Abstract
A total of 717 ticks collected from southern China were examined by nested PCR for the presence of Ehrlichia chaffeensis. Sixteen (55.2%) of 29 adult Amblyomma testudinarium ticks and 28 (11.7%) of 240 adult and at least 4.2% of 215 nymphal (pooled specimens) Haemaphysalis yeni ticks tested positive. Four other species of ticks were negative. Selected positive amplicons were confirmed by DNA sequencing.
Human monocytic ehrlichiosis is an acute febrile illness characterized by nonspecific clinical manifestations, mainly including fever, headache, myalgia, chills, malaise, anorexia, and vomiting and sometimes with leukopenia, thrombocytopenia, and elevated hepatic aminotransferase levels. It is usually moderate to severe and sometimes fatal (17). Since it was first reported in 1987 (12), the disease has been diagnosed in more than 30 states of the United States (17), as well as in Europe (14) and Africa (16). The etiologic agent, Ehrlichia chaffeensis has been associated with ticks, including Amblyomma americanum and Dermacentor variabilis (2, 11, 15) and Ixodes pacificus (8), which may serve as vectors. Wild white-tailed deer (Odocoileus virginianus) are believed to be the natural reservoirs of E. chaffeensis (10, 11). Although human monocytic ehrlichiosis has never been reported in Asia, there is immunoserologic evidence of exposure to E. chaffeensis among some individuals from Thailand (7) and southern China (9). The main purpose of this study was to determine the presence of E. chaffeensis in ticks from China.
Tick collection.
Adult and nymphal ticks were collected from three provinces in southern China during the period of 1996 to 1998. Ticks were collected from domestic and wild animals, including cattle, dog, southern China hare (Caprologus sinensis), goat-like deer (Muntiacus reevesi), short-eared rabbit (Lepus sinensis), and white-abdomened grant rat (Rattus edwardsi) (Table 1). In the laboratory, ticks were examined morphologically and sorted by species, developmental stage, and collection site. Tick specimens were then stored at −20°C until DNA extraction was performed.
TABLE 1.
Results of nested PCR for the identification of E. chaffeensis in ticks collected in southern China
| Tick species | Collection site (province) | Animal host(s) | No. tested | No. (%) positive |
|---|---|---|---|---|
| A. testudinarium | Yunnan | Cattle | 29 | 16 (55.2) |
| H. yeni | Fujian | M. reevesi and L. sinensis | 185 | 22 (11.9) |
| H. yeni | Fujian | Cattle | 37 | 2 (5.4) |
| H. yeni | Fujian | Caprologus sinensis | 18 | 4 (22.2) |
| H. yeni (nymph) | Fujian | M. reevesi and L. sinensis | 215 | 9 (4.2a) |
| H. hystricis | Fujian | M. reevesi and L. sinensis | 54 | 0 |
| I. granulatus | Fujian | Rattus edwardsi and dog | 50 | 0 |
| I. sinensis | Fujian | M. reevesi | 9 | 0 |
| H. longicornis | Zhejiang | Rattus edwardsi and cattle | 120 | 0 |
Minimum infection rate.
DNA extraction.
DNA was extracted from ticks by a modification of a previously described method (13). Briefly, the ticks were placed in microtubes and mechanically crushed with sterile scissors in 50 μl of DNA extraction buffer (10 mM Tris [pH 8.0], 2 mM EDTA, 0.1% sodium dodecyl sulfate, 500 μg of proteinase K per ml). The samples were incubated for 2 h at 56°C and then boiled at 100°C for 10 min to inactivate proteinase K. After centrifugation, the supernatant was used directly for PCR or purified by extraction twice with an equal volume of phenol-chloroform before use.
Nested PCR.
Nested PCR was performed using primers derived from the 16S rRNA gene of E. chaffeensis. For the initial amplification, 3 μl of each template sample was amplified in a 30-μl reaction mixture containing the primers HE1 (5′-CAATTGCTTATTACCTTTTGGTTATAAAT-3′) (3) and PER2 (5′-CTCTACACTAGGAATTCCGCTAT-3′) (5). For the nested amplification, 1 μl of the primary PCR product was used as the template in a second 30-μl reaction mixture with specific primers HE1 and HE3 (5′-TATAGGTACCGTCATTATCTTCCCTAT-3′) (3). The PCR amplifications were performed in a Perkin-Elmer model 480 thermal cycler, using the following protocol: preheating at 95°C for 3 min; followed by 35 cycles of 94°C for 1 min, 56°C for 75 s, and 72°C for 1 min; and then a final extension at 72°C for 7 min. In each set of amplifications, both a negative control (distilled water) and a positive control (plasmid containing the E. chaffeensis 16S rRNA gene) were included. PCR products were separated by agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light.
PCR product cloning and DNA sequencing.
PCR products were purified and then ligated into the plasmid vector pGEM-T (Promega Corp.) according to the manufacturer's instructions. The ligation products were transformed into Escherichia coli XL1-Blue, and white colonies were selected after growth on Luria-Bertani agar with IPTG (isopropyl-β-d-thiogalactopyranoside), X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and ampicillin. Recombinant plasmids were extracted and purified from overnight cultures using a Qiagen plasmid kit (QIAGEN GmbH). The nucleotide sequence of the plasmid insert was determined by a dideoxynucleotide cycle sequencing method with an automated fluorescent ABI PRISM 377 DNA sequencer (Perkin-Elmer, Inc.).
Results.
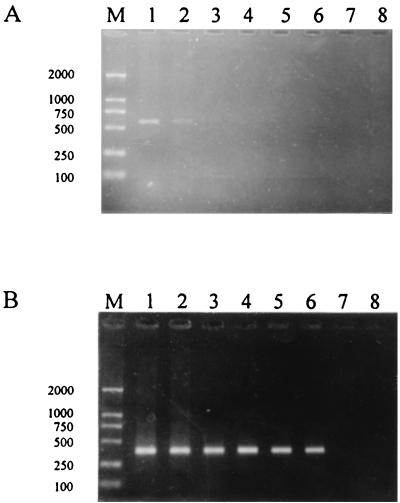
The sensitivity of the nested PCR was assessed by a spiking experiment with dilution of a plasmid containing the E. chaffeensis 16S rRNA gene sequence. The linearized plasmid DNA was diluted with DNA extracted from uninfected adult ticks. Serial dilutions of the quantified plasmid DNA were tested by the nested PCR assay. To control matrix effects, the same amount of uninfected tick background DNA was included in each initial amplification. Under these conditions, four copies of the double-stranded DNA could be identified (Fig. 1). The result was the same when the original tick DNA preparations purified from different uninfected (PCR-negative) tick species (Haemaphysalis yeni, Haemaphysalis longicornis, and Ixodes persulcatus) were used as nonspecific competitors (data not shown). Inhibitory effects did not appear when 10% of the background tick DNA amount was used. To assess the specificity of the assay, DNA templates extracted from various ehrlichial species (including E. canis, E. platys, E. ewingii, E. equi, and E. risticii) and possible tick infectious agents (including Rickettsia rickettsii, Rickettsia conorii, Rickettsia sibirica, Rickettsia japonica, Borrelia burgdorferi senso stricto, Borrelia garinii, and Borrelia afzelii) were tested by the nested PCR, and no products were amplified.
FIG. 1.
Analytical sensitivity of nested PCR for detection of E. chaffeensis 16S rRNA genes in Chinese ticks. Lanes M, molecular standards. Sizes (in base pairs) are indicated on the left. (A) Products of the primary amplification using serial dilutions of plasmids containing the E. chaffeensis 16S rRNA gene as templates. Lanes 1 through 7, template copy numbers of 8 × 104, 8 × 103, 8 × 102, 80, 8, 4, and 2, respectively. Lane 8, negative (water) control. The expected size of the primary amplified product is 587 bp. (B) Products of the nested PCR using 1 μl of the corresponding primary product as the template. The expected product size is 389 bp.
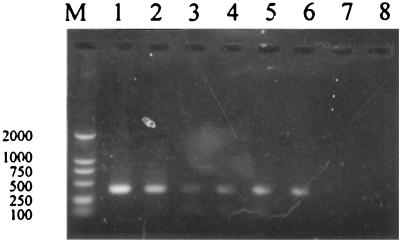
A total of 717 ticks were tested for the presence of ehrlichial DNA. The species and origin of ticks are shown in Table 1. After the first round of amplification with initial primers HE1 and PER2, 12 specimens, including 2 Amblyomma testudinarium and 10 H. yeni adults, generated characteristic 587-bp products. The nested PCR detected ehrlichial DNA in A. testudinarium and adult and nymphal H. yeni ticks, evidenced by the presence of a 389-bp band (Table 1 and Fig. 2). Of 29 A. testudinarium ticks from Monla county of Yunnan province, 16 (55.2%) tested positive. Among the ticks collected from Wuyishan city and Ninghua county of Fujian province, the positive rate of H. yeni adults was 11.7% (28 of 240) on average and varied from 5.4 to 22.2% with host origin. A total of 215 H. yeni nymphs from the same area were examined in pools (each containing five ticks), and nine pools were positive at a minimum frequency of 4.3%. PCR tests were negative for all of 54 Haemaphysalis hystricis, 50 Ixodes granulatus, and 9 Ixodes sinensis ticks from Wuyishan city of Fujian province and 120 H. longicornis ticks from Longyan county of Zhejiang province (Table 1).
FIG. 2.
Nested PCR products amplified from representative tick samples. Lane M, DNA marker. Sizes (in base pairs) are indicated on the left. Lane 1, positive control (plasmid containing the E. chaffeensis 16S rRNA gene); lane 2, A. testudinarium adult from cattle; lane 3, H. yeni adult from M. reevesi; lane 4, H. yeni adult from cattle; lane 5, H. yeni adult from Caprologus sinensis; lane 6, H. yeni nymph from L. sinensis; lane 7, water (as a negative control). The expected product size is 389 bp.
The nucleotide sequences determined for the 587-bp PCR products from two A. testudinarium and two adult H. yeni positive ticks after initial amplification were identical to each other and to the corresponding part of the 16S rRNA gene sequence of the E. chaffeensis agent previously described by Anderson et al. (GenBank accession number M73222) (1). Furthermore, the sequences of the 389-bp nested PCR amplicons from representative positive samples were all identified as partial sequence of the E. chaffeensis 16S rRNA gene.
Discussion.
The results of the present study demonstrate the presence of E. chaffeensis in A. testudinarium and H. yeni. To our knowledge, this is the first evidence of E. chaffeensis in ticks from China and the first finding of ehrlichial infection in Haemaphysalis species in the world. E. chaffeensis has been detected in a variety of ticks including A. americanum (2, 11, 15), D. variabilis (11, 15), and I. pacificus (8). Our findings, together with the evidence previously accumulated, suggest that E. chaffeensis is probably widespread and that a variety of tick species may be involved in transmission of the infectious agent. A. testudinarium is commonly seen in farmland and mountainous areas of southern and southwestern China. H. yeni is a dominant species in Fujian province which accounts for more than 80% of adult ticks and 85% of immature ticks collected from host animals such as M. reevesi and L. sinesis (18). Further studies are needed to determine the competence of A. testudinarium and H. yeni as vectors of E. chaffeensis.
Nested PCR may enhance sensitivity of detection of target nucleotide sequences (6). This technique has been shown to be sensitive for direct identification of ehrlichiae in ticks (4, 8, 15). In this study, the ability of the assay to detect ehrlichial DNA in ticks was assessed by using a plasmid containing the E. chaffeensis 16S rRNA gene, and the sensitivity was four copies. This method may be minimally sufficient to identify ehrlichiae in individual ticks and could be useful for field surveys. The specificity of the nested PCR was also evaluated, and no products were amplified from various ehrlichial species other than E. chaffeensis and other possible tick-harbored organisms, demonstrating the high specificity of the assay. The specificity of the assay was also confirmed by sequencing the PCR amplicons. All of the resulting sequences of selected positive specimens were identified as partial sequence of the E. chaffeensis 16S rRNA gene.
This study provides primary data regarding the prevalence of E. chaffeensis in ticks from southern China. The infection rate of A. testudinarium was 55.2% (16 of 29) and seems to be higher than that of A. americanum (15), a closely related species found in North America. In addition, 11.7% adult and at least 4.3% nymphal H. yeni ticks were positive for E. chaffeensis. Attempts to detect the agent in other tick species were unsuccessful. This study is not intended as a comprehensive survey of the ehrlichia distribution in ticks; rather, it was designed to investigate the presence of E. chaffeensis in China. Because the number of ticks examined was limited, the infection rates found in the present study could be biased. A randomized sampling scheme should be made and further collection of ticks should be done to obtain a reliable estimate.
It is known that large domestic and wild animals such as cattle, horse, sheep, and deer are hosts for adult A. testudinarium. H. yeni often parasitizes a variety of animals, as listed in Table 1. However, it is not so clear to what extent the two tick species feed on humans as alternate hosts. It remains to be determined whether the agent found in ticks in this study causes human disease. Isolation and identification of causative agents from patients will eventually provide direct evidence for human infection. However, ehrlichiosis should be considered when a patient has an unexplained fever with thrombocytopenia, leukopenia, and elevated hepatic aminotransferase levels and recent history of tick bite, especially in the areas where A. testudinarium or H. yeni is abundant.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been deposited in GenBank under accession no. AF147752.
Acknowledgments
This study was supported by a grant (no. 39970655) from the Natural Science Foundation of China.
We thank Rong-Man Xu for identification of ticks.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Sims K G, Olson J G, Childs J E, Piesman J F, Happ C M, Maupin G O, Johnson B J B. Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 3.Anderson B E, Sumner J W, Dawson J E, Tzianabos T, Greene C R, Olson J G, Fishbein D B, Olsen-Rasmussen M, Holloway B P, George E H, Azad A F. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30:775–780. doi: 10.1128/jcm.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlough J E, Madigan J E, Kramer V L, Clover J R, Hui L T, Webb J P, Vredevoe L K. Ehrlichia phagocytophila genotype rickettsiae in ixodid ticks from California collected in 1995 and 1996. J Clin Microbiol. 1997;35:2018–2021. doi: 10.1128/jcm.35.8.2018-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman J L, Nelson C, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloh U G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- 6.Haff L A. Improved quantitative PCR using nested primers. PCR Methods Appl. 1994;3:332–337. doi: 10.1101/gr.3.6.332. [DOI] [PubMed] [Google Scholar]
- 7.Heppner D G, Wongsrichanalai C, Walsh D S, McDaniel P, Eamsila C, Hanson B, Paxton H. Human ehrlichiosis in Thailand. Lancet. 1997;350:785–786. doi: 10.1016/S0140-6736(05)62571-8. [DOI] [PubMed] [Google Scholar]
- 8.Kramer V L, Randolph M P, Hui L T, Irwin W E, Gutierrez A G, Vugia D J. Detection of the agents of human ehrlichioses in ixodid ticks from California. Am J Trop Med Hyg. 1999;60:62–65. doi: 10.4269/ajtmh.1999.60.62. [DOI] [PubMed] [Google Scholar]
- 9.Li Q J, Zhi N, Rao X C, Yu G Q, Yu S R. Detection of antibody to E. chaffeensis in dogs and persons from Yunnan. Chin J Zoonoses. 1993;9(2):33–34. . (In Chinese.) [Google Scholar]
- 10.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E, Howerth E W. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus viginianus) J Clin Microbiol. 1997;35:1681–1686. doi: 10.1128/jcm.35.7.1681-1686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E, Little S E. Natural history of Ehrlichia chaffeensis (Rickettsiales: Ehrlichiae) in the Piedmont physiographic province of Georgia. J Parasitol. 1997;83:887–894. [PubMed] [Google Scholar]
- 12.Maeda K, Markowitz N, Hawley R, Ristic M, Cox D, McDade J. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 13.Magnarelli L A, Stafford III K C, Mather T N, Yeh M H, Horn K D, Dumler J S. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710–2714. doi: 10.1128/jcm.33.10.2710-2714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morais J D, Dawson J E, Greene C, Filipe A R, Galhardas L C, Bacellar F. First European case of ehrlichiosis. Lancet. 1991;388:633–634. doi: 10.1016/0140-6736(91)90644-5. [DOI] [PubMed] [Google Scholar]
- 15.Roland W E, Everett E D, Cyr T L, Hasan S Z, Dommaraju C B, McDonald G A. Ehrlichia chaffeensis in Missouri ticks. Am J Trop Med Hyg. 1998;59:641–643. doi: 10.4269/ajtmh.1998.59.641. [DOI] [PubMed] [Google Scholar]
- 16.Uhaa I J, Maclean J D, Greene C R, Fishbein D B. A case of human ehrlichiosis acquired in Mali: clinical and laboratory findings. Am J Trop Med Hyg. 1992;46:161–164. doi: 10.4269/ajtmh.1992.46.161. [DOI] [PubMed] [Google Scholar]
- 17.Walker D H, Dumler J S. Emergence of the ehrlichioses as human health problems. 1996. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu R M, Luo G L. Ticks parasitic on Muntiacus reevesi and Lepus sinensis in Wuyi Mountains, Fujian, China. System Appl Acarol. 1998;3:197. [Google Scholar]