Abstract
The aim of this study was to explore the effects of early weaning on growth performance, intestinal morphology, digestive enzyme activity, antioxidant status, and cytokine status in domestic pigeon squabs (Columba livia). The conclusion is based on body weight (BW) and average daily gain (ADG), length index and weight index of small intestine, small intestinal morphology, activity of digestive enzymes in duodenum content, the concentrations of jejunal antioxidant status and cytokines. A completely randomized design with 2 treatments, the control group (CON) and early weaning (EW) group, was utilized. Eight squabs per treatment were sampled at the age of 25 d. The results showed that early weaning reduced BW (P < 0.05), ADG (P < 0.05), ileac length index (P < 0.05), and weight index (P < 0.01). Compared with the CON group, small intestinal morphology was altered in the EW group. Ileac crypt depth (CD) increased significantly (P < 0.01). The villus area was decreased in the duodenum (P < 0.05), jejunum (P < 0.01), and ileum (P < 0.05). The ileac ratio of villus height to crypt depth (VCR) in the EW group was lower than the ileac ratio of villus height to VCR in the CON group (P < 0.01). The activity of trypsin (P < 0.05), sucrase (P < 0.01) and aminopeptidase-N (APN) (P < 0.01) in the duodenum was reduced. Jejunal malondialdehyde (MDA) (P < 0.01) was increased and total superoxide dismutase (T-SOD) (P < 0.01) was reduced significantly. Early weaning decreased the concentrations of interferon-γ (IFN-γ) (P < 0.01), interleukin-4 (IL-4) (P < 0.05) and interleukin-10 (IL-10) (P < 0.01) but induced significant upregulation of interleukin-2 (IL-2) (P < 0.05). In conclusion, our results suggested that early weaning did harm the BW and ADG, intestinal length index and weight index, intestinal morphology, activity of digestive enzymes, and antioxidant and cytokine status.
Key words: pigeon squabs, early weaning, growth performance, intestinal morphology, cytokine status
INTRODUCTION
Pigeons, as altricial birds, are different from precocial species such as chickens, ducks, and geeses in terms of nesting instincts, reproduction, rearing newly hatched offspring and the growth rate of the young (Xie et al., 2020). The growth and survival of their squabs is dominant in comparison to other poultry species, which contributes to pigeon milk, a sort of nourishing regurgitation from parental pigeons (Sales and Janssens, 2003; Zhang et al., 2016). Crop milk is distinct from breast milk from mammalian adults such as pigs and cattle with respect to nutrient substances (Abdel-Azeem and Abdel-Azeem, 2010). During the growth period of squabs, the ingredients of pigeon milk change consistently. In the early stage, crop milk is liquid, and then mixed with grains. Early stage crop milk is replaced by grain only gradually. Hegde (1973) and Ding et al. (2020) demonstrated that crop milk, composed of mainly lipids and proteins, was secreted from crops of both male and female pigeons. After hatching, parental pigeons take care of the young squabs in turn, because squabs cannot take care of themselves until they are ready to leave nests at a market weight of 500 g (at an age of approximately 25 d) (Lea et al., 1986; Gillespie et al., 2011; Hu et al., 2016; Ge et al., 2020). This gradual process is called ‘weaning’ for pigeons, similar to weaning piglets.
With the development of living standards, because of high nutrient quality and delicacy, pigeon meat has become more popular, especially in southern China and even in southeastern Asian markets (Mao et al., 2021). To satisfy the increasing demand for pigeon meat, pigeons are reared in China as a sort of poultry (Chen et al., 2008; Dong et al., 2013). As mentioned above, during the immediately posthatching period, the growth of young pigeons (squabs) depends on crop milk from parental pigeons, which severely limits the production of female pigeons and restricts the development of the pigeon industry. Young pigeons continuously stay in the nest and are dependent on their parents for feed intake. Early weaning of squabs can evidently relieve the burden of parental pigeon feeding, and shorten the interval between laying eggs, thus shortening the reproductive cycle, which is conducive to the improvement of economic benefits. However, very early weaning may cause adaptation problems. Weaning, as a stressful and important course (Yang and Vohra, 1987; Abdel-Azeem et al., 2016), has many effects on the health of individuals including growth performance, organic immunity and gut barrier integrity (Cao et al., 2018; Modina et al., 2019). Most effects on the gastrointestinal tract have received attention from researchers. The gastrointestinal tract plays an essential role in the digestion and absorption of nutrients, providing barrier functions, and the quality and taste of animal products. Piglets are subjected to the stress of early weaning at the age of 21 d under commercial conditions to satisfy the increasing requirements for animal products such as pork (Moeser et al., 2007; Smith et al., 2010; Wijtten et al., 2011). Hu et al. (2013b) elucidated the mechanism for the weaning stress of intestinal barrier function in piglets at the age of 28 d. In addition, Kai et al. (2018) studied whether weaning stress also influences gut barrier health in Chinese Hu sheep.
In summary, considering the influences of early weaning on intestinal health in vitro and in mammals such as piglets and sheep and because very little information has been published regarding the effects on intestinal health in squabs, we hypothesize that early weaning could also impact the small intestine health of domestic pigeon squabs. Therefore, this study was conducted to determine the effects of early weaning on growth performance, small intestinal morphology, digestive enzyme activity of duodenum content, antioxidant status, and immune cytokines of jejunum of squabs.
MATERIALS AND METHODS
All experimental protocols involving animals were approved by the Animal care and welfare Committee of Animal Science College and the scientific Ethical Committee of Zhejiang University (NO. ZJU2013105002) (Hangzhou, China).
Birds and Housing
A total of 160 White King Pigeon squabs (mixed sex, 1 d of age) and 80 pairs of parental White King pigeons (80 males and 80 females, 1-year-old) were obtained from a commercial farm (Baixiang Pigeon Breeding Co., Ltd., Fuyang, China). Each pair of parent pigeons with 2 young squabs was housed as a family. Each cage (50 cm width × 55 cm depth × 55 cm height) was equipped with a perch and a nest throughout the experiment. Squabs were divided into 2 treatments, which consisted of eight replications of 10 squabs. Squabs in the same treatment were randomly pair-matched and allocated into nests of parental pigeons. All adult pigeons were supplied with a mixed-grain diet of cereals and pulses (13.50% protein and energy content of 12.31 MJ/kg) supplemented with vitamin minerals and sand, and the squabs were fed crop milk (Hegde, 1973; Hullar et al., 1999). The ingredients and analyzed and calculated nutrient levels of the experimental diets for parent pigeons are shown in Table 1. The two treatment groups were as follows: control group (CON) and early weaning group (EW).
Table 1.
Ingredient composition and nutrient levels of the basic diet of parental pigeons1.
| Items | Content, % |
|---|---|
| Corn | 42.55 |
| Wheat | 12.77 |
| Pea | 25.53 |
| Sorghum | 12.77 |
| Green bean | 6.38 |
| Total | 100.00 |
| Calculated nutrients2, % | |
| Metabolizable energy, MJ/kg | 12.31 |
| Crude protein | 13.50 |
| Crude fat | 2.67 |
| Calcium | 0.08 |
| Total phosphorous | 0.31 |
| Analyzed nutrients, % | |
| Crude protein | 13.43 |
| Crude fat | 2.94 |
| Calcium | 0.18 |
| Total phosphorous | 0.39 |
| Ingredients of grit meal (%) | |
| Limestone | 52.93 |
| Shell meal | 28.10 |
| Yellow mud | 14.05 |
| Salt | 1.41 |
| Ferrous sulfate (monohydrate) | 0.23 |
| Premix3 | 3.28 |
| Total | 100.00 |
All feeds were fed in a whole-grain form at 8.00 and 16.00 hours each day, and grit meal was offered to pigeons consistently. The feeds were offered to adult pigeons in both control groups and EW group.
Nutrition values and metabolizable energy values determined in pigeons were calculated from table of feed composition and nutritive values in China (31st edition, 2020). A previous study reported that there was not significant difference between chicken and pigeons on metabolizable energy values (Hullar et al., 1999).
The premixes provided the following per kg of diet: Vitamin A 5,000 IU, Vitamin D3 2,000 IU, Vitamin E 500 IU; CU (as copper sulphate) 15 mg, Fe (as ferrous sulfate) 100 mg, Mn (as manganese sulfate) 45 mg, Zn (as zinc sulfate) 90 mg.
The control group squabs were fed pigeon milk for only 25 d after hatching. The parental birds were fed twice a day (8:00 a.m. and 4:00 p.m.) and provided with free access food and water. The squabs from the EW group were fed by parental pigeons during the first 6 d after hatching and then fed by hand from d 7 to 25. The mean initial weight of squabs at the age of 7 days was similar in the two groups at approximately 130 g. The average initial BW of squabs at the age of 7 d in the CON group and EW group was 130.28 g and 129.11 g, respectively. The ingredients of artificial crop milk (17.77% protein and energy content of 13.04 MJ/kg), were utilized during the experiment for 18 d, and the analyzed and calculated nutrient levels of artificial crop milk for the squabs are shown in Table 2. Artificial crop milk was a kind of self-developed product by our group. The powdered solid feeds were mixed with warm water (37°C) and fed using a 100 mL syringe.
Table 2.
Ingredient composition and nutrient levels of the basic diet of artificial crop milk1.
| Items | Content, % |
|---|---|
| Corn | 40.55 |
| Wheat | 14.67 |
| Pea | 6.01 |
| Sorghum | 18.77 |
| Soybean meal | 10.00 |
| Fish meal | 1.00 |
| Premixs2 | 2.00 |
| Canola oil | 2.00 |
| Casein | 5.00 |
| Total | 100.00 |
| Calculated nutrients3, % | |
| Metabolizable energy (MJ/kg) | 13.04 |
| Crude protein | 17.77 |
| Crude fat | 4.69 |
| Calcium | 0.15 |
| Total phosphorous | 0.38 |
| Analyzed nutrients, % | |
| Crude protein | 17.89 |
| Crude fat | 4.83 |
| Calcium | 0.27 |
| Total phosphorous | 0.54 |
All feeds were fed at 8.00, 12.00 and 18.00 hours each day from 7 D to 25 D. The feeds were offered to young pigeons in EW group by hand.
The premixes provided the following per kg of diet: Vitamin A 2,500 IU, Vitamin D3 500 IU, Vitamin E 2,500 IU, Vitamin k3 500 IU, Vitamin B1 500 IU, Vitamin B3 500 IU, Vitamin B3 500 IU; CU (as copper sulphate) 10 mg, Fe (as ferrous sulfate) 100 mg, Mn (as manganese sulfate) 50 mg, Zn (as zinc sulfate) 80 mg.
Nutrition values and metabolizable energy values determined in pigeons were calculated from table of feed composition and nutritive values in China (31st edition, 2020). A previous study reported that there was not significant difference between chicken and pigeons on metabolizable energy values (Hullar et al., 1999).
The squabs in the 2 groups were placed in the same room in to reduce the error of environment. The indoor temperature and the relative humidity were 18 to 26°C and 60 to 70%, respectively. The photoperiod was 12 h light-12 h dark during the experimental period.
Birds Slaughter and Sample Collection
These 80 family units were allocated into 2 groups of 8 replicates of one pair and 2 squabs. Eight squabs per treatment (one of each replication) were selected for sampling at the age of 25 d. The squabs were fasted for 12 h before weighing and slaughter. Before all squabs from each treatment were killed by cervical dislocation, the BW of each bird was measured and recorded. The length and weight of the small intestine were calculated individually without content, including the duodenum, jejunum and ileum. Duodenum content and jejunum content were collected into sterile plastic tubes with a sterile spatula, immediately frozen in liquid N2 and then stored at −80°C for measuring digestive enzymes. Approximately one centimeter of length of intestinal tubes from the duodenum (from the pylorus to distal parts of the duodenum loop) and jejunum (from the distal part of the duodenal loop to Meckel's diverticulum) were sampled and immediately frozen in liquid N2 and then stored at −80°C for utilization.
Growth Performance
Body weight (BW) of squabs was recorded as a pen basis on d 7 and 25. Average daily gain (ADG) and survival rate were calculated by period and cumulatively.
Intestinal Tract Histopathology
Tissue samples (approximately 1 cm), excised from the duodenum, jejunum, and ileum, were flushed with normal saline and fixed in 4% neutral-buffered formalin solution for histopathology. These samples were dehydrated in ethanol, cleared in xylene and embedded in paraffin. Serial sections (approximately 5 μm) per intestinal site were placed on glass slides, stained with hematoxylin and eosin, and finally sealed with neutral resin. Sections were deparaffinized in xylene, rehydrated in a graded alcohol series, examined by light microscopy (Mshot, MF52, China) and analyzed with Mshot Image Analysis System 1.1.4 software (Mshot Corporation). Villus height was measured from the tip of the villi to the villus crypt junction and width was at half height (Dong et al., 2012). Crypt depth was defined as the depth of the invagination between adjacent villi. The ratio of villus height to crypt depth (VCR) was also calculated. Each replicate per segment recorded data from at least 30 villi or crypts (Xie et al., 2020). The villus surface area was calculated by the formula [(2π) × (villus width/2) × (villus height)] (Sakamoto et al., 2000).
Determination of Intestinal Enzymatic Activity
The sample of intestinal content from the duodenum was frozen at −80°C to measure enzymatic activity. Briefly, approximately 0.1 g of sample was measured and homogenized in 10 volumes of ice-cold PBS (pH 7.0) by a homogenizer at 4°C. The homogenates were centrifuged at 2,500 rpm/min for 10 min, and then the supernatant was collected to test the activity of intestinal digestive enzymes including α-amylase, lipase (LPS), trypsin, maltase, and sucrase. The operation instruments followed an assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The supernatant was also used for measuring the activity of aminopeptidase-N (APN) and leucine aminopeptidase (LAP) by ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd, China) at 450 nm.
α-Amylase activity was determined according to a previous study (Somogyi, 1960). One α-amylase activity unit was defined as 10 mg starch hydrolyzed within 30 min at 37°C with enzymes in 1 mg protein. LPS activity was determined following Tietz and Fiereck (1966). The lipase activities were defined as the amount of enzyme consumed to liberate 1 μmol free fatty acids from the olive oil emulsion per min. Trypsin activity was assayed according to the method of Lainé et al. (1993) by an assay kit (Nanjing Jiancheng Bioengineering Institute). One activity unit was defined as every 0.003 change in absorbance per minute caused by trypsin in 1 mg protein from tissues at 37°C and pH 8.0. At 37°C and pH 6.0, one maltase or sucrase activity unit was defined as 1 mmol maltase and sucrose hydrolyzed by 1 mg protein per minute. The total protein concentration of the supernatant was determined by a Coomassie Brilliant Blue protein assay kit (Nanjing Jiancheng Bioengineering Institute).
One unit of APN and LAP was expressed as 1 ng of standard antibody per milliliter solution measured at 37°C. Enzyme activity of APN and LAP was calculated with the standard curve.
Jejunal Antioxidant Status
The concentrations of GSH-Px, The total superoxide dismutase (T-SOD), malondialdehyde (MDA), total antioxidant capacity (T-AOC), and catalase (CAT) were determined according to the manufacturer's instructions accompanying the assay kit, which involved a GPx assay kit, SOD assay kit, MDA assay kit, T-AOC assay kit, and CAT assay kit (Nanjing Jiancheng Bioengineering Institute; Wang et al., 2019).
Jejunum Cytokine Analysis
The concentrations of TNF-α, TNF-β, IFN-γ, IL-2, IL-4, IL-6, IL-10, and IL-1β in jejunal segments were determined by absorbance changes at 450 nm with ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd) according to the manufacturer's protocol. The final concentrations were expressed as units per mg of protein (Xu et al., 2020).
Statistical Analyses
All data were analyzed with SPSS statistical software (version 20.0, SPSS Inc., Chicago, IL) as reported by Xu et al. (2019) and subjected to GraphPad Prism Statistical software (GraphPad Software 8.0.2 Inc., La Jolla, CA) for statistical analysis using a two-tailed t test (values presented are the means of 8 sample squabs per treatment). The significant differences between two treatment means were expressed by using unpaired t test data. The results are presented as the means with standard error of the mean (SEM). Significance was attained if the P value was less than 0.05. In contrast, there was no significant difference between the two treatments when the P value was more than 0.05. In addition, very significant differences were considered when the P value < 0.01.
RESULTS
Growth Performance
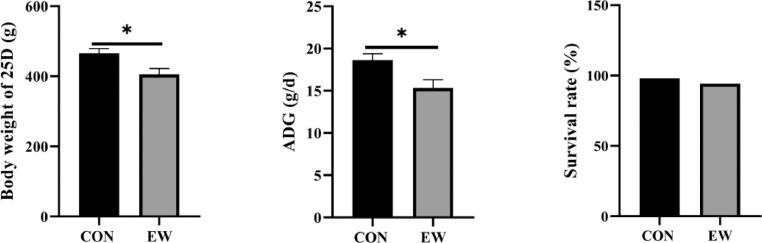
From Figure 1, the squabs grew worse during the complete experimental period in the EW group than in the CON group, which means that early weaning has harmful impacts on growth performance (P < 0.05). The average body weights on d 25 were 465.50 g and 405.00 g, respectively. The ADG in the two groups was 16.94 g/d and 15.33 g/d, respectively. ADG in the EW group decreased significantly (P < 0.05) compared to pigeons in the control group. The survival rate in the two groups was 98.00 and 94.29%, respectively. When comparing the survival rate between CON and EW, EW was 3.71% lower than CON.
Figure 1.
Effects of early weaning on BW (A), ADG (B), and survival rate (C) of squabs in two groups between 7 D and 25 D. BW of early weaning squabs was recorded at the age of 25 and compared with CON group. ADG was calculated by period and expressed as g/d. Rate of survival was calculated as the percentage of alive squabs to total number of squabs in CON group and EW group representatively. CON group: control group; EW group: test group, squabs were fed by parental pigeons for first 6 d after hatching, and then fed by hand. Data represents the mean value for each treatment (8 birds per treatment).
Small Intestine Length and Weight
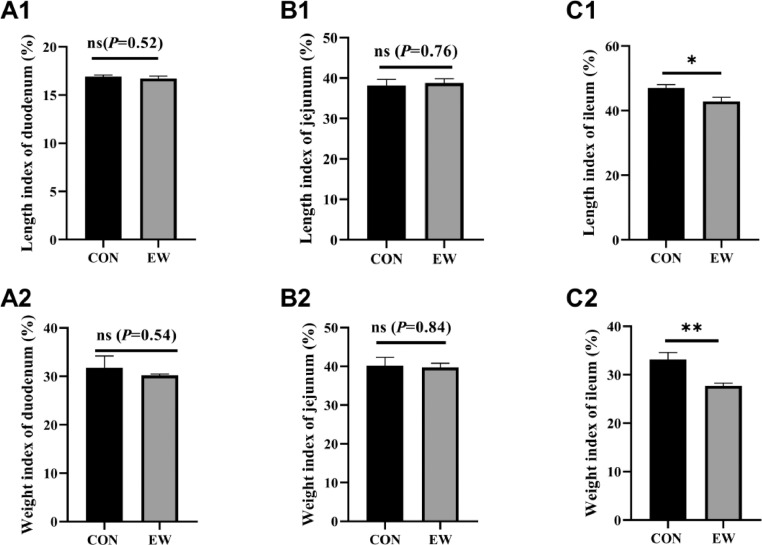
As shown in Figure 2, the effects of early-weaning squabs on the small intestine length index and weight index are presented. There was no significant difference in the length indices of the duodenum and jejunum in the two groups (Figures 2A1 and B1). However, the length index of the ileum in the squabs in the experimental group (EW) was significantly decreased (P < 0.05) compared with the length index of the ileum in the squab in the control group. In addition, early weaning stress did not significantly change the weight index of the duodenum and jejunum, as shown in Figures 2 A2 and B2. In contrast, the weight index of the ileum in the control group was significantly higher (P < 0.01) than the weight index of the ileum in the EW group (Figure 2C2).
Figure 2.
Effects of early weaning on length index (A1, B1, C1) and weight index (A2, B2, C2) of small intestine of squabs in two groups at 25 days old. Length index of duodenum, jejunum, and ileum was shown in Figure A1, B1, and C1 individually. Weight index was presented in Figure A2, B2, and C2. Length index was calculated as the percentage of segment length of duodenum, jejunum, and ileum to full length of small intestine; weight index was calculated as the percentage of segment weight of duodenum, jejunum and ileum to overall weight of small intestine (Xu et al., 2019). CON group: control group; EW group: test group, squabs were fed by parental pigeons for first 6 d after hatching, and then fed by hand. Values means n = 8 for the analysis of the intestine length index and weight index. Data are means ± SEM. * means P < 0.05; ** means P < 0.01.
Small Intestine Morphology Examination
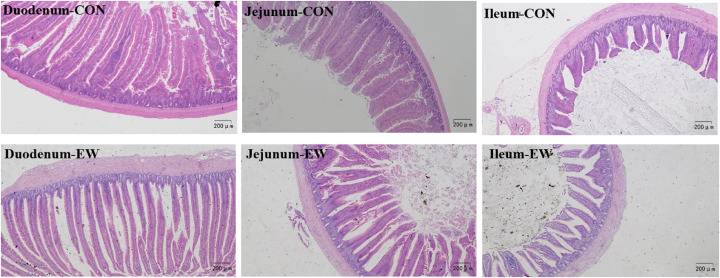
Hematoxylin-eosin staining of the small intestine involving the duodenum, jejunum, and ileum of the two treatment groups showed the influence of early weaning on the intestinal morphology examination in squabs (Figure 3). The duodenum, jejunum and ileum of squabs in the control group revealed a normal appearance with regular and complete intestinal villus and crypt structure. In contrast, visible damage to the intestinal villi was observed in the EW group (Figure 3).
Figure 3.
Effects of early weaning on intestinal morphology of duodenum, jejunum, and ileum in squabs of two groups. Sections were stained with hematoxylin and eosin. Bar = 200 μm. CON group: control group; EW group: test group, squabs were fed by parental pigeons for first 6 d after hatching, and then fed by hand.
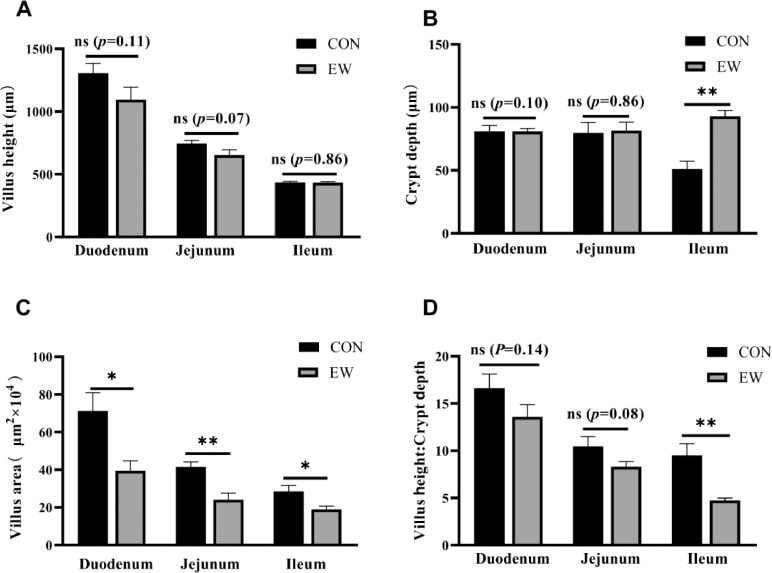
Observation and measurement throughout the light microscope and the effects of early weaning on squab small intestine morphometric traits of three segments (duodenum, jejunum and ileum) of the two groups are shown in Figure 4. In the duodenum, the area of the duodenal villi in the control group was significantly larger (P < 0.05) than the area of the duodenal villi in the EW group. There was no significant influence on villus height, crypt depth or VCR. In the jejunum, contrasted with the control group, the villus area in the jejunum was significantly decreased (P < 0.01) in the EW group. In the ileum, early weaning led to a significant change in crypt depth (P < 0.01), the villus area (P < 0.05), and VCR (P < 0.01) by comparison with the control group.
Figure 4.
Effects of early weaning on small intestine morphometric trait of three segments (duodenum, jejunum and ileum) of squabs of two groups at the age of 25 d. (A) Villus height; (B) crypt depth; (C) villus area; (D) the ratio of villus height to crypt depth (VCR). CON group: control group; EW group: test group, squabs were fed by parental pigeons for first 6 d after hatching, and then fed by hand. Values means n = 8 for the analysis of the intestine form. Data are means ± SEM. * means P < 0.05; ** means P < 0.01.
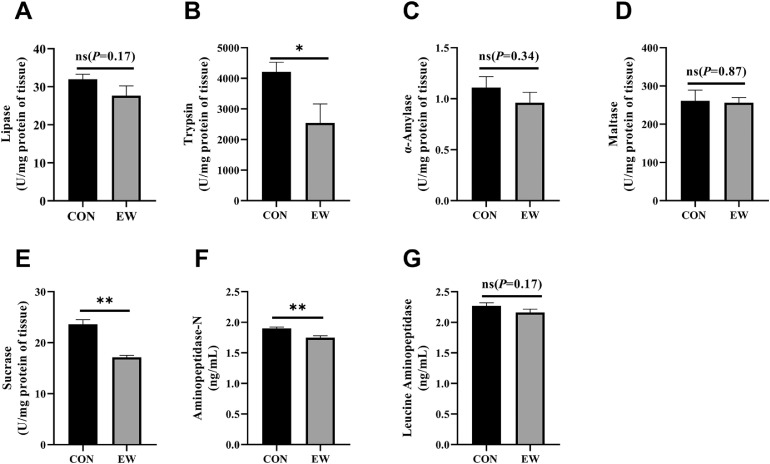
Determination of Enzymatic Activity
Digestive enzyme activity varies significantly in the duodenum content with the different methods of breeding, as shown in Figure 5. Clearly, there was a significant decreasing trend in the activity of trypsin (P < 0.05), sucrase (P < 0.01), and APN (P < 0.01). Early weaning in squabs had no significant influence on the enzymes of lipase, α-amylase, and LAP between the two groups.
Figure 5.
Effects of early weaning on digestive enzyme activities ([A] α-amylase, [B] trypsin, [C] lipase, [D] maltase, [E] sucrase, [F] Aminopeptidase-N, and [G] leucine aminopeptidase) of duodenum content of 25-day-old squabs in two groups. CON group: control group; EW group: test group, squabs were fed by parental pigeons for first 6 d after hatching, and then fed by hand. Values means n = 8 for the analysis of the intestine form. Data are means ± SEM. * means P < 0.05; ** means P < 0.01.
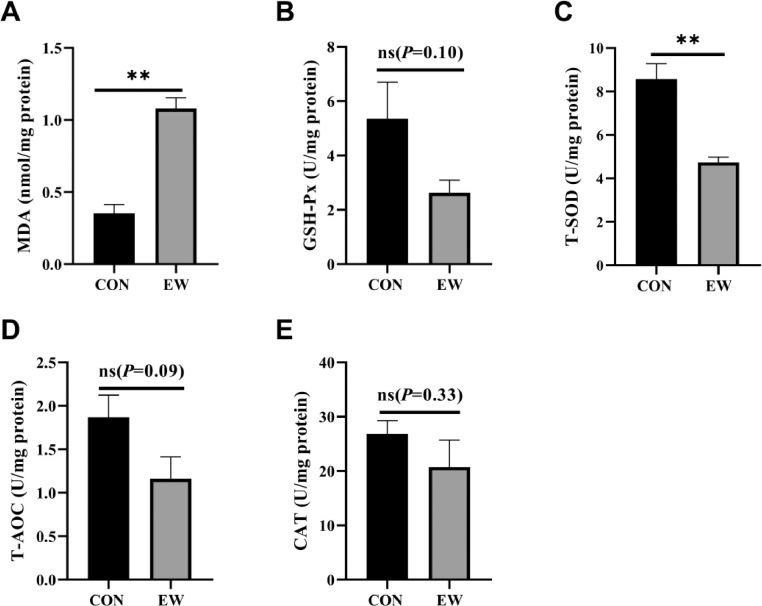
Determination of Intestinal Mucosal Oxidant Status
Figure 6 shows the effects of early weaning on the concentration of antioxidants status including MDA, GSH-Px, T-SOD, T-AOC, and CAT. As shown in Figure 6A, MDA levels in the EW group was significantly higher (P < 0.01) than MDA levels in the control group. The concentration of T-SOD in the jejunum content extremely decreased in the EW group compared with the control group (P < 0.01, Figure 6C). Although the enzyme activities of GSH-Px, T-AOC, and CAT in the jejunum decreased in the EW group, there were no significant differences between the two groups.
Figure 6.
Effects of early weaning on the concentration of antioxidant status ([A] MDA, [B] GSH-Px, [C] T-SOD, [D] T-AOC, and [E] CAT) of duodenum content of 25-day-old squabs in two groups. CON group: control group; EW group: test group, squabs were fed by parental pigeons for first 6 d after hatching, and then fed by hand. Values means n = 8 for the analysis of the intestine form. Data are means ± SEM. * means P < 0.05; ** means P < 0.01.
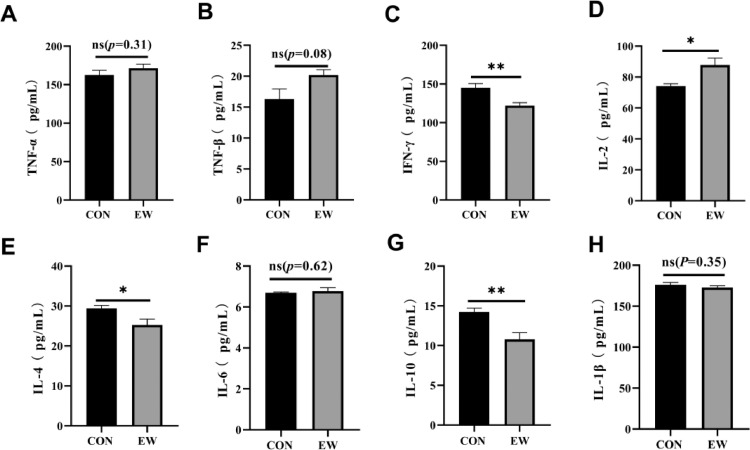
Intestinal Inflammatory Cytokines Analysis
The influence of early weaning on the levels of jejunum cytokines is shown in Figure 7. Compared with the CON group, early weaning stress increased jejunum tissue IL-2 levels, as shown in Figure 7D (P < 0.05), whereas the IFN-γ and IL-10 concentrations decreased significantly (P < 0.01). In addition, the level of IL-4 was lower in the control group than it in the EW group (P < 0.05, Figure 7E). Early weaned squabs expressed higher TNF-α, TNF-β, and IL-6 (P > 0.05, Figures 7A, B, and F), but there was no significant difference between the two groups.
Figure 7.
Effects of early weaning on inflammatory cytokine (TNF-α [A], TNF-β [B], IFN-γ [C], IL-2 [D], IL-4 [E], IL-6 [F], IL-10 [G], and IL-1β [H]) of small intestine of 25-day-old squabs in two groups. CON group: control group; EW group: test group, squabs were fed by parental pigeons for first 6 d after hatching, and then fed by hand. Values means n = 8 for the analysis of the intestine form. Data are means ± SEM. * means P < 0.05; ** means P < 0.01. Abbreviations: IFN-γ, interferon-γ; IL-2, interleukin-2; IL-4, interleukin-4; IL-6, interleukin-6; IL-10, interleukin-10; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; TNF-β, tumor necrosis factor-β.
DISCUSSION
The squabs obtain nutrients from the yolk during embryogenesis and at hatch transitions to acquire energy and nutrition from crop milk in the small intestine (Gillespie et al., 2013; Zhang and Wong, 2017). In addition, pigeons, altricial birds, exhibit rapid growth after hatching, which means that they need more energy and nutrition from feed. Hence, it requires a strong and healthy digestive system (Dong et al., 2012). The small intestine is a vital digestive and absorptive organ and innate barrier that maintains the balance of the inner environment (Li et al., 2017). Similar to piglets, the squab stage is an essential intestinal development period that affects growth performance, such as BW, ADG and rate of survival, later in adult pigeons. Therefore, the development of the small intestine is associated with the health of the young (Metzler-Zebeli et al., 2018).
A previous study showed that small intestinal length is related to growth state. Sometimes, the length index and weight index are regarded as essential and direct indicators of the development of the small intestine (Wang et al., 2019). The longer the small intestine is, the larger the surface area of the small intestine is to some extent. Better insight into the digestive and absorptive capabilities of small intestines of piglets of different lengths may provide important information for improving feed digestibility in pigs by nutritional intervention or genetic selection (Gao et al., 2010). Similar studies have been reported in poultry husbandry (Li et al., 2017). To explore the influence of early weaning on squabs, we studied the changes of in the length index and weight index of small intestine in young squabs at the age of 25 d. The results showed that early weaning was negative for the length index and weight index of the small intestine including the duodenum, jejunum, and ileum. We found that early weaning would decrease the length index of the small intestine, especially the segment of the ileum. Other studies reported that dietary addition relieved the stress of weaning rabbits, in terms of increasing the length of the small intestine (Liu et al., 2019b). Furthermore, King et al. (2008) stated that early weaning could lead to a decrease in the weight index of the small intestine, which was similar to our results.
As is well-known, compromising alterations in villus–crypt structure are universal in weaned animals and are characterized by a decrease in villus height and VCR and an increase in crypt depth (Hampson, 1986; Kim et al., 2001; Hu et al., 2013a). In our current work, hand rearing of early-weaned squabs resulted in a smaller villus area compared with the villus area of the control group at 3 sites. Villus height, crypt depth, and VCR in all segments were stable, and only in the ileum was crypt depth enlarged and VCR decreased. These worsening results were similar to previous studies, which revealed the effects of early weaning on intestinal changes in rabbits and piglets (Liu et al., 2019b; Wang et al., 2020b). Some studies have suggested that histology that is more integrated and morphology are conducive to digestion and absorption in animals. Decreasing villus height is associated with the weakened health status of pigeons. Similarly, deeper crypt depth and higher VCR harm the stimulation of enterocyte proliferation. In addition, the increased villus area allows pigeons to absorb more nutrients into the bodies (Wang et al., 2020b; Xu et al., 2020). A study by Tang et al. (1999) also indicated that weaning would induce a reduction in villus height and VCR and an increase in crypt depth. Liu et al. (2019a) stated that weaning caused changes in jejunal villus height and crypt depth, for which intestinal villi were damaged and the capacity of digestion and absorption was hindered. Some explanations were that villus changes might be reduced due to cell apoptosis caused by changes in feed composition after weaning (Van der Peet-Schwering et al., 2007). Taken together, deeper crypt depth and increasing VCR in ileum obviously result from early weaning obviously, and increasing villus area in all segments of intestine in pigeon squabs. It has also been stated that early weaning could influence intestinal morphology not only in mammals such as piglets and rabbits but also in poultry such as pigeons. The difference between mammals and altricial birds needs to be explored in the future.
As mentioned above, enterocytes acquire differentiated functions for digestion, including the expression of enzymes such as disaccharidase, peptidases and phosphatase, during their migration from the crypt toward the villus tip (Fan et al., 2001; Dong et al., 2012). The growth and development of animals are usually regulated by the digestibility of gastrointestinal digestive enzymes (Yang et al., 2017). The activities of digestive enzymes are useful criteria for reflecting the capacity of digestion and metabolism (Shih and Hsu, 2006). In this study, the decrease in the duodenum digestive enzyme activity with early weaning was consistent with a previous study on weaner piglets (Fan et al., 2001; Wang et al., 2020a). The data clearly suggest significantly lower activity of enzymes including trypsin, sucrase, and APN in the duodenum content of squabs in the EW group. First, as stated above, lower activity of enzymes might be due to the negative effects on intestinal morphology, which in turn inhibits the secretion of endogenous enzymes (Shang et al., 2020).
For example, APN is important in protein digestion and belongs to brush border hydrolases, is present in both membrane-bound and cytosolic (soluble) forms in “suckling animals” and is bound mainly on the apical surface of the mature enterocyte (Fan et al., 2002). Furthermore, the decreased activity of trypsin, sucrase, and APN is accepted, since they are mainly influenced by the protein content, carbohydrate content, and amino acid profile of the diet. This refer to Dong et al. (2012) suggested that changes in digestive enzyme activity in crop milk may not be totally free of carbohydrates. Previous studies also showed that the contents of pigeon milk would change instantly with the growth of squabs (Dong et al., 2012. In the end, we assumed that the secretory processes of enzymes are immature. Early weaning probably delays individual development and, in particular, the onset of secretory functions. That is, we speculated that organ development was also a potential cause for the decrease in digestive enzyme activity (Cahu and Infante, 1994). In summary, we assumed that three hypotheses were mentioned about the effects of weaning on digestive enzyme activity in squabs. Whether and how digestive enzyme activity in squabs was influenced by these factors warrants further investigation.
Numerous studies have reported that weaning stress could damage the antioxidant system and functions, and then lead to deterioration of the intestinal immune barrier (Zheng et al., 2013; Xu et al., 2014). After weaning, oxidative stress caused by weaning stress might damage DNA, biomembrane lipids, and proteins in tissues (Qian et al., 2011). Antioxidant enzymes, such as T-SOD and CAT, could help alleviate the impact of oxidative stress (Qian et al., 2011). MDA is a product of lipid peroxidation, and its content can directly reflect the degree of lipid oxidative injury (Pirinccioglu et al., 2010). In addition, barrier function is differently affected after weaning and is worse in the proximal and mid-jejunum than in the ileum (Peter et al., 2011). In the current study, we found that early weaning stress had an impact on the concentrations of antioxidants including MDA and T-SOD. The results showed a higher concentration of MDA and a decreased content of T-SOD in the jejunum in squabs. The extreme influences on these 2 antioxidant statuses were similar to the influences in studies on weaner piglets (Cao et al., 2018). Xu et al. (2014) reported that early weaning led to a sharp drop in intestinal antioxidant capacity and a steep increase in the level of MDA. Researchers demonstrated that early weaning was one major factor suppressing the expression of T-SOD and elevating the content of MDA in the serum of piglets (Zhu et al., 2012). According to previous studies on weaner piglets, we hypothesized that there were two crucial reasons for these results regarding changes in the content of antioxidant status in the jejunum. One reason was the change in diet in squabs; the other was related gene expression. Furthermore, based on previous studies, increasing levels of T-SOD were reported to be consistent with increasing mRNA expression of related genes during weaning (Yin et al., 2014). Therefore, hand-fed weaner squabs showed lower levels of T-SOD and higher concentrations of MDA. Early weaning stress was stated to have a negative influence on the intestinal antioxidant system and intestinal immune barrier.
Oxidative damage plays a proinflammatory role in inflammation. Cytokines play a central role in the regulation of the inflammatory response, but they also take part in the maintenance of intestinal barrier integrity (Al-Sadi et al., 2009; Zhang et al., 2020). Hence, changes in the cytokines of animals might be expected at weaning or other stresses, since changes in diet and environment result in significant morphological and functional adaptations in the small intestine (Pié et al., 2004). The responsibilities of proinflammatory cytokines are mediators of the inflammatory response, endothelial cell permeability, and organ dysfunction. Common examples include TNF-α, INF-γ, and IL-6. In addition, IL-4 and IL‐10 are classical anti-inflammatory cytokines because they are necessary in the containment of inflammatory responses, regulate the metabolism of tissues, promote the synthesis and release of other cytokines, and thus maintain immune processes (Al-Sadi et al., 2009; Zhang et al., 2020). For this study, IL-2 in the jejunum was significantly higher than those in the intestine of squabs in the EW group, whereas IL-4 and IL-10 were lower in the jejunum. These results could be accepted since higher expression of proinflammatory cytokines and lower concentrations of anti-inflammatory cytokines were simultaneous extinctions, indicating that early weaning was one of the most important reasons and stress to induce inflammation in young squabs. The anti-inflammatory functions were decreased and the negative influences of proinflammatory factors were increased. However, the levels of IFN-γ in the jejunum were reduced significantly. Most of the research suggested that the expression of IFN-γ was higher in the mid-jejunum of weaning piglets (Upadhaya et al., 2019). Nevertheless, similar results were shown for weaning Holstein calves. The study stated that weaning stress led to a reduction in the levels of IFN-γ. The reasons they analyzed may be the secretion of glucocorticoids. Weaning, can cause both physical and psychological stress, and has been shown to suppress blastogenesis of IFN-γ production (Cunnick et al., 1990). Glucocorticoids also suppress the synthesis and release of cytokines (Kim et al., 2011). Furthermore, the levels of IL-2 in spleen cells were lower in the weaned group than in the unweaned control group. Decreasing levels of IL-2 have been reported to possibly be associated with the occurrence of immune responses within the intestine (Bailey et al., 1992).
In conclusion, early weaning impaired intestinal development and intestinal health of young pigeons. Intestinal development was hindered, such as the length index and weight index of the ileum. The small intestine health was also impacted, including the activity of enzymes, the concentration of antioxidant status and the content of inflammatory cytokines. Damaged gut structure and function influence the digestion and absorption capacity of individuals. Therefore, damagedture and function would eventually harm growth performance, including BW and ADG. Our results were similar to the results of studies on early-weaned stress in mammals. This study will provide information and a reference for our further study on nutrition regulation in early-weaned squabs, and will be more productive.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (31902173); the Fundamental Research Funds for the Central Universities (2021FZZX002-08).
DISCLOSURES
The author declares that there is no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101613.
Appendix. Supplementary materials
REFERENCES
- Abdel-Azeem and F. Abdel-Azeem. 2010. The composition of the crop milk in Egyptian baladi pigeons and its role in growth of squabs. Egypt. Poult. Sci. 30(4):755–782.
- Abdel-Azeem A.F., Ame A.A., Shama T.A., Abbas W.A. Early weaning of pigeon squabs. Egypt. Poult. Sci. 2016;36:205–232. [Google Scholar]
- Al-Sadi R., Boivin M., Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci (Landmark Ed) 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M., Clarke C.J., Wilson A.D., Williams N.A., Stokes C.R. Depressed potential for interleukin-2 production following early weaning of piglets. Vet. Immunol. Immunopathol. 1992;34:197–207. doi: 10.1016/0165-2427(92)90164-l. [DOI] [PubMed] [Google Scholar]
- Cahu C.L., Zambonino Infante J.L. Early weaning of sea bass (Dicentrarchus labrax) larvae with a compound diet: effect on digestive enzymes. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 1994;109:213–222. [Google Scholar]
- Cao S.T., Wang C.C., Wu H., Zhang Q.H., Jiao L.F., Hu C.H. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018;96:1073. doi: 10.1093/jas/skx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.L., Zhao G.P., Zheng M.Q., Wen J., Yang N. Estimation of genetic parameters for contents of intramuscular fat and inosine-5ʹ-monophosphate and carcass traits in Chinese Beijing-You chickens. Poult. Sci. 2008;87:1098–1104. doi: 10.3382/ps.2007-00504. [DOI] [PubMed] [Google Scholar]
- Cunnick J.E., Lysle D.T., Kucinski B.J., Rabin B.S. Evidence that shock-induced immune suppression is mediated by adrenal hormones and peripheral β-adrenergic receptors. Pharmacol. Biochem. Behav. 1990;36:645–651. doi: 10.1016/0091-3057(90)90270-r. [DOI] [PubMed] [Google Scholar]
- Ding J., Liao N., Zheng Y., Yang L., Meng H. The composition and function of pigeon milk microbiota transmitted from parent pigeons to squabs. Front. Microbiol. 2020;11:1789. doi: 10.3389/fmicb.2020.01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X.Y., Wang Y.M., Dai L., Azzam M., Wang C., Zou X.T. Posthatch development of intestinal morphology and digestive enzyme activities in domestic pigeons (Columba livia) Poult. Sci. 2012;91:1886–1892. doi: 10.3382/ps.2011-02091. [DOI] [PubMed] [Google Scholar]
- Dong X.Y., Zhang M., Jia Y.X., Zou X.T. Physiological and hormonal aspects in female domestic pigeons (Columba livia) associated with breeding stage and experience. J. Anim. Physiol. Anim. Nutr. 2013;97:861–867. doi: 10.1111/j.1439-0396.2012.01331.x. [DOI] [PubMed] [Google Scholar]
- Fan M.Z., Adeola O., Asem E.K., King D. Postnatal ontogeny of kinetics of porcine jejunal brush border membrane-bound alkaline phosphatase, aminopeptidase N and sucrase activities. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2002;132:599–607. doi: 10.1016/s1095-6433(02)00102-2. [DOI] [PubMed] [Google Scholar]
- Fan M.Z., Stoll B., Jiang R., Burrin D.G. Enterocyte digestive enzyme activity along the crypt-villus and longitudinal axes in the neonatal pig small intestine. J. Anim. Sci. 2001;2:371–381. doi: 10.2527/2001.792371x. [DOI] [PubMed] [Google Scholar]
- Gao J., Ren J., Zhou L.H., Ren D.R., Li L., Xiao S.J., Yang B., Huang L.S. A genome scan for quantitative trait loci affecting the length of small intestine in a White Duroc x Chinese Erhualian intercross resource population. J. Anim. Breed. Genet. 2010;127:119–124. doi: 10.1111/j.1439-0388.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- Ge P., Ma H., Li Y., Ni A., Chen J. Identification of microrna-associated-cerna networks regulating crop milk production in pigeon (Columbia livia) Genes. 2020;12:39. doi: 10.3390/genes12010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M.J., Crowley T.M., Haring V.R, Wilson S.L., Harper J.A., Payne J.S., Green D., Monaghan P., Donald J.A., Nicholas K.R, Moore R.J. Transcriptome analysis of pigeon milk production - role of cornification and triglyceride synthesis genes. BMC Genomics. 2013;14:169–181. doi: 10.1186/1471-2164-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M.J., Haring V.R., Mccoll K.A., Monaghan P., Crowley T.M. Histological and global gene expression analysis of the 'lactating' pigeon crop. BMC Genomics. 2011;12:452. doi: 10.1186/1471-2164-12-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson D.J. Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 1986;40:32–40. [PubMed] [Google Scholar]
- Hegde S.N. Composition of pigeon milk and its effect on growth in chicks. Indian J. Exp. Biol. 1973;11:238. [PubMed] [Google Scholar]
- Hu C., Song J., Li Y., Luan Z., Zhu K. Diosmectite–zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br. J. Nutr. 2013;110:681–688. doi: 10.1017/S0007114512005508. [DOI] [PubMed] [Google Scholar]
- Hu C.H., Xiao K., Luan Z.S., Song J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013;91:1094–1101. doi: 10.2527/jas.2012-5796. [DOI] [PubMed] [Google Scholar]
- Hu X.C., Gao C.Q., Wang X.H., Yan H.C., Chen Z.S., Wang X.Q. Crop milk protein is synthesised following activation of the irs1/akt/tor signalling pathway in the domestic pigeon (Columba livia) Br. Poult. Sci. 2016;57:855–862. doi: 10.1080/00071668.2016.1219694. [DOI] [PubMed] [Google Scholar]
- Hullar I., Meleg I., Fekete S., Romvari R. Studies on the energy content of pigeon feeds I. Determination of digestibility and metabolizable energy content. Poult. Sci. 1999;78:1757–1762. doi: 10.1093/ps/78.12.1757. [DOI] [PubMed] [Google Scholar]
- Kai C., Wang B., Zhang N., Yan T., Tao M., Diao Q., Manuel P. Itraq-based quantitative proteomic analysis of alterations in the intestine of Hu sheep under weaning stress. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.C., Hansen C.F., Mullan B.P., Pluske J.R. Nutrition and pathology of weaner pigs: nutritional strategies to support barrier function in the gastrointestinal tract. Anim. Feed Sci. Technol. 2001;173:3–16. [Google Scholar]
- Kim M.H., Yang J.Y., Upadhaya S.D., Lee H.J., Yun C.H., Ha J.K. The stress of weaning influences serum levels of acute-phase proteins, iron-binding proteins, inflammatory cytokines, cortisol, and leukocyte subsets in Holstein calves. J. Vet. Sci. 2011;12:151–157. doi: 10.4142/jvs.2011.12.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M.R., Morel P., Revell D.K. Dietary bovine colostrum increases villus height and decreases small intestine weight in early-weaned pigs. Asian Australas. J. Anim. Sci. 2008;21:567–573. [Google Scholar]
- Lainé J., Beattie M., Lebel D. Simultaneous kinetic determinations of lipase, chymotrypsin, trypsin, elastase, and amylase on the same microtiter plate. Pancreas. 1993;8:383. doi: 10.1097/00006676-199305000-00016. [DOI] [PubMed] [Google Scholar]
- Lea R.W., Vowles D.M., Dick H.R. Factors affecting prolactin secretion during the breeding cycle of the ring dove (streptopelia risoria) and its possible role in incubation. J. Endocrinol. 1986;110:447–458. doi: 10.1677/joe.0.1100447. [DOI] [PubMed] [Google Scholar]
- Li S.M., Wang X.G., Qu L., Dou T.C., Ma M., Shen M.M., Guo J., Hu Y.P., Wang K.H. Genome-wide association studies for small intestine length in an F2 population of chickens. Ital. J. Anim. Sci. 2017;17:294–300. [Google Scholar]
- Liu G., Zheng J., Wu X., Xu X., Jia G., Zhao H., Chen X., Wu C., Tian G., Wang J. Putrescine enhances intestinal immune function and regulates intestinal bacteria in weaning piglets. Food Funct. 2019;10:4134–4142. doi: 10.1039/c9fo00842j. [DOI] [PubMed] [Google Scholar]
- Liu L., Zuo W., Li F. Dietary addition of Artemisia argyi reduces diarrhea and modulates the gut immune function without affecting growth performances of rabbits after weaning1. J. Anim. Sci. 2019;97:1693–1700. doi: 10.1093/jas/skz047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H.G., Xu X.L., Cao H.Y., Dong X.Y., Zou X.T., Xu N.Y., Yin Z.Z. H-FABP gene expression and genetic association with meat quality traits in domestic pigeons (Columba livia) Br. Poult. Sci. 2021;62:172–179. doi: 10.1080/00071668.2020.1839016. [DOI] [PubMed] [Google Scholar]
- Metzler-Zebeli B.U., Magowan E., Hollmann M., Ball M.E.E., Molnar A., Witter K., Ertl R., Hawken R.J., Lawlor P.G., O'Connell N.E., Aschenbach J., Zebeli Q. Differences in intestinal size, structure, and function contributing to feed efficiency in broiler chickens reared at geographically distant locations. Poult. Sci. 2018;97:578–591. doi: 10.3382/ps/pex332. [DOI] [PubMed] [Google Scholar]
- Modina S.C., Polito U., Rossi R., Corino C., Giancamillo A. Nutritional regulation of gut barrier integrity in weaning piglets. Animals. 2019;9:1045. doi: 10.3390/ani9121045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeser A.J., Ryan K.A., Nighot P.K., Blikslager A.T. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol.-Gastroint. Liver Physiol. 2007;293:G413–G421. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- Peetschwering C., Jansman A., Smidt H., Yoon I. Effects of yeast culture on performance, gut integrity, and blood cell composition of weanling pigs. J. Anim. Sci. 2007;85:3099. doi: 10.2527/jas.2007-0110. [DOI] [PubMed] [Google Scholar]
- Peter J. A. W., J. van der Meulen, and M. W. A. Verstegen. 2011. Intestinal barrier function and absorption in pigs after weaning: a review. Br. J. Nutr. 105:967–981. [DOI] [PubMed]
- Pié S., Lallès J.P., Blazy F., Laffitte J., Sève B., Oswald I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004;134:641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- Pirinccioglu A.G., Gokalp D., Pirinccioglu M., Kizil G., Kizil M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin. Biochem. 2010;43:1220–1224. doi: 10.1016/j.clinbiochem.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Qian Z., Piao X.L., Piao X.S., Lu T., Wang D., Kim S.W. Preventive effect of coptis chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food Chem. Toxicol. 2011;49:61–69. doi: 10.1016/j.fct.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Hirose H., Onizuka A., Hayashi M., Ezaki T. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 2000;94:99–106. doi: 10.1006/jsre.2000.5937. [DOI] [PubMed] [Google Scholar]
- Sales J., Janssens G. Nutrition of the domestic pigeon (Columba livia domestica) World Poult. Sci. J. 2003;59:221–232. doi: 10.1093/ps/82.9.1457. [DOI] [PubMed] [Google Scholar]
- Shang Q., Ma X., Liu H., Liu S., Piao X. Effect of fibre sources on performance, serum parameters, intestinal morphology, digestive enzyme activities and microbiota in weaned pigs. Arch. Anim. Nutr. 2020;74:121–137. doi: 10.1080/1745039X.2019.1684148. [DOI] [PubMed] [Google Scholar]
- Shih B.L., Hsu J.C. Development of the activities of pancreatic and caecal enzymes in White Roman goslings. Br. Poult. Sci. 2006;47:95–102. doi: 10.1080/00071660500475079. [DOI] [PubMed] [Google Scholar]
- Smith F., Clark J.E., Overman B.L., Tozel C.C., Huang J.H., Rivier J.E., Blikslager A.T., Moeser A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol.-Gastroint. Liver Physiol. 2010;298:G352–G363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi M. Modifications of two methods for the assay of amylase. Clin. Chem. 1960;6:23–35. [PubMed] [Google Scholar]
- Tang M., Laarveld B., Van Kessel A.G., Hamilton D.L., Estrada A., Patience J.F. Effect of segregated early weaning on postweaning small intestinal development in pigs. J. Anim. Sci. 1999;77:3191–3200. doi: 10.2527/1999.77123191x. [DOI] [PubMed] [Google Scholar]
- Tietz N.W., Fiereck E.A. A specific method for serum lipase determination. Clin. Chim. Acta. 1966;13:352–355. doi: 10.1016/0009-8981(66)90215-4. [DOI] [PubMed] [Google Scholar]
- Upadhaya S.D., Laguna F., Bertaud B., Kim I. Multi-strain yeast fraction product supplementation can alleviate weaning stress and improve performance and health of piglets raised under low sanitary conditions. J. Sci. Food Agric. 2019;99:6076–6083. doi: 10.1002/jsfa.9885. [DOI] [PubMed] [Google Scholar]
- Wang H., Li S., Xu S., Feng J. Betaine improves growth performance by increasing digestive enzymes activities, and enhancing intestinal structure of weaned piglets. Anim. Feed Sci. Technol. 2020;267 [Google Scholar]
- Wang M., Yang C., Wang Q.Y., Li J.Z., Yin Y.L. The growth performance, intestinal digestive and absorptive capabilities in piglets with different lengths of small intestines. Animal. 2019;14:1–8. doi: 10.1017/S175173111900288X. [DOI] [PubMed] [Google Scholar]
- Wang Z., Li J., Wang Y., Wang L., Yin Y., Yin L., Yang H., Yin Y. Dietary vitamin A affects growth performance, intestinal development, and functions in weaned piglets by affecting intestinal stem cells. J. Anim. Sci. 2020;98 doi: 10.1093/jas/skaa020. skaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijtten P., Meulen J., Verstegen M. Intestinal barrier function and absorption in pigs after weaning: a review. Br. J. Nutr. 2011;105:967–981. doi: 10.1017/S0007114510005660. [DOI] [PubMed] [Google Scholar]
- Xie P., Wan X.P., Yang C.X., Zhu J.G., Xu Y.G., Gong D.Q. Effects of incubation and chick rearing on intestinal morphology, digestive enzyme activities, and mrna expression of nutrient transporter genes in the pigeon (Columba livia) under artificial farming conditions. Poult. Sci. 2020;99:2785–2797. doi: 10.1016/j.psj.2019.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Xu C., Chen X., Cai X., Yang S., Sheng Y., Wang T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition. 2014;30:584–589. doi: 10.1016/j.nut.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Xu Q.Q., Zhang X.Y., Zou X.T., Dong X.Y. Effects of in ovo injection of L-histidine on hatch performance and post-hatch development in domestic pigeons (Columba livia) Poult. Sci. 2019;98:3194–3203. doi: 10.3382/ps/pez046. [DOI] [PubMed] [Google Scholar]
- Xu Q.Q., Wen J.S., Wang X.M., Zou X.T., Dong X.Y. Maternal dietary linoleic acid altered intestinal barrier function in domestic pigeons (Columba livia) Br. J. Nutr. 2020;126:31–34. doi: 10.1017/S0007114520004973. [DOI] [PubMed] [Google Scholar]
- Yang J., Yang L., Wang Y.C., Zhai S., Wang S., Yang Z., Wang W. Effects of dietary protein and energy levels on digestive enzyme activities and electrolyte composition in the small intestinal fluid of geese. Anim. Sci. J. 2017;88:294–299. doi: 10.1111/asj.12557. [DOI] [PubMed] [Google Scholar]
- Yang M.C., Vohra P. Protein and metabolizable energy requirements of early-weaned squabs from hatching to 28 days of age. Poult. Sci. 1987;66:2017–2023. doi: 10.3382/ps.0662017. [DOI] [PubMed] [Google Scholar]
- Yin J., Wu M.M., Xiao H., Ren W.K., Duan J.L., Yang G., Li T.J., Yin Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014;92:612. doi: 10.2527/jas.2013-6986. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wong E.A. Spatial transcriptional profile of PepT1 mRNA in the yolk sac and small intestine in broiler chickens. Poult. Sci. 2017;96:2871–2876. doi: 10.3382/ps/pex056. [DOI] [PubMed] [Google Scholar]
- Zhang H.Y., Li J.Z., Cao C.Y., Zhang B.R., Yang W., Shi B.M., Shan A.S. Pyrroloquinoline quinone inhibits the production of inflammatory cytokines via the sirt1/nf-κb signal pathway in weaned piglet jejunum. Food Funct. 2020;11:2137–2153. doi: 10.1039/c9fo02609f. [DOI] [PubMed] [Google Scholar]
- Zhang X.Y., Zhang N.N., Wan X.P., Li L.L., Zou X.T. Gene expression of amino acid transporter in pigeon (columbia livia) intestine during post-hatch development and its correlation with amino acid in pigeon milk. Poult. Sci. 2016;96:1120–1131. doi: 10.3382/ps/pew320. [DOI] [PubMed] [Google Scholar]
- Zheng P., Yu B., He J., Tian G., Luo Y.H., Mao X.B., Zhang K.Y., Che L.Q., Chen D.W. Protective effects of dietary arginine supplementation against oxidative stress in weaned piglets. Br. J. Nutr. 2013;109:2253–2260. doi: 10.1017/S0007114512004321. [DOI] [PubMed] [Google Scholar]
- Zhu L.H., Zhao K.L., Chen X.L., Xu J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012 doi: 10.2527/jas.2012-4444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.