Abstract
Background
Pulsed field ablation (PFA) is a promising technology based on electroporation. It is unclear if different catheter designs imply efficacy and safety differences.
Objective
To vary geometry, blood exposure, and energy delivery methods among 3 representative catheter designs, and then compare lesion transmurality, extra-atrial safety, and embolic risk.
Methods
A computed tomography–derived computer model was used. Balloon, flexible-circuit splined, and circular catheters were placed near the left pulmonary veins. Four energy delivery methods were tested: multi-unipolar, sequential unipolar, interlaced, and wide interlaced. A posterior wall target was defined. Efficacy was defined as percent target with >600 V/cm. Safety aspects included aortic/esophageal electroporation damage and a bubble-generation surrogate (electrode current density), with 90% transmurality requirement.
Results
Balloon catheters had highest efficacy, followed by flexible polymer splined and circular catheters. On energy delivery methods, the multi-unipolar one was most efficacious, followed by interlaced bipolar and sequential-unipolar ones. Electroporation risks to aorta and esophagus were highest with multi-unipolar energy delivery. Bubble risk was lowest with balloon catheters.
Conclusion
Computer models show that catheters with electrodes on a balloon surface or on flexible circuit splines are about 4 times more efficacious than circular catheters with electrodes exposed to atrial blood. Multi-unipolar energy delivery methods have a higher risk of electroporating aortic and esophageal tissue, when compared to bipolar interlaced methods. Considering embolic risks, circular catheters had the highest bubble-generating potential. A balloon or flexible circuit splined system with a wide interlaced delivery method showed the best balance in efficacy and safety.
Keywords: Pulsed field ablation, Electroporation, Pulmonary vein isolation, Catheters
Graphical abstract
Key Findings.
-
▪
Circular catheters are about 4 times less efficacious (lesion transmurality per unit current) and less safe (embolic risk) than balloon or flexible splined catheters, which have less electrode exposure to atrial blood. The loss of efficacy results from wasteful current shunting through that blood, between the exposed electrodes.
-
▪
Using a more efficacious catheter system improves safety, since less current is required to effect a transmural lesion, and less bubble generation is induced. Bubble formation potentially leading to embolic risk is mediated by electrolysis reactions. These directly relate circulating electrons (ie, current) to gas formation.
-
▪
A balloon or flexible circuit splined catheter with a bipolar, wide interlaced delivery method showed the best balance in efficacy and safety. Unipolar energy delivery methods are more likely to cause aortic and esophageal damage.
Introduction
Pulsed field ablation (PFA) is a re-newed technology based on electroporation that is promising to simplify and shorten pulmonary vein isolation procedures.1,24 Various catheter designs are appearing in study publications and on the market. One obvious difference among them is the level of electrode exposure to atrial blood. Another important difference is the method of energizing the electrodes. It is unclear how such differences translate into meaningful efficacy and safety differences. Therefore, the focus of this comparative study was to vary geometry, blood exposure, and energy delivery among 3 representative catheter designs, and then compare efficacy outcomes of lesion transmurality. In addition, safety outcomes related to extra-atrial tissue damage and embolic risk were also compared among the selected catheter types.
Methods
Catheters compared
With the goal of varying electrode proximity to atrial blood, 3 types of catheters were selected, as shown in Figure 1. All systems were 22 mm in diameter. The designs are similar to currently studied or available commercial devices used for radiofrequency and PFA ablation.2, 3, 4 Electrode dimension and spacing were chosen to be roughly similar to these devices, according to their published manuals or studies.
Figure 1.
The 3 catheter designs selected for comparison. Top row: A balloon (in situ) with surface electrodes, a spherical design (shown with a cutoff) using flexible circuit splines with back-insulated electrodes, and a circular decapolar design with ring electrodes. Bottom row: Balloon deployed at antrum of left pulmonary veins, and circular catheter at the same position. The spline electrodes (deployment not shown) were also at same position.
The balloon has 3.6 × 3.6 mm electrodes (area 13 mm2) contacting wall tissue without proximity to blood. The interelectrode spacing was 2.4 mm (6 mm on center). The balloon was modeled with a nonconductive filling fluid (eg, 5% dextrose), which was preliminarily tested to reduce high fields that could rupture the thin membrane.
The second design had flexible circuit splines, each 4 mm wide and 0.5 mm thick, insulating from atrial blood, since electrodes are mounted on polymer. Dimensions and spacing of electrodes were the same as the balloon above. Hypothetically, isolation is inferior to that of the balloon, since current could potentially flow through spaces between splines.
The third design was a circular catheter using ring electrodes, each 3 mm long and 1.6 mm diameter (area 15 mm2); interelectrode spacing was 3.7 mm.
All catheters had their electrodes placed on the same precise position, just contacting wall tissue tangentially, so as to control position as a source of variability affecting comparisons. To reduce the confounding effect of unequal indentation into tissue, a current-controlled scheme was employed, so that the same current was delivered regardless of electrode-tissue impedance.
Energy delivery methods
A second variable studied was the method of energizing electrodes. Figure 2 shows the 4 selected methods. These were based in part on emerging reports of PFA devices from some manufacturers. The multi-unipolar method was reported by Loh and colleagues.5 The interlaced method has been used by Stewart and colleagues.6
Figure 2.
Four methods of energizing electrodes. The first 2 are unipolar methods, with the negative electrode being a pad on back of the patient. In the multi-unipolar method, all electrodes are energized positively with simultaneous current flow from all electrodes to the back electrode. In the sequential unipolar method, each electrode is energized in turn, one at a time, while others float. For interlaced bipolar methods, there is no electrode on the patient’s back. The standard interlaced method has electrodes with alternating polarities. The wide interlaced method has nonenergized floating electrodes between alternating polarity ones; 2 sequential steps are used.
With interlaced bipolar energy deliveries, separating adjacent, opposite polarity electrodes avoids short circuits and thus reduces arcing. Separation can be realized with a circular catheter, given its relative rigidity. But with flexible balloon or splines, separation of adjacent electrodes may be difficult to maintain when positioning near pulmonary veins, as the balloon is flexible and may wrinkle. Thus, the standard interlaced system was not studied in balloons and splined catheters. Instead, a wide-interlaced system was used, with intervening floating electrodes (Figure 2).
Each individual electrode participating in a PFA simulation sourced or sank the same constant current. Current constancy, as opposed to a constant-voltage system, facilitates comparison of electrode designs with different electrode-tissue impedances, since the same current is injected regardless of variable contact impedance with tissue. Impedances are reported for readers interested in voltages, and are obtained by multiplying current by impedance, per Ohm’s law.
Imaging source and computer model
Three-dimensional, anatomically true computer models of the thorax were built from a publicly available computed tomography data set (Agecanonix; Pixmeo-Osirix, Geneva, Switzerland). Computer modeling is a known methodology in which an anatomy is represented in 3D by many small elements with particular tissue conductivities (described in the next section). The model allows the quantification and visualization of electric voltages, currents, and fields. Computer models are used, for example, in defibrillation7 as well as in ablation8 studies. Electrodes are placed in the virtual anatomy, and voltage or current is applied to energize them. The computer sees this as a large set of simultaneous equations—a generalization of Ohm’s law—and solves them using mathematical methods. Voltages, currents, and electric fields are then available for analysis at any point in the anatomy.
All organs and tissues near the left pulmonary veins—the target region—were included in detail, including atria, aortic wall, pulmonary arteries and veins, lung, airways, esophagus, and coronary sinus, among others. The author has training and experience in interpreting radiology imaging and anatomical segmentation.7,9 The left atrium had a volume of 66.42 cc including the left atrial appendage, and excluding pulmonary veins. The anteroposterior dimension of the left atrium was 3.46 cm, and the superoinferior dimension was 5.38 cm, according to echocardiographic conventional measurement methods,10 taken in a parasternal long-axis view. Figure 3 shows the finite-element computer model representing the anatomy. The atrial wall thickness of 2.5 mm is approximately 1 standard deviation above the average posterior wall thickness found across 60 pulmonary vein isolation patients.11 Comsol™ software version 5.6 (Comsol AB, Stockholm, Sweden) was used to solve for the stationary electric potential (and thus the electric field) throughout the thorax.
Figure 3.
Partial view of computed tomography–derived anatomy as modeled. Left panel: Posterior view with segment of the descending aorta (red), part of esophagus (yellow), airways and left lung (blue), left atrium (brown), and pulmonary trunk (magenta). The venae cavae, right atrium, and coronary sinus are shown in purple. Right panel: Superior view of the same, with more organs removed. The left atrial wall (gray) is 2.5 mm thick. A 6-mm-wide target at the left pulmonary vein antrum is just visible anterior to the aorta and esophagus (see text and Figure 4).
Electrical tissue properties
Tissue properties at 100 kHz (representative frequency for typical pulse field ablation pulses) were primarily sourced from a well-known database.12 Conductivity, the inverse of resistivity, expresses how easily current flows through a material. Tissues in the vicinity of the target were modeled with nonlinear conductivity, since it changes as a function of the electric field strength. That is, instead of being constant, tissue conductivity increases at higher electric fields as the cellular membranes permeabilize or break down under greater electric stress. For example, blood, which has a base (low electric field) conductivity of 0.667 S/m. At higher field strengths, it becomes more conductive, at 1.05 S/m. The conductivity change begins at field strengths of 400 V/cm and levels off at about 1100 V/cm,13 with the behavior of a smoothed S-shaped step. Other relevant tissue properties were as follows, listed in order of base, final, initial field threshold, final field threshold: myocardium, esophagus, and vessel walls 0.5 S/m, 1.0 S/m, 400 V/cm, 800 V/cm; pericardial and intervessel fat 0.1 S/m, 0.3 S/m, 350 V/cm, 700 V/cm. The lung was modeled linearly with a conductivity of 0.0714 S/m.14
Measurement of efficacy, target selection
The catheters to be compared were placed so as to create a continuous, circumferential lesion at the antrum of the left pulmonary veins, with electrode contact extending anteriorly from the left lateral ridge to the posterior wall of the left atrium, and vertically from floor to roof, as seen in Figures 1 and 4. Left-sided placement allowed for study of adverse effects on the nearby aorta and esophagus. To measure efficacy of a transmural lesion, a target volume was selected as a 6-mm-wide, 38-mm-long segment of the posterior wall, as depicted in Figure 4. The volume of this target was 0.569 cc. Efficacy was conservatively defined as the percentage of this volume that was energized with an electric field of more than 600 V/cm.15 Such percentage can be understood as a metric of transmurality of the lesion, with 100% meaning that the entire target volume had sufficient irreversible electroporation energy. This efficacy was calculated as a function of current, so as to discern which systems could achieve transmurality with less current.
Figure 4.
Target used for measurement of efficacy of lesion transmurality. A posterior, rectangular segment of left atrial myocardium is used. It is 6 mm wide and, like the rest of the atrial wall, 2.5 mm thick. Two of the catheters compared are shown here positioned against the target. Efficacy is defined as the percentage of this target’s volume that has more than 600 V/cm field strength. 100% represents a completely transmural lesion.
Strength duration, pulse width, repetition, and waveform
Current is one of the main variables affecting a durable lesion, as it determines the electroporating electric field (in V/cm). Other relevant variables include pulse width, waveform, and repetition rate.16 To control these, an equal myocardial electroporation threshold (the cited 600 V/cm) was assumed for all catheters compared. This magnitude is associated to a particular pulse width, waveform, and repetition number, all of which are assumed to be equal across the systems compared.
Of course, any or all of the individual efficacy results presented here could improve if, for example, a longer pulse width is used, since electroporation is known to have strength-duration behavior,17 meaning that the electroporation threshold is lower with longer pulse widths. Increasing pulse repetition could also have effects on efficacy, as could a biphasic waveform. To summarize, efficacy values reported here will vary from reality depending on the particular PFA parameters used, but this limitation will not affect the comparative observations presented, since parameters were equal across the comparisons.
Measurement of safety
Two quantities were used to assess safety: (1) electroporated volume of aortic and esophageal tissues that had an electric field of greater than 600 V/cm; and (2) an electrochemical and embolic safety measure: the electrode current density required to achieve 90% target transmurality, defined previously. Current density (with units of amperes per square cm) is defined as the electrical current at each electrode divided by its area. Such density is well understood in the field of electrochemistry to be a principal rate driver of gas bubbles when electrodes drive current through an aqueous electrolyte.18 The mechanism is the classical electrolysis reaction relating electric current to gas, eg, the flow of 2 electrons causes a molecule of hydrogen gas to be released according to 2H2O + 2e− → H2↑ + 2OH−.
The above safety metrics were calculated as a function of electrode current (or its density), so that safety could be assessed with relation to efficacy.
Results
Electrode impedances
There are many impedances available to report, since there are multiple electrodes on each catheter with varying tissues near them, and variable energy delivery methods. Further adding to the multiplicity of impedances, they varied nonlinearly with current, as described previously. Therefore, only some notable impedances at a current of 6 A per electrode are reported here.
For the multi-unipolar delivery method, a posterior wall electrode close to the aorta at a current of 6 amperes had impedances of 856, 821, and 630 ohms for the balloon, splined, and circular catheters, respectively. Voltages were 5135, 4929, and 3777 volts, respectively. The impedance numbers are per electrode, not system ones, which would be lower when all electrodes are considered. For example, for the circular catheter, the system impedance with the multi-unipolar method was approximately 63 ohms = average voltage 3803 V / (10 electrodes × 6 A). The balloon catheter, with more electrodes but better insulated by the balloon, yielded a slightly higher system impedance of approximately 68 ohms = average voltage 5299 V / (13 electrodes × 6 A).
When energized alone (sequential unipolar delivery method) at 6 amperes, the same posterior electrode had impedances of 184, 153, and 120 ohms for the balloon, splined, and circular catheters, respectively. Voltages were 1103, 919, and 718 volts, respectively. Impedances near fatty regions were slightly higher, for instance, 199 ohms for a roof electrode in the balloon system.
For an interlaced delivery method, the posterior electrode at 6 amperes had impedances of 257, 214, and 105 ohms for the balloon, splined, and circular catheters, respectively. Voltages were 1539, 1284, and 631 volts, respectively. It should be noted that the circular catheter had a standard interlaced delivery method, while the balloon and splined system had a sequential-wide-interlaced one, so their impedances are not strictly comparable.
Efficacy results
Figure 5 shows current distribution in atrial blood for 2 contrasting catheters compared. Figure 5 qualitatively shows how efficacy depends on electrode exposure and energy delivery method. Quantitative results for efficacy vs current for all catheter types and delivery methods are shown in Figure 6.
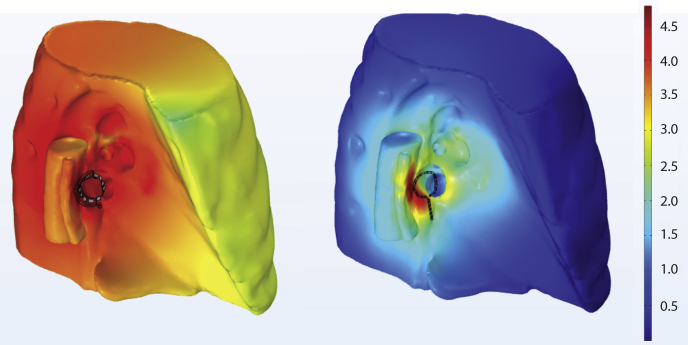
Figure 5.
Pulsed field ablation efficacy and safety aspects of two catheter designs: circular catheter (left), and balloon catheter (right). Right superior view of left atrium, aorta, and esophagus. With the circular catheter (left), current densities of 10 to 100 Amps/sq cm are subtantially present in atrial blood. This is less efficacious and favors bubble generation and consequent embolic risk.
Figure 6.
Efficacy vs current for the catheter designs and energizing methods that were compared. Efficacy is the percent of the target volume that is treated with more than 600 V/cm, with 100% representing a completely transmural lesion. In general, one can see that the balloon and flex-circuit splined catheters have the best efficacy, while the circular catheters require much higher currents to achieve similar transmural lesions. This makes sense if one considers that current is shunted via atrial blood as electrodes are closer to it. Seq = sequential.
Safety results
Figure 7 shows the results for electroporated volume in the aorta and esophagus, a measure of extracardiac tissue safety. One result is noted here: the circular catheter with interlaced energy delivery electroporated 0.011 cc of aortic and esophageal tissue, at a current of 20 A per electrode, the current to achieve 100% efficacy (this data point is not visible in Figure 7 but is available from the model’s solution).
Figure 7.
Aortic and esophageal safety vs current for the catheter designs and energizing methods compared. The curves show the volume of aorta and esophagus subject to more than 600 V/cm (ie, electroporated, damaged). A lower electroporated volume is safer. The circular catheter with an interlaced energy method is the safest, while multi-unipolar systems were the worst. (Safety from electrochemical processes is not depicted here; see Figure 9.) Seq = sequential.
Figure 8 is a qualitative view of the left lung, aorta, and esophagus showing electric fields that could result in irreversibly electroporated tissue. Figure 9 shows embolic safety results with efficacy considered. Namely, the chart shows the electrode current density (an embolic generator surrogate) required to achieve 90% transmurality on the defined target.
Figure 8.
Qualitative comparison of electroporation safety outside the heart: electric fields on the left lung, and segments of the aorta and esophagus. The balloon catheter (left) is energized in multi-unipolar fashion with 2 A at each electrode, whereas the circular catheter (right) is energized in an interlaced manner with 10 A at each electrode. The scale is logarithmic, so that a brown color corresponds to log (600 V/cm) = 4.8, a qualitative electroporation safety limit. The balloon system has large regions in orange-red, since current is flowing from all electrodes towards the back of the patient. The circular catheter, with an interlaced delivery, is much better at keeping high fields confined near the target, even with the higher current.
Figure 9.
Emboli-related electrochemical safety, in terms of current density to achieve 90% transmurality. Less current density means a lower rate of bubble generation, and greater safety. Relative to catheter designs that have electrodes with low exposure to blood, the circular catheter has about 4–5 times higher bubble generation, as expected, given its electrode proximity to blood electrolytes. Seq = sequential.
Discussion
Impedance
Impedances generally reflected the proximity of electrodes to atrial blood, as expected. The balloon catheter, which had the most isolated electrodes from blood, had the highest impedance, followed by the splined system, and then the circular catheter, which had the lowest one. Impedance also varied depending on proximity to the aorta, with electrodes closer to it having lower impedances.
With respect to energy delivery method, the multi-unipolar method had the highest voltage and impedance per electrode, a result of the electrode “guarding” effect that results when all electrodes are energized simultaneously.
Efficacy aspects
Per the results of Figure 6, the balloon catheter performed best, followed by the splined one. The circular catheter systems had substantially lower efficacy (about 4×), regardless of energy delivery method. These results show, as common sense suggests, that catheters with electrode proximity or exposure to atrial blood had lower efficacy. Observation of current flow and density in 3D showed that there is indeed greater current shunting through blood in systems with lower efficacy, Figure 5. This current distribution factor was also reflected in the ranking of energy delivery methods: the multi-unipolar one was the most efficacious, followed by interlaced and sequential-unipolar ones.
Thermal safety aspects
Thermal risks were evaluated as very low, but are not presented here, given that the nonthermal nature of PFA’s action is well established by bench experiments and by direct, clinical in vivo temperature measurements during PFA.19
Electroporation safety aspects
Conservatively neglecting tissue selectivity20,21 (see limitations section), this study compared electroporating exposure of extra-atrial tissues (Figure 7). A qualitative view is shown in Figure 8, illustrating how different catheters and energy deliveries can have very different exposures.
Multi-unipolar energy delivery, with a balloon design, produced the greatest electroporated volume of aortic and esophageal tissue: about 0.45 cc with an electrode current of 6 A, which is the current necessary to obtain 100% target efficacy (per Figure 6). The multi-unipolar splined system had 1.0 cc affected at 10 A, for the same efficacy. These volumes of electroporated aortic and esophageal tissue may be large enough to be clinically significant, as they roughly compare to the size of esophageal fistulas upon pathology examination. On the other hand, the circular catheter with interlaced energy delivery electroporated 0.011 cc of aortic and esophageal tissue, at a current of 20 A, the current required to achieve 100% transmurality. This benefit is achieved at the expense of significant current shunting through blood, given the closeness of electrodes to blood (Figure 5). This may increase embolic risks, as discussed in a later section below.
The balloon catheter using a sequential unipolar or a wide-interlaced delivery method showed the best electroporation safety vs efficacy profile: at a current of 6 A required for 100% transmurality, the volume of electroporated aortic and esophageal tissue was less than 0.05 cc (Figure 7).
As suggested by the above findings, at present there is a need for continued caution of extra-atrial safety with PFA. Cochet and colleagues22 reported aortic lesions visible in magnetic resonance imaging (MRI) in 6 of 18 (33%) patients undergoing PFA. Loh (Loh Peter. Personal communication, email August 13, 2021) and colleagues23 reported esophageal lesions in 1 of 20 patients, evident on endoscopy 1 day after PFA procedures.
Embolic and electrochemical safety aspects
PFA energy delivery generates a shower of gas bubbles emanating from the catheter that are obvious with intracardiac echocardiography, a concerning observation that is more than 36 years old,24 and evident again today with PFA's re-emergence. The primary rate driver of these bubbles is the electrode current density (eg, in A/cm2 units), as is well understood in the field of electrochemistry.18 Some of the reactions involved in PFA are those of classical electrolysis, and similarly produce gas and metal dissolution.
Evidence that gas bubbles generated from PFA persist and embolize downstream is in the recent study by Groen and colleagues.25 They used a femoral extracorporeal arterial bypass in pigs to measure bubbles originating from 200 J multi-unipolar PFA in the left atrium. Their study found that PFA-generated bubbles (worst case 85 μL volume per PFA application) embolized to the femoral shunt, and their volume was significantly reduced if the anode, not the cathode, is the pole in the atrium. That would restrict such benefit to unipolar systems, since interlaced and bipolar ones have both anodes and cathodes in the atrium. An opposite polarity-gas relationship was found by Bardy and colleagues26 and Holt and Boyd,27 but they used much higher currents with arcing that may have consumed hydrogen.
Using a different PFA system and a longer-duration waveform on a bench-saline setup, Woods and colleagues28 found bubble volume of 0.00152 μL per application. This is about 56,000 times smaller than that of Groen and colleagues25 in a live pig.
The small bubble volume measurements above notwithstanding, a shower of bubbles during PFA applications was still quite obvious in intracardiac echocardiography with the system used by Woods, as recently presented in live clinical conference cases.29 Other manufacturers’ devices also show a shower of bubbles in echocardiography during PFA.30
Goldfarb and Bahnson31 found that injecting 100 μL air bubbles at the coronary os in dogs can cause lasting myocardial damage. Loh23 reported 5 asymptomatic brain lesions on MRI in 3 out of 20 patients following PFA. Natale and colleagues32 reported microembolic signals coincident with PFA deliveries in 6 patients, using transcranial Doppler. An editorial in the Journal of the American College of Cardiology criticized the paucity of postprocedure brain MRI data in clinical studies of PFA.33
In summary, considering the electrochemically expected bubbles, their downstream persistence, and the reports of embolic lesions, there is an argument for reducing bubble generation.
The results of Figure 9 address this need, showing the current density required to achieve 90% target transmurality for the systems compared. It is not surprising that catheters with electrodes exposed to blood will have 4–5 times higher bubble gas generation, and thus higher embolic risk, than those with unexposed electrodes. The risk is compounded with energy delivery methods that drive current between nearby electrodes, such as the interlaced bipolar methods. In those cases, current will preferably flow through the least resistive proximal path. That is, it will flow through blood over myocardium or pericardial fat (Figure 5) and will, per electrochemical laws, directly relate flowing electrons to gas molecules.
Limitations
The conclusions presented are limited by an N = 1 sample size, typical of computer modeling studies. It is possible that the results would be different with inclusion of anatomies from a larger patient cohort. However, it is unlikely to change the major findings, not only because this is a comparative study, but mainly because electrical laws imply that current will be preferably shunted (and thus wasted) through the conductive atrial blood pool available as more exposed electrodes are used. Shunting will be reduced, regardless of patient variation, when electrode exposure is impeded by an insulating barrier or balloon. This impediment is unlikely to vary in substance from patient to patient.
The parameters in the simulations (eg, current amplitude injected by the electrodes) may not necessarily correlate with actual electrophysiologic effects for various reasons. True tissue electroporation threshold may vary from the assumed 600 V/cm (though this value is similar to that used by other investigators, as cited in the methods). However, the degree of said correlation is unlikely to change the main comparative conclusions found in the study, since equal parameters were assumed across all catheter types and energy delivery methods compared.
The data on the risk of electroporation of the aorta and esophagus should be taken as hypothesis-generating. In part, this is because there are conflicting clinical reports of tissue selectivity with PFA sparing these tissues, as cited in the discussion section. Also, the computer model did not include a higher electroporation threshold for these tissues, which would represent tissue selectivity, because of an absence of well-controlled, in vivo threshold data for the aorta and esophagus, to the best knowledge of this author, at writing time. Instead, the model conservatively assumed their thresholds were the same as for myocardium. Including the selective (ie, higher) aortic and esophageal thresholds would reduce the absolute volumes of the electroporated tissue, across all catheters compared. But the relative ranking of extracardiac electroporation risk according to catheter type or energy delivery method is unlikely to change, owing to the comparative nature of the study.
Finally, embolic events arising from thrombotic material from the catheter cannot be assessed by the computer model presented. Other investigators have recently reported that typical PFA field amplitudes are unlikely to cause red blood cell electrofusion.34
Conclusion
Computer models show that catheters with electrodes on a balloon surface or on flexible circuit splines are substantially more efficacious than conventional circular catheters with electrodes exposed to atrial blood. Multi-unipolar energy delivery methods have a higher risk of electroporating aortic and esophageal tissue, when compared to bipolar interlaced methods. Considering embolic risks, circular catheters had the highest bubble-generating potential owing to their required higher currents that undesirably shunt through atrial blood. A balloon or splined system with a wide interlaced delivery method had the best balance in efficacy and safety.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Andres Belalcazar has related patent pending on balloon dextrose fill, energy delivery methods.
Authorship
The author attests he meets the current ICMJE criteria for authorship.
Ethics Statement
No patient data or animals were used in this study. The computer models were built from a publicly available computed tomography data set.
References
- 1.Reddy V.Y., Neuzil P., Koruth J.S., et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74:315–326. doi: 10.1016/j.jacc.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 2.Gianni C., Chen Q., Della Rocca D., et al. Radiofrequency balloon devices for atrial fibrillation ablation. Card Electrophysiol Clin. 2019;11:487–493. doi: 10.1016/j.ccep.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Kottkamp H., Hindricks G., Pönisch C., et al. Global multielectrode contact-mapping plus ablation with a single catheter in patients with atrial fibrillation: Global AF study. J Cardiovasc Electrophysiol. 2019;30:2248–2255. doi: 10.1111/jce.14172. [DOI] [PubMed] [Google Scholar]
- 4.Stewart M.T., Haines D.E., Miklavčič D., et al. Safety and chronic lesion characterization of pulsed field ablation in a porcine model. J Cardiovasc Electrophysiol. 2021;32:958–969. doi: 10.1111/jce.14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loh P., van Es R., Groen M.H., et al. Pulmonary vein isolation with single pulse irreversible electroporation: a first in human study in 10 patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13:1083–1091. doi: 10.1161/CIRCEP.119.008192. [DOI] [PubMed] [Google Scholar]
- 6.Stewart M.T., Haines D.E., Verma A., et al. Intracardiac pulsed field ablation: proof of feasibility in a chronic porcine model. Heart Rhythm. 2019;16:754–764. doi: 10.1016/j.hrthm.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Heist E.K., Belalcazar A., Stahl W., Brouwer T.F., Knops R.E. Determinants of subcutaneous implantable cardioverter-defibrillator efficacy: a computer modeling study. Clin Electrophysiol. 2017;3:405–414. doi: 10.1016/j.jacep.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Pérez J.J., D’Avila A., Aryana A., Berjano E. Electrical and thermal effects of esophageal temperature probes on radiofrequency catheter ablation of atrial fibrillation: results from a computational modeling study. J Cardiovasc Electrophysiol. 2015;26:556–564. doi: 10.1111/jce.12630. [DOI] [PubMed] [Google Scholar]
- 9.Belalcazar A., Patterson R.P. Improved lung edema monitoring with coronary vein pacing leads: a simulation study. Physiol Meas. 2004;25:475. doi: 10.1088/0967-3334/25/2/007. [DOI] [PubMed] [Google Scholar]
- 10.Triulzi M., Gillam L.D., Gentile F., Newell J.B., Weyman A.E. Normal adult cross-sectional echocardiographic values: linear dimensions and chamber areas. Echocardiography. 1984;1:403–426. [Google Scholar]
- 11.Beinart R.O., Abbara S., Blum A., et al. Left atrial wall thickness variability measured by CT scans in patients undergoing pulmonary vein isolation. J Cardiovasc Electrophysiol. 2011;22:1232–1236. doi: 10.1111/j.1540-8167.2011.02100.x. [DOI] [PubMed] [Google Scholar]
- 12.Hasgall P.A., Di Gennaro F., Baumgartner C., et al. May 15, 2018. ITIS Database for thermal and electromagnetic parameters of biological tissues, Version 4.0. [DOI] [Google Scholar]
- 13.Marčan M., Kos B., Miklavčič D. Effect of blood vessel segmentation on the outcome of electroporation-based treatments of liver tumors. PloS One. 2015;10 doi: 10.1371/journal.pone.0125591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witsoe D.A., Kinnen E. Electrical resistivity of lung at 100 kHz. Med Biol Eng. 1967;5:239–248. doi: 10.1007/BF02474533. [DOI] [PubMed] [Google Scholar]
- 15.Pucihar G., Krmelj J., Reberšek M., Napotnik T.B., Miklavčič D. Equivalent pulse parameters for electroporation. IEEE Trans Biomed Eng. 2011;58:3279–3288. doi: 10.1109/TBME.2011.2167232. [DOI] [PubMed] [Google Scholar]
- 16.Pirc E., Reberšek M., Miklavčič D. In: Dosimetry in Bioelectromagnetics. Markov M., editor. CRC Press; 2017. Dosimetry in electroporation-based technologies and treatments; pp. 233–268. [Google Scholar]
- 17.Weaver J.C., Smith K.C., Esser A.T., Son R.S., Gowrishankar T.R. A brief overview of electroporation pulse strength–duration space: a region where additional intracellular effects are expected. Bioelectrochemistry. 2012;87:236–243. doi: 10.1016/j.bioelechem.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pletcher D.A. 2nd ed. Royal Society of Chemistry; Cambridge, UK: 2019. First Course in Electrode Processes. [Google Scholar]
- 19.Verma A., Boersma L.V., Haines D.E., et al. Pulsed field ablation and heat generation: electrode-tissue temperature analysis from the Pulsed-AF trial. Heart Rhythm. 2021;18:S70. [Google Scholar]
- 20.Maor E., Sugrue A., Witt C., et al. Pulsed electric fields for cardiac ablation and beyond: a state-of-the-art review. Heart Rhythm. 2019;16:1112–1120. doi: 10.1016/j.hrthm.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Reddy V.Y., Koruth J., Jais P., et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol. 2018;4:987–995. doi: 10.1016/j.jacep.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Cochet H., Nakatani Y., Sridi-Cheniti S., et al. Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace. 2021;23:1391–1399. doi: 10.1093/europace/euab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loh P. Single pulse PFA with a circular mapping catheter. 26th Annual International AF Symposium, 2021.
- 24.Rowland E.D., Foale R.O., Nihoyannopoulos P., Perelman M., Krikler D.M. Intracardiac contrast echoes during transvenous His bundle ablation. Heart. 1985;53:240–242. doi: 10.1136/hrt.53.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groen M.H., van Es R., van Klarenbosch B.R., et al. In vivo analysis of the origin and characteristics of gaseous microemboli during catheter-mediated irreversible electroporation. Europace. 2021;23:139–146. doi: 10.1093/europace/euaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardy G.H., Coltorti F.E., Ivey T.D., et al. Some factors affecting bubble formation with catheter-mediated defibrillator pulses. Circulation. 1986;73:525–538. doi: 10.1161/01.cir.73.3.525. [DOI] [PubMed] [Google Scholar]
- 27.Holt P.M., Boyd E.G. Hematologic effects of the high-energy endocardial ablation technique. Circulation. 1986;73:1029–1036. doi: 10.1161/01.cir.73.5.1029. [DOI] [PubMed] [Google Scholar]
- 28.Woods C.E., Viswanathan R., Reddy V. Electrolytic effects from a clinical endocardial pulsed field ablation system in a Benchtop model: a comparison of gas formation with focal RF ablation. J Cardiovasc Electrophysiol. 2021;32:1501–1502. [Google Scholar]
- 29.Reddy V, Neuzil P. Pulsed field ablation for persistent AF using single-shot and focal catheters. 26th Annual International AF Symposium, January 29-31, 2021
- 30.Verma A. Pulsed field ablation with an over-the-wire catheter system. 26th Annual International AF Symposium, January 29-31, 2021.
- 31.Goldfarb D., Bahnson H.T. Early and late effects on the heart of small amounts of air in the coronary circulation. J Thorac Cardiovasc Surg. 1963;46:368–378. [PubMed] [Google Scholar]
- 32.Natale A., Della Rocca D.G., Gianni C., et al. Cerebral microembolic signal burden during pulsed filed ablation: preliminary results from robotically assisted transcranial doppler. Heart Rhythm. 2021;18:S5–S6. doi: 10.1016/j.hrthm.2021.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Tomlinson D.R., Mandrola J. Pulsed field ablation for persistent atrial fibrillation (PersAFOne): hope or hype? J Am Coll Cardiol. 2020;76:1081–1083. doi: 10.1016/j.jacc.2020.07.032. [DOI] [PubMed] [Google Scholar]
- 34.Reddy V., Viswanathan R., Jais P. Dielectrophoretic red blood cell fusion by pulsed electric fields: ex vivo and porcine in vivo experiments. J Cardiovasc Electrophysiol. 2021;32:1514–1515. [Google Scholar]