Abstract
Background: The worldwide outbreak of carbapenem-resistant Klebsiella pneumoniae (CRKP) has become an urgent public health problem. High mortality and lack of effective treatments further pose new challenges to control this infection. However, studies about the evaluation of available antibiotics for CRKP infection are limited. The present study aimed to compare the efficacy of polymyxin B versus ceftazidime-avibactam (CAZ/AVI) in Chinese patients with CRKP infections and to identify risk factors affecting 7-day bacterial eradication and 28-day all-cause mortality.
Methods: From January 8, 2018, to July 6, 2020, a total of 115 adult CRKP infected patients from two tertiary teaching hospitals in Shanghai, China were enrolled based on the inclusion and exclusion criteria. By reviewing electronic medical records of these patients, demographic and clinical data were extracted. The selected patients were divided into polymyxin B and CAZ/AVI groups according to primary antibiotic exposure to compare therapeutic effects. Binary logistic and cox’s regression analysis were performed to identify risk factors for 7-day bacterial eradication and all-cause mortality.
Results: One hundred and five patients were treated with polymyxin B (67.8%) or CAZ/AVI (32.2%). Patients in the CAZ/AVI group had significantly lower rates of 28-day mortality (8.1 vs 29.5%, p = 0.013), higher microbiological eradication and 28-day clinical success. Multivariate analysis showed that Charlson comorbidity index (≥3) and prior antibiotic use within 90 days were independent risk factors for poor microbiological eradication. Cox’s regression analysis indicated that the length of hospitalization after CRKP infection and baseline creatinine clearance negatively affected 28-day mortality.
Conclusion: CAZ/AVI was more effective than polymyxin B and appeared to be a promising drug for CRKP infection, especially for critically ill patients.
Keywords: carbapenem-resistant klebsiella pneumoniae, polymyxin B, Ceftazidime-avibactam, microbiological clearance, 28-day mortality
Introduction
In 1982, Carl Friedlander first described Klebsiella pneumoniae (K. pneumoniae), which belongs to the Enterobacteriaceae family and is ubiquitous in the environment, plant surface, or the animal mucosal surface. In humans, it is present within the gastrointestinal tract or the nasopharynx. It can cause many healthcare-associated infections, including pneumonia, urinary tract infections (UTIs), and bloodstream infections in immunocompromised and healthcare-exposed patients (Zhang et al., 2014; Martin and Bachman, 2018). Hypervirulent K. pneumoniae (hvKP), a distinct subtype of K. pneumoniae, commonly found in the Asian Pacific countries, can cause community-acquired and metastatic infections in immunocompetent and young healthy individuals (Thomas and Russo, 2019). These infections can lead to a higher incidence of liver abscesses, sepsis, pneumonia, necrotizing fasciitis, and meningitis. In China, the prevalence of hvKP is high, ranging from 8.33 to 73.9%, with a varied geographic distribution, suggesting an increasing level of concern and need for effective management (Zhang et al., 2016). Besides hvKP, the rate of carbapenem-resistant Klebsiella pneumonia (CRKP) has also dramatically increased worldwide in the past 20 years. The global dissemination of CRKP accounts for 60–90% carbapenem-resistant Enterobacteriaceae (CRE) infections in United States, Europe, and China. Significantly in China, the prevalence of CRKP has rapidly increased from 2.9% in 2005 to 25% in 2021 according to China Antimicrobial Surveillance Network (http://www.chinets.com/data/GermYear). A multicenter study, which covered 25 tertiary hospitals in 14 provinces of China, found that K. pneumoniae caused 73.9% of 664 CRE cases (Zhang et al., 2018a). Due to the limited therapeutic options, the mortality rate of CRKP-infected patients also increased to 40–50%. The predominant CRKP clone in China is ST11, and its high evolutionary rate led to the development of ST11-KL47 and ST11-KL64 strains with high virulence features and increased mortality rates (Zhou et al., 2020a).
Only a few antibiotics are active against CRKP. Historically, the best available therapies for CRKP include polymyxins, in combination with meropenem, imipenem, ceftazidime, or tigecycline. However, concerns for severe toxicity, such as nephrotoxicity and neurotoxicity, limit polymyxins use to a last-line option. In 2015 and 2016, the United States. Food and Drug Administration (FDA) approved ceftazidime/avibactam (CAZ/AVI) to treat complicated intra-abdominal infections (cIAI), complicated urinary tract infections (cUTI), hospital-acquired and ventilator-associated pneumonia (HAP/VAP). CAZ/AVI is an intravenously administered combination of the third-generation cephalosporine ceftazidime and the synthetic non-β-lactam β-lactamase inhibitor avibactam at a fixed ratio of 4:1. Ceftazidime exerts its antibacterial effect mainly by inhibiting peptidoglycan cross-linking in cell wall synthesis, inducing cell lysis and death. Avibactam has activity against Ambler class A, class C, and some class D β-lactamase, including Klebisella pneumonia carbapenemase (KPC), but does not inhibit metallo-β-lactamase (MBL) enzymes (Zhanel et al., 2013). In 2019, the National Medical Products Administration of China approved CAZ/AVI to treat cIAI, HAP, and VAP caused by multidrug-resistant Gram-negative bacteria.
The worldwide outbreak of CRKP, especially in China, high mortality rate, and lack of effective therapies have brought new clinical practice challenges. Several retrospective observational studies showed that CAZ/AVI-based regimens had greater mortality benefits against CRE isolates, predominately KPC-producing Klebisella pneumonia, than best available regimens including tigecycline, aminoglycosides, and polymyxins (Shields et al., 2017a; van Duin et al., 2018; Tumbarello et al., 2019; Tsolaki et al., 2020; Zheng et al., 2021). Most studies with polymyxins have compared the clinical outcomes of colistin-based therapy with CAZ/AVI. Colistin is administered as an inactive prodrug, colistin methanesulfonate (CMS). In contrast, polymyxin B is administered as an active drug. To our knowledge, the effective comparison between CAZ/AVI and polymyxin B for CRKP infection is scarce. In addition, data comparing the use of CAZ/AVI in the treatment of CRKP infection are limited in Chinese patient populations. This retrospective study aimed to evaluate the efficacy of CAZ/AVI with polymyxin B-based regimens in CRKP-infected patients and identify risk factors for 7-day microbiologic clearance and 28-day mortality.
Methods
Study Design, Setting, and Participants
We conducted a multicenter retrospective observational study at Ruijin Hospital (Shanghai, China), a 2100-bed tertiary teaching hospital, and Shanghai Ninth People Hospital (Shanghai, China), an 1800-bed tertiary teaching hospital, between January 8, 2018, and July 6, 2020. Inclusion criteria were as follows (Zhang et al., 2014): patients with a culture-confirmed CRKP infection and (Martin and Bachman, 2018) treated with polymyxin B or CAZ/AVI monotherapy or combination therapy. Exclusion criteria were: (Zhang et al., 2014): patients aged less than 16 years, and (Martin and Bachman, 2018) received antibiotic treatment for less than 24 h. This study involving human participants was reviewed and approved by the Research Ethics Commission of Ruijin Hospital (KY 2021–227). The Research Ethics Committee approved the study protocol and waived the informed consent because of the study’s retrospective nature. All demographic, clinical, and microbiological data were retrospectively extracted from the electronic medical records.
Eligible patients with CRKP infection were divided into the polymyxin B and CAZ/AVI groups based on primary antibiotic exposure. CAZ/AVI 2.5 g was administered intravenously every 8 h, and dose adjustments were made for patients with decreased renal function. Polymyxin B was administered at a dose of 1.25–1.5 mg/kg every 12 h after a loading dose of 2.0–2.5 mg/kg based on the International Consensus Guidelines for the Optimal Use of the Polymyxins. The primary outcomes include mortality on day 28 after the onset of the study infection, microbiological eradication, and clinical success rate. Risk factors for 7-day microbiological clearance and 28-day mortality of patients with CRKP infections were also analyzed.
Definitions
Fever was defined as an axillary temperature of at least 38.3°C. Primary antibiotic exposure was defined as polymyxin B or CAZ/AVI exposure in patients with CRKP infection during the hospital stay. The severity of the disease was assessed by the Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II score on admission day (Knaus et al., 1985). Infection onset (day 1) was defined as the day that sample culture was drawn. Microbiological eradication/clearance was defined as the absence of the initially isolated pathogen from the site of index infection. Clinical success was defined as symptom resolution or significant improvement after the completion of antibiotic therapy on day 28 (Krapp et al., 2017). Hospital-acquired CRKP infection occurs 48 h or more after admission in non-intubated patients. The Charlson comorbidity index scores were used to predict the risk of mortality from disease (Charlson et al., 1994).
Microbiology
K. pneumoniae were identified by matrix-assisted laser desorption ionization-time of flight mass spectrometer (bioMérieux, Marcyl’Étoile, France). Then imipenem, meropenem were used to screen for carbapenem resistant. Once non-susceptible to ≥1 carbapenem antibiotics, K. pneumoniae was identified as carbapenem resistant. Antimicrobial susceptibilities of meropenem, imipenem, ceftazidime, colistin, tigecycline and ceftazidime were tested by broth microdilution method using the VITEK 2 COMPACT (bioMérieux, Marcy-l’Étoile, France). Antimicrobial susceptibilities of ceftazidime-avibactam were acquired by the standard broth microdilution method following the criteria of the Clinical and Laboratory Standard Institute (CLSI) 2020 guideline. Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and K. pneumoniae ATCC 700603 were used as quality control strains in the antibiotics susceptibility assay. The MIC breakpoint for meropenem, imipenem, ceftazidime and ceftazidime-avibactam were interpreted based on CLSI 2020 criteria (CaLS institute, 2020). The minimum inhibitory concentration (MIC) breakpoint for tigecycline and colistin was interpreted in accordance with European Committee on Antimicrobial Susceptibility Testing (EUCAST) 2020 criteria (Satlin et al., 2020).
Statistical Analysis
Statistical analyses were performed with SPSS software (version 25; SPSSInc., Chicago, IL, United States). Continuous variables with a normal distribution were expressed as mean ± SEMs and were analyzed by the Student’s t-test. Nonnormally distributed continuous variables were presented as median (interquartile range IQR) and analyzed by the Mann-Whitney U test. Categorical variables were expressed as n (%). The chi-square test or two-tailed Fisher’s exact test was used to compare the differences. A multivariate regression analysis model in a backward stepwise manner was performed to identify risk factors for microbiological clearance on day 7. Variables with a p-value ≤ 0.1 in univariate analysis were used in the binary logistic regression analysis. The Kaplan-Meier method was used for the survival analysis. All Variables with a p-value ≤ 0.1 were further used in Cox regression analysis model in a backward stepwise manner. Variables with a p-value ≤ 0.2 in polymyxin B and CAZ/AVI groups were included in the binary and Cox logistic regression analysis for adjustment of potential confounding factors. In contrast, variables with a p-value ≤ 0.1 were maintained in regression models. In addition, a propensity score for CAV/AVI group was calculated by the logistic regression models covering the variables with a p-value ≤ 0.1 as mentioned above. The propensity score was also included in the regression models. A p-value < 0.05 was considered statistically significant.
Results
Comparison of the Efficacy Between Polymyxin B and CAZ/AVI on CRKP Infected Patients
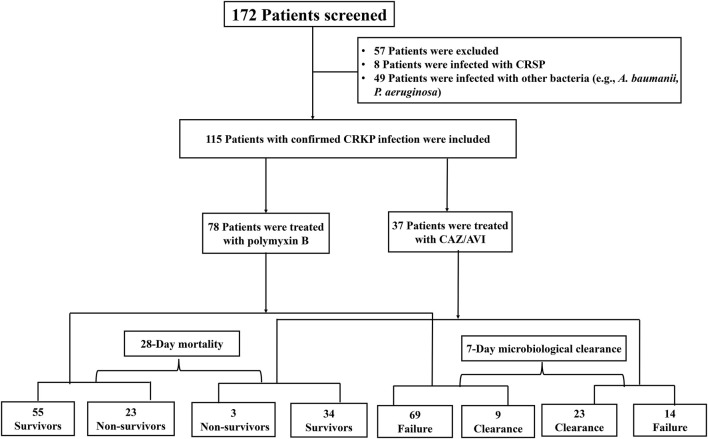
During the study period, 115 eligible patients with CRKP infections who received either polymyxin B (n = 78, 67.8%) or CAZ/AVI (n = 37, 32.2%) were included in the analysis (Figure 1). Seventy-five and forty patients were from Ruijin Hospital and Shanghai Ninth People Hospital, respectively. The antibiotic susceptibility characteristics of K. pneumoniae were shown in Table1. Almost all K. pneumoniae isolates were resistant to meropenem, imipenem, ceftazidime but susceptible to colistin, tigecycline and CAZ/AVI.
FIGURE 1.
Flowchart of the patients included in the study. Abbreviations: CRSP, carbapenem-susceptible Klebsiella pneumonia; A. baumannii, Acinetobacter baumannii; P. aeruginosa, Pseudomonas aeruginosa; CRKP, carbapenem-resistant Klebsiella pneumonia; CAZ/AVI, Ceftazidime-avibactam.
TABLE 1.
Antibiotic susceptibility characteristics of K. pneumoniae isolates.
| Antimicrobial agent | MIC range (μg/ml) | S (%) | I (%) | R (%) |
|---|---|---|---|---|
| Colistin | 0.125–0.5 | 100 | N/A | 0 |
| CAZ/AVI | ≤8/4−≥16/4 | 92.2 | N/A | 7.8 |
| Tigecycline | ≤0.5 | 100 | N/A | 0 |
| Ceftazidime | 4−≥64 | 0.88 | 6.19 | 93.81 |
| Imipenem | ≥16 | 1.77 | 2.65 | 95.58 |
| Meropenem | ≥16 | 0 | 0 | 100 |
Abbreviations: MIC, minimum inhibitory concentration; S, susceptible; I, intermediate; R, resistance; N/A, Not Applicable; CAZ/AVI, Ceftazidime-avibactam.
Table 2 summarizes the demographic and clinical characteristics of the study cohort. Patients ranged in age from 51 to 72 years. More than 90% of patients presented with hospital-acquired infection. Common infection types included pneumonia (57.4%) and bloodstream infections (50.4%). CAZ-AVI was most commonly used in combination with carbapenems (35.1%), aminoglycosides (13.5%), or tigecycline (20.9%). Polymyxin B group included carbapenem in 45.2%, aminoglycosides in 11.5%, and tigecycline in 5.1% of patient cases.
TABLE 2.
Demographic and clinical characteristics of patients with CRKP infections.
| Total | Polymyxin B | CAZ/AVI | p-value | |
|---|---|---|---|---|
| (n = 115) | (n = 78) | (n = 37) | ||
| Demographic characteristics | ||||
| Age | 63 (51–72) | 62.5 (52.5–70.5) | 64 (47–72) | 0.832 |
| Gender | 0.817 | |||
| Female | 27 (34.6%) | 12 (32.4%) | 39 (33.9%) | |
| Male | 51 (65.4%) | 25 (67.6%) | 76 (66.1%) | |
| Weight (Kg) | 65 (55–70) | 65 (55–70) | 65 (55–79.55) | 0.672 |
| BMI | 23.11 (19.92–25.35) | 22.72 (19.80–24.80) | 23.7 (20.67–26.60) | 0.424 |
| Comorbidity | ||||
| Hypertension | 51 (44.3%) | 33 (42.3%) | 18 (48.6%) | 0.523 |
| Malignancy | 33 (28.7%) | 26 (33.3%) | 7 (18.9%) | 0.11 |
| Diabetes mellitus | 31 (27%) | 18 (23.1%) | 13 (35.1%) | 0.173 |
| Cerebrovascular diseases | 19 (16.5%) | 10 (12.8%) | 9 (24.3%) | 0.121 |
| Chronic kidney disease | 19 (16.5%) | 13 (16.7%) | 6 (16.2%) | 0.952 |
| Chronic pulmonary disease | 18 (15.7%) | 13 (16.7%) | 5 (13.5%) | 0.664 |
| Coronary disease | 18 (15.7%) | 11 (14.1%) | 5 (18.9%) | 0.507 |
| Peptic ulcer | 15 (13%) | 12 (15.4%) | 3 (8.1%) | 0.279 |
| Immunocompromised | 12 (10.4%) | 6 (7.7%) | 6 (16.2%) | 0.284 |
| Chronic liver disease | 10 (8.7%) | 5 (6.4%) | 5 (13.5%) | 0.288 |
| Solid organ transplantation | 6 (5.2%) | 1 (1.3%) | 5 (13.5%) | 0.021 |
| Autoimmune disease | 5 (4.3%) | 4 (5.1%) | 1 (2.7%) | 0.915 |
| Creatinine clearance (ml/min) | 80.02 (44.37–124.08) | 88.88 (49.12–140.91) | 74.55 (39.26–112.38) | 0.084 |
| Severity variables | ||||
| Charlson comorbidity index | 1 (0–3) | 1 (0–2) | 2 (1–4) | 0.004 |
| APACHE II score | 15 ± 5.34 | 14.1 ± 5.51 | 13.68 ± 5.56 | 0.231 |
| ICU administration | 85 (73.9%) | 56 (71.8%) | 29 (78.4%) | 0.453 |
| Length of hospital stay before CRKP infection (days) | 20 (7–35) | 20 (8.75–34.25) | 21 (2–40) | 0.602 |
| Length of hospital stay after CRKP infection (days) | 31 (17–61) | 30 (16.75–57.25) | 40 (15–70.5) | 0.76 |
| Hospital stay (days) | 60 (40–97) | 56.5 (41.5–101.75) | 64 (34.5–92) | 0.94 |
| Infection variables | ||||
| Hospital-acquired infection | 109 (94.8%) | 75 (96.2%) | 34 (91.9%) | 0.609 |
| Number of infection site | 2 (1–2) | 2 (1–2) | 1 (1–2.5) | 0.75 |
| Pneumonia | 66 (57.4%) | 43 (55.1%) | 23 (62.2%) | 0.476 |
| Bloodstream | 58 (50.4%) | 44 (56.4%) | 14 (37.8%) | 0.063 |
| Abdominal | 32 (27.8%) | 18 (23.1%) | 14 (37.8%) | 0.099 |
| Urinary tract | 12 (10.4%) | 4 (5.1%) | 8 (21.6%) | 0.017 |
| Other sites | 15 (13.0%) | 12 (15.4%) | 3 (8.1%) | 0.432 |
| Concurrent fungal infection | 29 (25.2) | 22 (28.2%) | 7 (18.9%) | 0.284 |
| Fever | 80 (69.6%) | 55 (70.5%) | 25 (67.6%) | 0.72 |
| White blood cell count, x109 per L | 10.57 (7.04–15.74) | 11.42 (7.33–15.90) | 10.02 (6.45–14.29) | 0.215 |
| Neutrophils count, x109 per L | 8.77 (5.97–13.38) | 9.23 (6.11–13.87) | 8.7 (5.06–10.87) | 0.288 |
| Procalcitonin (μg/L) | 2.3 (0.77–7.19) | 2.695 (0.84–7.87) | 1.8862 (0.35–4.48) | 0.264 |
| C-reactive Protein (mg/L) | 90 (25.42–168) | 95.2103 (25.70–174.82) | 86.72 (23–164.3) | 0.724 |
| Prior healthcare history within 90 days of admission | ||||
| Hospitalization | 74 (64.3%) | 51 (65.4%) | 23 (62.2%) | 0.736 |
| Surgery | 54 (47%) | 34 (43.6%) | 20 (54.1%) | 0.294 |
| Antibiotic use | 65 (56.5%) | 42 (53.8%) | 23 (62.2%) | 0.401 |
| Treatment characteristic | ||||
| Carbapenems | 52 (45.2%) | 39 (50%) | 13 (35.1%) | 0.135 |
| Tigecycline | 24 (20.9%) | 19 (22.9%) | 5 (15.6%) | 0.39 |
| Fosfomycin | 19 (16.5%) | 13 (16.7%) | 6 (16.2%) | 0.952 |
| Cephalosporins | 23 (20%) | 19 (24.4%) | 4 (10.8%) | 0.09 |
| Aminoglycosides | 14 (12.2%) | 9 (11.5%) | 5 (13.5%) | 0.762 |
| Quinolones | 13 (11.4%) | 10 (13%) | 3 (8.1%) | 0.651 |
| SMZ | 8 (7.1%) | 4 (5.1%) | 4 (11.4%) | 0.417 |
| 1 active antibiotic | 20 (17.4%) | 9 (11.5%) | 11 (29.7%) | 0.016 |
| 2 active antibiotic | 51 (44.3%) | 36 (46.2%) | 15 (40.5%) | 0.571 |
| ≥3 active antibiotic | 44 (36.5%) | 33 (42.3%) | 11 (29.7%) | 0.195 |
| Duration of treatment (days) | 16 (10–25) | 17.5 (11–26) | 15 (9–18) | 0.047 |
| Healthcare interventions | ||||
| Mechanical ventilation | 74 (64.3%) | 49 (62.8%) | 25 (67.6%) | 0.62 |
| Vasoactive drugs | 83 (72.2%) | 55 (70.5%) | 28 (75.7%) | 0.564 |
| ECMO | 1 (0.9%) | 1 (1.3%) | 0 (0%) | 0.678 |
| CRRT | 17 (14.8%) | 9 (11.5%) | 8 (21.6%) | 0.155 |
Abbreviations: BMI, Body mass index; CRKP, carbapenem-resistant Klebsiella pneumonia; CAZ/AVI, Ceftazidime-avibactam; SMZ, compound sulfamethoxazole; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy.
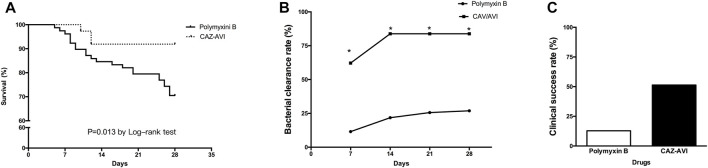
The 28-day mortality rates were 8.1 and 29.5% in the CAZ/AVI and polymyxin B groups, respectively. Survival analysis indicated that polymyxin B therapy was associated with a higher 28-day mortality rate than CAZ/AVI therapy (χ2 = 6.190, p = 0.013) (Figure 2A). 7-day microbiological clearance was accomplished in 62.2% of patients in the CAZ/AVI group and 11.5% of patients in the polymyxin B group (p < 0.001). 28-day microbiological clearance was accomplished in 83.8% of patients in the CAZ/AVI group, and 26.9% in the polymyxin B group (p < 0.001) (Figure 2B). The clinical success rate was observed in 51.4 and 11.5% in the CAZ/AVI and polymyxin B groups on day 28, respectively (Figure 2C).
FIGURE 2.
Efficacy of polymyxin B and CAZ/AVI on CRKP infected patients. Comparison of Kaplan-Meier survival curves (A), 28-day bacterial clearance rate (B) and clinical success rate (C) between CRKP infected patients treated with polymyxin B and CAZ/AVI.
Risk Factors for 7-Day Bacterial Clearance in Patients With CRKP Infection
The microbiological clearance rates on day 7 were 62.2% for CAZ/AVI and 11.5% for polymyxin B groups, respectively. To identify the risk factors for bacterial eradication rate, we performed univariate and multivariable analyses in all enrolled patients. All CRKP infected patients were divided into failure and clearance groups according to the 7-day outcome of bacterial clearance. The demographic and clinical characteristics of these two groups were shown in Table 3. Statistically significant differences were found between Charlson comorbidity index (≥3), prior antibiotic use within 90 days and CAZ/AVI-based regimen. After adjusting the propensity score in binary logistic regression model evaluating risk factors for 7-day microbiological clearance, all variables remained in the model without significant difference (Table 4).
TABLE 3.
Univariate analysis of factors associated with 7-Day microbiological clearance in the 115 patients with CRKP infection.
| Total | Failure | Clearance | p-value | |
|---|---|---|---|---|
| (n = 115) | (n = 83) | (n = 32) | ||
| Demographic characteristics | ||||
| Age | 63 (51–72) | 64 (53–74) | 61.5 (51–69) | 0.851 |
| Gender | 0.948 | |||
| Female | 39 (33.9%) | 21 (65.6%) | 11 (34.4%) | |
| Male | 76 (66.1%) | 55 (66.3%) | 28 (33.7%) | |
| Weight (Kg) | 65 (55–70) | 65 (55–70) | 65 (55–71.13) | 0.544 |
| BMI | 23.11 (19.92–25.35) | 23.11 (19.76–25.39) | 23.26 (20.42–25.25) | 0.844 |
| Comorbidity | ||||
| Hypertension | 51 (44.3%) | 25 (39.7%) | 8 (53.3%) | 0.336 |
| Malignancy | 33 (28.7%) | 30 (36.1%) | 3 (9.4%) | 0.004 |
| Diabetes mellitus | 31 (27%) | 22 (26.5%) | 9 (28.1%) | 0.861 |
| Cerebrovascular diseases | 19 (16.5%) | 13 (15.7%) | 6 (18.8%) | 0.69 |
| Chronic kidney disease | 19 (16.5%) | 12 (14.5%) | 7 (21.9%) | 0.337 |
| Chronic pulmonary disease | 18 (15.7%) | 17 (20.5%) | 1 (3.1%) | 0.022 |
| Coronary disease | 18 (15.7%) | 13 (15.7%) | 5 (15.6%) | 0.996 |
| Peptic ulcer | 15 (13%) | 13 (15.7%) | 2 (6.3%) | 0.353 |
| Immunocompromised | 12 (10.4%) | 9 (10.8%) | 3 (9.4%) | 1 |
| Chronic liver disease | 10 (8.7%) | 6 (7.2%) | 4 (12.5%) | 0.461 |
| Solid organ transplantation | 6 (5.2%) | 2 (2.4%) | 4 (12.5%) | 0.05 |
| Autoimmune disease | 5 (4.3%) | 5 (6%) | 0 (0%) | 0.32 |
| Creatinine clearance (ml/min) | 80.01 (44.37–124.08) | 89.78 (53.31–137.30) | 67.83 (34.19–97.85) | 0.007 |
| Severity variables | ||||
| Charlson comorbidity index (≥3) | 45 (39.1%) | 41 (49.4%) | 4 (12.5%) | <0.001 |
| APACHE II score | 14.1 ± 5.51 | 13.81 ± 5.72 | 14.88 ± 4.92 | 0.354 |
| ICU administration | 85 (73.9%) | 63 (75.9%) | 22 (68.8%) | 0.434 |
| Length of hospital stay before CRKP infection (days) | 20 (7–35) | 20 (8–34) | 20 (4–41.5) | 0.309 |
| Length of hospital stay after CRKP infection (days) | 31 (17–61) | 27 (11–56) | 42 (19.5–77.5) | 0.722 |
| Hospital stay (days) | 60 (40–97) | 56 (36–104) | 68 (47–91.5) | 0.897 |
| Infection variables | ||||
| Hospital-acquired infection | 79 (95.2%) | 30 (93.8%) | 109 (94.8%) | 0.67 |
| Number of infection site | 2 (1–2) | 2 (1–2) | 1 (1–2.75) | 0.601 |
| Pneumonia | 66 (57.4%) | 47 (56.6%) | 19 (59.4%) | 0.789 |
| Bloodstream | 58 (50.4%) | 47 (56.6%) | 11 (34.4%) | 0.032 |
| Urinary tract | 12 (10.4%) | 8 (9.6%) | 4 (12.5%) | 0.736 |
| Abdominal | 32 (27.8%) | 20 (24.1%) | 12 (37.5%) | 0.151 |
| Other sties | 15 (13%) | 9 (10.8%) | 6 (18.8%) | 0.353 |
| Concurrent fungal infection | 29 (25.2%) | 26 (31.3%) | 3 (9.4%) | 0.015 |
| Fever | 80 (69.6%) | 57 (68.7%) | 23 (71.9%) | 0.738 |
| White blood cell count, x109 per L | 10.57 (7.04–15.74) | 11.09 (6.74–16.19) | 10.23 (7.29–14.54) | 0.328 |
| Neutrophils count, x109 per L | 8.77 (5.97–13.38) | 9.22 (5.97–14.07) | 8.49 (5.96–10.94) | 0.258 |
| Procalcitonin (μg/L) | 2.3 (0.77–7.19) | 2.57 (0.85–7.43) | 1.96 (0.44–6.55) | 0.561 |
| C-reactive Protein (mg/L) | 90 (25.4173–168) | 91 (26.85–161.00) | 86.36 (20.18–183.75) | 0.279 |
| Prior healthcare history within 90 days of admission | ||||
| Hospitalization | 74 (64.3%) | 58 (69.9%) | 16 (50%) | 0.046 |
| Surgery | 54 (47%) | 38 (45.8%) | 16 (50%) | 0.05 |
| Antibiotic use | 65 (56.5%) | 52 (62.7%) | 13 (40.6%) | 0.033 |
| Treatment characteristic | ||||
| CAZ/AVI | 37 (32.2%) | 14 (16.9%%) | 23 (71.9%) | <0.001 |
| Carbapenems | 52 (45.2%) | 40 (48.2%) | 12 (37.5%) | 0.302 |
| Tigecycline | 24 (20.9%) | 19 (22.9%) | 5 (15.6%) | 0.39 |
| Fosfomycin | 19 (16.5%) | 14 (16.9%) | 5 (15.6%) | 0.872 |
| Cephalosporins | 23 (20%) | 19 (22.9%) | 3 (12.5%) | 0.212 |
| Aminoglycosides | 14 (12.2%) | 10 (12%) | 4 (12.5%) | 1 |
| Quinolones | 13 (11.4%) | 12 (14.6%) | 1 (3.1%) | 0.107 |
| SMZ | 8 (7.1%) | 5 (6.1%) | 3 (9.7%) | 0.508 |
| 1 active antibiotic | 20 (17.4%) | 13 (15.7%) | 7 (21.9%) | 0.431 |
| 2 active antibiotic | 51 (44.3%) | 35 (42.2%) | 16 (50.0%) | 0.449 |
| ≥3 active antibiotic | 44 (38.3%) | 35 (42.3%) | 9 (28.1%) | 0.165 |
| Duration of treatment (days) | 16 (10–25) | 17 (10–26) | 15 (8.25–23.25) | 0.256 |
| Healthcare interventions | ||||
| Mechanical ventilation | 74 (64.3%) | 52 (62.7%) | 22 (68.8%) | 0.541 |
| Vasoactive drugs | 83 (72.2%) | 60 (72.3%) | 23 (71.9%) | 0.965 |
| ECMO | 1 (0.9%) | 1 (1.2%) | 0 (0%) | 1 |
| CRRT | 17 (14.8%) | 9 (10.8%) | 8 (25%) | 1 |
Abbreviations: BMI, Body mass index; CRKP, carbapenem-resistant Klebsiella pneumonia; CAZ/AVI, Ceftazidime-avibactam; SMZ, compound sulfamethoxazole; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy.
TABLE 4.
Binary logistic regression analysis of risk factors for patients with CRKP infection for 7-day microbiological clearance.
| Variables | Without propensity score adjustment | Adjusted for the propensity score for CAZ-AVI-based treatment | ||
|---|---|---|---|---|
| p-value | OR (95% CI) | p-value | OR (95% CI) | |
| charlson comorbidity index (≥3) | <0.001 | 0.061 (0.013–0.294) | 0.002 | 0.074 (0.015–0.376) |
| Prior antibiotic use within 90 days | 0.013 | 0.185 (0.048–0.704) | 0.024 | 0.214 (0.056–0.820) |
| CAZ/AVI-based regimen | <0.001 | 44.094 (9.744–199.546) | <0.001 | 25.227 (5.278–120.573) |
Abbreviations: CRKP, carbapenem-resistant Klebsiella pneumonia; OR, odds ration; CI, confidence interval, CAZ/AVI, Ceftazidime-avibactam.
All these statistically significant variables with p < 0.05 in univariate analysis were used for multivariate analysis. The results of binary logistic regression analysis indicated that Charlson comorbidity index (≥3) (OR = 0.074, p = 0.002) and prior antibiotic use within 90 days (OR = 0.214, p = 0.024) were independently associated with a lower rate of 28-day bacterial clearance. But patients treated with CAZ/AVI-containing regimens were associated with a higher 7-day microbiological clearance than polymyxin B, which was similar to our previous findings (OR = 25.227, p < 0.001). The results were shown in Table 3.
Risk Factors for 28-Day All-Cause Mortality in CRKP Infected Patients
A total of 115 patients with CRKP infection were included in this analysis. These patients were classified into the survivor and non-survivor groups by the 28-day outcome. The overall all-cause 28-day mortality rate in CRKP-infected patients was 22.6% (26/115). The detailed analysis of demographic and clinical characteristics was summarized in Table 5. Kaplan-Meier survival analysis was used to distinguish statistically significant variables between survivor and non-survivor groups, which included age, chronic pulmonary disease, creatinine clearance, length of hospital stay after CRKP infection, hospital stay, 28-day microbiological clearance, CAZ/AVI-based regimen, and vasoactive drugs.
TABLE 5.
Univariate analysis of factors associated with 28-Day mortality in the 115 patients with CRKP infection.
| Total | Survivors | Non-survivors | p-value | |
|---|---|---|---|---|
| (n = 115) | (n = 89) | (n = 26) | ||
| Demographic characteristics | ||||
| Age | 63 (51–72) | 61 (49–69) | 69 (63.75–76) | 0.002 |
| Gender | 0.304 | |||
| Female | 39 (33.9%) | 28 (31.5%) | 11 (42.3%) | |
| Male | 76 (66.1%) | 61 (68.5%) | 15 (57.7%) | |
| Weight (Kg) | 65 (55–70) | 65 (55–71.25) | 63.5 (54.75–70) | 0.618 |
| BMI | 23.11 (19.92–25.35) | 23.11 (20.34–25.37) | 22.55 (19.70–25.18) | 0.642 |
| Comorbidity | ||||
| Hypertension | 51 (44.3%) | 36 (40.4%) | 15 (57.7%) | 0.119 |
| Malignancy | 33 (28.7%) | 24 (27%) | 9 (34.6%) | 0.448 |
| Diabetes mellitus | 31 (27%) | 23 (25.8%) | 8 (30.8%) | 0.618 |
| Cerebrovascular diseases | 19 (16.5%) | 17 (19.1%) | 2 (7.7%) | 0.281 |
| Chronic kidney disease | 19 (16.5%) | 14 (15.7%) | 5 (19.2%) | 0.902 |
| Chronic pulmonary disease | 18 (15.7%) | 10 (11.2%) | 8 (30.8%) | 0.035 |
| Coronary disease | 18 (15.7%) | 14 (15.7%) | 4 (15.4%) | 0.966 |
| Peptic ulcer | 15 (13.0%) | 12 (13.5%) | 3 (11.5%) | 0.545 |
| Immunocompromised | 12 (10.4%) | 9 (10.1%) | 3 (11.5%) | 1 |
| Chronic liver disease | 10 (8.7%) | 9 (10.1%) | 1 (3.8%) | 0.547 |
| Solid organ transplantation | 6 (5.2%) | 6 (6.7%) | 0 (0%) | 0.391 |
| Autoimmune disease | 5 (4.3%) | 3 (3.4%) | 2 (7.7%) | 0.686 |
| Creatinine clearance rate (ml/min) | 80.01 (44.37–124.08) | 137.92 (90.99–200.49) | 74.38 (54.03–111.88) | <0.001 |
| Severity variables | ||||
| Charlson comorbidity index (≥3) | 45 (39.1%) | 34 (38.2%) | 11 (42.3%) | 0.706 |
| APACHE II score | 14 (10–18) | 13 (10–18) | 15 (10–18.25) | 0.533 |
| ICU administration | 85 (73.9%) | 63 (70.8%) | 22 (84.6%) | 0.158 |
| Length of hospital stay before CRKP infection (days) | 20 (7–35) | 19 (6–34.5) | 27 (16–49) | 0.158 |
| Length of hospital stay after CRKP infection (days) | 31 (17–61) | 46 (23.5–77.5) | 10.5 (7.75–25) | <0.001 |
| Hospital stay (days) | 60 (40–97) | 67 (47–110) | 41 (25–59.5) | <0.001 |
| Infection variables | ||||
| Hospital-acquired infection | 109 (94.8%) | 85(95.5%) | 24 (92.3%) | 0.886 |
| Number of infection site | 2 (1–2) | 1 (1–2) | 2 (1–2) | 0.673 |
| Pulmonary | 66 (57.4%) | 49 (55.1%) | 17 (65.4%) | 0.349 |
| Bloodstream | 58 (50.4%) | 44 (49.4%) | 14 (53.8%) | 0.692 |
| Urinary tract | 12 (10.4%) | 10 (11.2%) | 2 (7.7%) | 0.877 |
| Abdominal | 32 (27.8%) | 26 (29.2%) | 6 (23.1%) | 0.539 |
| Other sites | 15 (13.0%) | 12 (13.5%) | 3 (11.5%) | 1 |
| Concurrent fungal infection | 29 (25.2) | 25 (28.1%) | 4 (15.4%) | 0.189 |
| 28-day microbiological clearance | 52 (45.2%) | 46 (51.7%) | 6 (23.1%) | 0.011 |
| Fever | 80 (70.2%) | 63 (71.6%) | 17 (65.4%) | 0.543 |
| White blood cell count, x109 per L | 10.57 (7.04–15.74) | 10.42 (6.88–14.68) | 12.23 (8.24–16.49) | 0.152 |
| Neutrophils count, x109 per L | 8.77 (5.97–13.38) | 8.7 (6.01–11.99) | 9.645 (5.7–14.87) | 0.336 |
| Procalcitonin PCT (μg/L) | 2.3 (0.77–7.19) | 1.89 (0.62–7.31) | 2.94 (1.18–6.54) | 0.44 |
| C-reactive Protein (mg/L) | 90 (25.42–168) | 91 (29.93–177.24 | 85.33 (12.88–136.81) | 0.22 |
| Prior healthcare history within 90 days of admission | ||||
| Hospitalization | 74 (64.3%) | 58 (65.2%) | 16 (61.5%) | 0.734 |
| Surgery | 54 (47%) | 43 (48.3%) | 11 (42.3%) | 0.589 |
| Antibiotic use | 65 (56.5%) | 50 (56.2%) | 15 (57.7%) | 0.891 |
| Antibiotic exposure during hospital stay | ||||
| CAZ/AVI-based regimen | 37 (32.2%) | 34 (38.2%) | 3 (11.5%) | 0.01 |
| Carbapenems | 52 (45.2%) | 41 (46.1%) | 11 (42.3%) | 0.735 |
| Tigecycline | 5 (4.3%) | 3 (3.4%) | 2 (7.7%) | 0.342 |
| Fosfomycin | 19 (16.5%) | 14 (15.7%) | 5 (19.2%) | 0.902 |
| Cephalosporins | 23 (20%) | 17 (19.1%) | 6 (23.1%) | 0.656 |
| Aminoglycosides | 14 (12.2%) | 14 (15.7%) | 0 (0%) | 0.069 |
| Quinolones | 13 (11.4%) | 12 (13.5%) | 1 (4%) | 0.336 |
| SMZ | 8 (7.1%) | 7 (8%) | 1 (3.8%) | 0.767 |
| 1 active antibiotic | 20 (17.4%) | 17 (19.1%) | 3 (11.5%) | 0.368 |
| 2 active antibiotic | 51 (44.3%) | 38 (42.7%) | 13 (50.0%) | 0.422 |
| ≥3 active antibiotic | 44 (38.3%) | 34 (38.2%) | 10 (38.5%) | 0.921 |
| Duration of treatment (days) | 16 (10–25) | 17 (11–26) | 10.5 (8.75–22.5) | 0.016 |
| Healthcare interventions | ||||
| Mechanical ventilation | 74 (64.3%) | 54 (60.7%) | 20 (76.9%) | 0.128 |
| Vasoactive drugs | 83 (72.2%) | 60 (67.4%) | 23 (88.5%) | 0.035 |
| ECMO | 1 (0.9%) | 1 (1.1%) | 0 (0%) | 1 |
| CRRT | 17 (14.8%) | 12 (13.5%) | 5 (19.2%) | 0.68 |
Abbreviations: BMI, Body mass index; CRKP, carbapenem-resistant Klebsiella pneumonia; CAZ/AVI, Ceftazidime-avibactam; SMZ, compound sulfamethoxazole; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy.
Variables with p < 0.1 were further analyzed using Cox regression analysis and presented in Table 5. The results suggested that the following factors independently decreased the 28-day mortality rate of CRKP infection: CAZ/AVI-based regimen, length of hospital stay after CRKP Infection, and creatinine clearance (OR = 0.989, p = 0.23). After adjusting the propensity score in Cox regression model evaluating risk factors for mortality, all variables remained in the model without significant difference (Table 6).
TABLE 6.
Cox regression analysis of risk factors for patients with CRKP infection for 28-day mortality.
| Variables | Without propensity score adjustment | Adjusted for the propensity score for CAZ-AVI-base treatment | ||
|---|---|---|---|---|
| p-value | OR (95% CI) | p-value | OR (95% CI) | |
| CAZ/AVI-based regimen | 0.005 | 0.178 (0.053–0.601) | 0.003 | 0.153 (0.045–0.521) |
| Length of hospital stay after CRKP infection (days) | <0.001 | 0.928 (0.896–0.961) | <0.001 | 0.927 (0.893–0.926) |
| Creatinine clearance (ml/min) | 0.019 | 0.99 (0.981–0.998) | 0.023 | 0.989 (0.98–0.998) |
Abbreviations: CRKP, carbapenem-resistant Klebsiella pneumonia; OR, odds ration; CI, confidence interval, CAZ/AVI, Ceftazidime-avibactam.
Discussion
Infections attributed to CRKP are being increased and spread worldwide, posing significant threat to public health. According to two national surveillance reports from China, the prevalence of CRKP has been markedly increased since 2005 from 3.0 to 20.9% in 2017 (Hu et al., 2018). Risk factors for the development of CRKP infections included longer length of hospital stay, admission to ICU, previous antibiotic use, exposure to carbapenems (Liu et al., 2018). With the emergence and rapid expansion of carbapenem-resistant pathogens, including K. pneumonia, limited antibiotics (CAZ/AVI, polymyxins or tigecycline) are effective for treatment (van Duin et al., 2013). Clinical data comparing the effectiveness of ploymyxins versus CAZ/AVI in treating CRKP infection is limited. Van Duin and colleagues prospectively analyzed 137 CRE-infected patients to compare the clinical outcomes between colistin and CAZ/AVI-based regimens. The authors found that all-cause 30-days hospital mortality for CAZ/AVI and colistin-based regimens were 9 vs. 32%, respectively (van Duin et al., 2018). To our knowledge, the comparison between CAZ/AVI and polymyxin B-based regimens has not been reported in other retrospective studies9-13. The results of this retrospective study demonstrated that CAZ/AVI-containing regimen significantly decreased the 28-day mortality rate, increased 7-day microbiological clearance, and 28-day clinical success rate, compared with polymyxin B in patients with CRKP infection. Approximately 74% of patients included in the study were in the ICU. Our results revealed that CAZ/AVI was superior to the best available therapies, including carbapenem plus aminoglycoside, carbapenem plus colistin against CRKP infection, which were similar to other multicenter retrospective cohort studies9-13. Tsolaki and his colleagues conducted a retrospective observational study on 77 mechanically ventilated patients with CRE infections in ICU to evaluate clinical, microbiological, and safety outcomes. The authors showed that the CAZ/AVI-containing regime was more effective than other available antibiotics for treating CRE infections by increasing survival rate, microbiological eradication, and clinical cure rate (Tsolaki et al., 2020). In addition, a series of case reports also demonstrated that CAZ/AVI achieved treatment success in vertebral osteomyelitis, bloodstream, and pulmonary infections caused by CRKP in kidney transplant patients (Cani et al., 2018; Wang et al., 2020). Our results, retrospective cohort studies, and case reports indicated that CAZ/AVI was superior to polymyxin B for CRKP infected patients, especially for critically ill patients.
Studies are conducted in vitro and vivo to compare CAZ/AVI monotherapy with combination therapy. Zheng and his colleagues suggested that CAZ/AVI combined with another in vitro non-susceptible antimicrobials could significantly decrease the 30-day mortality rate of critically ill patients with CRKP infections compared with CAZ/AVI monotherapy (Zheng et al., 2021). An in vitro study indicated that polymyxin B and CAZ/AVI combinations could improve the antimicrobial activity, delay or suppress the regrowth of CRKP resistant subpopulation (Ma et al., 2019). However, results of meta-analysis indicated that there were no siginifaicant difference in mortality rate, microbiologically negative and clinical success between CAZ/AVI-based combination tehrapy and CAZ/AVI monotherapy (Li et al., 2021). Therefore, the actual effect of combination therapy on CRKP infection in humans is unknown. Future studies to compare the efficacy of monotherapy (polymyxin B or CAZ/AVI) with combination therapy in CRKP infected patients are warranted.
Although CAZ/AVI is a novel antibiotic to CRKP infection, 23.3% of CRKP strains isolated from neonates were resistant to CAZ/AVI (Zhou et al., 2020b). And the resistance rate of CAZ/AVI was about 3.7% (32/872) in China. Among the 32 resistant isolates, 53.1% were MBL-producing K. pneumoniae, 40.6% were carbapenemase-producing K. pneumoniae, the remaining were a mix of both (Zhang et al., 2020a). CAZ/AVI resistant K. pneumoniae usually emerged in patients after 10–19 days of CAZ/AVI treatment. By whole-genome sequencing, the mutations of plasmid-bone blakpc-3 in CAZ/AVI resistant K. pneumoniae were not detected in control baseline isolates (Shields et al., 2017b). The increased use of tigecycline and colistin also led to the emergence of tigecycline- and colistin-resistant CRKP strains (Zhang et al., 2018b). A cohort study of 264 patients with CRKP infection found that colistin-resistant is up to 13% (Rojas et al., 2017). Nearly all CRKP strains isolated in our study are susceptible to colistin and CAZ/AVI. Once CRKP strains are resistant to these “last-line” treatments, the mortality would sharply increase. In order to control the worldwide outbreak of CRKP, prompt and appropriate antibiotic therapy for CRKP infection is necessary.
Several studies indicated the higher rates of treatment failure among people infected with CRKP isolated. In a multivariate analysis of risk factors for treatment failure of polymyxin B monotherapy, baseline renal insufficiency was associated with increased clinical failure in treating CRKP infection (Dubrovskaya et al., 2013). Risk factors for CAZ/AVI treatment failure and resistance among patients with CRE infection included pneumonia and renal replacement therapy (Shields et al., 2018). The present study showed that Charlson comorbidity index (≥3) and prior antibiotic use within 90 days were associated with decreased 7-day microbiological clearance. On the contrary, CAZ/AVI-based regimen increased the 7-day microbiological clearance rate and reduced the 28-day mortality rate. Moreover, the lower rate of 28-day all-cause mortality was positively related to the length of hospital stay after CRKP infection and baseline creatinine clearance. One retrospective analysis suggested that imipenem MICs of >8 mg/L, tigecycline therapy, and inappropriate treatment were associated with higher 28-day mortality in nontransplant patients infected with CRKP (Xiao et al., 2020). Therefore, appropriate antibiotics and an adequate treatment period would increase the rate of treatment success. A systematic review and meta-analysis of the mortality of patients infected with CRKP suggest that bloodstream infection, ICU administration, or solid organ transplantation were also strongly associated with higher mortality of CRKP infection (Xu et al., 2017).
As reported, carbapenem resistance is often associated with resistance to all traditional β-lactans and other antibiotics. The major mechanisms of carbapenem resistance are production of carbapenemases, production of efflux pumps, porin mutation or loss (Durante-Mangoni et al., 2019). The rapid increase of carbapenems resistance is associated with the most potent mechanism of carbapenemase such as New Delhi metallo-β-lactamase (NDM) especially NDM-1 which belong to Ambler class B1 superfamily. The percentage of NDM producer in MBL-producing CRKP is as high as 83.3% (Qamar et al., 2021). Molecular analysis of NDM-1gene in clinical isolates of Enterobacteriaceae form two tertiary hospital in Sakaka, Saudi Arabia found that bla NDM-1 and bla NDM harbored isolates were respectively accounted for 63.2 and 36.8% (Ejaz et al., 2020). In asian padiatric patients, carbapenem resistance were accounted for 24% of total E. coli and the NDM producing E. coli strains harbored more NDM-5 (50%) than NDM-1 (46%) and NDM-4 (3.5%)variants (Nosheen et al., 2020; Nosheen et al., 2021). Until now, multiple NMD-1 inhibitors have been designed and tested, but there is still no inhibitor on market. In China, the primary resistant mechanism can be attributed to the production K. pneumoniae carbapenemase by ST11 clones (Wang et al., 2018; Tian et al., 2019; Wyres et al., 2020). These strains are hypervirulent, multidrug-resistant, and highly transmissible, posing a greater threat to public health (Gu et al., 2018) and dominantly harbored the carbapenemase gene blakpc-2 (Yu et al., 2019; Lin et al., 2021). Among the KPC-2-producing ST11, carbapenem-resistant hypervirulent were more frequently identified than previously assumed, increasing from 2.1% in 2015 to 7.0% in 2017 and posed further infection control (Zhang et al., 2020b). But in the United States and Europe, the dominant sequence type of carbapenem-resistant strains of the K. pneumoniae are ST258, which are associated with high mortality by adopting two opposing infection programs through easily acquired gain- and loss-of-function mutations (Ernst et al., 2020). Therefore, genotypic identification of CRKP is beneficial to explore the resistant mechanism. We did not perform CRKP genotyping in this study. Thus, the prevalent strains responsible for CRKP infection in the two study sites were not identified. This was one limitation for our study. CAZ/AVI was not covered by most insurance plans and has not been widely used for critically ill patients in China. Only 32% of patients were in the CAZ/AVI group, which was another limitation for this study. Not all reported risk factors were identified given a relatively small number of non-survivor in the study. Further studies with a larger group of patients are warranted to identify additional risk factors associated with the 28-day mortality rate and 7-day microbiological clearance.
Conclusions
In conclusion, our study showed that CAZ/AVI was more effective in treating CRKP infection than polymyxin B therapy, especially for critically ill patients. Charlson comorbidity index (≥3) and prior antibiotic use within 90 days were independent risk factors for inadequate bacterial eradication. Patients receiving CAZ/AVI-based regimens presented more rapid bacterial clearance and, most importantly, have increased survival compared to patients in the polymyxin B group. The length of hospitalization after CRKP infection and baseline creatinine clearance negatively affected 28-day mortality. Further large-scale clinical trials are needed to determine optimal CAZ/AVI therapies in treating CRE infections.
Acknowledgments
We thank all patients who participated in this study.
Data Availability Statement
The data supporting the conclusion of this article will be made available from the corresponding author upon on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by The Research Ethics Commission of Ruijin Hospital (KY 2021-227). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JF, HL, GCS, and XLB conceived and designed this study. JF contributed to data analysis and interpretation, and revised the manuscript. HL contributed to data analysis and interpretation, wrote and revised the manuscript. MZ contributed to data analysis and interpretation, and revised the manuscript critically for important intellectual content. MYL contributed to data collection. YJW contributed to data collection.
Funding
This work was partially supported by The National Key R&D Program of China (2020YFC2008304).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- CaLS Institute. (2020). Performance Standards for Antimicrobial Susceptibility Testing. United States: CLSI. [Google Scholar]
- Cani E., Moussavi F., Ocheretyaner E., Sharma R., Brown C., Eilertson B. (2018). Carbapenem-resistant Klebsiella pneumoniae Vertebral Osteomyelitis in a Renal Transplant Recipient Treated with Ceftazidime-Avibactam. Transpl. Infect. Dis. 20 (2), e12837. Epub 2018/01/24. 10.1111/tid.12837 [DOI] [PubMed] [Google Scholar]
- Charlson M., Szatrowski T. P., Peterson J., Gold J. (1994). Validation of a Combined Comorbidity index. J. Clin. Epidemiol. 47 (11), 1245–1251. 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- Dubrovskaya Y., Chen T. Y., Scipione M. R., Esaian D., Phillips M. S., Papadopoulos J., et al. (2013). Risk Factors for Treatment Failure of Polymyxin B Monotherapy for Carbapenem-Resistant Klebsiella pneumoniae Infections. Antimicrob. Agents Chemother. 57 (11), 5394–5397. Epub 2013/08/21. 10.1128/AAC.00510-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante-Mangoni E., Andini R., Zampino R. (2019). Management of Carbapenem-Resistant Enterobacteriaceae Infections. Clin. Microbiol. Infect. 25 (8), 943–950. Epub 2019/04/21. 10.1016/j.cmi.2019.04.013 [DOI] [PubMed] [Google Scholar]
- Ejaz H., Alzahrani B., Hamad M. F. S., Abosalif K. O. A., Junaid K., Abdalla A. E., et al. (2020). Molecular Analysis of the Antibiotic Resistant NDM-1 Gene in Clinical Isolates of Enterobacteriaceae. Clin. Lab. 66 (3), 409–417. 10.7754/Clin.Lab.2019.190727 [DOI] [PubMed] [Google Scholar]
- Ernst C. M., Braxton J. R., Rodriguez-Osorio C. A., Zagieboylo A. P., Li L., Pironti A., et al. (2020). Adaptive Evolution of Virulence and Persistence in Carbapenem-Resistant Klebsiella pneumoniae . Nat. Med. 26 (5), 705–711. Epub 2020/04/15. 10.1038/s41591-020-0825-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu D., Dong N., Zheng Z., Lin D., Huang M., Wang L., et al. (2018). A Fatal Outbreak of ST11 Carbapenem-Resistant Hypervirulent Klebsiella pneumoniae in a Chinese Hospital: a Molecular Epidemiological Study. Lancet Infect. Dis. 18 (1), 37–46. 10.1016/s1473-3099(17)30489-9 [DOI] [PubMed] [Google Scholar]
- Hu F., Zhu D., Wang F., Wang M. (2018). Current Status and Trends of Antibacterial Resistance in China. Clin. Infect. Dis. 67 (Suppl. l_2), S128–S34. Epub 2018/11/14. 10.1093/cid/ciy657 [DOI] [PubMed] [Google Scholar]
- Knaus W. A., Draper E. A., Wagner D. P., Zimmerman J. E. (1985). Apache II: a Severity of Disease Classification System. Crit. Care Med. 13, 818–829. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- Krapp F., Sutton Sh J. L., Barr E. A., Barr V. O. (2017). Treating Complicated Carbapenem-Resistant Enterobacteriaceae Infections with Ceftazidime/avibactam: a Retrospective Study with Molecular Strain Characterisation. Int. J. Antimicrob. Agents 49, 770–773. 10.1016/j.ijantimicag.2017.01.018 [DOI] [PubMed] [Google Scholar]
- Li D., Fei F., Yu H., Huang X., Long S., Zhou H., et al. (2021). Ceftazidime-Avibactam Therapy versus Ceftazidime-Avibactam-Based Combination Therapy in Patients with Carbapenem-Resistant Gram-Negative Pathogens: A Meta-Analysis. Front. Pharmacol. 12, 707499. Epub 2021/10/02. 10.3389/fphar.2021.707499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Wu M., Yu H., Jia X., Zou H., Ma D., et al. (2021). Clinical and Microbiological Characterization of Carbapenem-Resistant Enterobacteriales: A Prospective Cohort Study. Front. Pharmacol. 12, 716324. Epub 2021/10/26. 10.3389/fphar.2021.716324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Li X., Luo M., Xu X., Su K., Chen S., et al. (2018). Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Infection: A Meta-Analysis. Microb. Drug Resist. 24 (2), 190–198. Epub 2017/07/28. 10.1089/mdr.2017.0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., He Y., Yu X., Cai Y., Zeng J., Cai R., et al. (2019). Ceftazidime/avibactam Improves the Antibacterial Efficacy of Polymyxin B against Polymyxin B Heteroresistant KPC-2-Producing Klebsiella pneumoniae and Hinders Emergence of Resistant Subpopulation In Vitro . Front. Microbiol. 10, 2029. Epub 2019/09/26. 10.3389/fmicb.2019.02029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. M., Bachman M. A. (2018). Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae . Front Cel Infect Microbiol 8, 4. Epub 2018/02/07. 10.3389/fcimb.2018.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosheen S., Bukhari N. I., Ejaz H., Abbas N. (2020). Antibiogram and Recent Incidence of Multi-Drug Resistant Carbapenemase Producing Escherichia coli Isolated from Paediatric Patients. Pak J. Med. Sci. 36 (2), 246–250. Epub 2020/02/18. 10.12669/pjms.36.2.928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosheen S., Irfan Bukhari N., Junaid K., Anwar N., Ahmad F., Younas S., et al. (2021). Phylogenetic Diversity and Mutational Analysis of New Delhi Metallo-β-Lactamase (NDM) Producing E. coli Strains from Pediatric Patients in Pakistan. Saudi J. Biol. Sci. 28 (10), 5875–5883. Epub 2021/10/01. 10.1016/j.sjbs.2021.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamar M. U., Ejaz H., Walsh T. R., Shah A. A., Al Farraj D. A., Alkufeidy R. M., et al. (2021). Clonal Relatedness and Plasmid Profiling of Extensively Drug-Resistant New Delhi Metallo-β-Lactamase-Producing Klebsiella pneumoniae Clinical Isolates. Future Microbiol. 16, 229–239. 10.2217/fmb-2020-0315 [DOI] [PubMed] [Google Scholar]
- Rojas L. J., Rojas M. S., Salim M., Cober E., Richter S. S., Perez F., et al. (2017). Colistin Resistance in Carbapenem-Resistant Klebsiella pneumoniae: Laboratory Detection and Impact on Mortality. Clin. Infect. Dis. 64, 711–718. 10.1093/cid/ciw805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satlin M. J., Lewis J. S., Weinstein M. P., Patel J., Humphries R. M., Kahlmeter G., et al. (2020). Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing Position Statements on Polymyxin B and Colistin Clinical Breakpoints. Clin. Infect. Dis. 71 (9), e523–e9. Epub 2020/02/14. 10.1093/cid/ciaa121 [DOI] [PubMed] [Google Scholar]
- Shields R. K., Chen L., Cheng S., Chavda K. D., Press E. G., Snyder A., et al. (2017). Emergence of Ceftazidime-Avibactam Resistance Due to Plasmid-Borne blaKPC-3 Mutations during Treatment of Carbapenem-Resistant Klebsiella pneumoniae Infections. Antimicrob. Agents Chemother. 61 (3), 16. 10.1128/AAC.02097-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R. K., Nguyen M. H., Chen L., Press E. G., Kreiswirth B. N., Clancy C. J. (2018). Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance Among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 62 (5). 10.1128/AAC.02497-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R. K., Nguyen M. H., Chen L., Press E. G., Potoski B. A., Marini R. V., et al. (2017a). Ceftazidime-Avibactam Is Superior to Other Treatment Regimens against Carbapenem-Resistant Klebsiella pneumoniae Bacteremia. Antimicrob. Agents Chemother. 61 (8). 10.1128/AAC.00883-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A., Russo C. M. M. (2019). Hypervirulent Klebsiella pneumoniae . Clin. Microbiol. Rev. 32 (3), 1–43. 10.1128/CMR.00001-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Huang C., Ye X., Jiang H., Zhang R., Hu X., et al. (2019). Molecular Epidemiology of and Risk Factors for Extensively Drug-Resistant Klebsiella pneumoniae Infections in Southwestern China: A Retrospective Study. Front. Pharmacol. 10, 1307. Epub 2019/11/19. 10.3389/fphar.2019.01307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolaki V., Mantzarlis K., Mpakalis A., Malli E., Tsimpoukas F., Tsirogianni A., et al. (2020). Ceftazidime-Avibactam to Treat Life-Threatening Infections by Carbapenem-Resistant Pathogens in Critically Ill Mechanically Ventilated Patients. Antimicrob. Agents Chemother. 64 (3), 19. 10.1128/AAC.02320-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello M., Trecarichi E. M., Corona A., De Rosa F. G., Bassetti M., Mussini C., et al. (2019). Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients with Infections Caused by Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae . Clin. Infect. Dis. 68 (3), 355–364. Epub 2018/06/13. 10.1093/cid/ciy492 [DOI] [PubMed] [Google Scholar]
- van Duin D., Kaye K. S., Neuner E. A., Bonomo R. A. (2013). Carbapenem-resistant Enterobacteriaceae: a Review of Treatment and Outcomes. Diagn. Microbiol. Infect. Dis. 75 (2), 115–120. Epub 2013/01/08. 10.1016/j.diagmicrobio.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin D., Lok J. J., Earley M., Cober E., Richter S. S., Perez F., et al. (2018). Colistin versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin. Infect. Dis. 66 (2), 163–171. Epub 2017/10/12. 10.1093/cid/cix783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang X., Wang J., Ouyang P., Jin C., Wang R., et al. (2018). Phenotypic and Genotypic Characterization of Carbapenem-Resistant Enterobacteriaceae: Data from a Longitudinal Large-Scale CRE Study in China (2012-2016). Clin. Infect. Dis. 67 (Suppl. l_2), S196–S205. Epub 2018/11/14. 10.1093/cid/ciy660 [DOI] [PubMed] [Google Scholar]
- Wang Z., Ma K., Chen Z., Guo Z., Zhao G., Guo H., et al. (2020). Successful Treatment of Early Post-Transplant Bloodstream and Pulmonary Infection Caused by Carbapenem-Resistant Klebsiella pneumoniae with a Combination of Ceftazidime-Avibactam and Carbapenem: A Case Report. Transpl. Proc 52 (9), 2742–2746. Epub 2020/08/31. 10.1016/j.transproceed.2020.08.006 [DOI] [PubMed] [Google Scholar]
- Wyres K. L., Lam M. M. C., Holt K. E. (2020). Population Genomics of Klebsiella pneumoniae . Nat. Rev. Microbiol. 18 (6), 344–359. Epub 2020/02/15. 10.1038/s41579-019-0315-1 [DOI] [PubMed] [Google Scholar]
- Xiao T., Zhu Y., Zhang S., Wang Y., Shen P., Zhou Y., et al. (2020). A Retrospective Analysis of Risk Factors and Outcomes of Carbapenem-Resistant Klebsiella pneumoniae Bacteremia in Nontransplant Patients. J. Infect. Dis. 221 (Suppl. 2), S174–S83. Epub 2020/03/17. 10.1093/infdis/jiz559 [DOI] [PubMed] [Google Scholar]
- Xu L., Sun X., Ma X. (2017). Systematic Review and Meta-Analysis of Mortality of Patients Infected with Carbapenem-Resistant Klebsiella pneumoniae . Ann. Clin. Microbiol. Antimicrob. 16 (1), 18. Epub 2017/03/31. 10.1186/s12941-017-0191-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Zhang W., Zhao Z., Ye C., Zhou S., Wu S., et al. (2019). Molecular Characterization of Carbapenem-Resistant Klebsiella pneumoniae Isolates with Focus on Antimicrobial Resistance. BMC Genomics 20 (1), 822. Epub 2019/11/09. 10.1186/s12864-019-6225-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhanel G. G., Lawson C. D., Adam H., Schweizer F., Zelenitsky S., Lagacé-Wiens P. R., et al. (2013). Ceftazidime-avibactam: a Novel Cephalosporin/β-Lactamase Inhibitor Combination. Drugs 73 (2), 159–177. Epub 2013/02/02. 10.1007/s40265-013-0013-7 [DOI] [PubMed] [Google Scholar]
- Zhang P., Shi Q., Hu H., Hong B., Wu X., Du X., et al. (2020a). Emergence of Ceftazidime/avibactam Resistance in Carbapenem-Resistant Klebsiella pneumoniae in China. Clin. Microbiol. Infect. 26 (1), 124–e4. Epub 2019/09/09. 10.1016/j.cmi.2019.08.020 [DOI] [PubMed] [Google Scholar]
- Zhang R., Dong N., Huang Y., Zhou H., Xie M., Chan E. W., et al. (2018b). Evolution of Tigecycline- and Colistin-Resistant CRKP (Carbapenem-resistant Klebsiella pneumoniae) In Vivo and its Persistence in the GI Tract. Emerg. Microbes Infect. 7 (1), 127. Epub 2018/07/10. 10.1038/s41426-018-0129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Jin L., Ouyang P., Wang Q., Wang R., Wang J., et al. (2020b). Evolution of Hypervirulence in Carbapenem-Resistant Klebsiella pneumoniae in China: a Multicentre, Molecular Epidemiological Analysis. J. Antimicrob. Chemother. 75 (2), 327–336. Epub 2019/11/13. 10.1093/jac/dkz446 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Q., Yin Y., Chen H., Jin L., Gu B., et al. (2018a). Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: Report from the China CRE Network. Antimicrob. Agents Chemother. 62 (2). 10.1128/AAC.01882-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yao Z., Zhan S., Yang Z., Wei D., Zhang J., et al. (2014). Disease burden of Intensive Care Unit-Acquired Pneumonia in China: a Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 29, 84–90. Epub 2014/12/03. 10.1016/j.ijid.2014.05.030 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhao C., Wang Q., Wang X., Chen H., Li H., et al. (2016). High Prevalence of Hypervirulent Klebsiella pneumoniae Infection in China: Geographic Distribution, Clinical Characteristics, and Antimicrobial Resistance. Antimicrob. Agents Chemother. 60 (10), 6115–6120. Epub 2016/08/03. 10.1128/AAC.01127-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Zhang J., Wang B., Cai J., Wang L., Hou K., et al. (2021). Ceftazidime-Avibactam in Combination with In Vitro Non-susceptible Antimicrobials versus Ceftazidime-Avibactam in Monotherapy in Critically Ill Patients with Carbapenem-Resistant Klebsiella Pneumoniae Infection: A Retrospective Cohort Study. Infect. Dis. Ther. 10 (3), 1699–1713. Epub 2021/07/10. 10.1007/s40121-021-00479-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Yang J., Hu F., Gao K., Sun J., Yang J. (2020). Clinical and Molecular Epidemiologic Characteristics of Ceftazidime/Avibactam-Resistant Carbapenem-Resistant Klebsiella pneumoniae in a Neonatal Intensive Care Unit in China. Infect. Drug Resist. 13, 2571–2578. Epub 2020/08/18. 10.2147/IDR.S256922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K., Xiao T., David S., Wang Q., Zhou Y., Guo L., et al. (2020). Novel Subclone of Carbapenem-Resistant Klebsiella pneumoniae Sequence Type 11 with Enhanced Virulence and Transmissibility, China. Emerg. Infect. Dis. 26 (2), 289–297. Epub 2020/01/22. 10.3201/eid2602.190594 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusion of this article will be made available from the corresponding author upon on reasonable request.