Abstract
Glycopeptide-resistant enterococci (GRE) from broilers and pigs were characterized to investigate the background for the persistence of GRE in pig herds. All porcine isolates belonged to closely related pulsed-field gel electrophoretic (PFGE) types, with the ermB and vanA genes located on the same transferable genetic element. Broiler isolates belonged to different PFGE types. The persistence of GRE in Danish pig herds after the ban of glycopeptides may be explained by the genetic link between ermB and vanA and coselection by use of macrolides for treatment and growth promotion.
Glycopeptide-resistant enterococci (GRE) have emerged as important pathogens since the late 1980s (6, 22). Resistance to glycopeptides in Enterococcus faecium and Enterococcus faecalis is mediated by the vanA, vanB, vanD, or vanE gene cluster (12, 15, 16).
In 1993, vanA-positive GRE were isolated from food animals in England (10), and since then, GRE containing the vanA gene have been found among food animals in several countries worldwide (2, 14, 19, 31). It has been shown that the occurrence of GRE among food animals is associated with the use of the glycopeptide avoparcin for growth promotion (1, 7, 19), and a number of studies have shown that similar E. faecium clones and vanA gene clusters can be found among food animals and humans (14, 17, 26, 27, 29, 30).
Because GRE can be transferred from food animals to humans and because of the risk of severe infections in humans, the use of avoparcin was banned in Denmark in 1995. A ban followed in Germany in 1996 and in all European Union countries in 1997.
Whereas several studies have shown an association between the use of antimicrobial agents and the occurrence of resistance, only a limited number of studies have reported changes in resistance among natural populations after the discontinuation of antimicrobial agent usage.
Since the discontinuation of avoparcin usage in animal husbandry, a decline in the occurrence of GRE in poultry meat has been observed in Germany (20) and Italy (24) and in fecal samples from humans in the community in Germany (20). Similarly, in Denmark, a decline from 80% to 5% in the occurrence of GRE isolated from broilers was observed from 1995 to 1998, whereas the occurrence of GRE in pigs remained at about 20% (8). The reason for the persistence of GRE in pig herds is not known but may be a consequence of coselection through the use of other antimicrobial agents. Thus, a previous study found almost all GRE from pigs to be simultaneously resistant to tetracycline and macrolides (8).
In this study, GRE isolated from broilers and pigs in Denmark from 1995 through 1998 were characterized by pulsed-field gel electrophoresis (PFGE), hybridization analysis, plasmid profiling, and transferability studies. Studies of genetic markers may provide an explanation for the persistence of GRE in pig herds in contrast to broiler flocks.
Bacterial isolates.
Bacterial isolates were obtained from the Danish surveillance program for antimicrobial resistance (3, 8). A total of 35 glycopeptide-resistant E. faecium isolates obtained from pigs and 34 isolates obtained from broilers from 1995 through 1998 were included. The isolates from broilers were distributed as follows: 8 from 1995, 10 from 1996, 13 from 1997, and 3 from 1998. The isolates from pigs were distributed as follows: 3 from 1995, 11 from 1996, 12 from 1997, and 9 from 1998. All isolates from pigs were simultaneously resistant to glycopeptides, erythromycin, and tetracycline, whereas 19 (56%) and 3 (9%) of the isolates from broilers were resistant to erythromycin and tetracycline, respectively.
PCR detection of resistance genes.
All isolates were examined for the presence of vanA, and all isolates resistant to erythromycin and tetracycline were examined for the presence of ermB and tet(M), respectively, using PCR as previously described (2, 5, 18).
PFGE.
DNA purification and enzyme digestion for PFGE analysis of the isolates were performed as previously described (17). All isolates were examined with SmaI as a restriction enzyme. In addition, eight selected isolates (two from each year) from pigs were examined with ApaI. Electrophoresis was performed with a CHEF-DR III system (Bio-Rad Laboratories, Hercules, Calif.) and 1.2 or 1.4% SeaKem agarose in 0.5× Tris-borate-EDTA at 180 V. Running conditions consisted of two phases used in sequence. Phase 1 was 2 to 8 s with a run time of 20 h. Phase 2 was 8 to 22 s with a run time of 20 h.
Transferability of resistance.
Conjugation of vancomycin resistance was performed with the eight isolates from pigs, which were also examined with ApaI using the filter mating procedure as described by Clewell et al. (13). E. faecium BM4105RF, resistant to rifampin and fusidin, was used as a recipient. Transconjugants were selected on Mueller-Hinton II agar plates containing rifampin (50 μg/ml), fusidin (10 μg/ml), and vancomycin (20 μg/ml). Conjugation of erythromycin and tetracycline resistance was attempted with two isolates and erythromycin (20 μg/ml) and tetracycline (10 μg/ml), respectively, instead of vancomycin.
Hybridization.
Digoxigenin-labeled DNA probes were prepared by PCR amplification with primers for vanA, ermB, and tet(M) and labeled with a Boehringer Mannheim Biochemicals DNA labeling kit. Southern transfer and hybridization of all PFGE profiles of the isolates from pigs were performed as previously described (17) with the vanA, ermB, and tet(M) probes. Southern transfer and hybridization of the PFGE profiles of all transconjugants were also performed with the ermB and vanA probes.
Plasmid profiling.
Plasmid profiling of the eight isolates from pigs was performed with S1 nuclease as previously described (9).
All isolates yielded positive PCR products for the vanA gene. All 35 isolates from pigs and 17 (89%) of the 19 erythromycin-resistant isolates from broilers yielded positive PCR products for the ermB gene. All isolates from pigs and the three tetracycline-resistant isolates from broilers yielded positive PCR products for the tet(M) gene.
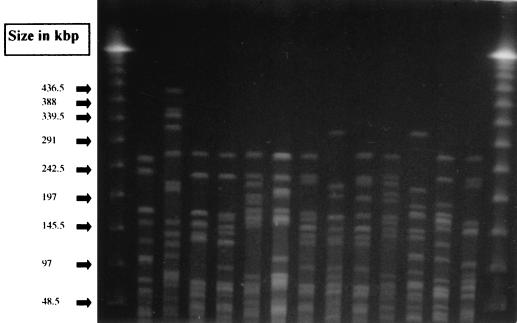
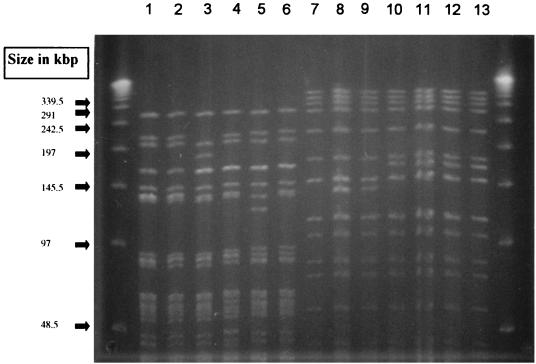
All 34 GRE isolates from broilers belonged to different PFGE types (more than three band differences), whereas all 35 isolates from pigs belonged to three closely related PFGE types, with up to two band differences to the most common type. Representative types from broilers are shown in Fig. 1, and the three closely related types from pigs are shown in Fig. 2. Twenty-eight isolates from pigs belonged to the most common type, 4 belonged to a closely related type, and the remaining 3 belonged to another closely related type.
FIG. 1.
Representative SmaI PFGE types observed among glycopeptide-resistant E. faecium isolates from broilers in Denmark.
FIG. 2.
Representative SmaI PFGE types observed among glycopeptide-resistant E. faecium isolates from pigs in Denmark and transfer of glycopeptide resistance to E. faecium BM4105RF. Lanes 1 to 6, isolates from pigs; lane 7, E. faecium BM4105RF; lanes 8 to 13, glycopeptide-resistant transformants of E. faecium BM4105RF corresponding to isolates in lanes 1 to 6; lanes 1, 2, 4, and 6, the most common PFGE type from pigs; lane 3, a closely related type, represented by four isolates; lane 5, another closely related type, represented by three isolates.
The tet(M) probe hybridized to a SmaI fragment of approximately 135 kb in all pig isolates. The vanA and ermB probes hybridized to the same SmaI fragment in all isolates from pigs; this fragment was either approximately 135 or 160 kb. The tet(M) probe hybridized to an ApaI fragment of approximately 200 kb; however, the vanA and ermB probes did not hybridize to fragments with this digest.
Transfer of vancomycin resistance from all eight porcine isolates was achieved at a frequency of 0.75 × 10−5 to 12 × 10−5 (transconjugant/donor). Transfer of vancomycin resistance was followed in all cases by transfer of erythromycin resistance, but not resistance to tetracycline, and by transfer of the relevant SmaI fragment (Fig. 2). The ermB and vanA probes hybridized to the transferred element in all transconjugants. Transfer of tetracycline resistance was not achieved, whereas transfer of erythromycin resistance from two isolates was achieved at approximately the same frequency as vancomycin resistance and was followed by transfer of vancomycin resistance.
S1 nuclease digestion yielded a band of the same size as the SmaI fragment to which the vanA and ermB probes hybridized.
The present study showed that the occurrence of glycopeptide resistance in the pig population in Denmark was caused by the presence of a single E. faecium clone, as indicated by closely related PFGE profiles, that was resistant to glycopeptides, erythromycin, and tetracycline. Among broilers, on the contrary, several different clones of GRE were found. Furthermore, among the porcine isolates, the genes encoding resistance to glycopeptides (vanA) and erythromycin (ermB) were located on the same transferable DNA element, probably a plasmid, as indicated by S1 nuclease digestion. Large plasmids containing the vanA gene have previously been observed among GRE (28).
The linkage of resistance to macrolides and glycopeptides has also been observed in other studies (21, 25). Furthermore, Noble et al. (23) cotransferred vanA-mediated glycopeptide resistance from E. faecium to Staphylococcus aureus using macrolides for selection, and Bozdogan et al. (11) found the vanA and ermAM (synonymous with ermB) genes to be present on the same plasmid.
It can be speculated that the linkage of resistance genes can play an important role in the coselection or persistence of antimicrobial agent resistance. However, only a very few epidemiological data support such a hypothesis. This investigation, together with a previous study (8), provides evidence that vanA-mediated resistance to glycopeptides has persisted among E. faecium in Danish pig herds because of genetic linkage to the ermB gene, conferring resistance to macrolides on the same mobile DNA elements, most likely large plasmids. The persistence is most probably due to the continued use of tylosin, which was used in very large amounts for growth promotion in Danish pig herds. The use of tylosin for growth promotion decreased in Denmark during the end of 1998 and was banned in all European Union countries from July 1999. Thus, it is expected that the occurrence of GRE among pigs will decrease in the future. If this happens, it will provide further evidence for the hypothesis of coselection being responsible for the persistence of GRE in pigs. However, since all GRE from pigs belonged to the same clone, another explanation could be that this clone was especially successful in establishing in and spreading between pig herds in Denmark. This clone was also resistant to tetracycline, which has been widely used for therapy for food animals in Denmark (4), and this usage also could have contributed to the successful persistence of this clone.
The reason for the occurrence of multiple GRE clones among the broiler population compared to the presence of only a single clone among GRE from the pig population is not known. It might be due to differences in production or feeding or having GRE introduced several times in the broiler population compared to the pig population.
In conclusion, this study showed that GRE from broilers belonged to different clones, while the isolates from pigs belonged to a single clone resistant to glycopeptides, macrolides, and tetracycline. The ermB and vanA genes were located on the same transferable DNA elements, most likely large plasmids. The persistence of GRE in Danish pig herds was most likely a consequence of coselection by the continued use of tylosin for growth promotion.
Acknowledgments
I thank Anja Dahl and René Hendriksen for technical assistance.
This study was supported by a grant from the Danish Directorate for Development (98-3324).
REFERENCES
- 1.Aarestrup F M. Occurrence of glycopeptide resistance among Enterococcus faecium isolates from conventional and ecological poultry farms. Microb Drug Resist. 1995;1:255–257. doi: 10.1089/mdr.1995.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Aarestrup F M, Ahrens P, Madsen M, Pallesen L V, Poulsen R L, Westh H. Glycopeptide susceptibility among Danish Enterococcus faecium and Enterococcus faecalis isolates of animal and human origin and PCR identification of genes within the VanA cluster. Antimicrob Agents Chemother. 1996;40:1938–1940. doi: 10.1128/aac.40.8.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarestrup F M, Bager F, Jensen N E, Madsen M, Meyling A, Wegener H C. Surveillance of antimicrobial resistance in bacteria isolated from food animals to antimicrobial growth promoters and related therapeutic agents in Denmark. APMIS. 1998;106:606–622. doi: 10.1111/j.1699-0463.1998.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 4.Aarestrup F M, Bager F, Jensen N E, Madsen M, Meyling A, Wegener H C. Resistance to antimicrobial agents used for animal therapy in pathogenic-, zoonotic- and indicator bacteria isolated from different food animals in Denmark: A baseline study for the Danish Integrated Antimicrobial Resistance Monitoring Programme (DANMAP) APMIS. 1998;106:745–770. doi: 10.1111/j.1699-0463.1998.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 5.Aarestrup, F. M., Y. Agersø, M. Madsen, P. G. Smith, and L. B. Jensen. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers and pigs in Denmark. Diagn. Microbiol. Infect. Dis., in press. [DOI] [PubMed]
- 6.Anonymous. National nosocomial infection surveillance system report. Atlanta, Ga: Centers for Disease Control; 1997. [Google Scholar]
- 7.Bager F, Madsen M, Christensen J, Aarestrup F M. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev Vet Med. 1997;31:95–112. doi: 10.1016/s0167-5877(96)01119-1. [DOI] [PubMed] [Google Scholar]
- 8.Bager F, Aarestrup F M, Madsen M, Wegener H C. Glycopeptide resistance in Enterococcus faecium from broilers and pigs following discontinued use of avoparcin. Microb Drug Resist. 1999;5:53–56. doi: 10.1089/mdr.1999.5.53. [DOI] [PubMed] [Google Scholar]
- 9.Barton B M, Harding G P, Zuccarelli A J. A general method for detecting and sizing large plasmids. Anal Biochem. 1995;226:235–240. doi: 10.1006/abio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 10.Bates J, Jordens J Z, Griffiths D T. Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man. J Antimicrob Chemother. 1994;34:507–514. doi: 10.1093/jac/34.4.507. [DOI] [PubMed] [Google Scholar]
- 11.Bozdogan B, Leclercq R, Lozniewski A, Weber M. Plasmid-mediated coresistance to streptogramins and vancomycin in Enterococcus faecium HM1032. Antimicrob Agents Chemother. 1999;43:2097–2098. doi: 10.1128/aac.43.8.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casadewall B, Courvalin P. Characterization of the vanD glycopeptide resistance gene cluster from Enterococcus faecium BM4339. J Bacteriol. 1999;181:3644–3648. doi: 10.1128/jb.181.12.3644-3648.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clewell D B, An F Y, White B A, Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918) J Bacteriol. 1985;162:1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Descheemaeker P R, Chapelle S, Devriese L A, Butaye P, Vandamme P, Goossens H. Comparison of glycopeptide-resistant Enterococcus faecium isolates and glycopeptide resistance genes of human and animal origins. Antimicrob Agents Chemother. 1999;43:2032–2037. doi: 10.1128/aac.43.8.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evers S, Quintiliani R, Jr, Courvalin P. Genetics of glycopeptide resistance in enterococci. Microb Drug Resist. 1996;2:219–223. doi: 10.1089/mdr.1996.2.219. [DOI] [PubMed] [Google Scholar]
- 16.Fines M, Perichon B, Reynolds P, Sahm D F, Courvalin P. VanE, a new type of acquired glycopeptide resistance in Enterococcus faecalis BM4405. Antimicrob Agents Chemother. 1999;43:2161–2164. doi: 10.1128/aac.43.9.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen L B, Ahrens P, Dons L, Jones R N, Hammerum A M, Aarestrup F M. Molecular analysis of Tn1546 in Enterococcus faecium isolated from animals and humans. J Clin Microbiol. 1998;36:437–442. doi: 10.1128/jcm.36.2.437-442.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen L B, Frimodt-Moller N, Aarestrup F M. Presence of erm gene classes in gram-positive bacteria of animal and human origin in Denmark. FEMS Microbiol Lett. 1999;170:151–158. doi: 10.1111/j.1574-6968.1999.tb13368.x. [DOI] [PubMed] [Google Scholar]
- 19.Klare I, Heier H, Claus H, Reissbrodt R, Witte W. vanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiol Lett. 1995;125:165–171. doi: 10.1111/j.1574-6968.1995.tb07353.x. [DOI] [PubMed] [Google Scholar]
- 20.Klare I, Badstubner D, Konstabel C, Bohme G, Claus H, Witte W. Decreased incidence of VanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microb Drug Resist. 1999;5:45–52. doi: 10.1089/mdr.1999.5.45. [DOI] [PubMed] [Google Scholar]
- 21.Leclercq R, Derlot E, Weber M, Duval J, Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989;33:10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison D, Woodford N, Cookson B. Enterococci as emerging pathogens of humans. Soc Appl Bacteriol Symp Ser. 1997;26:89–99. [PubMed] [Google Scholar]
- 23.Noble W C, Virani Z, Cree R G. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992;72:195–198. doi: 10.1016/0378-1097(92)90528-v. [DOI] [PubMed] [Google Scholar]
- 24.Pantosi A, Del Grosso M, Tagliabue S, Macri A, Caprioli A. Decrease of vancomycin-resistant enterococci in poultry meat after avoparcin ban. Lancet. 1999;354:741–742. doi: 10.1016/S0140-6736(99)02395-8. [DOI] [PubMed] [Google Scholar]
- 25.Uttley A H C, George R C, Naidoo J, Woodford N, Johnson A P, Collins C H, Morrison D, Gilfillan A J, Fitch L E, Heptonstall J. High-level vancomycin-resistant enterococci causing hospital infections. Epidemiol Infect. 1989;103:173–181. doi: 10.1017/s0950268800030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Bogaard A E, Jensen L B, Stobberingh E E. Vancomycin-resistant enterococci in turkeys and farmers. N Engl J Med. 1997;337:1558–1559. doi: 10.1056/NEJM199711203372117. [DOI] [PubMed] [Google Scholar]
- 27.Werner G, Klare I, Witte W. Arrangement of the vanA gene cluster in enterococci of different ecological origin. FEMS Microbiol Lett. 1997;155:55–61. doi: 10.1111/j.1574-6968.1997.tb12685.x. [DOI] [PubMed] [Google Scholar]
- 28.Werner G, Klare I, Witte W. Large conjugative vanA plasmids in vancomycin-resistant Enterococcus faecium. J Clin Microbiol. 1999;37:2383–2384. doi: 10.1128/jcm.37.7.2383-2384.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willems R J, Top J, van den Braak N, van Belkum A, Mevius D J, Hendriks G, van Santen-Verheuvel M, van Embden J D. Molecular diversity and evolutionary relationships of Tn1546-like elements in enterococci from humans and animals. Antimicrob Agents Chemother. 1999;43:483–491. doi: 10.1128/aac.43.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodford N, Adebiyi A M, Palepou M F, Cookson B D. Diversity of VanA glycopeptide resistance elements in enterococci from humans and nonhuman sources. Antimicrob Agents Chemother. 1998;42:502–508. doi: 10.1128/aac.42.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshimura H, Ishimaru M, Endoh Y S, Suginaka M, Yamatani S. Isolation of glycopeptide-resistant enterococci from chicken in Japan. Antimicrob Agents Chemother. 1998;42:3333. doi: 10.1128/aac.42.12.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]