Abstract
Atrioesophageal fistula is a life-threatening complication of ablation treatment for atrial fibrillation. Methods to reduce the risk of esophageal injury have evolved over the last decade, and diagnosis of this complication remains difficult and therefore challenging to treat in a timely manner. Delayed diagnosis leads to treatment occurring in the context of a critically ill patient, contributing to the poor prognosis associated with this complication. The associated mortality risk can be as high as 70%. Recent important advances in preventative techniques are explored in this review.
Preventative techniques used in current clinical practice are discussed, which include high-power short-duration ablation, esophageal temperature probe monitoring, cryotherapy and laser balloon technologies, and use of proton pump inhibitors. A lack of randomized clinical evidence for the effectiveness of these practical methods are found. Alternative methods of esophageal protection has emerged in recent years, including mechanical deviation of the esophagus and esophageal temperature control (esophageal cooling). Although these are fairly recent methods, we discuss the available evidence to date. Mechanical deviation of the esophagus is due to undergo its first randomized study. Recent randomized study on esophageal cooling has shown promise of its effectiveness in preventing thermal injuries. Lastly, novel ablation technology that may be the future of esophageal protection, pulsed field ablation, is discussed.
The findings of this review suggest that more robust clinical evidence for esophageal protection methods is warranted to improve the safety of atrial fibrillation ablation.
Keywords: Atrioesophageal fistula, Atrial fibrillation ablation, Complications, Esophageal protection, Thermal injury
Key Findings.
-
▪
Atrioesophageal fistula (AEF) remains a potentially serious complication from atrial fibrillation ablation. Although the estimated risk is low, the mortality rate is high to those that sustain this injury. We review the current strategies used to prevent this complication and future methods still undergoing research study.
-
▪
Current clinical strategies to prevent esophageal injury suffer from a lack of randomized clinical evidence of its effectiveness. So far, randomized trials on esophageal temperature monitoring probes, a common standard of care in many cardiac centers, show that there is no reduction in thermal injury compared to control ablations.
-
▪
Advances in radiofrequency (RF) and alternative ablation methods are discussed, including cryotherapy and laser balloon technology. There are limitations on assessing the true incidence or risk of AEF with each ablation modality. Laser balloon is relatively new technology compared to RF or cryotherapy, with limited clinical experience on safety and efficacy.
-
▪
Novel methods of esophageal protection are explored, including mechanical deviation of the esophagus, esophageal cooling, and advanced ablation modalities such as ultra-low-temperature cryotherapy and pulsed field ablation and the implications for esophageal protection.
Introduction
Atrial fibrillation (AF) is a common problem, affecting an estimated 1%–2% of the world’s population1 across a range of age groups, including persons with structurally normal or athletic hearts and those with underlying cardiomyopathies. AF is an important condition resulting in symptoms that erode functional ability and quality of life; it reduces cardiac function, predisposing to stroke and heart failure.2 It creates a large burden of healthcare and socioeconomic costs.3,4
AF ablation is an increasingly important procedure with growing demand globally. Recent randomized trials have confirmed that ablation is overwhelmingly more effective than traditional pharmacological therapies for AF, with a strong trend to superior safety. As referral patterns respond to this balance of evidence, healthcare systems are expected to accommodate this demand, with increasing provision of ablation services. AF ablation is a safe procedure with a mortality rate of <0.1%.5 Most potentially fatal complications can be avoided by careful technique and meticulous periprocedural control of coagulation, but one lethal complication has been stubbornly difficult to eliminate: esophageal thermal injury, potentially leading to symptomatic esophageal ulceration, upper gastrointestinal bleeding, and atrioesophageal fistulation.6 The esophagus is vulnerable to this injury because of its anatomical proximity to the areas of the left atrium that are targeted in AF ablation.7,8 Gillinov and colleagues9 reported the first case in 2001 after a surgical ablation using radiofrequency (RF) and similar reports followed, after percutaneous AF catheter ablation.10,11 The majority of the cases described in these reports did not survive. Following these reports, there has been increasing study on the risk factors, diagnosis, and treatment of this serious complication and the strategies that can be used to prevent this.
Risks associated with AF ablation
In recent series, the risk of stroke during or soon after AF ablation is 0.1%, the risk of cardiac tamponade is at 0.9%, and risk of vascular trauma requiring repair or intervention is <0.5%.5 All of these risks can be minimized by care and expertise on behalf of the operator and by careful attention to periprocedural anticoagulation. The risk of tamponade is falling in response to modern equipment and increasingly experienced institutions and operators; access site complications are falling in response to these same factors and to the increased use of ultrasound guidance and suture-assisted hemostasis.
Among the life-threatening complications, atrioesophageal fistula remains uniquely difficult to avoid. It is an axiom of AF ablation that all lesions must be transmural (Figure 1); as the atrial myocardium abuts other organs with a minimal layer of fat insulation, lesions can potentially extend into adjacent structures. The pericardium and the pleura, the diaphragmatic and intercostal muscle, ribs, lung, bronchi, phrenic nerves, the aorta, and the esophagus all lie within reach. For most of these, the injury is unimportant. In most locations, the tissue of the extracardiac lesion becomes necrotic and is then converted safely to scar tissue. In the esophagus it can be lethal because the esophagus differs in being exposed to bacterial action and to regurgitated stomach contents that include hydrochloric acid and sometimes bile and digestive enzymes. It is probably a combination of these factors that can cause necrosis in this location to extend and destroy the esophageal wall, permitting the formation of ulcers, perforations, or fistulae into the pericardial space or the left atrial cavity12, 13, 14, 15 (Figure 2).
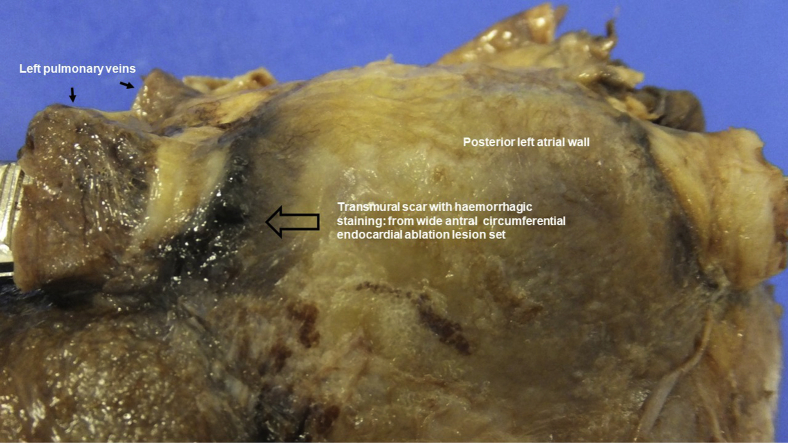
Figure 1.
Postmortem photograph of the dissected left atrium: the posterior aspect is viewed from the epicardial perspective. All 4 pulmonary veins are present. Dark hemorrhagic scarring is seen around the pulmonary veins, which represent transmural ablation lesions caused by endocardial applications of wide antral circumferential ablation lesions during pulmonary vein isolation.
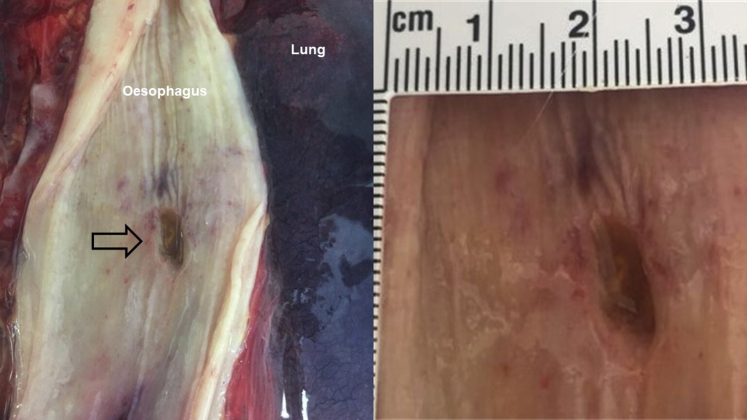
Figure 2.
Postmortem gross anatomical dissection study from a fatal case of atrioesophageal fistula caused by atrial fibrillation ablation. Hemorrhagic staining of the esophageal wall is evident surrounding the esophageal perforation lesion, measuring approximately 1 cm length × 0.5 cm width (black arrow).
Anatomical basis
The vulnerability of the esophagus to collateral damage is due to the close anatomical relationship to the posterior left atrium. Computed tomography (CT) has shown that in 50% of human subjects, the esophagus lies close to the left pulmonary venous ostia, on average 3.3 mm from the left atrial myocardium,7 whereas in a different but similar study, 91.3% of the subjects had the esophagus close to the left pulmonary vein ostia.12 Cadaveric studies confirm the relationship8: The esophagus was <5 mm away from the left atrial wall in 40% of the 15 specimens studied by Sánchez-Quintana and colleagues,8 with a posterior wall that varied in thickness along its length. Beyond the layer of fibrous pericardium is a layer of fibrofatty tissue that carries esophageal arteries and the vagal plexus. The portion of the esophagus in contact with the posterior left atrium was 42 ± 7 mm in length and 13.5 ± 5 mm in breadth.
The spectrum of esophageal injury
RF energy delivered from a catheter in contact with the myocardium produces heating of the tissue that is maximal at a depth of several millimeters, from whence it is conducted in all directions, including outward to the esophagus. Cryoablation produces maximal cooling at the balloon surface, with the effect transmitted outward. Whether injury is by heating or freezing, the progression from an acutely inflamed thermal injury to the formation of atrioesophageal fistula or pericardial-esophageal fistula typically takes around 2–6 weeks after the ablation procedure, although there have also been reports of its presentation within days.13,14
Paradoxically, although ablation-related thermal injury to the esophagus progresses from outside to inside, it is followed by a progression of damage from lumen outward: Inflamed areas can progress to ulceration, occasionally perforation, and even fistula formation.16
Risk factors for atrioesophageal fistula
Patient factors are of limited use in predicting the risk of esophageal injury. Although esophageal injury and complications in general may be perceived to occur more often at extremes of the spectrum of body weight, atrioesophageal fistulas were seen across a wide range of body habitus and not confined to those with low body mass index.17 A lack of epicardial fat between the left atrium and the esophagus may increase the risk of thermal energy transmission to the esophagus.7 Fat is a relatively poor conductor of thermal energy and so a thicker layer of fatty tissue between these 2 structures provides greater insulation and esophageal protection. Within the fatty tissue there are esophageal arteries.8 A thin fat layer between the atrium and esophagus is a nonmodifiable risk factor between individual patients. Atrioesophageal fistula does not seem to be strongly linked with any pre-existing or at least previously recognized esophageal pathology.
Equipment factors are equally unhelpful as predictors of risk: The risk appears to be less for cryoballoon ablation than for RF, but all modalities of ablation and all ablation catheters currently in routine clinical use are capable of causing atrioesophageal fistula. An experimental multielectrode RF catheter appeared to carry a risk that was at least as great as that for single-point RF catheters.18
Contemporary studies consistently support the fact that esophageal injury is a risk to all those that receive AF ablation and is not confined to methodologies where linear ablation is performed at the low posterior wall.6 Owing to the lack of patient predictive factors and rigidity of most procedural characteristics, usual care is currently limited in preventing this serious complication.
Pulsed field ablation (PFA) is nonthermal ablation technology in which myocardial lesions are created by electroporation.19 The process is dependent on cell size, making myocardial tissue more vulnerable than adjacent structures, including the esophagus. It is hoped that this can reduce collateral injury. Initial studies have shown a low risk of injury compared to RF ablation. Clinical experience with the technique is still limited.
Clinical features and incidence
Mild esophageal injuries and superficial ulcers are generally asymptomatic but may cause pain, dysphagia, or reflux-type symptoms. Symptoms may still be mild or insidious, even with deep ulceration or severe thermal injury. Atrioesophageal fistula causes neurological symptoms owing to embolism of air or septic material but may also cause new-onset chest pain, acid reflux, or dysphagia. These symptoms may be accompanied by typical signs of sepsis.18
The insidious progression and protean nature of the symptoms create diagnostic difficulty and delayed diagnosis. CT is usually the test that reveals the diagnosis, with endoscopy being contraindicated owing to risk of air embolization from insufflation during the examination.20 Preferred treatment is surgical repair with a limited role for esophageal stenting if the process is recognized in its early stages.
The risk of atrioesophageal fistula is commonly quoted at between 0.1% and 0.3%6,13,14,18; this is likely to be an underestimate, as the diagnosis can easily be missed. The mortality rate may be as high as 70%.18 It can occur regardless of ablation modality or methodology and even despite senior operating experience.
Diagnostic aids
A recent French nationwide survey21 on the reported cases of atrioesophageal fistulas outlined the patient, procedure, and event details, which included the diagnostic tests used to reach the diagnosis of this complication. The survey included 82 out of 103 cardiac centers, and 33 cases of confirmed fistulas were found over a period where more than 100,000 AF ablation procedures were performed. The diagnostic test most commonly used was CT, but the sensitivity from this dataset was only 81%. An initial normal scan therefore does not necessarily rule out the complication, and a recommendation for repeat interval CT scans was made for cases where the clinical suspicion was high. Other imaging may also identify the diagnosis—for example, a CT head or an echocardiogram showing air bubbles.13,21 Blood tests often yield nonspecific results but in the context of possible atrioesophageal fistula, early rise in white cell counts were previously reported as being an important marker.13,22 As fever is the most common presenting symptom, blood cultures are often performed and the organisms are typically gram positive.13 Certainly, blood tests and cultures are important adjuncts in the investigative process, providing pieces of evidence that may support the clinical diagnosis. As mentioned, endoscopy is dangerous in this situation and not recommended; barium swallow was also found to have been used in some cases, but with low diagnostic yield.13
Treatment approaches
Intervention for confirmed atrioesophageal fistula is either surgical repair or esophageal stenting, but the overall mortality rate nears 100% without any intervention.13,21,23 Early intervention is associated with improved outcomes. Various case series comparing outcomes post stenting vs surgery found a much lower mortality rate with surgical treatment. Mohanty and colleagues23 found that all 5 of their patients who had esophageal stenting died while 4 who had surgery survived. Surgery involves excision of the fistula and sealing off any inter-organ communication via an intercostal muscle patch. However, the esophageal stent may still allow communication from the open atrial end and continuation of embolization and sepsis. This may explain the poor outcomes post stenting. Esophageal stents, however, still have a role in the management of esophagopericardial fistulas. A recent review of esophagopericardial fistulas by Sternick and colleagues24 found that stenting in this situation with esophagomediastinal or pericardial fistulas (without breaching into the left atrium) had a favorable outcome. Either percutaneous or thoracotomy approaches to mediastinal, pleural, and pericardial drainage have been applied in these cases as part of the procedure. Antibiotic cover is a crucial part of management, in addition to general supportive therapies for sepsis.
Demonstrating benefit of preventative strategies
Atrioesophageal fistula is too rare an event to serve as an endpoint for a randomized trial; surrogate endpoints are needed, and endoscopically detected esophageal lesions serve this purpose. Endoscopically detected esophageal lesions (EDELs) are well-demarcated mucosal lesions that are highly characteristic of ablation-related injury. These lesions vary in severity from mild erythema to deep ulcers with clot formation to perforation and fistula formation. Grading systems used to quantify EDELs include the Kansas City Classification.16 Severe-grade lesions can progress to formation of atrioesophageal fistulas (with an estimated rate of nearly 10%25), so EDELs appear to be good surrogate markers for atrioesophageal fistulas in studies of this type.
Preventative strategies in usual care
Reduction of ablation power and contact force
From the first case reports,9, 10, 11 atrioesophageal fistula was associated with the use of higher ablation energy. Limitation of power has remained a cornerstone of avoiding this complication.26 With contact force–sensing catheters, limitation in contact force was also shown to reduce esophageal thermal injury. RESCUE-AF was a randomized study of AF ablation to either contact force limitation to <20 g or without contact force limitation or with ablation catheter without contact force measurement.27 In the group with force limitation there were no cases of esophageal injury vs 9 cases in those without contact force limitation and non–contact force–sensing catheters. The randomized groups in this study had similar procedural efficacy, but a policy that limits lesion size does run the risk of reducing efficacy.
Temperature monitoring probes
Commercially available esophageal temperature monitoring probes include single-sensor and multisensor probes, solid shaft designs, and acoustascopes, and so vary in the quality of data produced. A comparative study of commercially available probes by Turagam and colleagues28 showed potentially important differences in their ability to detect temperature changes. In water bath experiments, the time taken to register a temperature change of 8°C ranged from 6.2 to 19.7 seconds. This result is worrying, as a delay of nearly 20 seconds could permit a life-threatening temperature change.
Placement of the probe may be more important than its responsiveness. Single-sensor probes have to be sited carefully to ensure close proximity to the area of the esophagus that is at risk. Recent studies in the context of high-power short-duration (HPSD) ablation have shown that temperature rises may be undetected when the temperature sensors are >20 mm away from the site of ablation.29 An imprecisely positioned probe might not register the full severity of temperature change, fostering a false sense of security. Once a temperature probe is correctly sited, it instructs the operator to halt ablation whenever there is a significant temperature rise, usually 38 degrees and over, and to restart ablation in that region once temperatures fell back into acceptable limits. Application of consecutive lesions in close anatomical proximity at the posterior segments of the left atrium may cause “heat stacking,” and in clinical practice a “skip” strategy is applied during this part of the ablation procedure to avoid this phenomenon.30
Despite the widespread, indeed almost universal use of these probes and, in some cases, their considerable price, temperature monitoring probes have not proved to be an effective strategy for reducing esophageal thermal injury during AF ablations. The recent OPERA randomized controlled trial31 compared AF ablations with and without a temperature monitoring probe (SensithermTM; FIAB, Firenze, Italy), with some variation between the 2 randomized groups. Those without temperature probe monitoring had posterior left atrial ablation set at 25 W. In the group with the esophageal temperature monitoring probe, ablations started at 25 W and increased to 30 W if temperatures did not rise beyond 40 degrees. Endoscopy occurred within 72 hours of the ablation procedure and showed no difference in endoscopically detected lesions between the 2 randomized groups: 10 of 90 vs 8 of 90 (P = .62). This was the first randomized study to evaluate the effectiveness of esophageal monitoring probes against control group ablations.
Meininghaus and colleagues32 performed a study of similar design to OPERA but using a multisensor probe. They also showed that temperature monitoring did not reduce thermal injury but noted that a temperature rise of >42 degrees increased the likelihood of mucosal lesions: In this study 6 of 44 of the luminal esophageal temperature monitoring group vs 2 of 42 of the control group had mucosal lesions (P = ns). Earlier international guidelines have not supported local temperature monitoring beyond class IIa33 as a reasonable option. These more recent data showing a trend toward harm may warrant changes to this recommendation.
The evidence for esophageal temperature monitoring probes is summarized in Table 1.31,32,34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 In the observational studies alone, we find that in 1558 patients who had AF ablation (243 cryoablations; 1315 RF, including HPSD) with a type of commercially available esophageal temperature monitoring probe; 209 had an esophageal mucosal lesion. The studies occurred over a time period of 2009–2020. None showed clear benefit from the probes, although they offered insight into the vulnerability of the esophagus to injury based on degree of temperature rise correlating to severity of thermal lesions.
Table 1.
A summary of the studies in the esophageal temperature monitoring probes
| Study | Year | Type | RCT | Group 1 - type of LET probe | Group 1, n | Group 2 – control, n | Ablation method | Posterior settings | Total in study, n | Total in group 1 that had OGD, n | Group 1 positive EDEL results, n (%) | Group 2 positive EDEL results, n (%) | Study outcomes | Time of endoscopy (if known), days | Adverse event from LET probe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Di Biase et al | 2009 | RCT but randomization for GA vs LA. All had LET monitoring probes | 1 - GA vs LA | ER400-9, Smiths Medical ASD Inc, Rockland, MA. Single-sensor probe. | 50 | NA | RF | 35 W; 20 seconds | 50 | 50 | 13 (26%) | NA | GA increased risk of EDEL injury compared to LA | 1 - capsule | |
| Ahmed et al | 2009 | Prospective single-arm | NA | Vital Temp, Vital Signs Colorado Inc (Single thermocouple) | 67 | NA | Cryo | Cryo | 67 | 35 | 6 (17.1%) | NA | Cryoballoon ablation can cause significant LET decreases, resulting in reversible esophageal ulcerations in 17% of patients | 1 | |
| Di Biase et al | 2010 | Prospective single arm study | NA | ER400–9 Smiths Medical ASD, Inc, Rockland, MA | 88 | NA | RF | 35 W; 20 seconds | 88 | 88 | 15 (17%) | NA | Capsule endoscopy can be used to detect EDELs | 1 | |
| Sause et al | 2010 | Prospective single-arm | NA | Esotherm, FIAB, Florence, Italy (7F, 5 electrodes) | 184 | NA | RF | 30 W; 20 seconds | 184 | 184 | 3 (1.63%) | NA | Temperature limit of 40 degrees was associated with lowest incidence of EDEL at the time the study was published | 1 | |
| Halm et al | 2010 | Prospective single-arm | NA | Not specified | 185 | NA | RF | Not known | 185 | 185 | 27 (14.6%) | NA | Localized esophageal ulcer-like lesion is a frequent event after left atrial catheter ablation and can be found in patients whose intraluminal temperature has reached at least 41 degrees | Not known | |
| Leite et al | 2011 | Prospective single-arm | NA | EPT Blazer II temperature ablation catheter, Boston Scientific, Natick, MA | 45 | NA | RF | 25 W; if LET increased by 2 degrees from baseline then stop ablation | 45 | 45 | 0 | NA | A deflectable LET probe and stopping ablation after a 2-degree rise in LET may reduce esophageal injury | 1–2 | |
| Contreras et al | 2011 | Prospective single-arm | NA | Acoustascope, Smiths Medical ASD, Inc, Keene, NH | 219 | NA | RF | 25 W; 20 seconds | 219 | 82 | 22 (26.8%) | NA | The macroscopic severity of esophageal lesions detected on endoscopy the day after RF ablation can predict the time to resolution, with severe, deep ulcerations taking the longest to heal | 1, 10, 14 days until healed | |
| Furnkranz et al | 2013 | Prospective single-arm | NA | Sensitherm, St Jude Medical, Inc, St Paul, MN (3 thermocouples) | 32 | NA | Cryo | Cryo | 32 | 32 | 6 (18.75%) | NA | Second-generation 28 mm CB PVI is associated with significant esophageal cooling, resulting in lesion formation in 19% of the patients. LET measurement accurately predicts lesion formation. | 1–3 | |
| Knopp et al | 2014 | Prospective single-arm | NA | Sensitherm, St Jude Medical, Inc, St Paul, MN | 425 | NA | RF | 30 W | 425 | 425 | 47 (11%) | NA | Thermal injury including gastroparesis was common after AF ablation | 1–3 | |
| Furnkranz et al | 2014 | Prospective single-arm | NA | Sensitherm, St Jude Medical, Inc, St Paul, MN | 94 | NA | Cryo | Cryo | 94 | 32 | 6 (18.8%) | NA | Titration of CB PVI depending on LET temp fall to -15 degrees can reduce EDEL | Within 3 days | |
| Metzner et al | 2014 | Prospective single-arm | NA | Sensitherm, St Jude Medical, Inc, St Paul, MN | 50 | NA | Cryo | Cryo | 50 | 50 | 6 (12%) | NA | Using the second-generation 28-mm CB, EDEL was detected in 6 of 50 (12%) patients. All mucosal lesions were in the healing process on repeat EGD. | 2 | |
| Muller P et al | 2015 | Prospective double-arm – observational - nonrandomized | NA | Sensitherm, FIAB, Firenze, Italy (7F, 5 electrodes) | 40 | 40 | RF | 25 W | 80 | 40 | 12 (30%) | 1 (2.5%) | Use of temperature probes the only independent predictor of development of EDEL: Use of temperature probes was a risk factor for EDEL during AF ablation in this study | 2 | |
| Halbfass et al | 2017 | Observational | NA | S-Cath TM (Circa Scientific, LLC, Englewood, CO); esophageal temperature probe with insulated thermocouples: s-shaped and 12 electrodes | 40 | 40 | RF | 25 W | 80 | 40 | 3 (7.5%) | 4 (10%) | No reduction in EDELs with use of LET | 1–4 | |
| Deneke et al | 2018 | Prospective single-arm | NA | IRTS, Securus Medical Group, Inc, Cleveland, OH; 9F esophageal catheter connected to an external infrared detector | 63 | NA | RF | 25 W; 20 seconds; 5–20 g of contact force | 63 | 63 | 12 (19%) | NA | Peak temperature rise was associated with EDELs | 1 | |
| Daly et al | 2018 | Prospective single-arm | NA | IRTS, Securus Medical Group, Inc, Cleveland, OH | 16 | NA | RF | 20 W | 16 | 16 | 12.5 (78.1%) | NA | Infrared thermography provided dynamic, high-resolution mapping of esophageal temperatures during cardiac ablation. Esophageal thermal injury occurred with temperatures >50°C and was associated with large spatiotemporal gradients. | 1–2 | |
| Schoene et al | 2020 | RCT | 1 | Sensitherm, St Jude Medical, Inc, St Paul, MN | 90 | 90 | RF | 25–30 W | 180 | 90 | 10 (11.1%) | 8 (8.9%) | The Sensitherm LET probe does not affect the probability of developing EDEL | Within 3 days | |
| Chen S et al | 2020 | Prospective single-arm | NA | S-Cath TM (Circa Scientific, LLC, Englewood, CO) | 122 | NA | RF - AI-HP | 50 W/400 AI | 122 | 57 | 2 (3.5%) | NA | AI-HP ablation is associated with low incidence of EDELs; esophageal temperature probe monitoring was in use in these cases | 1 | |
| Meininghaus et al | 2021 | RCT | 1 | S-Cath TM (Circa Scientific, LLC, Englewood, CO) | 44 | 42 | RF | 25 W | 86 | 44 | 6 (13.6%) | 2 (4.8%) | LET monitoring does not prevent EDELs; temperatures >42 degrees were associated with increased likelihood of mucosal lesions | Within 3 days | 4 cases of epistaxis |
AF = atrial fibrillation; AI-HP = ablation index-high power; CB = cryoballoon; Cryo = cryoablation; EDEL = endoscopically detected esophageal lesion; EGD = esophago-gastroduodenoscopy; GA = general anaesthesia; LA = local anaesthesia; LET = luminal esophageal temperature; NA = not available; OGD = osophago-gastroduodenoscopy; PVI = pulmonary vein isolation; RCT = randomized controlled trial; RF = radiofrequency.
Carroll and colleagues50 compared multisensor esophageal temperature monitoring probes to single-sensor probes (88 were in the multisensor group and 455 in the single-sensor group) and found that although multisensor probes could detect temperature rises more effectively, there were a greater number of esophageal thermal lesions in this group. This was postulated to be due to exacerbation of the “antenna effect,” whereby the multisensor probes enhance the effect of bipolar thermal energy transmission. The antenna effect was described as a possible answer as to why some studies showed the use of these probes paradoxically increased esophageal lesions. The bipolar thermal ablation energy transmission is between the RF ablation catheter and the metallic component of the esophageal probe.
Proton pump inhibitors
It is common practice for all left atrial ablation patients to receive a proton pump inhibitor on the assumption that gastric acid could play a role in the progression of esophageal lesions.51 There is no evidence to support or disprove the hypothesis or the practice. However, its continued use is attractive, as the medication is low in cost and, in the majority of cases, proton pump inhibitors are well tolerated. As with most pharmacotherapy, it is not entirely harmless; even proton pump inhibitors may cause serious disruption to gut flora and affect absorption of other medications as well as promoting Clostridium difficile infections. The evidence for some effect in reducing esophageal thermal lesions was suggested by a substudy of the MADE-PVI trial.52 This study found that preprocedural use of proton pump inhibitors reduced esophageal/mediastinal lesions as assessed by endoscopic ultrasound as well as pre– and post–upper gastrointestinal endoscopy.
Alternative ablation strategies currently in use
The data on the incidence of atrioesophageal fistula with alternative ablation therapies such as cryoballoon were comparatively limited up until recently. Piccini and colleagues53 conducted a search of all cases of suspected or confirmed atrioesophageal fistula, reported directly to Medtronic’s global complaint database over the course of a decade, from 2009 to 2019 (covering all Arctic Front cryoballoons). With over 500,000 uses of the cryoballoon during this time, only 20 cases of atrioesophageal or pericardial esophageal fistula were reported. The calculated incidence rate was 0.004%. However, the mortality rate was 68.8%. The nature of the study meant that it was possible that the incidence rate was underestimated; in those where a fistula was suspected or confirmed, the median time to clinical presentation was 21 (interquartile range: 4–30) days. The delayed presentation means that it was possible to have missed patients who suffered an event and presented at a different hospital/region, where the information did not reach the treating hospital or the manufacturer. Nevertheless, this estimate from a global record over the past decade is the best figure we have at present. Comparisons on risk of esophageal thermal injuries between modalities are confounded by different monitoring and reporting for adverse events; the apparently lower rate of fistula formation with cryoballoon should not be interpreted as being “safer” compared to RF without further study. Laser balloon technology emerged in 2016 as an alternative method to provide durable pulmonary vein isolation (PVI); the real-world experience with laser balloon is increasing, but the data on its comparative efficacy and safety to other ablation methods are limited. Its unique technology uses the balloon “single-shot”–style ablation with an adjustable arc of laser energy, which can be applied via an endoscope. A systematic review and meta-analysis by Reynolds and colleagues54 reviewed 17 studies on laser balloon AF ablation, involving 1188 patients, mostly with paroxysmal AF. Only 2 of the included studies were randomized comparing to other methods of ablation. Acute success from the procedures was high (>98.8% achieving PVI) and the 12-month freedom from AF was at 72.9% for all AF subtypes. The pooled data showed that the most common complication was transient phrenic nerve injury, with no reported cases of atrioesophageal fistula.
High-power short-duration ablation
In addition to the refinement of RF technology and tools, methods of ablation lesion application were studied for determinants of efficacy and efficiency. The purpose of ablation treatment is ultimately to create transmural lesions to cause electrical isolation of either the pulmonary veins or atrial substrate where appropriate. Conventional RF applications fall within 20–35 W, usually at more than 20 seconds; but since 2006, clinical studies have investigated the method of “high-power short-duration” ablation as a method to improve efficiency of the procedure, without compromising safety. The definition of HPSD varies, but the power and duration typically range from 45 to 50 W and <10 seconds posteriorly and 5–15 seconds elsewhere.55,56 The more recently available catheter QDot micro and QMODE plus modality (not yet commercially available) allows 90 W lesion application at 3–4 seconds. Although there are preclinical studies showing that these lesions are broader-based and shallower,56 there remains lack of randomized comparison of the conventional approach to RF ablation vs HPSD ablation. Nevertheless, a recent meta-analysis of the available studies was conducted55 (a mix of prospective, retrospective, and propensity-matched studies with different power settings and time settings) where a total of 3718 patients were included, comparing outcomes of 2537 HPSD patients and 1361 patients who received conventional RF treatment. Despite the limitations of the types of studies included in the meta-analysis, overall study and subgroup analysis to review contact force–sensing catheters, power at 50 W or more and for paroxysmal and left atrial substrate ablation showed the HPSD ablation had improved efficacy and efficiency outcomes without significant difference in adverse events compared to conventional ablation.
Studies that investigated the incidence of esophageal thermal injuries after HPSD ablation have conflicting outcomes, with some reporting that the incidence of thermal injury was not significantly different compared to conventional ablation, while others noted that HPSD was associated with far greater incidence of injury (37% vs 22%; P = .011) after a PVI-only procedure and that it was an independent predictor of injury.57,58 Although the real-world practice of HPSD is increasing, there is currently no randomized evidence or registry data for its safety compared to conventional RF.
Novel methods of esophageal protection
Mechanical deviation of the esophagus
The esophagus is a mobile structure and mobility of the esophagus during AF ablation under conscious sedation has been demonstrated. Good and colleagues59 studied 51 patients who had a barium swallow at the start and end of the ablation and, using digital cine-fluoroscopic images, showed that the esophagus moved to a different extent at the superior, mid, and inferior segments of the esophagus. In 67% there were >2 cm of lateral movement and in 4% more than 4 cm. The study authors felt that this demonstrated that preprocedural imaging of the esophagus would be inadequate to “map” out the esophagus. Real-time imaging was felt to be more useful, but this also paved the way for further study on targeted manipulation of the esophagus during ablation.
From the perspective of the electrophysiologist, the esophagus is an organ that is badly designed, being demonstrably too delicate and inconveniently located. At a straightforward level of problem solving, strategies have been developed to move the esophagus out of harm’s way during ablation. The first tool used to mechanically deviate the esophagus away from the site of ablation was the transesophageal echocardiography (TEE) probe. Herweg and colleagues60 described this method: They turned the deflection wheel of the TEE probe, guiding the direction of angulation by fluoroscopy, and the degree by the feel of resistance. Deviation of the esophagus can work only if there is sufficient movement away from the site of ablation and if the esophagus moves in its entirety rather than leaving a “trailing edge,” where the wall of the esophagus closest to the left atrium remains at a neutral position while the opposite wall is stretched to the site of the deviating probe.
There are reservations about the concept of mechanical deviation as a protective method: Traumatic insertion or manipulation of a TEE probe is recorded as a cause of death in 1 in 3000 cardiac procedures performed under general anesthesia.61 In these TEE-related deaths, there is no reliable preprocedure marker of vulnerability, though there is an excess of risk in elderly females. Despite these reservations, dedicated devices have been developed.
An early study of the feasibility of lateral displacement of the esophagus used a malleable metal stylet within a plastic probe.62 This method was evaluated in a detailed follow-up study of 114 patients.63 The range of esophageal movement was recorded from the trailing esophageal edge to the site of ablation using fluoroscopy and a 3D electroanatomic mapping system. A distance of 0 to >20 mm was recorded and lesser esophageal deviation correlated with increased risk of elevation of temperature >38 degrees. A displacement of 9.1 ± 6.5 mm was still associated with a temperature rise, whereas a distance of 18 ± 7.6 mm was not.
In the original study protocol, progressively stiffer stylets were used to achieve the target deviation, but this aspect of the protocol was abandoned after 3 out of 4 patients experienced device-related trauma at the level of the oropharynx, 1 requiring cauterization for a pharyngeal laceration. Although no trauma was observed at an esophageal level, the potential for harm was clear. A modification of this design, the DV8 device,64 involved a dedicated esophageal balloon retractor, a polyurethane balloon enclosed in silicone. The device was studied in 200 patients, with an esophageal temperature monitoring probe used as an adjunct in most cases for the purpose of the study. The use of DV8 resulted in 2 episodes of oropharyngeal bleeding but no esophageal trauma. Instances of temperature rise were seen across all study groups, even when there was deviation of greater than 20 mm.
The DEFLECT-GUT study65 involved a nitinol stylet (EsoSure) used to deflect the esophagus. The stylet is advanced into an orogastric probe (18F tube) and the stylet curves to deflect the orogastric probe and hence the esophagus. This nonrandomized study involved 209 patients that had the EsoSure device and propensity-matched analysis was performed in 180 out of the 209. No device-related complication occurred. Esophageal temperature rise was used as a measure of effectiveness, and in this respect the device appeared effective: The use of the deviation device was associated with lower probability and severity of temperature rise. A randomized study has been listed for this device but not yet completed.
The design of the mechanical deviation devices has continued to evolve: Aguinaga and colleagues66 reported a first-in-man evaluation of the esolution device. This mechanical deviation device has a suctioning component to eliminate the trailing edge effect. In addition, there are inner stacking plates to ensure that deviation occurs exclusively in a medial-to-lateral plane, without anteroposterior movement; it also features a locking mechanism to keep the device at the deviated state for as long as required. In this preliminary series of 7 cases, no esophageal thermal injury occurred, but there was a case of pharyngeal hematoma. This device is due to undergo formal evaluation in a randomized study comparing the use of an esophageal temperature monitoring probe alone vs the esolution device and a temperature probe.
To date, there is no randomized evidence supporting the use of esophageal deviation in any form, although there is finally a listing for an upcoming randomized study (EASY-AF; NCT04659213). One randomized study (NCT01546168) was started but had to be terminated early after finding more lesions in the deviated group than in controls (13.9% lesion rate in the patients undergoing deviation, and 12.1% in the controls). Although posted in 2018, these results have yet to be published in full.67
Esophageal temperature control
Esophageal cooling during RF ablation was a topic of extensive evaluation before mechanical deviation devices were considered; attention then appeared to wane but has returned in the past few years. The first published study on esophageal cooling in 200568 led to a series of publications by other research groups. The study results were inconclusive in isolation, but a recent meta-analysis showed that esophageal cooling by direct water injection into the esophagus had a significant protective effect.69 The heat extraction capacity of this direct water instillation method is low, because the use of a larger volume of water could create a risk of fluid overload. However, the meta-analysis results of these early methods of esophageal cooling still showed promise—these studies provided a rationale for investigating how esophageal temperature might be controlled more effectively. This led to the investigation of an already available device for esophageal temperature control, albeit for other clinical reasons.
A temperature control device designed to regulate whole-body temperature by cooling or warming the esophagus (ensoETM®; Attune Medical, Chicago, IL) has been in routine clinical use in a critical care setting.70 With a baseline experience of thousands of real-world patient-days of clinical use with a good safety track record, this multilumen probe made of medical-grade silicone (dimensions: length 75 cm, 1.2 cm diameter) was an obvious candidate for controlling esophageal temperature. During operation, the water volume in the tubing is 55 mL and it flows at 2.4 L/min, exerting a maximum pressure of 103 kPa. Even at full pressure it is less rigid than a TEE probe, and it is designed to be inserted in the same manner. The distal end of the probe is soft and sits in the body of the stomach. The proximal end protruding from the mouth of the patient is connected to a mobile heat exchange console (Blanketrol III; Gentherm, Cincinnati, OH), allowing water to be pumped through the probe in a closed-loop system; the temperature of the irrigated water is controlled by the console, keeping the probe at a temperature from 4°C to 42°C, at the discretion of the operator.
Two small pilot studies suggested benefits of active cooling with the ensoETM. Clark and colleagues71 compared direct iced-water instillation to active cooling in 6 patients and found that the extent of esophageal injury was less severe with the active cooling device. Tschabrunn and colleagues72 compared local temperature monitoring to active cooling in 44 patients, and found a 67% reduction in the incidence of severe lesion, despite the use of more extensive lesion sets in patients randomized to active cooling.
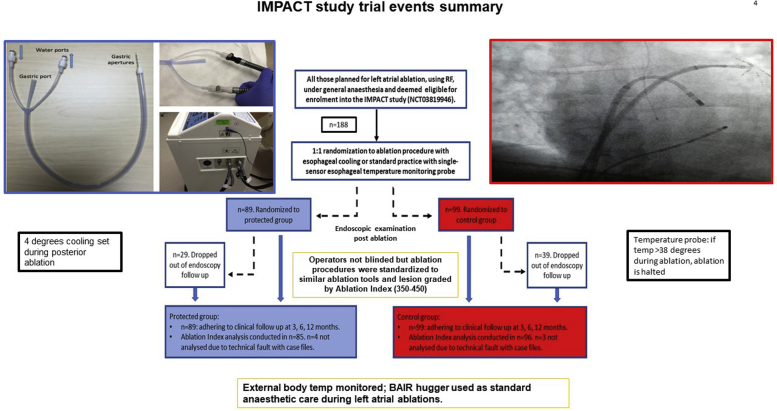
In the IMPACT study (Figure 3),73 a double-blind, prospective randomized controlled trial, 120 patients undergoing left atrial ablation under general anesthesia were randomly assigned in a 1:1 manner to either esophageal cooling using the ensoETM temperature control device or to a control group in which a single-sensor esophageal temperature monitoring probe was used. The study showed that esophageal cooling reduced thermal injury by 83.4% compared to control ablations using a single-sensor temperature monitoring probe. In a per-protocol analysis, the formation of severe esophageal thermal injury was reduced 100%. This does not prove that the device can eliminate the risk of atrioesophageal fistula, but it shows that it can reduce both the incidence and the severity of thermal injury, implying a capacity to minimize the risk. The study also showed that active thermal protection did not adversely affect ablation lesion formation in the left atrium or procedure efficacy in the short term.74
Figure 3.
IMPACT study summary.
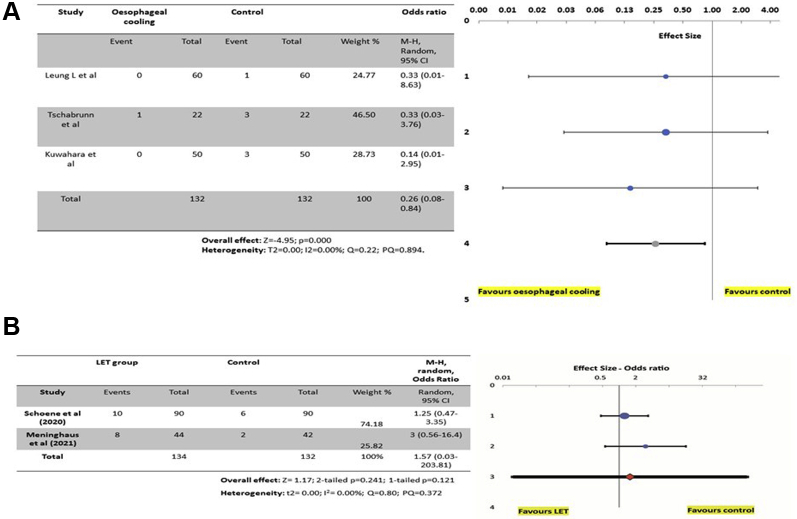
Endoscopy occurred a week after ablation in the IMPACT study, later than in most of the previous studies of ablation-related thermal injury, where endoscopy occurred within 12–72 hours of the ablation. There is no “correct” timing for postablation endoscopy, but from a pathophysiological point of view, the 7-day interval makes sense in allowing time for the resolution of the mildest lesions, potentially improving the specificity of the endoscopic findings. Thermal lesions identified at 7 days are less likely to represent trivial, non-specific findings that may be identified immediately post ablation and more likely to identify severe lesions that have had time to manifest endoscopically. Figure 4 summarizes the evidence so far from esophageal cooling and also for esophageal temperature monitoring probes.
Figure 4.
A: A forest plot of the randomized controlled trials on esophageal cooling or active thermal protection of the esophagus. A comparison is made from the studies where clinically significant injury was reported. The evidence so far favors esophageal cooling/active thermal protection; however, the numbers are low and further prospective study is required to confirm its effectiveness. B: A forest plot on randomized studies investigating the effectiveness of esophageal temperature monitoring probes. The evidence so far does not support the use of esophageal temperature monitoring, but more randomized studies may be required to clarify the situation.
A mathematical modeling analysis of the biophysical effect of the ensoETM device set at different temperatures during AF ablation confirmed the potential to protect the esophagus over a wide range of temperature settings.75 Projected tissue temperature rise and tissue damage were improved even if the circulating water within the probe was set at normal body temperature.
Of the methods evaluated by randomized trial, esophageal temperature control appears to be promising, showing significant reduction in thermal injury while maintaining an efficient procedural workflow and effective procedures. However, further evaluation is required in the form of a multicenter randomized trial to confirm its effectiveness and safety profile (Table 2, Figure 4).
Table 2.
A summary of randomized evidence in esophageal protection methods
| Study | Year | Group 1 - esophageal protection probe being investigated | Group 2 - control or comparison group | Ablation method | Posterior settings | Total in study, n | Total in group 1 that had endoscopy, n | Total in group 2 that had endoscopy, n | Group 1 positive EDEL results, n (%) | Group 2 positive EDEL results, n (%) | Outcomes | Time of endoscopy | Adverse event from study probe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schoene et al | 2020 | Sensitherm, (FIAB, Firenze Italy) | No esophageal probe | RF | 25–30 W | 180 | 90 | 90 | 10 (11.1%) | 8 (8.89%) | The Sensitherm LET probe does not affect the probability of developing EDEL | Within 72 hours | 0 |
| Leung et al | 2020 | EnsoETM (Attune Medical, Chicago IL) temperature control device for esophageal cooling | Single-sensor esophageal temperature monitoring probe (Level 1 Smiths Medical, Minneapolis, MN) | RF | 30 W with ablation index target of 350–400 | 188 | 60 | 60 | 2 (3.3%) | 12 (20%) | Controlled active thermal protection using the ensoETM device significantly reduces thermal injury during left atrial ablations compared to controls, using a single-sensor probe | 1 week postablation | 0 |
| Meininghaus et al | 2021 | S-Cath TM (Circa Scientific, LLC, Englewood, CO) | No esophageal probe | RF | 25 W | 86 | 44 | 42 | 6 (13.6%) | 2 (4.76%) | LET monitoring does not prevent EDELs; temperatures >42 degrees were associated with increased likelihood of mucosal lesions. | Within 72 hours | 4 epistaxis |
EDEL = endoscopically detected esophageal lesion; LET = luminal esophageal temperature; RF = radiofrequency.
Alternative ablation methods: Future applications
Ultra-cryotherapy
Cryotherapy of the left atrium in real practice has been limited to balloon technology mainly, with single-tip cryo-catheters reserved for treatment of other arrhythmias (eg, slow pathway or septal accessory pathways). Previous randomized study on multicatheter cryoablation showed that cryotherapy can be used to deliver linear lesions in the left atrium comparable to RF, but long-term outcomes were not significantly different, and so further study of this method using standard cryo-catheters was not recommended.76 Ultra-low-temperature cryotherapy (Adagio Medical, Laguna Hills, CA) may change the limitations associated with standard cryotherapy. It is a novel and advanced form of cryotherapy that has begun its initial clinical evaluations. It offers ultra-low cryotherapy temperatures that enables transmural cryoablation in under a minute. It has different stylet shapes to enable the operator to engage with both the left and right pulmonary veins and also to perform posterior wall and mitral line linear lesions; its technology uses near critical nitrogen. Owing to ultra-low temperatures (down to -196°C), an esophageal warming balloon is used in the company’s protocol for left atrial ablation work. The main published clinical evaluation using this technology so far has been to treat atrial flutter.77 Although acute success was obtained in 100% (17 patients), only 82.4% remained free from atrial flutter after 12 months of follow-up, which is a lower long-term success rate compared to established ablation methods.78 However, as the technology is new, there is scope for improvement. The complication rate was 1.44%, and this may change as further experience is gained.
Non-thermal ablation: Pulsed field ablation
Non-thermal ablation is continuing to develop a profile of evidence behind its clinical efficacy, efficiency, and safety. PFA is a non-thermal method of ablation that offers a single-shot-type ablation of myocardial tissue by delivering, through a catheter, DC ultrarapid energy pulses in milliseconds that cause irreversible cell death by electroporation. This was found in preclinical studies to be effective in delivering tissue-selective ablation to the myocardium with minimal effect on surrounding tissue.19 Therefore, this method may reduce collateral injury and improve the overall safety profile of AF ablation. A recent collation of 3 multicenter trials (IMPULSE, PEFCAT, PEFCAT II)79 confirmed its safety relative to cryoballoon or RF, but in the remap performed in 110 of 121 patients at 90 ± 30.1 days, 64.5% of patients had enduring PVI at this time (or 84.1% of those who had optimized biphasic energy waveform ablation). There were no cases of atrioesophageal fistula reported. Reviewing specifically for evidence of esophageal injury, noninvasively, Cochet and colleagues80 reported on their cardiac magnetic resonance study of 41 patients undergoing PVI via PFA, RF, or cryoballoon. This small non-randomized study noted that there were no cases of esophageal injury seen in the PFA group, although there was some aortic injury (33%). Comparatively, there were more aortic injuries in the combined thermal group (43%) and 43% rate of esophageal injury. All esophageal lesions resolved on repeat imaging study.
Although at present randomized data and long-term follow-up are required to confirm the efficacy and safety profile of PFA, it remains the most promising advance in the field of interventional electrophysiology in the last few years. Its technology has the potential to achieve vein isolation within seconds and, if proven to be robustly tissue-selective at the same time, this may be the future answer towards optimized esophageal protection.
Future directions in esophageal protection
Further work is required to confirm the value of active thermal protection during AF ablations and to compare this approach to more intensive monitoring of temperature or the use of mechanical deviation of the esophagus. The relevance of all of these methods will have to be re-evaluated for each new ablation methodology that is introduced. At present, none of the protection methods has been evaluated for cryotherapy, and it remains unclear whether any protection is required for PFA. No study has addressed the issue of esophageal protection in patients undergoing ablation under conscious sedation.
Conclusion
Injury to the esophagus is an important consideration in ablations for AF. Esophageal protection resulting in a reduction in esophageal complications would significantly improve the safety profile of AF ablation. To date, esophageal cooling appears to be the most promising option for esophageal protection; further research in this area is ongoing.
Acknowledgments
Meta-Essentials program (www.meta-essentials.com) by Suurmond and colleagues.81
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Dr Leung has received research support from Attune Medical. Dr Gallagher has received research funding from Attune Medical and has acted as a consultant and a paid speaker for Boston Scientific and Cook Medical. All of the other authors do not have any conflicts of interest to declare.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
References
- 1.Zoni-Berisso M., Lercari F., Carazza T., Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–220. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potpara T.S., Lip G.Y.H., Blomstrom-Lundqvist C., et al. The 4S-AF scheme (Stroke Risk; Symptoms; Severity of Burden; Substrate): A novel approach to in-depth characterization (rather than classification) of atrial fibrillation. Thromb Haemost. 2021;121:270–278. doi: 10.1055/s-0040-1716408. [DOI] [PubMed] [Google Scholar]
- 3.Bajpai A., Camm A.J., Savelieva I. Epidemiology and economic burden of atrial fibrillation. US Cardiology. 2007;4:14–17. [Google Scholar]
- 4.Chen L.Y., Chung M.K., Allen L.A., et al. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation. 2018;137:e623–e644. doi: 10.1161/CIR.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loring Z., Holmes D.N., Matsouaka R.A., et al. Procedural patterns and safety of atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapur S., Barbhaiya C., Deneke T., Michaud G.F. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation. 2017;136:1247–1255. doi: 10.1161/CIRCULATIONAHA.117.025827. [DOI] [PubMed] [Google Scholar]
- 7.Lemola K., Sneider M., Desjardins B., et al. Computed tomographic analysis of the anatomy of the left atrium and the esophagus: implications for left atrial catheter ablation. Circulation. 2004;110:3655–3660. doi: 10.1161/01.CIR.0000149714.31471.FD. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Quintana D., Cabrera J.-A., Climent V., Farré J., de Mendonça M.C., Ho S.Y. Anatomic relations between the esophagus and left atrium and relevance for ablation of atrial fibrillation. Circulation. 2005;112:1400–1405. doi: 10.1161/CIRCULATIONAHA.105.551291. [DOI] [PubMed] [Google Scholar]
- 9.Gillinov A.M., Pettersson G., Rice T.W. Esophageal injury during radiofrequency ablation for atrial fibrillation. J. Thorac Cardiovasc Surg. 2001;122:1239–1240. doi: 10.1067/mtc.2001.118041. [DOI] [PubMed] [Google Scholar]
- 10.Scanavacca M., D’avila A., Parga J., et al. Left atrial-esophageal fistula following radiofrequency catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:960–962. doi: 10.1046/j.1540-8167.2004.04083.x. [DOI] [PubMed] [Google Scholar]
- 11.Pappone C., Oral H., Santinelli V., et al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–2726. doi: 10.1161/01.CIR.0000131866.44650.46. [DOI] [PubMed] [Google Scholar]
- 12.Tsao H.-M., Wu M.-H., Higa S., et al. Anatomic relationship of the esophagus and left atrium. Chest. 2005;128:2581–2587. doi: 10.1378/chest.128.4.2581. [DOI] [PubMed] [Google Scholar]
- 13.Pappone C., Vicedomini G., Santinelli V. Atrio-esophageal fistula after AF ablation: pathophysiology, prevention and treatment. J Atr Fibrillation. 2013;6:860. doi: 10.4022/jafib.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez P., Messerli F.H., Casso Dominuez A., et al. Atrioesophageal fistula following ablation procedures for atrial fibrillation: systematic review of case reports. Open Heart. 2015;2 doi: 10.1136/openhrt-2015-000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodzlock G.M., Norton C.A., Montgomery J.A. Prevention and treatment of atrioesophageal fistula related to catheter ablation for atrial fibrillation. J Innov Cardiac Rhythm Manage. 2019;10:3634–3640. doi: 10.19102/icrm.2019.100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarlagadda B., Deneke T., Turagam M., et al. Temporal relationships between esophageal injury type and progression in patients undergoing atrial fibrillation catheter ablation. Heart Rhythm. 2019;16:204–212. doi: 10.1016/j.hrthm.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Glover B.M., Hong K.L., Dagres N., et al. on behalf of the ESC-EHRA Atrial Fibrillation Long-term Registry investigators. Impact of body mass index on the outcome of catheter ablation of atrial fibrillation. Heart. 2019;105:244–250. doi: 10.1136/heartjnl-2018-313490. [DOI] [PubMed] [Google Scholar]
- 18.Han H.C., Ha F.J., Sanders P., et al. Atrioesophageal fistula. Clinical presentation, procedural characteristics, diagnostic investigations, and treatment outcomes. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.117.005579. [DOI] [PubMed] [Google Scholar]
- 19.Wittkampf F.H.M., van Es R., Neven K. Electroporation and its relevance for cardiac catheter ablation. JACC Clin Electrophysiol. 2018;4:977–986. doi: 10.1016/j.jacep.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Ghannam M., Beran A., Ghazaleh D., et al. Cerebral air embolism after esophagogastroduodenoscopy: insight on pathophysiology, epidemiology, prevention and treatment. J Stroke Cerebrovasc Diseases. 2019;28:104403. doi: 10.1016/j.jstrokecerebrovasdis.2019.104403. [DOI] [PubMed] [Google Scholar]
- 21.Gandjbakhch E., Mandel F., Dagher Y., et al. Incidence, epidemiology, diagnosis and prognosis of atrio-oesophageal fistula following percutaneous catheter ablation: a French nationwide survey. Europace. 2021;23:557–564. doi: 10.1093/europace/euaa278. [DOI] [PubMed] [Google Scholar]
- 22.Dagres N., Kottkamp H., Piorkowski C., et al. Rapid detection and successful treatment of esophageal perforation after radiofrequency ablation of atrial fibrillation: lessons from five cases. J Cardiovasc Electrophysiol. 2006;17:1213–1215. doi: 10.1111/j.1540-8167.2006.00611.x. [DOI] [PubMed] [Google Scholar]
- 23.Mohanty S., Santangeli P., Mohanty P., et al. Outcomes of atrioesophageal fistula following catheter ablation of atrial fibrillation treated with surgical repair versus esophageal stenting. J Cardiovasc Electrophysiol. 2014;25:579–584. doi: 10.1111/jce.12386. [DOI] [PubMed] [Google Scholar]
- 24.Sternick E.B., Correa F.S., Drumond L.F., et al. Esophago-pericardial fistula after catheter ablation of atrial fibrillation: a review. J Cardiovasc Electrophysiol. 2020;31:2600–2606. doi: 10.1111/jce.14723. [DOI] [PubMed] [Google Scholar]
- 25.Halbfass P., Pavlov B., Müller P., et al. Progression from esophageal thermal asymptomatic lesion to perforation complicating atrial fibrillation ablation: a single-center registry. Circ Arrhythm Electrophysiol. 2017;10:e005233. doi: 10.1161/CIRCEP.117.005233. [DOI] [PubMed] [Google Scholar]
- 26.Bahnson T.D. Strategies to minimize the risk of esophageal injury during catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2009;32:248–260. doi: 10.1111/j.1540-8159.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X., Kuang X., Gao X., et al. RESCUE-AF in patients undergoing atrial fibrillation ablation: the RESCUE-AF Trial. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.118.007044. [DOI] [PubMed] [Google Scholar]
- 28.Turagam M.K., Miller S., Sharma S.P., et al. Differences in transient thermal response of commercial esophageal temperature probes. JACC Clin Electrophysiol. 2019;5:1280–1288. doi: 10.1016/j.jacep.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Barbhaiya C.R., Kogan E.V., Jankelson L., et al. Esophageal temperature dynamics during high-power short-duration posterior wall ablation. Heart Rhythm. 2020;17:721–727. doi: 10.1016/j.hrthm.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Perzanowski C., Teplitsky L., Hranitzky P.M., Bahnson T.D. Real-time monitoring of luminal esophageal temperature during left atrial radiofrequency catheter ablation for atrial fibrillation: observations about esophageal heating during ablation at the pulmonary vein ostia and posterior left atrium. J Cardiovasc Electrophysiol. 2006;17:166–170. doi: 10.1111/j.1540-8167.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 31.Schoene K., Arya A., Grashoff F., et al. Oesophageal Probe Evaluation in Radiofrequency Ablation of Atrial Fibrillation (OPERA): results from a prospective randomized trial. Europace. 2020;22:1487–1494. doi: 10.1093/europace/euaa209. [DOI] [PubMed] [Google Scholar]
- 32.Meininghaus D.G., Blembel K., Waniek C., et al. Temperature monitoring and temperature-driven irrigated radiofrequency energy titration do not prevent thermally-induced esophageal lesions in pulmonary vein isolation: a randomized study controlled by esophagoscopy before and after catheter ablation. Heart Rhythm. 2021;18:926–934. doi: 10.1016/j.hrthm.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Calkins H., Hindricks G., Cappato R., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Biase L., Saenz L.C., Burkhardt D.J., et al. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circ Arrhythm Electrophysiol. 2009;2:108–112. doi: 10.1161/CIRCEP.108.815266. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed H., Neuzil P., d’Avila A., et al. The esophageal effects of cryoenergy during cryoablation for atrial fibrillation. Heart Rhythm. 2009;6:962–969. doi: 10.1016/j.hrthm.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 36.Di Biase L., Dodig M., Saliba W., et al. Capsule endoscopy in examination of esophagus for lesions after radiofrequency catheter ablation: a potential tool to select patients with increased risk of complications. J Cardiovasc Electrophysiol. 2010;21:839–844. doi: 10.1111/j.1540-8167.2010.01732.x. [DOI] [PubMed] [Google Scholar]
- 37.Sause A., Tutdibi O., Pomsel K., et al. Limiting esophageal temperature in radiofrequency ablation of left atrial tachyarrhythmias results in low incidence of thermal esophageal lesions. BMC Cardiovasc Disord. 2010;10:52. doi: 10.1186/1471-2261-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halm U., Gaspar T., Zachäus M., et al. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol. 2010;105:551–556. doi: 10.1038/ajg.2009.625. [DOI] [PubMed] [Google Scholar]
- 39.Leite L.R., Santos S.N., Maia H., et al. Luminal esophageal temperature monitoring with a deflectable esophageal temperature probe and intracardiac echocardiography may reduce esophageal injury during atrial fibrillation ablation procedures: results of a pilot study. Circ Arrhythm Electrophysiol. 2011;4:149–156. doi: 10.1161/CIRCEP.110.960328. [DOI] [PubMed] [Google Scholar]
- 40.Contreras-Valdes F.M., Heist E.K., Danik S.B., et al. Severity of esophageal injury predicts time to healing after radiofrequency catheter ablation for atrial fibrillation. Heart Rhythm. 2011;8:1862–1868. doi: 10.1016/j.hrthm.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 41.Fürnkranz A., Bordignon S., Schmidt B., et al. Luminal esophageal temperature predicts esophageal lesions after second-generation cryoballoon pulmonary vein isolation. Heart Rhythm. 2013;10:789–793. doi: 10.1016/j.hrthm.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Knopp H., Halm U., Lamberts R., et al. Incidental and ablation-mediated findings during upper gastrointestinal endoscopy in patients after ablation of atrial fibrillation: a retrospective study of 425 patients. Heart Rhythm. 2014;11:574–578. doi: 10.1016/j.hrthm.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Fürnkranz A., Bordignon S., Bohmig M., et al. Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm. 2015;12:268–274. doi: 10.1016/j.hrthm.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 44.Metzner A., Burchard A., Wohlmuth P., et al. Increased incidence of esophageal thermal lesions using the second-ggeneration 28-mm cryoballoon. Circ Arrhythm Electrophysiol. 2013;6:769–775. doi: 10.1161/CIRCEP.113.000228. [DOI] [PubMed] [Google Scholar]
- 45.Muller P., Dietrich J.-W., Halbfass P., et al. Higher incidence of esophageal lesions after ablation of atrial fibrillation related to the use of esophageal temperature probes. Heart Rhythm. 2015;12:1464–1469. doi: 10.1016/j.hrthm.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Halbfass P., Muller P., Nentwich K., et al. Incidence of asymptomatic oesophageal lesions after atrial fibrillation ablation using an oesophageal temperature probe with insulated thermocouples: a comparative controlled study. Europace. 2017;19:385–391. doi: 10.1093/europace/euw070. [DOI] [PubMed] [Google Scholar]
- 47.Deneke T., Nentwich K., Berkovitz A., et al. high-resolution infrared thermal imaging of the esophagus during atrial fibrillation ablation as a predictor of endoscopically detected thermal lesions: results from the HEAT-AF Study. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.118.006681. [DOI] [PubMed] [Google Scholar]
- 48.Daly M.G., Melton I., Roper G., Lim G., Crozier I.G. High resolution infrared thermography of esophageal temperature during radiofrequency ablation of atrial fibrillation. Circ Arrhythm Electrophsyiol. 2018;11 doi: 10.1161/CIRCEP.117.005667. [DOI] [PubMed] [Google Scholar]
- 49.Chen S., Chun K.R.J., Tohoku S., et al. Esophageal endoscopy after catheter ablation of atrial fibrillation using ablation-index guided high-power: Frankfurt AI-HP ESO I. JACC Clin Electrophysiol. 2020;6:1253–1261. doi: 10.1016/j.jacep.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Carroll B.J., Contreras-Valdes F.M., Heist E.K., et al. Multi-sensor esophageal temperature probe used during radiofrequency ablation for atrial fibrillation is associated with increased intraluminal temperature detection and increased risk of esophageal injury compared to single-sensor probe. J Cardiovasc Electrophysiol. 2013;24:958–964. doi: 10.1111/jce.12180. [DOI] [PubMed] [Google Scholar]
- 51.Zellerhoff S., Lenze F., Eckardt L. Prophylactic proton pump inhibition after atrial fibrillation ablation: is there any evidence? Europace. 2011;13:1219–1221. doi: 10.1093/europace/eur139. [DOI] [PubMed] [Google Scholar]
- 52.Cordes F., Ellermann C., Dechering D.G., et al. Pre-procedural proton pump inhibition is associated with fewer peri-oesophageal lesions after cryoballoon pulmonary vein isolation. Sci Rep. 2021;11:4728. doi: 10.1038/s41598-021-83928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piccini J.P., Braegelmann K.M., Simma S., et al. Risk of atrioesophageal fistula with cryoballoon ablation of atrial fibrillation. Heart Rhythm O2. 2020;1:173–179. doi: 10.1016/j.hroo.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reynolds M.R., Zheng Q., Doros G. Laser balloon ablation for AF: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2018;29:1363–1370. doi: 10.1111/jce.13698. [DOI] [PubMed] [Google Scholar]
- 55.Ravi V., Poudyal A., Abid Q., et al. High-power short duration vs. conventional radiofrequency ablation of atrial fibrillation: a systematic review and meta-analysis. Europace. 2021;23:710–721. doi: 10.1093/europace/euaa327. [DOI] [PubMed] [Google Scholar]
- 56.Kotadia I.D., Williams S.E., O’Neill M. High-power, short-duration radiofrequency ablation for the treatment of AF. Arrhythm Electrophysiol Rev. 2019;8:265–272. doi: 10.15420/aer.2019.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin D.G., Lim H.E. Esophageal endoscopy after high-power and short-duration ablation in atrial fibrillation patients. Korean Circ J. 2021;51:154–156. doi: 10.4070/kcj.2020.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaneshiro T., Kamioka M., Hijioka N., et al. Characteristics of esophageal injury in ablation of atrial fibrillation using a high-power short-duration setting. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008602. [DOI] [PubMed] [Google Scholar]
- 59.Good E., Oral H., Lemola K., et al. Movement of the esophagus during left atrial catehter ablation for atrial fibrillation. J Am Coll Cardiol. 2005;46:2107–2110. doi: 10.1016/j.jacc.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 60.Herweg B., Johnson N., Postler G., Curtis A.B., Barold S.S., Ilercil A. Mechanical esophageal deflection during ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:957–961. doi: 10.1111/j.1540-8159.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- 61.Ramalingam G., Choi S.W., Agarwal S., et al. Complications related to peri-operative transoesophgeal echocardiography - a one-year prospective national audit by the Association of Cardiothoracic Anaesthesia and Critical Care. Anaesthesia. 2020;75:21–26. doi: 10.1111/anae.14734. [DOI] [PubMed] [Google Scholar]
- 62.Koruth J.S., Reddy V.Y., Miller M.A., et al. Mechanical esophageal displacement during catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:147–154. doi: 10.1111/j.1540-8167.2011.02162.x. [DOI] [PubMed] [Google Scholar]
- 63.Palaniswamy C., Koruth J.S., Mittnacht A.J., et al. The extent of mechanical esophageal deviation to avoid esophageal heating during catheter ablation of atrial fibrillation. JACC Clin Electrophysiol. 2017;3:1146–1154. doi: 10.1016/j.jacep.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 64.Bhardwaj R., Naniwadekar A., Whang W., et al. Esophageal deviation during atrial fibrillation ablation: clinical experience with a dedicated esophageal balloon retractor. JACC Clin Electrophysiol. 2018;4:1020–1030. doi: 10.1016/j.jacep.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Parikh V., Swarup V., Hantla J., et al. Feasibility, safety, and efficacy of a novel preshaped nitinol esophageal deviator to successfully deflect the esophagus and ablate left atrium without esophageal temperature rise during atrial fibrillation ablation: The DEFLECT GUT study. Heart Rhythm. 2018;15:1321–1327. doi: 10.1016/j.hrthm.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 66.Aguinaga L., Palazzo A., Bravo A., et al. Esophageal deviation with vacuum suction and mechanical deflection during ablation of atrial fibrillation: first in man evaluation. J Cardiovasc Electrophysiol. 2021;32:67–70. doi: 10.1111/jce.14801. [DOI] [PubMed] [Google Scholar]
- 67.Reddy V. Deviating the esophagus in atrial fibrillation ablation. 2021. https://clinicaltrials.gov/ct2/show/NCT01546168
- 68.Berjano E.J., Hornero F. A cooled intraesophageal balloon to prevent thermal injury during endocardial surgical radiofrequency ablation of the left atrium: a finite element study. Phys Med Biol. 2005;50:N269–N279. doi: 10.1088/0031-9155/50/20/N03. [DOI] [PubMed] [Google Scholar]
- 69.Leung L.W., Gallagher M.M., Santangeli P., et al. Esophageal cooling for protection during left atrial ablation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2020;59:347–355. doi: 10.1007/s10840-019-00661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hegazy A.F., Lapierre D.M., Butler R., Martin J., Althenayan E. The esophageal cooling device: a new temperature control tool in the intensivist’s arsenal. Heart Lung. 2017;46:143–148. doi: 10.1016/j.hrtlng.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Clark B, Alvi N, Hanks J, Suprenant B. A pilot study of an esophageal cooling device during radiofrequency ablation for atrial fibrillation. medRxiv 2020:2020.2001.2027.20019026.
- 72.Tschabrunn CM, Attalla S, Salas J, et al. Active esophageal cooling for the prevention of thermal injury during atrial fibrillation ablation: a randomized controlled pilot study [published online ahead of print February 23, 2021]. J Interv Card Electrophysiol. https://doi.org/10.1007/s10840-021-00960-w [DOI] [PubMed]
- 73.Leung L.W., Bajpai A., Zuberi Z., et al. Randomized comparison of oesophageal protection with a temperature control device: results of the IMPACT study. Europace. 2021;23:205–215. doi: 10.1093/europace/euaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leung L, El Batran A, Dhillon G, et al. Oesophageal thermal protection during AF ablation: effect on left atrial myocardial ablation lesions formation and patient outcomes. Europace 2021;23:euab116.253.
- 75.Mercado M., Leung L., Gallagher M., et al. Modeling esophageal protection from radiofrequency ablation via a cooling device: an analysis of the effects of ablation power and heart wall dimensions. Biomed Eng Online. 2020;19:77. doi: 10.1186/s12938-020-00821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gallagher M.M., Gang Y., Gonna H., et al. Multi-catheter cryotherapy compared with radiofrequency ablation in long-standing persistent atrial fibrillation: a randomized clinical trial. Europace. 2020;00:1–10. doi: 10.1093/europace/euaa289. [DOI] [PubMed] [Google Scholar]
- 77.Klaver M.N., De Potter T., Iliodromitis K., et al. Ultralow temperature cryoablation using near-critical nitrogen for cavotricuspid isthmus-ablation, first-in-human results. J Cardiovasc Electrophysiol. 2021;32:2025–2032. doi: 10.1111/jce.15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pérez F.J., Schubert C.M., Parvez B., et al. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter. Circ Arrhythm Electrophysiol. 2009;2:393–401. doi: 10.1161/CIRCEP.109.871665. [DOI] [PubMed] [Google Scholar]
- 79.Reddy V.Y., Dukkipati S.R., Neuzil P., et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. 2021;7:614–627. doi: 10.1016/j.jacep.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 80.Cochet H., Nakatani Y., Sridi-Cheniti S., et al. Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace. 2021;23:1391–1399. doi: 10.1093/europace/euab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suurmond R., van Rhee H., Hak T. Introduction, comparison, and validation of Meta-Essentials: a free and simple tool for meta-analysis. Res Synth Methods. 2017;8:537–553. doi: 10.1002/jrsm.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]