth | 6.3 |
Weighted and unweighted mean, median, and interquartile ranges for each parameter are reported in Table 4. Distributions for dosage parameters were positively skewed. In brief, the interquartile range (i.e., the middle 50%) of published aphasia interventions in our sample provided a median of 20 hr of treatment (interquartile range: 12–30 hr) over the course of a median of 15 sessions (interquartile range: 10–24 sessions) 2–5 times per week.
Table 4.
Scoping review dosage statistics averaged across studies (top) and weighted by study sample size (bottom).
| Variable | Mean | Median | Minimum | Q25 | Q75 | Maximum |
|---|---|---|---|---|---|---|
| Total sessions | 20.1 | 15.0 | 1.0 | 10.0 | 23.8 | 137.0 |
| Total hours | 25.1 | 20.0 | 1.0 | 12.0 | 30.0 | 151.3 |
| Hours per session | 1.3 | 1.0 | 0.2 | 0.9 | 1.5 | 4.0 |
| Hours per week | 4.7 | 3.0 | 0.5 | 2.0 | 5.0 | 22.9 |
| Sessions per week | 3.6 | 3.0 | 0.6 | 2.0 | 5.0 | 20.0 |
| Total weeks | 7.0 | 6.0 | 1.0 | 4.0 | 8.0 | 63.6 |
|
Total sessions |
21.6 |
15.0 |
1.0 |
10.0 |
24.1 |
137.0 |
| Total hours | 28.9 | 24.3 | 1.0 | 15.0 | 37.4 | 151.3 |
| Hours per session | 1.5 | 1.0 | 0.2 | 1.0 | 2.0 | 4.0 |
| Hours per week | 5.7 | 4.0 | 0.5 | 2.3 | 7.5 | 22.9 |
| Sessions per week | 4.1 | 3.0 | 0.6 | 2.0 | 5.0 | 20.0 |
| Total weeks | 7.0 | 6.0 | 1.0 | 3.5 | 9.0 | 63.6 |
Note. Dosage variables are calculated across studies. The number of treatment studies that reported sufficient information to calculate each variable varied, as follows: total sessions, 279/303; total hours, 260/303; hours per session, 250/303; hours per week, 254/303; sessions per week, 265/303; and total weeks, 280/303.
Question 3: To What Extent Is the Dosage in Contemporary Aphasia Treatment Research Aligned With Current Outpatient Clinical Practice Settings?
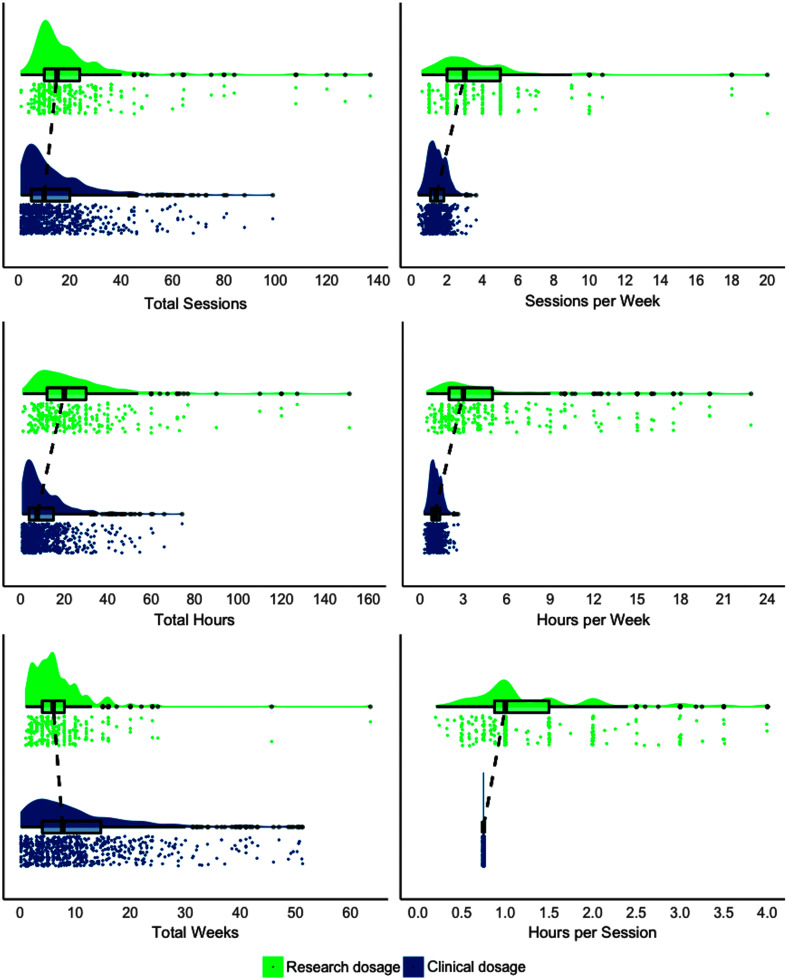
Permutation tests demonstrated that differences were significant for all dosage parameters (p < .0001) such that dosage was significantly greater in the scoping review versus clinic data for all parameters, except total treatment duration in weeks, which was significantly greater for clinic data. The distributions for each dosage parameter and the overlap between research and clinical estimates are visualized in Figure 2 using raincloud plots (Allen et al., 2019). Raincloud plots provide information about individual observations, patterns across observations, and the central tendency of a distribution. Unstandardized effect sizes, the difference in medians, are reported in Table 5. Given the relatively large sample size, which is powered to detect small differences, we emphasize interpreting the results in terms of the effect size, the difference in medians, and not p values alone (Wasserstein et al., 2019).
Figure 2.
Distributions for clinical and research dosage are visualized using raincloud plots. Raincloud plots include a density plot (top), a box plot with the median, interquartile range, and whiskers extending 1.5 times the interquartile range. Observations beyond 1.5 times the interquartile range are shown as solid gray dots. Dashed lines visualize differences between the median between clinical and research distributions. Vertically jittered, raw observations are plotted below each density and box plot (Allen et al., 2019). Research distributions are averaged across studies. Differences between distributions are significant in all cases (p < .0001).
Table 5.
Difference in medians between research and clinical dosage and associated p values from nonparametric permutation tests.
| Variable | Difference in medians | p |
|---|---|---|
| Total sessions | 5.0 | < .0001 |
| Total hours | 12.5 | < .0001 |
| Hours per session | 0.3 | < .0001 |
| Hours per week | 2.0 | < .0001 |
| Sessions per week | 1.6 | < .0001 |
| Total weeks | −1.7 | < .0001 |
Note. Difference in medians reflects research dosage − clinical dosage. p value was estimated using two-sample difference-in-medians permutation test. Hours per session was estimated using one-sample difference-in-medians permutation test, relative to a 45-min (0.75-hr) treatment session.
Discussion
The overarching purpose of this study was to quantify a potential dosage research–practice gap between clinical aphasia research and outpatient clinical practice settings. Such a gap, if it exists, threatens the external validity and resulting effectiveness of aphasia interventions in outpatient clinical settings. To accomplish this aim, we first evaluated billing data from a major regional outpatient neurorehabilitation provider to estimate clinical treatment received by people with aphasia in outpatient clinical settings. Second, we systematically extracted key parameters of treatment dosage from the aphasia treatment literature from 2009 to 2019 and calculated summary statistics describing the typical treatment dosage administered in contemporary aphasia treatment research. Ultimately, we compared the distributions of dosage between clinical practice and the aphasia treatment research. In the following, we discuss the results for each of these research questions and the implications for future research and clinical practice settings.
Question 1: What Is the Typical Treatment Dose Received by People With Aphasia in an Episode of Care in Outpatient Rehabilitation Clinical Settings?
To answer Question 1, we analyzed clinical billing data as a proxy for clinical treatment dosage. In general, the distributions derived from billing data appear to reasonably approximate expectations, given the clinical experience of the authorship team within CRS and similarly sized outpatient rehabilitation clinics, and existing reports in the literature (e.g., Katz et al., 2000; Simmons-Mackie, 2018; Skolarus et al., 2017).
In general, we found that treatment dosage for stroke survivors with aphasia is likely to range between five and 20 sessions 1–2 times a week over the course of 4–14 weeks. Given that the typical treatment duration in CRS clinics for aphasia is 45 min, the middle 50% of people with aphasia receive 3.75–15 hr of treatment per encounter, with a median of 7.5 hr. These findings approximate previously reported hours of treatment from Skolarus et al. (2017) for stroke survivors who are Medicare beneficiaries, which found that stroke survivors receive approximately 8 hr within the first year after stroke. The weekly session frequency is consistent with the one to two sessions reported by Simmons-Mackie (2018). Additionally, we note that the distributions of these dosage variables are both skewed and highly distributed, such that a subset of people with aphasia received an extended period of rehabilitation that is well outside of the interquartile range. However, even for these people with aphasia who receive treatment for more than 3–4 months, session frequency was around two sessions per week.
To date, information on clinical dosage/utilization have been estimated based on clinician survey, studies focused on general stroke survivors, and our general knowledge of insurance limitations, all of which have constrained our understanding of clinical dosage. While there are a number of nontrivial limitations to this data set (discussed below), we feel confident that this study represents a much-needed empirical approximation of real-world clinical dosage for people with aphasia in outpatient clinical settings within the U.S. healthcare system.
Question 2: What Is the Typical Treatment Dose Administered to People With Aphasia in Contemporary Clinical Aphasia Studies?
To answer Question 2, we systematically extracted key parameters of treatment dosage from the aphasia treatment literature from 2009 to 2019. Consistent with recent work in this domain, reporting of treatment dose in aphasia rehabilitation research was highly variable (Harvey et al., 2020b; Pierce et al., 2020). The clarity and transparency of reporting on treatment dosage are not ideal, such that researchers wishing to evaluate or replicate the dosage of a nontrivial number of published treatment studies may be unable to do so. We did not attempt to collect any information regarding dose form (e.g., the number of repetitions of an active ingredient) within treatment sessions as this parameter is not consistently reported and difficult to summarize across the wide variety of interventions (Harvey et al., 2020b). To this end, we echo previous recommendations that authors explicitly and transparently describe treatment dosage when reporting aphasia interventions using existing frameworks (Hoffmann et al., 2014).
Not surprisingly, the results of the scoping review reveal a wide distribution of treatment dosage across the aphasia literature, from interventions delivered weekly to intensive protocols that administer treatment for multiple hours per day, 5 days per week. In this review, we applied a relatively broad definition of aphasia intervention, so as not to bias the results from our preconceptions of what constitutes an aphasia treatment study. Consequently, we included a number of interventions that were focused more on theoretical questions rather than clinical use at the prescribed dosage. For example, studies by Middleton and colleagues (e.g., Middleton et al., 2015) have advanced our understanding of test-related practice effects and effortful retrieval in anomia in such a way that they qualified for inclusion but are unlikely to be intended to be implemented in clinical settings in such small dosages (i.e., approximately 1 hr of treatment in one session). On the other hand, we also included a number of case studies that provided treatment to one or two people with aphasia for extended durations that are impractical in larger trials. In the most extreme case, Webster and Gordon (2009) provided therapy to a person with aphasia for approximately 150 total hours. We account for these outliers by focusing our interpretation of robust summary statistics (median and interquartile range) rather than statistics subject to skewing and outliers. Furthermore, to address concerns that larger studies constitute stronger evidence for existing interventions, we also calculated dosage parameters weighted by study sample size. In this case, dosage estimates are modestly larger for the weighted dosage parameters (median: 24.3 total hours, interquartile range: 15–37.4 total hours).
It is worth noting that some studies intentionally provided intensive treatment protocols, whether to evaluate the effects of high-dosage aphasia interventions or, often, with the goal of producing quick and robust treatment effects sufficient to identify neurobehavioral correlates of treatment response. These studies are in contrast to those explicitly provided at dosages consistent with clinical rehabilitation practice patterns or studies that administer treatment via technology that does not require constant face-to-face contact with a provider. Broad summary statistics are agnostic to these nuances in administered treatment dosage. Still, all studies, regardless of dosage, serve to substantiate the evidence base for clinical aphasia practice.
Question 3: To What Extent Is the Dosage in Contemporary Aphasia Treatment Research Aligned With Current Outpatient Clinical Practice Settings?
Having estimated the typical range of dosage in both outpatient clinical practice settings and quantified the typical dosage in clinical aphasia research, we turn to a general comparison of data from these two sources. In the following, we discuss the implications for current clinical aphasia services, models of care provision for people with chronic aphasia, and suggestions for future critical needs that need to be addressed by the aphasia research and clinical communities.
On the whole, dosage parameters in clinical practice are less than what is typically employed in treatment studies, with the notable exception of total treatment duration. Such findings are not surprising and are consistent with anecdotal reports and scarce clinical data on outpatient therapy utilization. The total number of treatment hours and the number of treatment hours per week appear to be particularly disparate between research and clinical practice, driven by additive differences in treatment session duration and weekly session frequency.
On the other hand, the total duration of treatment provided was robustly greater in clinical practice (median: 7.7 weeks; interquartile range: 4–14.6 weeks) than in clinical research (median: 6 weeks, interquartile range: 4–8 weeks). These differences likely reflect the pragmatic constraints of clinical practice in terms of reimbursement and provider availability. In general, this finding suggests that people with aphasia receive a more distributed treatment schedule than much of the aphasia treatment literature and that more distributed practice schedules may be more compatible with outpatient clinical practice settings. Ongoing work continues to compare intensive and distributed practice schedules in aphasia rehabilitation (Conlon et al., 2020; Dignam et al., 2015), and these schedules are more or less compatible with different clinical practice settings. For example, intensive schedules are more compatible with inpatient rehabilitation settings in the United States, as they provide clinical services for up to 3 hr/day across therapy disciplines for 5–6 days/week. Intensive, comprehensive aphasia programs are also able to provide high-dose, high-intensity treatment. However, intensive schedules are rarely compatible with traditional outpatient settings similar to those described in this study.
This broad comparison between clinical research and outpatient clinical practice does not consider differences in research and clinical priorities. For instance, research sessions are, by design, entirely dedicated to the provision of the target intervention to equate dosage across participants. Clinicians manage a more extensive administrative burden and provide ongoing counseling and goal setting—all of which limit the time available for a single intervention. The time spent on a single intervention likely constitutes a fraction of the total treatment time available. On the other hand, while home practice does not appear to be the norm in aphasia interventions in this study, it is utilized relatively often in clinical settings (Brown et al., 2020). It is possible that home practice can augment a limited number of sessions in outpatient settings, but there are little data available to evaluate whether or not it is sufficiently prescribed or completed to evaluate this hypothesis.
The notion that clinical providers may experience difficulty implementing the aphasia evidence base in real-world clinical settings is not novel. We hope the data presented in this study begin to quantify clinicians' challenges in applying current aphasia rehabilitation research in current clinical practice settings. In pointing out this research–practice gap, we offer several recommendations that may help optimize the translation of treatment dosage to clinical settings.
First, aphasia researchers should be thoughtful about their selection of dosage regarding the stage of research and long-term objectives of the research agenda. Treatment dosage in early-stage research is likely to reflect the underlying research question, statistical power, and funding constraints. However, for later-stage research focused on improving clinical outcomes, aphasia researchers should provide a clear justification for deviating from a dosage that is attainable in clinical settings. For example, high-intensity treatments might add to the increasing evidence base for intensive, comprehensive aphasia programs, a critical step to justifying the cost of these programs to people with aphasia and funding sources (Hula et al., 2013). Otherwise, administering research interventions in dosages attainable in clinical practice reduces the risk of significant voltage drop when they are ultimately applied by clinicians.
Second, few large-scale studies have experimentally examined the effects of dosage on treatment outcomes (e.g.,Dignam et al., 2015). We reiterate previous assertions that determining the optimal dosage (in terms of treatment intensity and cumulative treatment dose) of effective treatments should remain a priority in aphasia research (Harvey et al., 2020a).
Third, if treatments intended for clinical use are more effective at higher dosages, researchers should take steps to provide easily accessible materials to facilitate effective home practice and augment limited face-to-face time with clinicians. While software and app development might constitute an ideal method for delivering effective home practice, low-tech options can be similarly effective. For example, Beeson et al. (2003) have made templates for Copy and Recall Treatment home practice freely available online. Edmonds (2014) has published clinical tutorials describing verb network strengthening treatment in detail, including the dosage of the evidence base. When designing treatment plans, clinicians should consider dosage differences between the evidence base for a given treatment and what amount of total treatment time (face-to-face and home practice) is feasible for each individual client with aphasia when making decisions about treatment selection. Similarly, we encourage clinicians to consider the difference between their face-to-face time with a client and the published dosage of a selected treatment as a starting point for the amount of home practice they might prescribe.
Fourth, once treatment effectiveness has been established in research settings, it is crucial that researchers further evaluate treatment effectiveness in real-world clinical settings through pragmatic trials. Such studies should be stakeholder driven, incorporating perspectives of people with aphasia, caregivers, clinicians, and researchers to identify pragmatic solutions to the challenges of implementing aphasia interventions in clinical settings. Currently, implementation work in aphasia rehabilitation and communication disorders is rare. For example, a recent study found that less than 1% of all clinical practice studies published in journals from the American Speech-Language-Hearing Association evaluated the implementation of clinical research (Roberts et al., 2020). At the moment, the strongest evidence for current practice patterns and use of evidence-based practice in clinical settings is likely found in “usual care” arms of large trials (e.g., Brogan, Ciccone, & Godecke, 2020; Brogan, Godecke, & Ciccone, 2020).
The current findings suggest two diverging directions for addressing a research–practice dosage gap between clinical research and clinical practice. As discussed, it is clear that further research is needed to optimize the translation of aphasia interventions to current clinical practice settings. However, if ongoing research establishes that treatments provided at higher treatment dosage engender superior outcomes, advocacy will be increasingly critical to promote access to high-intensity clinical services, whether in outpatient settings, community aphasia groups, or intensive, comprehensive aphasia programs.
While our focus was to evaluate a potential research–practice dosage gap between clinical aphasia research and outpatient clinical practice settings, it is worth noting that additional factors need to be considered to improve the quality of translational and implementation aphasia research. Aphasia intervention research must continue to work toward specifying the essential, active ingredients (i.e., dose form) of interventions. Without a clear specification of the active ingredients, the true dosage of an intervention is unknown. Understanding which treatment components are critical and which components are unrelated to treatment response will make aphasia interventions more efficient for clinical settings where time is a major limitation (Turkstra et al., 2016). The importance of treatment dosage also does not supersede the need for treatments to hold therapeutic value in the first place. No increase in dosage will make an ineffective treatment worth using.
While this study compared dosage during a single episode of care compared to a single instance of research participation, it is also worth noting that aphasia is a chronic condition that is rarely completely addressed in a single episode of care. Therefore, a successful long-term rehabilitation program likely requires multiple sequential episodes of care, building upon and augmenting successive gains in function over time (e.g., Beeson et al., 2019). However, long-term sequential treatment paradigms are rare in aphasia research. While people with aphasia respond positively to rehabilitation years and decades into the chronic phase (e.g., Smania et al., 2010), there is limited evidence supporting sequential long-term care models specifically. A more comprehensive understanding of dosage should take these longer-term temporal considerations into account.
Limitations
We believe that the clinical and research data generated by this work provide a much-needed comparison of real-world clinical dosage in outpatient clinical practice settings to published treatment research. However, we would like to be transparent with regard to the limitations of this data set and the comparisons made in this study.
First, therapy utilization was estimated from a single provider in a single region of the United States, and it is not clear how representative these data are to the U.S. healthcare system as a whole. Insurance rules and provision often vary from state to state depending on the insurers that offer coverage in the state. Western Pennsylvania is also not as diverse as many other regions of the United States, which may positively or negatively affect utilization. Additionally, CRS is a large rehabilitation provider that may not be representative of outpatient clinics nationally. The present findings should be replicated at the national level.
There are inherent limitations to information provided by billing data. Speech-language pathologists primarily use a nonspecific billing code for evaluations provided to people with aphasia (92523), likely because their evaluations include assessment of domains outside of language. Thus, the 92523 CPT code may be preferable over 96105 because 92523 is both a better representation of the evaluation and because it reimburses at a higher rate. The CPT treatment code typically used for aphasia is similarly a catch-all for most adult speech-language pathology services inclusive of speech, voice, and language. As such, even though we identified people with diagnoses of aphasia, it is entirely possible treatment was provided for other poststroke communication disorders such as dysarthria or apraxia of speech. However, treatment for dysphagia and cognition do have separate CPT codes, and so we suspect it is unlikely that the sessions captured here were primarily focused on dysphagia or cognitive-communication disorders.
We do not know the reasons for short treatment encounters in this data set. Utilization may be limited due to problems of access to therapy, such as transportation, insurance coverage, or financial status. It may be affected by aphasia severity or time postonset. In our experience, it is likely that some encounters reflect instances where a person with aphasia attended only a few follow-up sessions and subsequently declined further therapy due to a quick recovery. With the current approach, we can carefully quantify the amount of treatment people with aphasia actually receive, but not whether this dosage was sufficient for their individual needs. Future work could address this limitation by incorporating a detailed chart review of individual patient records to identify reasons for discharge, which is outside the scope of this work.
We also have no way of empirically evaluating diagnosis date and diagnosis accuracy. Previous research has indicated that ICD codes are relatively accurate at “ruling in” stroke diagnosis (Birman-Deych et al., 2005). However, we are not aware of any evidence to date pertaining to the accuracy of ICD codes as they pertain to the diagnosis of aphasia. Still, the presence of stroke and aphasia diagnosis codes, speech-language evaluations, and the exclusion of dysphagia and cognitive treatment codes should provide some confidence that the estimates reported in this study are reasonably accurate for people with poststroke aphasia.
With regard to the scoping review, because of the presence of recently published, comprehensive work, we did not evaluate the quality of each included study. Instead, we converged on clear inclusion criteria for aphasia treatment studies that we felt would set a minimum standard for inclusion. These criteria excluded studies that were explicitly targeted toward inpatient rehabilitation settings or applied some adjuvant, whether stimulation or pharmaceutical, to maximize the applicability to current outpatient clinical services. Studies that did not explicitly mention aphasia in the title or abstract were also excluded (e.g., Beeson et al., 2018). The time span for the scoping review was limited to 2009–2019 to match the anticipated date range of clinical data and characterize current trends in dosage over the past 10 years. However, this shortened date range also excluded many influential aphasia treatment studies published prior to 2009. It is not clear whether these excluded studies might have administered different dosages than those included in this review. There were differences in the included articles in contrast to the recent scoping review from Harvey et al. (2020b), which we attribute to differences in our literature search strategy and inclusion and exclusion criteria.
Conclusions
Successful implementation of aphasia interventions in real-world clinical practice settings is key to maximizing treatment outcomes for people with aphasia. In this study, we compared treatment dosage in recent aphasia rehabilitation research with an estimate of treatment dosage in outpatient clinical practice settings. Results demonstrate that treatment dosage in outpatient clinical practice may be substantially less than what is typical of the aphasia treatment literature. This meaningful research–practice gap is particularly apparent in weekly treatment intensity and the total number of treatment hours. Because the estimated effect sizes demonstrated in the aphasia treatment literature are often based on greater dosage, this mismatch increases the potential for voltage drop in clinical practice (i.e., the attenuation of intervention effects from research to clinical settings). It also highlights the fact that the dosage utilized in current aphasia treatment literature is likely difficult for clinicians to implement with reasonable treatment fidelity. Expanding future research in clinical effectiveness, dissemination, and implementation science research is recommended to address the substantial research–practice gap identified in this study.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award TL1TR001858. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was supported by the SHRS Research Development Fund, School of Health and Rehabilitation Sciences, University of Pittsburgh. The Health Record Research Request Service is currently supported by the National Institutes of Health (Grant UL1-TR001857). We are grateful to Scott Rothenberger, PhD, Assistant Professor of Medicine and Statistician, Center for Research on Health Care Data Center, at the University of Pittsburgh School of Medicine, for his advice on statistical methods employed in this study. We would also like to thank the research team at the Health Record Research Request Service at the University of Pittsburgh for their assistance in procuring clinical data. Sincere thanks to Brandon Nguy and Joshua Peckman for their assistance in reliability scoring and article management in the scoping review.
Appendix
Inclusion Criteria for Study 1
Record must have a relevant CPT code billed in a Speech-Language Pathology Department:
CPT evaluation code: 92523, 92506, or 96105
CPT treatment code: 92507
CPT cognitive exclusion code: 97532, 97127, or G0515.
And a diagnosis of stroke and aphasia
ICD-9: Stroke and Aphasia
438.11, 438.12
ICD-10: Stroke and Aphasia
I69.020, I69.021, I69.120, I69.121, I69.220, I69.221, I69.320, I69.321, I69.820, I69.821, I69.920, I69.921.
Or a diagnosis of aphasia and a separate diagnosis of stroke
ICD-9 Aphasia: 784.3
ICD-10 Aphasia: R47.01, R47.02
ICD-9 Stroke:
362.3, 430, 431, 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91, 435, 435.0, 435.1, 435.2, 435.3, 435.8, 435.9, 436, 438, 438.0, 438.1, 438.10, 438.11, 438.12, 438.13, 438.14, 438.19, 438.2, 438.20, 438.21, 438.22, 438.3, 438.30, 438.31, 438.32, 438.4, 438.40, 438.41, 438.42, 438.5, 438.50, 438.51, 438.52, 438.53, 438.6, 438.7, 438.8, 438.81, 438.82, 438.83, 438.84, 438.85, 438.89, 438.9
ICD-10 Stroke:
G45, G45.0, G45.1, G45.2, G45.3, G45.4, G45.8, G45.9, I60, I60.0, I60.00, I60.01, I60.02, I60.1, I60.10, I60.11, I60.12, I60.2, I60.3, I60.30, I60.31, I60.32, I60.4, I60.5, I60.50, I60.51, I60.52, I60.6, I60.7, I60.8, I60.9, I61, I61.0, I61.1, I61.2, I61.3, I61.4, I61.5, I61.6, I61.8, I61.9, I62, I62.0, I62.00, I62.01, I62.02, I62.03, I62.1, I62.9, I63, I63.0, I63.00, I63.01, I63.011, I63.012, I63.013, I63.019, I63.02, I63.03, I63.031, I63.032, I63.033, I63.039, I63.09, I63.1, I63.10, I63.11, I63.111, I63.112, I63.113, I63.119, I63.12, I63.13, I63.131, I63.132, I63.133, I63.139, I63.19, I63.2, I63.20, I63.21, I63.211, I63.212, I63.213, I63.219, I63.22, I63.23, I63.231, I63.232, I63.233, I63.239, I63.29, I63.3, I63.30, I63.31, I63.311, I63.312, I63.313, I63.319, I63.32, I63.321, I63.322, I63.323, I63.329, I63.33, I63.331, I63.332, I63.333, I63.339, I63.34, I63.341, I63.342, I63.343, I63.349, I63.39, I63.4, I63.40, I63.41, I63.411, I63.412, I63.413, I63.419, I63.42, I63.421, I63.422, I63.423, I63.429, I63.43, I63.431, I63.432, I63.433, I63.439, I63.44, I63.441, I63.442, I63.443, I63.449, I63.49, I63.5, I63.50, I63.51, I63.511, I63.512, I63.513, I63.519, I63.52, I63.521, I63.522, I63.523, I63.529, I63.53, I63.531, I63.532, I63.533, I63.539, I63.54, I63.541, I63.542, I63.543, I63.549, I63.59, I63.6, I63.8, I63.81, I63.89, I63.9
Funding Statement
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award TL1TR001858. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was supported by the SHRS Research Development Fund, School of Health and Rehabilitation Sciences, University of Pittsburgh. The Health Record Research Request Service is currently supported by the National Institutes of Health (Grant UL1-TR001857).
Footnote
Zipse et al. (2012) included one 11-year-old individual with aphasia. Otherwise, the minimum age was 17 years.
References
- Allen, M. , Poggiali, D. , Whitaker, K. , Marshall, T. R. , & Kievit, R. A. (2019). Raincloud plots: A multi-platform tool for robust data visualization. Wellcome Open Research, 4, 63. https://doi.org/10.12688/wellcomeopenres.15191.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arksey, H. , & O'Malley, L. (2005). Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology, 8(1), 19–32. https://doi.org/10.1080/1364557032000119616 [Google Scholar]
- Bauer, M. S. , Damschroder, L. , Hagedorn, H. , Smith, J. , & Kilbourne, A. M. (2015). An introduction to implementation science for the non-specialist. BMC Psychology, 3(1), 32. https://doi.org/10.1186/s40359-015-0089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, E. (2012). Optimal intervention intensity. International Journal of Speech-Language Pathology, 1(5), 401–409. https://doi.org/10.3109/17549507.2012.700323 [DOI] [PubMed] [Google Scholar]
- Beeson, P. M. , Bayley, C. , Shultz, C. , & Rising, K. (2019). Maximising recovery from aphasia with central and peripheral agraphia: The benefit of sequential treatments. Neuropsychological Rehabilitation, 29(9), 1399–1425. https://doi.org/10.1080/09602011.2017.1417873 [DOI] [PubMed] [Google Scholar]
- Beeson, P. M. , Rising, K. , DeMarco, A. T. , Foley, T. H. , & Rapcsak, S. Z. (2018). The nature and treatment of phonological text agraphia. Neuropsychological Rehabilitation, 28(4), 568–588. https://doi.org/10.1080/09602011.2016.1199387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson, P. M. , Rising, K. , & Volk, J. (2003). Writing treatment for severe aphasia. Journal of Speech, Language, and Hearing Research, 46(5), 1038–1060. https://doi.org/10.1044/1092-4388(2003/083) [DOI] [PubMed] [Google Scholar]
- Birman-Deych, E. , Waterman, A. D. , Yan, Y. , Nilasena, D. S. , Radford, M. J. , & Gage, B. F. (2005). Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Medical Care, 43(5), 480–485. https://doi.org/10.1097/01.mlr.0000160417.39497.a9 [DOI] [PubMed] [Google Scholar]
- Bray, A. , Ismay, C. , Chasnovski, E. , Baumer, B. , & Cetinkaya-Rundel, M. (2020). infer: Tidy Statistical Inference (0.5.4) [Computer software] . https://CRAN.R-project.org/package=infer
- Brogan, E. , Ciccone, N. , & Godecke, E. (2020). An exploration of aphasia therapy dosage in the first six months of stroke recovery. Neuropsychological Rehabilitation. Advance online publication. https://doi.org/10.1080/09602011.2020.1776135 [DOI] [PubMed] [Google Scholar]
- Brogan, E. , Godecke, E. , & Ciccone, N. (2020). Behind the therapy door: What is “usual care” aphasia therapy in acute stroke management? Aphasiology, 34(10), 1291–1313. https://doi.org/10.1080/02687038.2020.1759268 [Google Scholar]
- Brown, E. D. , Wallace, S. , Liu, Q. , & Famularo, M. (2020). Creating home practice programs for persons with aphasia: A survey of speech language pathologists. Archives of Physical Medicine and Rehabilitation, 101(11), e72. https://doi.org/10.1016/j.apmr.2020.09.219 [Google Scholar]
- Carragher, M. , Sage, K. , & Conroy, P. (2013). The effects of verb retrieval therapy for people with non-fluent aphasia: Evidence from assessment tasks and conversation. Neuropsychological Rehabilitation, 23(6), 846–887. https://doi.org/10.1080/09602011.2013.832335 [DOI] [PubMed] [Google Scholar]
- Chambers, D. A. , Glasgow, R. E. , & Stange, K. C. (2013). The dynamic sustainability framework: Addressing the paradox of sustainment amid ongoing change. Implementation Science, 8(1), Article 117. https://doi.org/10.1186/1748-5908-8-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, L. , Wang, H. , Terdiman, J. , Hoffman, J. , Ciol, M. A. , Lattimore, B. F. , Sidney, S. , Quesenberry, C. , Lu, Q. , & Sandel, M. E. (2009). Disparities in outpatient and home health service utilization following stroke: Results of a 9-year cohort study in Northern California. PM&R, 1(11), 997–1003. https://doi.org/10.1016/j.pmrj.2009.09.019 [DOI] [PubMed] [Google Scholar]
- Cherney, L. R. (2012). Aphasia treatment: Intensity, dose parameters, and script training. International Journal of Speech-Language Pathology, 14(5), 424–431. https://doi.org/10.3109/17549507.2012.686629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon, E. L. , Braun, E. J. , Babbitt, E. M. , & Cherney, L. R. (2020). Treatment fidelity procedures for an aphasia intervention within a randomized controlled trial: Design, feasibility, and results. American Journal of Speech-Language Pathology, 29(1S), 412–424. https://doi.org/10.1044/2019_AJSLP-CAC48-18-0227 [DOI] [PubMed] [Google Scholar]
- Conroy, P. , Sotiropoulou Drosopoulou, C. , Humphreys, G. F. , Halai, A. D. , & Lambon Ralph, M. A. (2018). Time for a quick word? The striking benefits of training speed and accuracy of word retrieval in post-stroke aphasia. Brain, 141(6), 1815–1827. https://doi.org/10.1093/brain/awy087 [DOI] [PubMed] [Google Scholar]
- Dignam, J. , Copland, D. , McKinnon, E. , Burfein, P. , O'Brien, K. , Farrell, A. , & Rodriguez, A. D. (2015). Intensive versus distributed aphasia therapy: A nonrandomized, parallel-group, dosage-controlled study. Stroke, 46(8), 2206–2211. https://doi.org/10.1161/STROKEAHA.115.009522 [DOI] [PubMed] [Google Scholar]
- Edmonds, L. A. (2014). Tutorial for Verb Network Strengthening Treatment (VNeST): Detailed description of the treatment protocol with corresponding theoretical rationale. SIG 2 Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 24(3), 78–88. https://doi.org/10.1044/nnsld24.3.78 [Google Scholar]
- Evans, W. S. , Cavanaugh, R. , Gravier, M. L. , Autenreith, A. M. , Doyle, P. J. , Hula, W. D. , & Dickey, M. W. (2021). Effects of semantic feature type, diversity, and quantity on semantic feature analysis treatment outcomes in aphasia. American Journal of Speech-Language Pathology, 30(1S), 344–358. https://doi.org/10.1044/2020_AJSLP-19-00112 [DOI] [PubMed] [Google Scholar]
- Hall, M. J. , Levant, S. , & DeFrances, C. J. (2012). Hospitalization for stroke in US hospitals, 1989–2009. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Hardy, R. Y. , Lindrooth, R. C. , Peach, R. K. , & Ellis, C. (2019). Urban–rural differences in service utilization and costs of care for racial-ethnic groups hospitalized with poststroke aphasia. Archives of Physical Medicine and Rehabilitation, 100(2), 254–260. https://doi.org/10.1016/j.apmr.2018.06.033 [DOI] [PubMed] [Google Scholar]
- Harvey, S. , Carragher, M. , Dickey, M. W. , Pierce, J. E. , & Rose, M. L. (2020a). Dose effects in behavioural treatment of post-stroke aphasia: A systematic review and meta-analysis. Disability and Rehabilitation. Advance online publication. https://doi.org/10.1080/09638288.2020.1843079 [DOI] [PubMed] [Google Scholar]
- Harvey, S. , Carragher, M. , Dickey, M. W. , Pierce, J. E. , & Rose, M. L. (2020b). Treatment dose in post-stroke aphasia: A systematic scoping review. Neuropsychological Rehabilitation. Advance online publication. https://doi.org/10.1080/09602011.2020.1786412 [DOI] [PubMed] [Google Scholar]
- Hinckley, J. J. , Hasselkus, A. , & Ganzfried, E. (2013). What people living with aphasia think about the availability of aphasia resources. American Journal of Speech-Language Pathology, 22(2), S310–S317. https://doi.org/10.1044/1058-0360(2013/12-0090) [DOI] [PubMed] [Google Scholar]
- Hoffmann, T. C. , Glasziou, P. P. , Boutron, I. , Milne, R. , Perera, R. , Moher, D. , Altman, D. G. , Barbour, V. , Macdonald, H. , Johnston, M. , Lamb, S. E. , Dixon-Woods, M. , McCulloch, P. , Wyatt, J. C. , Chan, A.-W. , & Michie, S. (2014). Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ, 348, g1687. https://doi.org/10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- Hula, W. D. , Cherney, L. R. , & Worrall, L. E. (2013). Setting a research agenda to inform intensive comprehensive aphasia programs. Topics in Stroke Rehabilitation, 20(5), 409–420. https://doi.org/10.1310/tsr2005-409 [DOI] [PubMed] [Google Scholar]
- Ing, M. M. , Vento, M. A. , Nakagawa, K. , & Linton, K. F. (2014). A qualitative study of transportation challenges among intracerebral hemorrhage survivors and their caregivers. Hawai’i Journal of Medicine & Public Health, 73(11), 353–357. [PMC free article] [PubMed] [Google Scholar]
- Katz, R. C. , Hallowell, B. , Code, C. , Armstrong, E. , Roberts, P. , Pound, C. , & Katz, L. (2000). A multinational comparison of aphasia management practices. International Journal of Language & Communication Disorders, 35(2), 303–314. https://doi.org/10.1080/136828200247205 [DOI] [PubMed] [Google Scholar]
- Kendall, D. L. , Moldestad, M. O. , Allen, W. , Torrence, J. , & Nadeau, S. E. (2019). Phonomotor versus semantic feature analysis treatment for anomia in 58 persons with aphasia: A randomized controlled trial. Journal of Speech, Language, and Hearing Research, 62(12), 4464–4482. https://doi.org/10.1044/2019_JSLHR-L-18-0257 [DOI] [PubMed] [Google Scholar]
- Laska, A. C. , Hellblom, A. , Murray, V. , Kahan, T. , & Von Arbin, M. (2001). Aphasia in acute stroke and relation to outcome. Journal of Internal Medicine, 249(5), 413–422. https://doi.org/10.1046/j.1365-2796.2001.00812.x [DOI] [PubMed] [Google Scholar]
- Lee, J. B. , & Cherney, L. R. (2008). The changing “face” of aphasia therapy. SIG 2 Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 18(1), 15–23. https://doi.org/10.1044/nnsld18.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton, E. L. , Schwartz, M. F. , Rawson, K. A. , & Garvey, K. (2015). Test-enhanced learning versus errorless learning in aphasia rehabilitation: Testing competing psychological principles. Learning, Memory, and Cognition, 41(4), 1253–1261. https://doi.org/10.1037/xlm0000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & The PRISMA Group. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLOS Medicine, 6(7), e1000097. https://doi.org/10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozeiko, J. , Coelho, C. A. , & Myers, E. B. (2016). The role of intensity in constraint-induced language therapy for people with chronic aphasia. Aphasiology, 30(4), 339–363. https://doi.org/10.1080/02687038.2015.1070949 [Google Scholar]
- Nguyen, V. Q. , PrvuBettger, J. , Guerrier, T. , Hirsch, M. A. , Thomas, J. G. , Pugh, T. M. , & Rhoads, C. F., III (2015). Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Archives of Physical Medicine and Rehabilitation, 96(7), 1297–1303. https://doi.org/10.1016/j.apmr.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Ortolan, T. (2017). New congressional legislation finally eliminates the Medicare therapy cap. Journal of Health Care Finance, 44(2), Article 2. [Google Scholar]
- Ostwald, S. K. , Godwin, K. M. , Cheong, H. , & Cron, S. G. (2009). Predictors of resuming therapy within four weeks after discharge from inpatient rehabilitation. Topics in Stroke Rehabilitation, 16(1), 80–91. https://doi.org/10.1310/tsr1601-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, J. E. , O'Halloran, R. , Menahemi-Falkov, M. , Togher, L. , & Rose, M. L. (2020). Comparing higher and lower weekly treatment intensity for chronic aphasia: A systematic review and meta-analysis. Neuropsychological Rehabilitation. Advance online publication. https://doi.org/10.1080/09602011.2020.1768127 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing (4.0.2). R Foundation for Statistical Computing; . https://www.r-project.org/ [Google Scholar]
- Reeves, M. J. , Bushnell, C. D. , Howard, G. , Gargano, J. W. , Duncan, P. W. , Lynch, G. , Khatiwoda, A. , & Lisabeth, L. (2008). Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. The Lancet. Neurology, 7(10), 915–926. https://doi.org/10.1016/S1474-4422(08)70193-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, M. Y. , Sone, B. J. , Zanzinger, K. E. , Bloem, M. E. , Kulba, K. , Schaff, A. , Davis, K. C. , Reisfeld, N. , & Goldstein, H. (2020). Trends in clinical practice research in ASHA journals: 2008–2018. American Journal of Speech-Language Pathology, 29(3), 1629–1639. https://doi.org/10.1044/2020_AJSLP-19-00011 [DOI] [PubMed] [Google Scholar]
- Sarno, M. T. (2004). Aphasia therapies: Historical perspectives and moral imperatives. In Duchan J. F. & Byng S. (Eds.), Challenging aphasia therapies: Broadening the discourse and extending the boundaries (pp. 33–45). Psychology Press. [Google Scholar]
- Simmons-Mackie, N. (2018). Aphasia in North America. Aphasia Access. [Google Scholar]
- Skolarus, L. E. , Feng, C. , & Burke, J. F. (2017). No racial difference in rehabilitation therapy across all post-acute care settings in the year following a stroke. Stroke, 48(12), 3329–3335. https://doi.org/10.1161/STROKEAHA.117.017290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smania, N. , Gandolfi, M. , Aglioti, S. M. , Girardi, P. , Fiaschi, A. , & Girardi, F. (2010). How long is the recovery of global aphasia? Twenty-five years of follow-up in a patient with left hemisphere stroke. Neurorehabilitation and Neural Repair, 24(9), 871–875. https://doi.org/10.1177/1545968310368962 [DOI] [PubMed] [Google Scholar]
- Turkstra, L. S. , Norman, R. , Whyte, J. , Dijkers, M. P. , & Hart, T. (2016). Knowing what we're doing: Why specification of treatment methods is critical for evidence-based practice in speech-language pathology. American Journal of Speech-Language Pathology, 25(2), 164–171. https://doi.org/10.1044/2015_AJSLP-15-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, S. F. , Fey, M. E. , & Yoder, P. J. (2007). Differential treatment intensity research: A missing link to creating optimally effective communication interventions. Mental Retardation and Developmental Disabilities Research Reviews, 13(1), 70–77. https://doi.org/10.1002/mrdd.20139 [DOI] [PubMed] [Google Scholar]
- Wasserstein, R. L. , Schirm, A. L. , & Lazar, N. A. (2019). Moving to a world beyond “p < 0.05.” The American Statistician, 73(Suppl. 1), 1–19. https://doi.org/10.1080/00031305.2019.1583913 [Google Scholar]
- Webster, J. , & Gordon, B. (2009). Contrasting therapy effects for verb and sentence processing difficulties: A discussion of what worked and why. Aphasiology, 23(10), 1231–1251. https://doi.org/10.1080/02687030802246291 [Google Scholar]
- Winans-Mitrik, R. L. , Hula, W. D. , Dickey, M. W. , Schumacher, J. G. , Swoyer, B. , & Doyle, P. J. (2014). Description of an intensive residential aphasia treatment program: Rationale, clinical processes, and outcomes. American Journal of Speech-Language Pathology, 23(2), S330–S342. https://doi.org/10.1044/2014_AJSLP-13-0102 [DOI] [PubMed] [Google Scholar]
- Zipse, L. , Norton, A. , Marchina, S. , & Schlaug, G. (2012). When right is all that is left: Plasticity of right-hemisphere tracts in a young aphasic patient. Annals of the New York Academy of Sciences, 1252, 237–245. https://doi.org/10.1111/j.1749-6632.2012.06454.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.