Abstract
Background
Quadripolar left ventricular (LV) leads are capable of sensing and pacing the left ventricle from 4 different electrodes, which may potentially improve patient response to cardiac resynchronization therapy (CRT).
Objective
We measured 3 different time intervals: right ventricular (RV)-sensed to LV-sensed during intrinsic rhythm (RVs-LVs), RV-paced to LV-sensed (RVp-LVs), and LV-paced to LV-sensed (LVp-LVs, between distal [LV1] and proximal pole on a quadripolar LV lead), and assessed their association with CRT response in terms of LV end-systolic volume (LVESV) and a composite benefit index (CBI) comprising LVESV, LV ejection fraction (LVEF), brain natriuretic peptide level, and NYHA class.
Methods
A CRT-defibrillator system with quadripolar LV lead was implanted in 196 patients (mean age 69 years, mean LVEF 30%, left bundle-branch block [LBBB] 58%). Conduction intervals were measured before hospital discharge. At baseline and 7-month follow-up, echocardiographic and other components of CBI were determined.
Results
The mean RVs-LV1s, RVp-LV1s, and LVp-LVs delays were 68 ± 38 ms, 132 ± 34 ms, and 99 ± 31 ms, respectively. From baseline to 7 months, LVESV decreased by 17.3% ± 28.6%. The RVs-LV1s interval correlated stronger with CBI (R2 = 0.12, P < .00001) than with LVESV change (R2 = 0.05, P = .006). In contrast, RVp-LV1s did not correlate and LVp-LVs correlated only weakly with CRT response. The subgroup of patients (44%) with LBBB and RVs-LV1s above the lower quartile (≥34 ms) showed the greatest response to CRT.
Conclusion
The RVs-LVs interval during intrinsic rhythm is relevant for CRT success, whereas RVp-LVs and LVp-LVs intervals did not predict CRT response.
Keywords: Cardiac resynchronization therapy (CRT), Composite benefit index, CRT response, Interventricular electrical delay, Left ventricular end-systolic volume, Quadripolar left ventricular lead
Key Findings.
-
▪
The intrinsic interventricular delay (right ventricular sensed [RVs] – left ventricular sensed [LVs]) is relevant for cardiac resynchronization therapy (CRT) success, while RV-paced interventricular delay (RVp-LVs) and LV-paced intraventricular delay (LVp-LVs) did not predict CRT response.
-
▪
The subgroup of patients (44%) with left bundle branch block RVs-LVs above the lower quartile ≥34 ms) showed the greatest response to CRT.
-
▪
The observed variation in the RVs-LVs interval across the 4 poles of a quadripolar LV lead implies that the use of the latest-activated pole as LV pacing electrode for biventricular pacing may increase relative CRT benefit by ≈11% in terms of LV end-systolic volume reduction.
Introduction
Most left ventricular (LV) leads currently implanted as a part of cardiac resynchronization therapy (CRT) systems are quadripolar, capable of sensing and pacing from 4 different electrodes.1 Although the hemodynamic benefit of CRT may vary according to the chosen LV electrode, there are no established recommendations on how to program the optimal electrode configuration. Intracardiac electrogram channels in novel CRT devices allow measurement of electrical delays at all 4 LV electrodes, including right ventricular (RV)-sensed to LV-sensed time interval during intrinsic rhythm (RVs-LVs), RV-paced to LV-sensed time interval (RVp-LVs), and LV-paced to LV-sensed interval (pacing at any LV electrode and sensing by any other LV electrode, eg, LV1p-LV4s) (Figure 1).
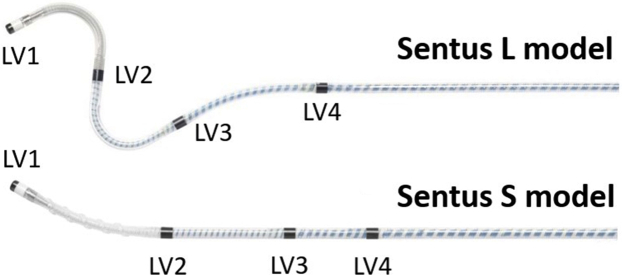
Figure 1.
The Sentus ProMRI OTW quadripolar left ventricular (LV) lead (Biotronik SE & Co. KG, Berlin, Germany) has a 2-D dual curve (“L” model) or a bend enhanced with a silicone screw (“S” model) for passive fixation in an LV coronary vein by stylet or by over-the-wire technique. The distances between adjacent electrodes are 21 mm (LV1tip-LV2ring), 20/15 mm (LV2ring-LV3ring, for L/S model), and 20/10 mm (LV3ring-LV4ring, for L/S model).
After 2 decades of routine use of CRT, the rate of responders is still limited (≈60%).2 It has been suggested that a prolonged intrinsic delay (RVs-LVs ≥70 ms) can predict CRT response3 and better clinical outcome,4,5 since simultaneous biventricular pacing synchronizes the cardiac contraction by completely eliminating this time interval. In a recent study, prolonged RVp-LVs interval also correlated with CRT benefit.1 No data exist on the predictive value of LVp-LVs interval, which may reflect scarring of the posterior or lateral LV wall or other conditions potentially influencing CRT response and clinical outcome.1,2,6
In the present study, we analyzed the interrelationship between intrinsic, RV-paced, and LV-paced conduction delays and assessed their association with CRT response and potential consequence on LV electrode selection in CRT patients with a quadripolar LV lead. Since single measures of CRT response, such as LV end-systolic volume (LVESV) decrease or LV ejection fraction (LVEF) increase, can be affected by a considerable measurement error, we combined 4 indicators of heart failure development—LVESV, LVEF, brain natriuretic peptide (BNP) level, and New York Heart Association (NYHA) functional class—to obtain a more stable composite index of CRT response.
Methods
Study design and patient selection
The Multicenter Prospective Pilot Study To Test LV Intracardiac Conduction Time as a Predictor of CRT Response (BIO|SELECT) was a single-arm study conducted at 28 hospitals in Japan. It enrolled patients eligible to receive a CRT-defibrillator (CRT-D) based on current guideline indications,7 who had no recent or planned cardiac surgery, had no previous CRT(-D) device, and were planned to receive Sentus ProMRI OTW quadripolar LV lead (Biotronik SE & Co. KG, Berlin, Germany) and a Biotronik CRT-D with multipole pacing feature (Intica or Inlexa 7 HF-T QP). Patients were followed for 7 months after implantation. The study was approved by appropriate ethics committees and performed in compliance with ISO 14155:2011 and Ethical Guidelines for Medical and Health Research Involving Human Subjects. All patients provided written informed consent (ClincialTrials.gov Identifier: NCT03337763).
Study protocol
Enrolled patients were evaluated at baseline, including echocardiography (see below) and biomarker BNP or N-terminal pro-B-type natriuretic peptide (NT-proBNP) level. Patients were scheduled to receive CRT-D and a steroid-eluting quadripolar LV lead designed for transvenous implantation in the coronary venous system. The investigators could choose between the 2 lead models depicted in Figure 1. Selection of the RV lead, bipolar atrial lead, and implantation sites of all 3 leads were chosen at the discretion of the investigators. The implantation procedure could be de novo or pacemaker or defibrillator upgrade to CRT-D.
Implantations were performed according to institutional standards. Two-dimensional right and left anterior oblique fluoroscopic projections were used to document locations of LV electrodes. Prehospital discharge (PHD) control included echocardiography and intracardiac conduction time measurements.
During the 1-, 4-, and 7-month follow-up visits, echocardiography was repeated and BNP or NT-proBNP level and NYHA class were determined. Decisions regarding device programming and patient treatment were at the discretion of investigators.
Echocardiography
At baseline and all follow-ups, LVEF, LVESV, LV end-diastolic volume, mitral regurgitation, and interventricular dyssynchrony were assessed by echocardiography. Cardiac volumes were estimated using the modified Simpson method (90% of patients) or the Teichholz method (10%).
Conduction times
At the PHD control, conduction times were measured according to a defined procedure, automatically or on the CRT-D programmer screen using calipers (Figure 2). The RVs-LVs and RVp-LVs intervals were determined between RV-tip electrode and each of the 4 LV electrodes. The LVp-LVs interval was calculated as the mean value of the distal-to-proximal and proximal-to-distal pole delays (Figures 1 and 2). The most proximal electrode suited for pacing was LV4 in 75% and LV3 in 25% of the patients. Thus, the LVp-LVs interval was in 75% of cases the mean of LV1p-LV4s and LV4p-LV1s, and in 25% of cases it was the mean of LV1p-LV3s and LV3p-LV1s conduction times.
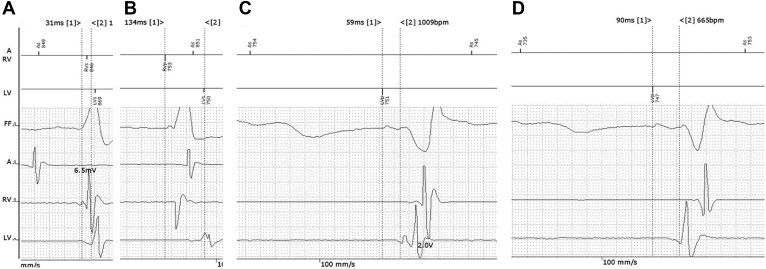
Figure 2.
Example of measurement of electrical delays on the Biotronik Renamic (Biotronik SE & Co. KG, Berlin, Germany) programmer screen. Atrial, right ventricular (RV), and left ventricular (LV) electrograms were displayed at a sweep speed of 100 mm/s. The distance between 2 calipers was indicated in ms. A: RVs-LVs (31 ms); calipers are manually positioned to measure interval from the peak of the first fragmentation of RV wave pattern to the peak of the first fragmentation of LV wave pattern. B: RVp-LVs (134 ms), automatically measured by the programmer between the RV-pace marker and the LV-sense marker (calipers are not used but shown to demonstrate the interval approximately). C: LV1 tip pacing – LV4 ring sensing (59 ms); calipers are manually positioned on the LV pace marker and on the peak of the first fragmentation of LV wave pattern. D: LV4 ring pacing – LV1 tip sensing (90 ms); calipers are positioned as in panel C. In panels B–D, wave pattern cannot be shown during the blanking period after RV pacing and LV pacing. A = atrial; FF = far-field signal; p = paced; s = sensed.
Estimation of CRT benefit
The individual CRT benefit at 7 months after implantation was calculated using the established indicator LVESV reduction (in %) and a composite benefit index (CBI) combining LVESV, LVEF, BNP, and NYHA class changes from initial values. To increase their precision, we averaged as initial echo parameters the baseline and PHD measurements, taken a median of 7 days apart. Missing data at 7 months (in <2% of patients) were imputed by 4-month data.
To determine CBI, the individual change in LVESV, LVEF, NYHA class (treated as numerical variable), and the log-transformed BNP value were divided by the corresponding threshold values commonly used in clinical practice for CRT response: 15% decrease in LVESV, 5% absolute increase in LVEF, 25% decrease in BNP, and improvement by 1 NYHA class.8,9 A patient’s composite CBI was then calculated as the sum of the 4 values. For example, in a patient with 10% LVESV reduction, 6% LVEF increase, 20% BNP decrease, and 1-class NYHA improvement, the CBI is 10/15+6/5+20/25+1/1=3.67. NT-proBNP was converted to BNP according to the following formula: log(BNP) = -0.127 + 0.815 × log(NT-proBNP).10
Study objectives
The main study objective was to assess if the LVp-LVs conduction time predicts CRT response. We also analyzed the interrelationship between RVs-LVs, RVp-LVs, and LVp-LVs conduction times, and assessed their association with CRT response and potential consequence on LV electrode selection.
Statistical Methods
In an exploratory study without primary hypothesis, the sample size was calculated based on the width of the confidence interval of an assumed correlation coefficient between LVp-LVs and hemodynamic indices. Assuming the coefficient r = 0.7 (arbitrarily, since no literature exists), a confidence interval width d = 0.2, a 99% confidence level, and a 10% patient dropout rate during the study, the calculated sample size was 198 patients.
Linear regression models were used to determine correlation coefficients. With a large number of data points, even weak correlations can be significant. The strength of a correlation is expressed by the R2 value, describing the share of the variability of the dependent variable that is explainable by the independent variable. We denote R2 < 0.10 as a weak correlation if the P value <.05 identifies statistical significance. Other data are presented as mean ± standard deviation, median (interquartile range [IQR]), or absolute and relative frequencies. Data were analyzed with the SAS (SAS Institute Inc, Cary, NC) and R (R Development Core Team, https://www.R-project.org/) statistical software.
Results
Patients
The study enrolled 201 patients between November 7, 2017 and March 12, 2019. Patient characteristics are shown in Table 1. One hundred seventy-two (86%) patients had de novo CRT-D implantation, 16 (8%) pacemaker upgrade, and 13 (7%) defibrillator upgrade. Two patients dropped out before implantation and 3 after LV lead implantation failure.
Table 1.
Baseline characteristics of enrolled patients
| Parameter | Data availability N |
Value |
|---|---|---|
| Age [years] | 201 | 69 ± 11 |
| Sex, male | 201 | 150 (74.6) |
| Ischemic heart disease | 201 | 84 (41.8) |
| Prior myocardial infarction | 201 | 54 (26.9) |
| NYHA functional class | 201 | |
| I | 3 (1.5) | |
| II | 91 (45.3) | |
| III | 103 (51.2) | |
| IV | 4 (2.0) | |
| History of ventricular tachyarrhythmia | 201 | 126 (62.7) |
| History of atrial fibrillation | 201 | 77 (38.3) |
| Bundle branch block | 200 | 161 (80.5) |
| Left bundle branch block | 115 (57.5) | |
| Right bundle branch block | 23 (12.5) | |
| Intraventricular conduction delay | 19 (9.5) | |
| Other | 4 (2.0) | |
| Intrinsic QRS duration [ms] | 178 | 152 ± 25 |
| Echocardiographic parameters | ||
| LVEF [%] | 199 | 30 ± 8 |
| LVESV [mL] | 198 | 141 ± 73 |
| LVEDV [mL] | 198 | 195 ± 83 |
| Severe or moderate MR | 199 | 74 (37.2) |
| BNP [pg/mL] | 159 | 628 ± 972 |
| NT-proBNP [pg/mL] | 41 | 4849 ± 10422 |
| Major cardiovascular medication | 195 | |
| ACE inhibitor or ARB | 150 (76.5) | |
| Beta blocker (excluding sotalol) | 168 (85.7) | |
| Diuretic | 165 (84.2) | |
| Mineralocorticoid receptor antagonists | 69 (35.2) | |
| Antiarrhythmic drug | 62 (31.6) |
Data are shown as N (%) or mean ± standard deviation.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BNP = brain natriuretic peptide; LV = left ventricular; LVEDV = LV end-diastolic volume; LVEF = LV ejection fraction; LVESV = LV end-systolic volume; MR = mitral regurgitation; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association.
LV leads
The LV lead was successfully implanted in 196 patients (60% Sentus QP L, 40% Sentus QP S). Distal poles (LV1, LV2) were often placed in the apical and mid segments. The proximal pole (LV4) remained in the basal region and occasionally in the coronary sinus. The distribution of anterior/lateral/posterior positions was similar across 4 LV electrodes. Supplemental Tables S1 and S2 summarize implant positions and pacing thresholds for different LV electrodes.
Follow-up
The number of patients at follow-up controls was 195 (PHD), 191 (1 month), 181 (4 months), and 178 (7 months); the main reasons for attrition were patient death (n = 4) and moving away (n = 5). After the 1-month follow-up, conventional biventricular pacing was used in 71% of patients, multipole LV pacing in 25%, and LV-only pacing in 4%.
Conduction times
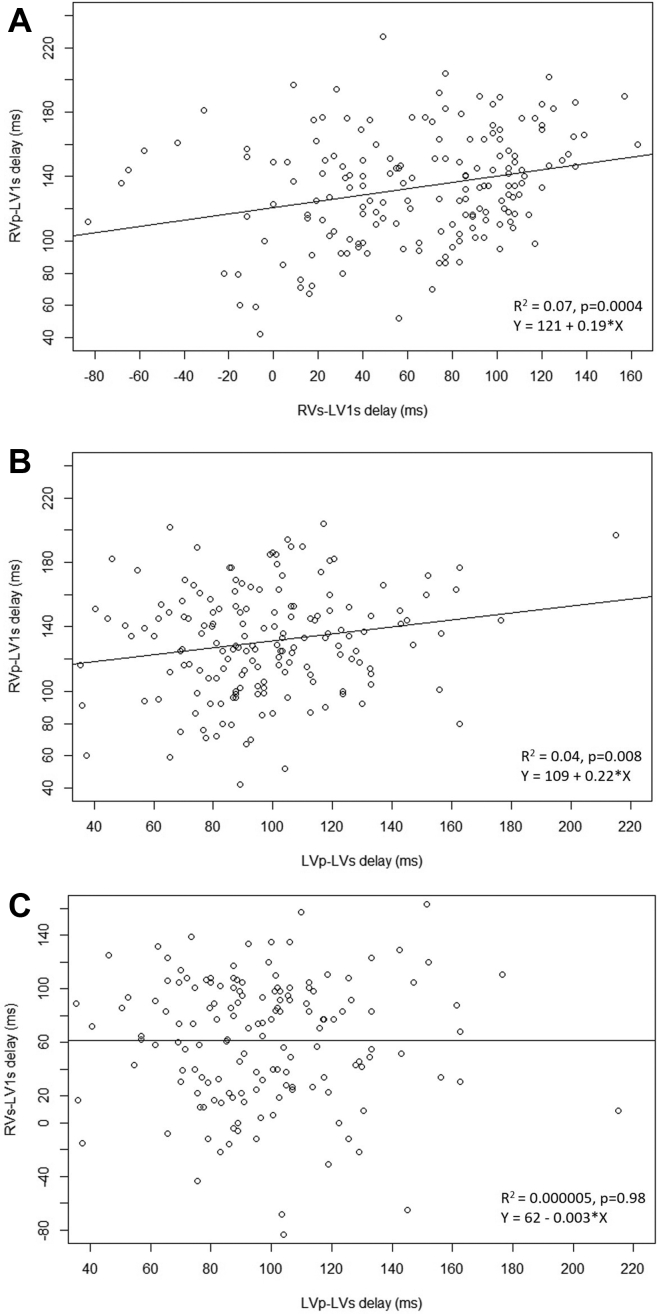
The mean RVs-LV1s interval was 68 ± 38 ms (median 74 ms [IQR 34–100]), based on data in 174 patients with preserved atrioventricular conduction. The mean RVp-LV1s was 132 ± 34 ms (median 133 ms [IQR 110–153]; n = 193), and the mean LVp-LVs was 98 ± 31 ms (median 95 ms [IQR 80–114]; n = 169). There was a weak positive correlation between RVs-LV1s and RVp-LV1s (R2 = 0.07, P = .0004) and between RVp-LV1s and LVp-LVs (R2 = 0.04, P = .008), but not between RVs-LV1s and LVp-LVs conduction times (Figure 3). Reproducibility of the measurements is summarized in the Supplemental Results.
Figure 3.
Scatterplots showing interrelationship between the following: A: intrinsic and right ventricular (RV)-paced, B: left ventricular (LV)-paced and RV-paced, and C: LV-paced and intrinsic electrical delays. Correlation parameters (R2, P value) and the formula of the regression line are indicated. p = paced; s = sensed.
CRT benefit
The LVESV was reduced from 137 ± 73 mL (initial value) to 112 ± 69 mL (7 months), a mean relative change of -17% ± 29% (median -15% [IQR -37 to +1]; n = 178 paired data points). The LVEF increased from 30% ± 8% to 35% ± 12%, corresponding to a +5% ± 9% absolute change (median 4% [IQR 1–11]; n = 178).
BNP and NT-proBNP levels in pg/mL decreased from 617 ± 978 (n = 154) to 374 ± 599 (n = 138) and from 4849 ± 10,422 (n = 41) to 3220 ± 11,952 (n = 38), respectively. In the pooled data set, with both parameters transformed into log(BNP), the mean difference between 7-month and baseline value was -0.24 log units (n = 180), equivalent to a mean reduction of 43%. The NYHA classes (I/II/III/IV) improved from 3/89/99/4 to 50/116/13/2; the mean numerical improvement was 0.7 classes (n = 181).
The mean CBI for all patients was 4.8 ± 5.6 (median 4.5 [IQR 1.0–7.8]; n = 181). In the subgroup of patients who approached 7-month follow-up control with the CRT-D programmed to conventional biventricular pacing, the mean index was 5.3 ± 5.7 (n = 126), and in patients with multipole LV pacing it was 3.7 ± 4.9 (n = 44). The respective LVESV change was -20.3% ± 27.9% for biventricular and -8.9% ± 29.1% for multipole pacing.
Correlations between the 4 CBI components are described in the Supplement.
Correlation of CRT benefit and electrical delays
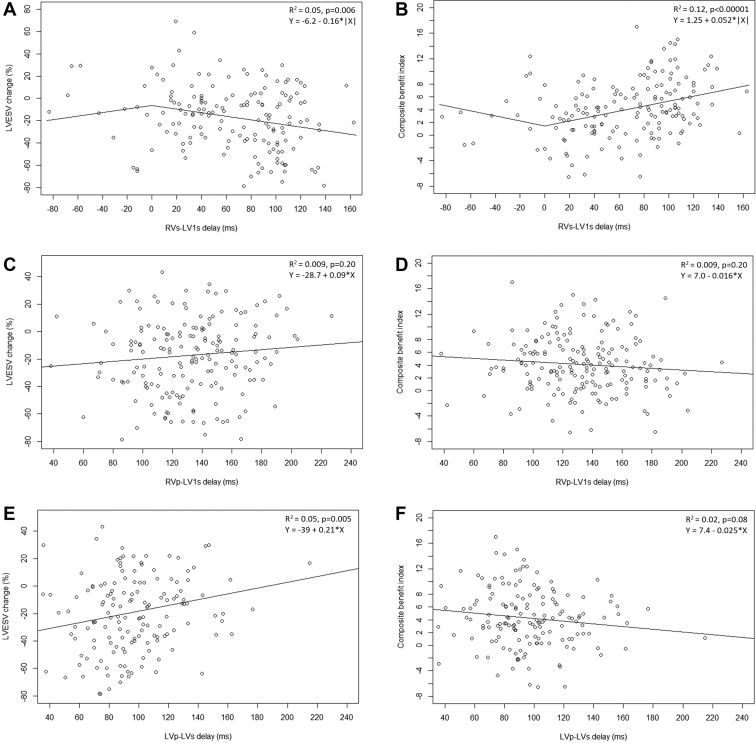
Figure 4 illustrates correlations of CRT benefit and conduction times in order of measurements. LVESV reduction and RVs-LV1s were weakly correlated (R2 = 0.05, P = .006). The slope of the regression line of -0.16 means that each 10-ms increase in RVs-LV1s translates into 1.6% greater LVESV reduction. A stronger correlation was found between CBI and RVs-LV1s (R2 = 0.12, P < .00001); a regression slope of 0.052 indicates that a 10-ms increase in RVs-LV1s is associated with ≈11% greater CBI. The higher R2 value suggests that the CBI is less affected by measurement error than the LVESV alone. Supplemental Table S3 shows correlation of CRT benefit and RVs-LV1s in subgroups of patients (eg, with vs without ischemic disease).
Figure 4.
Interrelationship between left ventricular end-systolic volume (LVESV) change or composite cardiac resynchronization therapy benefit index and intrinsic (A,B), right ventricular (RV)-paced (C,D), and left ventricular (LV)-paced (E,F) electrical delays. Correlation parameters (R2, P value) and the formula of the regression line are indicated. For intrinsic delay, regression was determined using absolute values of the conduction time, which converted negative values (sensing at LV1 occurring earlier than at RV) to positive values. The regression lines in the plots are therefore kinked at 0 ms. p = paced; s = sensed.
In contrast, CRT benefit did not correlate with RVp-LV1s (Figure 4C and 4D).
Finally, the LVp-LVs interval correlated weakly only with LVESV reduction (R2 = 0.05, P = .005) but not with CBI (R2 = 0.02, P = .08) (Figure 4E and 4F), suggesting that RVs-LV1s is an overall better predictor than LVp-LVs.
Possible effect of LV pacing electrode choice
The mean RVs-LVs conduction time differed slightly for different LV electrodes (Table 2). The maximum time difference between excitation at LV1 and excitation at any other LV electrode was 12 ± 21 ms. Since the regression slope for LVESV change vs RVs-LV1s was -0.16 (Figure 4A), a longer intrinsic delay by 12 ms (if the latest-activated LV electrode is used as pacing electrode instead of LV1) may yield an additional LVESV reduction of ≈1.9% (= 12 ms × 0.16 %/ms). On top of the aforementioned mean LVESV reduction of 17.3% after 7 months of follow-up in all patients, an additional LVESV reduction of 1.9% would increase the CRT benefit by ≈11% (= 1.9/17.3).
Table 2.
Intrinsic interventricular delays for different left ventricular electrodes
| N | Mean (SD) | Median (IQR) | |
|---|---|---|---|
| Intrinsic interventricular delay, ms | |||
| RVs-LV1s | 174 | 63 (47) | 73 (31-100) |
| RVs-LV2s | 174 | 66 (47) | 71 (33-101) |
| RVs-LV3s | 172 | 67 (49) | 76 (31-106) |
| RVs-LV4s | 140 | 71 (46) | 74 (43-105) |
| Maximum difference between excitations, ms | |||
| At LV1 and at any other LV electrode | 174 | 12 (21) | 11 (0-21) |
| At any 2 LV electrodes | 174 | 23 (18) | 18 (11-28) |
IQR = interquartile range; LV = left ventricular; LV1 to LV4 = 4 electrodes of quadripolar LV lead (see Figure 1); RV = right ventricular; s = sensed; SD = standard deviation.
For the CBI vs RVs-LV1s, the regression slope was 0.052 (Figure 4B), and a 12-ms longer conduction interval for the latest-activated LV electrode (when used as pacing electrode) translates into a CBI increase by 0.62 (= 12 ms × 0.052/ms), or ≈13% on top of the mean CBI of 4.8 after 7 months of follow-up in all patients.
CRT benefit in left bundle branch block and non–left bundle branch block patients depending on RVs-LVs
Kosztin and colleagues5 suggested that CRT patients with left bundle branch block (LBBB) and an RVs-LVs interval above the lower quartile gain the largest long-term clinical benefit from CRT in terms of heart failure and death, whereas patients with LBBB and an RVs-LVs below the lower quartile and patients without LBBB irrespective of RVs-LVs had significantly less benefit. We checked if similar group definitions in our study would result in similar difference in CRT response.
The lower quartile for RVs-LV1s interval in our study was 34 ms. In LBBB patients, both CBI (6.7 ± 5.7) and LVESV change (-24.9% ± 29.4%) were remarkably higher for RVs-LVs ≥34 ms than for RVs-LVs <34 ms (2.7 ± 6.3 and -9.6% ± 28.7%, respectively). In non-LBBB patients, the results for RVs-LVs ≥34 ms were similar to those for RVs-LVs <34 ms and clearly worse than in LBBB patients (Table 3).
Table 3.
Cardiac resynchronization therapy benefit in left bundle branch block and non–left bundle branch block patients depending on intrinsic interventricular delay
| Patient subgroup | N | CBI, mean (SD) | LVESV change, mean (SD) |
|---|---|---|---|
| LBBB | |||
| RVs-LV1s ≥34 ms (above the lower quartile) | 93 | 6.7 (5.7) | -24.9 (29.4) |
| RVs-LV1s <34 ms (below the lower quartile) | 13 | 2.7 (6.3) | -9.6 (28.7) |
| No LBBB | |||
| RVs-LV1s ≥34 ms | 34 | 2.6 (4.3) | -8.4 (21.1) |
| RVs-LV1s <34 ms | 34 | 2.8 (5.1) | -5.0 (28.7) |
CBI = composite benefit index; CRT, cardiac resynchronization therapy; LBBB = left bundle branch block; LVESV = left ventricular end-systolic volume; RVs-LV1s = intrinsic interventricular delay; SD = standard deviation.
Discussion
Major findings of the present study are that RVs-LVs is relevant for CRT success, while RVp-LVs and LVp-LVs did not predict CRT response. We confirm that patients with LBBB and prolonged RVs-LVs interval derive the greatest benefit from CRT.
Intrinsic interventricular delay (RVs-LVs)
Reducing the nonresponder rate continues to be an important goal of CRT.2 The interplay between the LV lead position and the underlying substrate is a major determinant of CRT outcomes.1 Recent subanalyses of the SMART-AV trial demonstrated that CRT response, defined as LVESV reduction by either >15-mL3 or >15%11 from implantation to 6 months, increases significantly for LV lead positions associated with longer RVs-LVs conduction times. We confirm this result with a different definition of CRT benefit, using LVESV and the newly designed CBI. Furthermore, in a subanalysis of the PEGASUS study, a prolonged RVs-LVs interval at CRT implantation was also associated with improved clinical outcomes at 1 year, including the composite endpoint of hospitalization for heart failure and death.4 This increasing evidence and a recent combined clinical-computational study12 support the strategy of evaluating intrinsic interventricular delay to position the LV lead in an area of the latest electrical activation.1,3,4,11,12 This strategy might replace alternative methods for LV lead positioning based on anatomical criteria13,14 or on LV lead electrical delay measured from the onset of the surface electrocardiogram QRS complex to the onset of the sensed electrogram on the LV lead.11,15 However, only observational data are available.
The impact of RVs-LVs interval on the CRT success in LBBB vs non-LBBB patients was studied by Kosztin and colleagues.5 Patients with LBBB and an RVs-LVs delay above the lower quartile (≥86 ms) showed the greatest improvement in all outcome measures: NT-proBNP concentration, LVEF, and clinical outcome (heart failure or death) after a median follow-up of >2 years.5 In contrast, there was no significant difference in outcomes by RVs-LVs interval in non-LBBB patients. This result supports the notion that LBBB is an electrical disease for which CRT is a potent therapy, while non-LBBB patients have a more complex disease process and a less discernible or no CRT benefit regardless of the RVs-LVs conduction time or baseline QRS duration.5,16, 17, 18 Our findings in Table 3 confirm the difference in CRT benefit between LBBB and non-LBBB patients also for the novel CBI.
RV-paced interventricular delay (RVp-LVs)
Moubarak and colleagues1 found a stronger correlation (R2 = 0.27) between RVp-LVs and RVs-LVs than we did (R2 = 0.07). This is probably related to the fact that they assumed data for 4 different poles of the same quadripolar LV lead to be independent, allowing each of 32 patients to contribute 4 pairs of values.1 In contrast, 174 patients in our study each contributed only 1 pair of values, providing a larger set of truly independent data.
Moubarak and colleagues suggested that RVp-LVs may guide the selection of LV pacing site in patients requiring RV pacing.1 The idea that the RVp-LVs is relevant is theoretically not convincing (see Supplemental Discussion), and more evidence is needed before its practical use. In our study, RVp-LVs did not have any influence on CRT response.
LV-paced intraventricular delay (LVp-LVs)
Higher myocardial scar burden (eg, after myocardial infarction) near the LV lead tip or at the lateral or posterior LV wall can delay LV intraventricular conduction and impair CRT response and clinical outcome.1,5,6,19
The correlation between LVp-LVs and other variables was assessed with the LV1-LV4 or LV1-LV3 pole configuration, where any of these 2 distances can be shorter or longer, depending on the anatomy and tortuosity of the veins. We did not find any clinically meaningful interrelationship between LVp-LVs and other conduction times or CRT benefit. This is probably because the scar may be anywhere in the ventricle, affecting the conduction between 2 specific points only in a small subset of patients, and therefore not visible in pooled data for all patients.
Based on the observed variation of the RVs-LVs delay across the 4 poles of the quadripolar LV lead, our study generates a hypothesis that the use of the latest-activated pole during intrinsic conduction as LV pacing electrode instead of LV1 (if reliable pacing is possible at an acceptable output setting) may increase CRT benefit by 13% on average, corresponding to an additional LVESV reduction by ≈1.9%.
Wisnoskey and Varma19 were first to measure the LVp-LVs intervals by an implanted device. In an intraprocedural study of 120 heart failure patients with LBBB and QRS >120 ms receiving CRT with quadripolar LV leads, they studied reactions to LV stimulation and whether they were affected by QRS duration/morphology, LV substrate, or electrode choice.19 They found that the area of latest LV activation was large and spanned by a multipolar lead, but functional conduction barriers existed only in a minority.19 Responses to LV pacing varied widely and were unpredictable from baseline QRS morphology or LV stimulation site.19
Two recent studies suggest that a new parameter named LV-paced conduction disturbance6 (or paced LV dyssynchrony20), defined as present when LV-paced interventricular delay (LVp-RVs) is larger than RV-paced interventricular delay (RVp-LVs), predicts nonresponse to CRT6,20 and poor outcome (death and/or heart failure hospitalizations).6 These patients also present with higher perfusion defects in the anterolateral region and significantly smaller QRS narrowing after CRT than patients without LV-paced conduction disturbance.6 Owing to late publication of these 2 studies, we were not aware of the potential merits of LVp-RVs. A note on the use of multipole LV pacing in our study is provided in the Supplemental Discussion.
Composite benefit index
Single parameters used to assess patient response to CRT, such as LVESV, LVEF, BNP, NYHA class, quality of life, and exercise capacity,2,5,8,9,11,14,21 may be affected by measurement errors and noise. For example, a seemingly unchanged LVEF after several months of CRT in an individual patient may be caused by a low follow-up value owing to inaccurate border detection by echocardiography. Similarly, a BNP increase may be triggered by atrial fibrillation at the day of measurement despite an improved LVEF value.
We therefore constructed CBI by combining LVESV, LVEF, BNP, and NYHA class. An inspection of Figure 4 indicates a generally good concordance between LVESV reduction and CBI, despite several apparent outliers for LVESV in the scatterplots. In the established correlation of CRT response and the intrinsic interventricular delay, the R2 values suggest that CBI performs better than LVESV. In Supplemental Table S3, both LVESV and CBI resulted in statistically significant correlations in the same 5 subgroups of patients and nonsignificant correlation in the other 3 subgroups. But when there was a statistically significant correlation, it was more pronounced for CBI (R2 value about twice as high, P value consistently lower) than for LVESV, as if CBI strengthened the correlation by removing the outliers. Overall, this novel 4-component index performed up to our satisfaction, seemingly enabling a more precise estimate of the true benefit and increasing our ability to draw conclusions.
Study limitations
This study has several limitations worth noting. First, we did not measure and consider the LVp-RVs delay as a potential predictor of CRT response, as this parameter was largely unknown at the time of our study design. Second, conduction delays were measured on the programmer screen and no core lab was used for it or for echocardiography. Third, we did not collect data on RV lead implant location. Fourth, the decision to use multipole LV pacing in individual patients and selection of pacing poles were at the discretion of the investigators. Since the knowledge of the delays may have influenced a decision to employ multipole LV pacing, and multipole pacing with wide anatomic separation has been recently suggested to improve CRT response (especially in patients with LV enlargement),22 this constitutes a possible confounder. Furthermore, we used an untypical and not validated CBI along with LVESV, and based our conclusions on both indicators.
Conclusion
The intrinsic interventricular delay (RVs-LVs) is relevant for CRT success, while RV-paced interventricular delay (RVp-LVs) and LV-paced intraventricular delay (LVp-LVs) did not predict CRT response. Patients with LBBB and prolonged RVs-LVs interval derive the greatest benefit from CRT. The observed variation in the RVs-LVs interval across the 4 poles of a quadripolar LV lead implies that the use of the latest-activated pole as LV pacing electrode for biventricular pacing may increase relative CRT benefit by ≈11% (LVESV) to ≈13% (CBI).
Acknowledgments
The authors are thankful to Yukiko Matsumura for study management, Ulrich Gauger for statistical analysis, and Dejan Danilovic, PhD, for critical reading and editing of the manuscript.
Footnotes
ClincialTrials.gov Identifier: NCT03337763.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2021.09.007.
Funding Sources
This work was supported by BIOTRONIK SE & Co. KG (Berlin, Germany) and BIOTRONIK JAPAN, INC (Tokyo, Japan; grant/contract numbers: CR023).
Disclosures
YK, SS, FM, NN, and KA serve as a consultant or provide substantial speaker services to Biotronik Japan. All clinical authors received research grants in the context of this study. HS and JS are employees of Biotronik.
Authorship
All authors attest they meet the current ICMJE criteria for authorship. The principal investigator designed the protocol with the sponsor. The sponsor managed the trial and performed the analysis. The authors take full responsibility for the result and the decision to submit the article.
Patient Consent
All patients provided written informed consent.
Ethics Statement
The study was approved by appropriate ethics committees and performed in compliance with ISO 14155:2011 and Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Appendix. Supplementary data
References
- 1.Moubarak G., Sebag F.A., Socie P., Villejoubert O., Louembe J., Ferchaud V. Interrelationships between interventricular electrical delays in cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2020;31:2405–2414. doi: 10.1111/jce.14629. [DOI] [PubMed] [Google Scholar]
- 2.Sieniewicz B.J., Gould J., Porter B., et al. Understanding non-response to cardiac resynchronisation therapy: common problems and potential solutions. Heart Fail Rev. 2019;24:41–54. doi: 10.1007/s10741-018-9734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold M.R., Singh J.P., Ellenbogen K.A., et al. Interventricular electrical delay is predictive of response to cardiac resynchronization therapy. JACC Clin Electrophysiol. 2016;2:438–447. doi: 10.1016/j.jacep.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Gold M.R., Yu Y., Wold N., Day J.D. The role of interventricular conduction delay to predict clinical response with cardiac resynchronization therapy. Heart Rhythm. 2017;14:1748–1755. doi: 10.1016/j.hrthm.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Kosztin A., Kutyifa V., Nagy V.K., et al. Longer right to left ventricular activation delay at cardiac resynchronization therapy implantation is associated with improved clinical outcome in left bundle branch block patients. Europace. 2016;18:550–559. doi: 10.1093/europace/euv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda N., Noda T., Nakajima I., et al. Clinical impact of left ventricular paced conduction disturbance in cardiac resynchronization therapy. Heart Rhythm. 2020;17:1870–1877. doi: 10.1016/j.hrthm.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Tsutsui H., Isobe M., Ito H., et al. JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure - Digest Version. Circ J. 2019;83:2084–2184. doi: 10.1253/circj.CJ-19-0342. [DOI] [PubMed] [Google Scholar]
- 8.Rinkuniene D., Bucyte S., Ceseviciute K., et al. Predictors of positive response to cardiac resynchronization therapy. BMC Cardiovasc Disord. 2014;14:55. doi: 10.1186/1471-2261-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakos Z., Chatterjee N.C., Reitan C., Singh J.P., Borgquist R. Prediction of clinical outcome in patients treated with cardiac resynchronization therapy - the role of NT-ProBNP and a combined response score. BMC Cardiovasc Disord. 2018;18:70. doi: 10.1186/s12872-018-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taki M., Hoshide S., Kono K., Kario K. Correlation between B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide in a large Japanese population at risk of stage A heart failure. Pulse (Basel) 2018;6(1-2):1–8. doi: 10.1159/000485660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Field M.E., Yu N., Wold N., Gold M.R. Comparison of measures of ventricular delay on cardiac resynchronization therapy response. Heart Rhythm. 2020;17:615–620. doi: 10.1016/j.hrthm.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Huntjens P.R., Ploux S., Strik M., et al. Electrical substrates driving response to cardiac resynchronization therapy: a combined clinical-computational evaluation. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.005647. [DOI] [PubMed] [Google Scholar]
- 13.Thebault C., Donal E., Meunier C., et al. Sites of left and right ventricular lead implantation and response to cardiac resynchronization therapy observations from the REVERSE trial. Eur Heart J. 2012;33:2662–2671. doi: 10.1093/eurheartj/ehr505. [DOI] [PubMed] [Google Scholar]
- 14.Behon A., Schwertner W.R., Merkel E.D., et al. Lateral left ventricular lead position is superior to posterior position in long-term outcome of patients who underwent cardiac resynchronization therapy. ESC Heart Fail. 2020;7:3374–3382. doi: 10.1002/ehf2.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh J.P., Fan D., Heist E.K., et al. Left ventricular lead electrical delay predicts response to cardiac resynchronization therapy. Heart Rhythm. 2006;3:1285–1292. doi: 10.1016/j.hrthm.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Zareba W., Klein H., Cygankiewicz I., et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 17.Birnie D.H., Ha A., Higginson L., et al. Impact of QRS morphology and duration on outcomes after cardiac resynchronization therapy: results from the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT) Circ Heart Fail. 2013;6:1190–1198. doi: 10.1161/CIRCHEARTFAILURE.113.000380. [DOI] [PubMed] [Google Scholar]
- 18.Goldenberg I., Kutyifa V., Klein H.U., et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370:1694–1701. doi: 10.1056/NEJMoa1401426. [DOI] [PubMed] [Google Scholar]
- 19.Wisnoskey B.J., Varma N. Left ventricular paced activation in cardiac resynchronization therapy patients with left bundle branch block and relationship to its electrical substrate. Heart Rhythm O2. 2020;1:85–95. doi: 10.1016/j.hroo.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauthey A., Willemen E., Lumens J., et al. Impact of paced left ventricular dyssynchrony on left ventricular reverse remodeling after cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2020;31:494–502. doi: 10.1111/jce.14330. [DOI] [PubMed] [Google Scholar]
- 21.Daubert C., Behar N., Martins R.P., Mabo P., Leclercq C. Avoiding non-responders to cardiac resynchronization therapy: a practical guide. Eur Heart J. 2017;38:1463–1472. doi: 10.1093/eurheartj/ehw270. [DOI] [PubMed] [Google Scholar]
- 22.Varma N., Baker J., Tomassoni G., et al. Left ventricular enlargement, cardiac resynchronization therapy efficacy, and impact of multipoint pacing. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.