Abstract
Primary ventricular fibrillation (PVF) may occur in the early phase of ST-elevation myocardial infarction (STEMI) prior to primary percutaneous coronary intervention (PCI). Multiple electrocardiographic STEMI patterns are associated with PVF and short-term mortality including the tombstone, Lambda, and triangular QRS-ST-T waveform (TW). We aimed to compare the predictive value of different electrocardiographic STEMI patterns for PVF and 30-day mortality. We included a consecutive cohort of 407 STEMI patients (75% males, median age 66 years) presenting within 12 h of symptoms onset. At first medical contact, 14 (3%) showed the TW or Lambda ECG patterns, which were combined in a single group (TW-Lambda pattern) characterized by giant R-wave and downsloping ST-segment. PVF prior to primary PCI occurred in 39 (10%) patients, significantly more often in patients with the TW-Lambda pattern than those without (50% vs. 8%, p < 0.001). For the multivariable analysis, Killip class ≥3 (OR 6.19, 95% CI 2.37–16.1, p < 0.001) and TW-Lambda pattern (OR 9.64, 95% CI 2.99–31.0, p < 0.001) remained as independent predictors of PVF. Thirty-day mortality was also higher in patients with the TW-Lambda pattern than in those without (43% vs. 6%, p < 0.001). However, only LVEF (OR 0.86, 95% CI 0.82–0.90, p < 0.001) and PVF (OR 4.61, 95% CI 1.49–14.3, p = 0.042) remained independent predictors of mortality. A mediation analysis showed that the effect of TW-Lambda pattern on mortality was mediated mainly via the reduced LVEF. In conclusion, among patients presenting with STEMI, the electrocardiographic TW-Lambda pattern was associated with both PVF before PCI and 30-day mortality. Therefore, this ECG pattern may be useful for early risk stratification of STEMI.
Keywords: acute myocardial infarction, electrocardiogram, ST-segment elevation, ventricular fibrillation
1. Introduction
Primary ventricular fibrillation (PVF) occurs commonly during the early phase of ST-elevation myocardial infarction (STEMI) before primary percutaneous coronary intervention (PCI), and has been associated with an increased short- [1] and long-term risk of death [2,3].
Several risk factors for PVF are known including clinical, hemodynamic, and electrocardiographic parameters, and their recognition is useful to optimize the early management of STEMI patients [4,5,6]. Besides its diagnostic value and the information regarding the culprit coronary vessel and extension of the ischemic area, the electrocardiogram (ECG) may identify subgroups of patients at higher risk of acute complications. In particular, multiple ECG patterns have been found to be associated with PVF [7,8,9,10,11]. Recently, we described the “triangular QRS-ST-T waveform” (TW), a distinctive STEMI pattern characterized by a single, giant, triangular wave, resulting from the fusion of the QRS complex, the ST-segment, and the T-wave, and we observed that patients presenting with the TW pattern more frequently had PVF, cardiogenic shock, and higher in-hospital mortality [10]. Similarly, the “lambda-like” pattern, characterized by an ST elevation resembling the Greek letter lambda, was described in patients with acute myocardial infarction (AMI) complicated with multiple episodes of polymorphic ventricular tachycardia and PVF [8]. These STEMI patterns have two features in common: the giant R wave (amplitude > 1 mV) and the steep downsloping of the ST segment, each of which have been described in association with PVF [9,11]. However, a specific quantitative risk analysis has never been performed.
The aim of the study was to assess the predictive value of different electrocardiographic patterns for the occurrence of PVF during the early phase of STEMI and for 30-day mortality.
2. Materials and Methods
In this observational single-center study, we enrolled all consecutive patients admitted to the Cardiology Unit of the University Hospital of Padua with a diagnosis of STEMI from January 2015 to July 2017. Data were collected from in-hospital and outpatient clinical evaluations and digital medical records of our hospital. The investigation was carried out following the rules of the Declaration of Helsinki of 1975; the ethics review board of our institution was notified of the study protocol. Given the retrospective and observational nature of the study, consent from the patients was not required.
Eligible patients were all STEMI patients with a readable 12-lead ECG at the time of the first medical contact (FMC). According to the 2012 European Society of Cardiology Guidelines [12], STEMI was diagnosed in the case of (1) symptoms consistent with myocardial ischemia; and (2) ST-segment elevation measured at the J point in at least two contiguous leads, ≥0.25 mV in men below the age of 40 years, ≥0.2 mV in men over the age of 40 years, or ≥0.15 mV in women in leads V2–V3 and/or ≥0.1 mV in other leads, in the absence of left ventricular hypertrophy or left bundle branch block, or ST-segment depression in lead V1–V3 confirmed by concomitant ST-segment elevation ≥0.05 mV recorded in leads V7–V9.
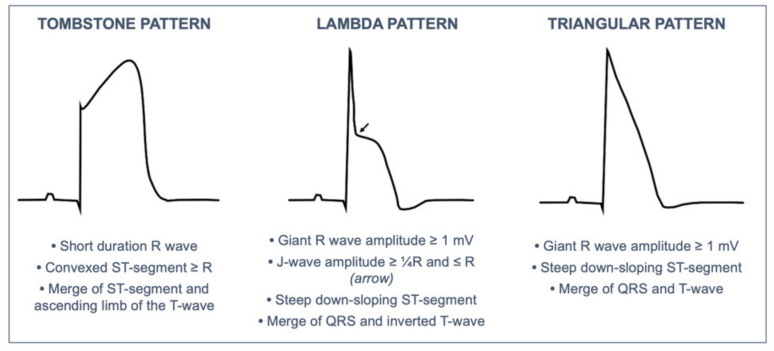
Data systematically collected included anamnestic data (especially those of special interest for cardiological risk stratification), Killip class on first medical contact, and ECG. The 12-lead ECGs (25 mm/s, 10 mm/mV, 0.05–150 Hz) were acquired in most cases in the pre-hospital setting by the emergency medical service for early identification of STEMI and were prospectively collected and analyzed by two investigators (M.G., G.M.V.); controversial cases were solved by consensus. The analysis focused on particular ECG patterns including the “tombstone”, “lambda”, and “TW”, which were defined according to previous publications [8,10,13]. Figure 1 provides a schematic representation of these STEMI ECG patterns. The TW and Lambda patterns were merged in a single group (TW-Lambda pattern) as proposed by Aizawa et al. [9]. Giant R wave was defined as R amplitude ≥1.0 mV.
Figure 1.
Definition of the electrocardiographic STEMI pattern analyzed in the study.
All patients underwent an echocardiography at admission to evaluate left ventricular ejection fraction (LVEF). The GRACE score on admission was calculated for all patients [14]. Two endpoints were considered: PVF (VF occurring before invasive coronary angiography and PCI) and mortality at 30 days.
Statistical analysis was performed using R-software Version 3.3.3 for Mac OS X (R Foundation for Statistical Computing, Vienna, Austria). As normality could not be assumed for any variables, continuous variables were presented as median (1st–3rd quartiles) and compared with non-parametric tests such as the Mann–Whitney test. Categorical variables were reported as number and percentage and compared using the χ2 test or Fisher’s exact test, as appropriate. Univariate logistic regression analysis was performed to investigate the relationship between the outcomes (PVF and 30-day mortality) and (1) variables that showed significant differences in medians or proportions between groups; and (2) other relevant variables based on previous study [4]. Among the significant variables at univariate analysis (p < 0.05), for each outcome, we chose to enter into the multivariate logistic regression model the ones that we considered more clinically relevant for the scope of the study. The number of variables that were chosen was limited in order to maintain a ≈1:10 co-variates to outcome ratio to avoid the risk of overfitting. We then used a stepwise selection approach based on Akaike information criterion (AIC) to improve the model’s performance. A mediation analysis (Causal Mediation Analysis, package ‘mediation’ version 4.5.0, R-software Version 3.3.3, Vienna, Austria) was performed, to quantify the extent to which the PVF and LVEF ejection fraction participated in the relationship between the TW-Lambda pattern and 30-day mortality. We searched for the significance of the indirect effect of the independent variable on the dependent one using bootstrapping procedures. Unstandardized indirect effects were computed for each of 1000 bootstrapped samples, and the 95% confidence interval was computed by determining the indirect effects at the 2.5th and 97.5th percentiles. A two-sided p value of <0.05 was considered indicative of statistical significance.
3. Results
The study population included 407 patients (307 males, 75%) with a median age of 66 (56–75) years. The ECG of the first medical contact was recorded after a median of 169 (41–200) minutes from symptom onset, showing ST-segment elevation in anterior leads V1–V4 in 189 (46%) and in inferior leads II/aVF/III in 117 (29%). Left main coronary artery was the culprit vessel in 21 (5%) patients and left ventricular descending artery in 197 (48%). Primary PCI was performed in 346 (85%). Considering ECG STEMI patterns, 45 (11%) patients showed the tombstone pattern and 14 (3%) the TW-Lambda pattern. Giant R wave was observed in 125 (31%). Baseline characteristics, risk factors, clinical, and electrocardiographic features are depicted in Table 1.
Table 1.
Clinical and electrocardiographic features in the study population. Values are expressed as number of patients (%) or median [25 and 75% percentiles].
| Study Population (n = 407) | |
|---|---|
| Male sex | 307 (75) |
| Age, years | 66 (56–75) |
| FMC to PCI, min | 101 (55–120) |
| Primary PCI | 346 (85) |
| Medical history | |
| Arterial hypertension | 261 (64) |
| Dyslipidemia | 176 (43) |
| Diabetes mellitus | 75 (18) |
| Familiar CAD | 153 (38) |
| Smoke | 139 (34) |
| Previous AMI | 35 (9) |
| CKD | 36 (9) |
| Severe COPD | 13 (3) |
| ECG at first medical contact | |
| Anterior STEMI (V1–V4) | 189 (46) |
| Anterior (V1–V4) and lateral (V5- V5–V6, I, aVL V6, I, aVL) | 23 (6) |
| Lateral (V5–V6, I, aVL) | 14 (3) |
| Inferior (II/aVF/III) | 117 (29) |
| Inferior (II/aVF/III) and lateral (V5–V6, I, aVL) | 59 (14) |
| Posterior | 5 (1) |
| Tombstone pattern | 45 (11) |
| Triangular-Lambda pattern | 14 (3) |
| R-wave ≥ 1.0 mV | 125 (31) |
| Culprit coronary vessel | |
| Left main | 21 (5) |
| LAD | 197 (48) |
| Left circumflex | 52 (13) |
| Ramus intermedius | 3 (1) |
| Right coronary | 134 (33) |
| Clinical variables | |
| LVEF, % | 47 (40–55) |
| GFR, mL/min/1.73 m2 | 75 (61–90) |
| Killip class ≥ III | 24 (6) |
| Peak Troponin I, ug/L | 70 (26–150) |
| Events | |
| PVF | 39 (10) |
| 30-day mortality | 28 (7) |
AMI = acute myocardial infarction; CAD = coronary artery disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; FMC = first medical contact; GFR = glomerular filtration rate; LAD = left anterior descending; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention; PVF = primary ventricular fibrillation.
Primary ventricular fibrillation prior to PCI occurred in 39 (10%) patients. Table 2 shows the distribution of anamnestic, clinical, and ECG features among patients with or without PVF. PVF prior to primary PCI occurred in 39 (10%) patients, significantly more often in patients with the TW-Lambda pattern than those without (50% vs. 8%, p < 0.001). Of note, patients with the TW-Lambda pattern also showed a lower LVEF at admission (median 32%) than those without (48%, p < 0.001).
Table 2.
Distribution of anamnestic, clinical, and electrocardiographic features among the groups with and without PVF.
| No PVF n = 368 (90%) |
PVF n = 39 (10%) |
p Value | |
|---|---|---|---|
| Anamnestic and clinical variables | |||
| Age, years | 65.4 (52.1, 78.5) | 61.2 (50.2, 73.3) | 0.033 * |
| Sex, male | 273 (74.2) | 34 (87.2) | 0.073 |
| Arterial hypertension | 236 (64.1) | 25 (64.1) | 0.563 |
| Dyslipidemia | 157 (42.7) | 17 (43.6) | 0.288 |
| Diabetes mellitus | 68 (18.5) | 7 (17.9) | 0.569 |
| Familiar CAD | 141 (38.3) | 12 (30.8) | 0.228 |
| Smoking | 122 (33.2) | 17 (43.6) | 0.130 |
| Severe COPD | 12 (3.3) | 1 (2.6) | 0.641 |
| CKD | 33 (9.0) | 3 (7.7) | 0.539 |
| Previous AMI | 30 (8.2) | 5 (12.8) | 0.234 |
| Killip class ≥3 | 15 (4.1) | 9 (23.1) | <0.001 * |
| ECG variables | |||
| New onset AF | 24 (6.5) | 5 (12.8) | 0.132 |
| Advanced AV block | 11 (3.0) | 1 (2.6) | 0.678 |
| Anterior STEMI | 163 (44.3) | 25 (64.1) | 0.014 * |
| Lateral STEMI | 21 (5.7) | 2 (5.1) | 0.238 |
| Inferior STEMI | 108 (29.3) | 9 (23.1) | 0.267 |
| Posterior STEMI | 5 (1.4) | 0 (0) | - |
| Tombstone pattern | 38 (10.3) | 7 (17.9) | 0.175 |
| Triangular-Lambda pattern | 7 (1.9) | 7 (17.9) | <0.001 * |
| Giant R wave | 107 (29.1) | 18 (46.2) | 0.024 * |
* means p < 0.050. Values are expressed as number of patients (%) or median [25 and 75% percentiles]. AF = atrial fibrillation; AMI = acute myocardial infarction; AV = atrio-ventricular block; CAD = coronary artery disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; ECG = electrocardiography; FMC = first medical contact; LAD = left anterior descending; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention; STEMI = ST-elevated myocardial infarction; PVF = primary ventricular fibrillation.
Univariable logistic regression analysis showed that age, Killip class ≥3, anterior STEMI, giant R wave, and TW-Lambda pattern emerged as significant risk factors for PVF (Table 3).
Table 3.
Predictors of primary ventricular fibrillation at logistic regression analysis.
| Primary Ventricular Fibrillation |
Univariate Logistic Regression |
Multivariate Logistic Regression |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 0.74 | 0.57, 0.97 | 0.030 | |||
| Arterial hypertension | 0.99 | 0.50, 1.99 | 0.997 | |||
| Diabetes Mellitus | 0.76 | 0.41, 2.28 | 0.935 | |||
| Killip class ≥ 3 | 7.06 | 2.85, 17.50 | <0.001 | 6.19 | 2.37, 16.1 | 0.035 |
| Anterior STEMI | 2.26 | 1.15, 4.59 | 0.021 | |||
| Triangular-Lambda wave | 11.3 | 3.72, 34.2 | <0.001 | 9.64 | 2.99, 31.0 | 0.027 |
| R-wave ≥ 1.0 mV | 2.09 | 1.06, 4.08 | 0.030 | |||
STEMI = ST-segment elevation myocardial infarction.
Stepwise multivariate logistic regression analysis included four relevant variables, in other words, three ECG features (TW-Lambda pattern, giant R waves, anterior STEMI) and hemodynamic status (Killip class), Killip class ≥3 (OR 6.19, 95% CI 2.37, 16.1, p < 0.001), and TW-Lambda pattern (OR 9.64, 95% CI 2.99, 31.0, p < 0.001) remained independent predictors of PVF (Table 3).
At 30-day follow up, we recorded 28 deaths (7%). Table 4 shows the distribution of different variables in patients who died at 30 days. Mortality was significantly higher in patients with the TW-Lambda pattern than in those without (43% vs. 6%, p < 0.001). An increased risk of death was also identified for PVF, EF during hospitalization, GFR, arterial hypertension, Grace score, Killip class ≥3.
Table 4.
Distribution of anamnestic, clinical, and instrumental variables among the groups with and without death at 30 days. * means p < 0.050. Values are expressed as number of patients (%) or median [25 and 75% percentiles].
| Alive n = 379 (93%) |
Dead n = 28 (7%) |
p Value | |
|---|---|---|---|
| Anamnestic variables | |||
| Age, years | 64.6 (51.4, 79.1) | 70.1 (56.7, 83.2) | 0.043 * |
| Male sex | 284 (74.9) | 23 (82.1) | 0.273 |
| Arterial hypertension | 238 (62.8) | 23 (82.1) | 0.028 * |
| Dyslipidemia | 168 (44.3) | 8 (28.6) | 0.075 |
| Diabetes mellitus | 66 (17.4) | 9 (32.1) | 0.073 |
| Familiar CAD | 148 (39.1) | 5 (17.9) | 0.180 |
| Smoking | 129 (34.0) | 10 (35.7) | 0.503 |
| Previous AMI | 34 (9.0) | 1 (3.6) | 0.283 |
| CKD | 32 (8.4) | 4 (14.3) | 0.227 |
| Severe COPD | 10 (2.6) | 3 (10.7) | 0.052 |
| Clinical and instrumental variables | |||
| Killip class ≥3 | 15 (4.0) | 9 (32.1) | <0.001 * |
| Grace score | 140 (18, 210) | 191 (60, 250) | <0.001 * |
| GFR mL/min/1.73 m2 | 81 (62, 90) | 45 (36, 75) | <0.001 * |
| Peak Troponin I, ug/L | 67.2 (1, 579) | 115 (22, 400) | 0.005 * |
| Delayed FMC-PCI > 120 min | 39 (10.3) | 5 (17.9) | 0.283 |
| Culprit lesion | |||
| Left main | 16 (4.7) | 4 (22.2) | 0.013 * |
| LAD | 181 (52.6) | 11 (61.1) | 0.324 |
| Left Circumflex | 45 (13.1) | 5 (27.8) | 0.086 |
| RCA | 127 (36.9) | 3 (16.7) | 0.063 |
| Ramus Intermedius | 2 (0.6) | 1 (5.6) | 0.142 |
| EF (%) | 49 (20, 71) | 26.5 (9, 54) | <0.001 * |
| Tombstone pattern | 40 (10.6) | 5 (17.9) | 0.218 |
| Triangular-Lambda pattern | 8 (2.1) | 6 (21.4) | <0.001 * |
| Giant R wave | 114 (30.1) | 11 (39.3) | 0.208 |
| PVF | 31 (8.2) | 8 (28.6) | 0.003 * |
| AF | 24 (6.3) | 5 (17.9) | 0.054 |
| Advanced AV block | 10 (2.6) | 2 (7.1) | 0.197 |
AF = atrial fibrillation; AMI = acute myocardial infarction; AV = atrio-ventricular block; CAD = coronary artery disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; ECG = electrocardiography; FMC = first medical contact; GFR = glomerular filtration rate; LAD = left anterior descending; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention; STEMI = ST-elevated myocardial infarction; PVF = primary ventricular fibrillation.
Univariable logistic regression analysis showed that age, arterial hypertension, Grace score, Killip class ≥3, GFR, LVEF, peak Troponin I, PVF, and TW-Lambda pattern emerged as significant risk factors for 30-day death (Table 5).
Table 5.
Predictors of 30-day mortality at logistic regression analysis.
| 30 Days Death | Univariate Logistic Regression |
Multivariate Logistic Regression |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 1.03 | 1.00, 1.07 | 0.036 * | |||
| Arterial hypertension | 2.73 | 1.01, 7.33 | 0.047 * | |||
| Grace score | 1.03 | 1.02, 1.04 | <0.001 * | |||
| Killip class ≥3 | 11.5 | 4.46, 29.6 | <0.001 * | |||
| GFR | 0.96 | 0.94, 0.97 | <0.001 * | |||
| LVEF | 0.86 | 0.82, 0.90 | <0.001 * | 0.86 | 0.82, 0.90 | <0.001 * |
| Peak Troponin I | 1.02 | 1.01, 1.08 | 0.012 * | |||
| PVF | 4.49 | 1.63, 11.0 | 0.001 * | 4.61 | 1.49, 14.3 | 0.042 * |
| Triangular/Lambda pattern | 12.6 | 4.03, 39.6 | <0.001 * | |||
* means p < 0.050. GFR = glomerular filtration rate (mL/min/1.73 m2); LVEF = left ventricular ejection fraction; PVF = primary ventricular fibrillation.
After performing multivariate stepwise logistic regression analysis including the three most clinically (and rapidly assessable at patient’s admission) relevant factors (such as LVEF, Killip class, PVF, and TW-Lambda pattern), only LVEF (OR 0.86, 95% CI 0.82, 0.90, p < 0.001) and PVF (OR 4.61, 95% CI 1.49, 14.3, p = 0.042) were maintained in the regression model (Table 5).
Thus, a mediation analysis was performed to analyze the possible mediation effect of these latter variables in determining univariate significance of the ECG pattern on 30-day mortality (Table 6 and Table 7). The analysis confirmed that the effect of TW-Lambda pattern on 30-day mortality was mediated via the presence of reduced LVEF, but not of PVF. Indeed, the bootstrapped unstandardized indirect effect (i.e., the effect of TW-Lambda on 30-day mortality going through the considered mediators) was tested to be significant only when considering LVEF as a mediator (estimate 0.235 95% CI 0.113, 0.350, <0.001), though a trend toward significance was also found when considering PVF as the mediator (estimate 0.063, 95% CI −0.013, 0.140, p = 0.108).
Table 6.
Mediation analysis exploring the effect of triangular/lambda pattern on 30-day mortality mediated by primary ventricular fibrillation.
| Effect | Estimate | 95% CI | p | % Mediation |
|---|---|---|---|---|
| Indirect | 0.063 | −0.013, 0.140 | 0.108 | 17.0 |
| Direct | 0.305 | 0.058, 0.590 | 0.016 * | 83.0 |
| Total | 0.369 | 0.088, 0.650 | 0.008 * | 100.0 |
* means p < 0.050.
Table 7.
Mediation analysis exploring the effect of triangular/lambda pattern on 30-day mortality mediated by left ventricular function.
| Effect | Estimate | 95% CI | p | % Mediation |
|---|---|---|---|---|
| Indirect | 0.235 | 0.113, 0.350 | <0.001 | 62.7 |
| Direct | 0.139 | −0.039, 0.360 | 0.112 | 37.3 |
| Total | 0.374 | 0.114, 0.640 | 0.008 * | 100.0 |
* means p < 0.050.
4. Discussion
In the present study, we investigated the predictive value of specific electrocardiographic STEMI patterns—the tombstone, Lambda, and TW (Figure 1)—for the occurrence of PVF during the early phase of STEMI and for 30-day mortality. The main results of the study were: (1) ECG detection of a Lambda or TW STEMI pattern was a strong and independent predictor of PVF before primary PCI; (2) significant predictors of 30-day mortality were the occurrence of PVF, and lower LVEF; and (3) the effect of Lambda or TW STEMI pattern on 30-mortality was significantly mediated by a lower LVEF.
The ECG has a central role in the early management of AMI as it helps in providing diagnosis and guiding the appropriate therapy [15]. Additionally, the ECG can offer the potential to provide helpful information about the prognosis of AMI patients, in terms of arrhythmic or hemodynamic complications and mortality. The tombstone, Lambda, and TW STEMI patterns represent severe QRS-ST-T deformations that have been previously associated with PVF and poor outcome [9,10,12]. However, most of these observations are anecdotal and their predictive value for PVF and mortality has never been analyzed. Aizawa et al. classified the ECG of STEMI patients into three main types and found that the “Type 1” (namely the TW-Lambda pattern), defined as a QRS-ST-T pattern characterized by a downsloping J-ST segment toward T waves immediately after the R wave, without a flat or rising portion, was highly associated with PVF [11] (Figure 2). In keeping with this, our study demonstrated that the TW-Lambda pattern is an independent predictor of PVF, conferring to the STEMI patients a 6-fold increase in risk of PVF before primary PCI. The steep downsloping of the J-ST-segment appears to be the common denominator of these high-risk patterns and may be the expression of the diffuse dispersion of excitability, conduction, and refractoriness occurring in the myocardium during a severe and extensive ischemia, predisposing to PVF triggered by R-on-T phenomena (phase-2 re-entry) such as those observed in Brugada syndrome [16,17].
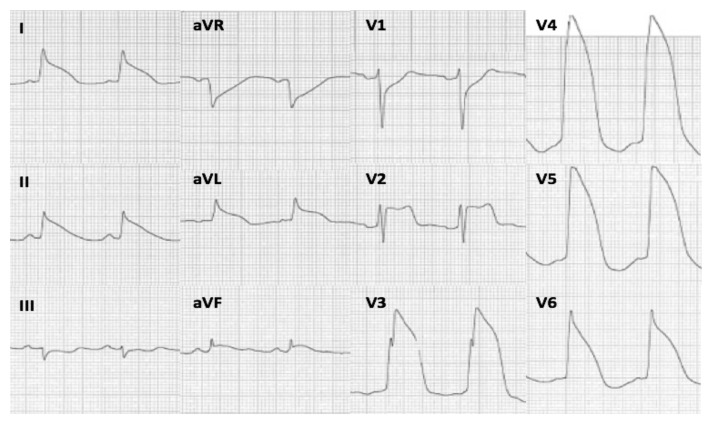
Figure 2.
Electrocardiogram of a 68-year-old male patient who presented to the emergency department for chest pain. A triangular QRS-ST-T waveform was appreciable on anterolateral and inferior leads. Shortly after the ECG recording, the patient had PVF, requiring urgent defibrillation. Coronary angiography showed an occlusion of the proximal left anterior descending coronary artery.
Other significant predictors of PVF were the younger age, a Killip class ≥3, the giant R-wave, and the anterior AMI. A trend toward an inverse relationship between age and PVF was also observed in a recent paper [18] and in a previous metanalysis [19], and may be related to the fact that younger STEMI patients more often suffer an acute coronary artery occlusion without pre-conditioning or collateral flow. The anterior location of AMI has also been found to be a major risk factor of PVF in previous investigations [20,21]. This finding may confirm that the ischemia of the anterior wall has a greater propensity for PVF, being more densely innervated by cardiac sympathetic fibers, which in the presence of ischemia may lead to a greater release of catecholamines and trigger ventricular arrhythmias [22,23].
In the pre-PCI era, the tombstone pattern, as its name suggests, was a marker of severe ischemia and extensive cardiac damage and was associated with fatal arrhythmias and poor outcome [7,13]. However, our data showed that the tombstone pattern was neither associated with PVF nor with higher mortality. This may be due to the devolvement of the primary PCI network, which enables aa reduction in reperfusion time, thereby reducing the occurrence of electrical and mechanical complications and improving outcome [24].
In our study, PVF before primary PCI occurred in 10% of STEMI patients, a prevalence consistent with recent reports [20,25]. Likewise, the 30-day mortality for STEMI in our hospital (28/407, 7%) was comparable to that of another region in Italy [26] and within the range of those reported for the U.S. and European populations [27,28,29,30]. The second endpoint of the study, 30-day mortality, was associated with the TW-Lambda pattern as well as PVF and lower LVEF, a finding in keeping with previous reports [31]. However, only PVF and lower LVEF, but not the TW-Lambda ECG pattern, remained independent predictors of death in the multivariable analysis. This is not surprising considering that the TW-Lambda is not a physiopathological mechanism directly linked to increased mortality, but rather an epiphenomenon of increased electrical instability (higher risk of PVF, as discussed above) and higher amount of ischemic myocardium (hence worse LVEF at admission). The fact that the occurrence of the TW-Lambda pattern often reflects an acute occlusion of a proximal coronary artery justifies why these patients typically show a severe impairment of LV systolic function [10]. Interestingly, the indirect effect of the TW-Lambda ECG pattern on mortality was predominantly mediated by reduced LVEF.
According to these findings, the observation of a TW-Lambda pattern should influence the management of STEMI patients, not only suggesting the need for a stricter pre-PCI monitoring, but also of a more aggressive management, for example, referring the patient to a hospital able to deliver a high-intensity level of care (e.g., capability of ventricular assist device implantation).
The limitations of the study include its observational nature and the fact that patients who died outside of hospitals were not included in the analysis; thus, our results may not be generalizable to all STEMI patients.
In conclusion, this study showed that STEMI patients presenting with the TW-Lambda pattern have a higher risk of PVF and 30-day mortality. These results have clinical implications in improving the early arrhythmic risk stratification of STEMI patients and in planning an adequate therapeutic strategy.
Author Contributions
Conceptualization, All Authors; Methodology, A.C., D.C. and A.Z.; Validation, A.Z., F.C. and L.C.; Formal analysis, A.C.; Writing, A.C., G.B., G.D. and A.Z.; Writing—review and editing, N.M., G.B., F.C., G.M.V. and F.D.; Visualization, G.D., S.I., M.G. and M.P.M.; Supervision, A.C., D.C. and A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The ethics review board of our institution was notified of the study protocol on 1 March 2015, procedure needed by our Institution for retrospective and observational studies.
Informed Consent Statement
Patient consent was waived for the retrospective and observational nature of the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alahmar A.E., Nelson C.P., Snell K.I., Yuyun M.F., Musameh M.D., Timmis A., Birkhead J.S., Chugh S.S., Thompson J.R., Squire I.B., et al. Resuscitated cardiac arrest and prognosis following myocardial infarction. Heart. 2014;100:1125–1132. doi: 10.1136/heartjnl-2014-305696. [DOI] [PubMed] [Google Scholar]
- 2.Kosmidou I., Embacher M., McAndrew T., Dizon J.M., Mehran R., Ben-Yehuda O., Mintz G.S., Stone G.W. Early Ventricular Tachycardia or Fibrillation in Patients with ST Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention and Impact on Mortality and Stent Thrombosis (from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction Trial) Am. J. Cardiol. 2017;120:1755–1760. doi: 10.1016/j.amjcard.2017.07.080. [DOI] [PubMed] [Google Scholar]
- 3.Mehta R.H., Starr A.Z., Lopes R.D., Hochman J.S., Widimsky P., Pieper K.S., Armstrong P.W., Granger C.B. APEX AMI Investigators. Incidence of and outcomes associated with ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention. JAMA. 2009;301:1779–1789. doi: 10.1001/jama.2009.600. [DOI] [PubMed] [Google Scholar]
- 4.Lawrie D.M., Higgins M.R., Godman M.J., Oliver M.F., Julian D.G., Donald K.W. Ventricular fibrillation complicating acute myocardial infarction. Lancet. 1968;2:523–528. doi: 10.1016/S0140-6736(68)92403-3. [DOI] [PubMed] [Google Scholar]
- 5.Volpi A., Cavalli A., Santoro L., Negri E. Incidence and prognosis of early primary ventricular fibrillation in acute myocardial infarction—Results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI-2) database. Am. J. Cardiol. 1998;82:265–271. doi: 10.1016/S0002-9149(98)00336-1. [DOI] [PubMed] [Google Scholar]
- 6.Thompson C.A., Yarzebski J., Goldberg R.J., Lessard D., Gore J.M., Dalen J.E. Changes over time in the incidence and case-fatality rates of primary ventricular fibrillation complicating acute myocardial infarction: Perspectives from the Worcester Heart Attack Study. Am. Heart J. 2000;139:1014–1021. doi: 10.1067/mhj.2000.106160. [DOI] [PubMed] [Google Scholar]
- 7.Balci B., Yesildag O. Correlation between clinical findings and the tombstoning electrocardiographic pattern in patients with anterior wall acute myocardial infarction. Am. J. Cardiol. 2003;92:1316–1318. doi: 10.1016/j.amjcard.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Kukla P., Jastrzebski M., Sacha J., Bryniarski L. Segment elevation in acute myocardial infarction: A new risk marker for ventricular fibrillation? Three case reports. Kardiol. Polska. 2008;66:873–877. [PubMed] [Google Scholar]
- 9.Aizawa Y., Jastrzebski M., Ozawa T., Kawecka-Jaszcz K., Kukla P., Mitsuma W., Chinushi M., Ida T., Aizawa Y., Ojima K., et al. Characteristics of electrocardiographic repolarization in acute myocardial infarction complicated by ventricular fibrillation. J. Electrocardiol. 2012;45:252–259. doi: 10.1016/j.jelectrocard.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Cipriani A., D’Amico G., Brunello G., Perazzolo Marra M., Migliore F., Cacciavillani L., Tarantini G., Bauce B., Iliceto S., Corrado D., et al. The electrocardiographic “triangular QRS-ST-T waveform” pattern in patients with ST-segment elevation myocardial infarction: Incidence, pathophysiology and clinical implications. J. Electrocardiol. 2018;51:8–14. doi: 10.1016/j.jelectrocard.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Madias J.E., Krikelis E.N. Transient giant R waves in the early phase of acute myocardial infarction: Association with ventricular fibrillation. Clin. Cardiol. 1981;4:339–349. doi: 10.1002/clc.4960040606. [DOI] [PubMed] [Google Scholar]
- 12.Steg P.G., James S.K., Atar D., Badano L.P., Blömstrom-Lundqvist C., Borger M.A., Di Mario C., Dickstein K., Ducrocq G., Fernandez-Aviles F., et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012;33:2569–2619. doi: 10.1016/j.rec.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Wimalaratna H.S.K. “Tombstoning” of ST segment in acute myocardial infarction (letter) Lancet. 1993;342:496. doi: 10.1016/0140-6736(93)91622-S. [DOI] [PubMed] [Google Scholar]
- 14.Eagle K.A., Lim M.J., Dabbous O.H., Pieper K.S., Goldberg R.J., Van de Werf F., Goodman S.G., Granger C.B., Steg P.G., Gore J.M., et al. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 15.Zimetbaum P.J., Josephson M.E. Use of the electrocardiogram in acute myocardial infarction. N. Engl. J. Med. 2003;348:933–940. doi: 10.1056/NEJMra022700. [DOI] [PubMed] [Google Scholar]
- 16.Yan G.X., Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.CIR.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 17.Fish J.M., Antzelevitch C. Brugada syndrome and ischemia-induced ST-segment elevation. Similarities and differences. J. Electrocardiol. 2005;38:14–17. doi: 10.1016/j.jelectrocard.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosugi S., Shinouchi K., Ueda Y., Abe H., Sogabe T., Ishida K., Mishima T., Ozaki T., Takayasu K., Iida Y., et al. Clinical and Angiographic Features of Patients with Out-of-Hospital Cardiac Arrest and Acute Myocardial Infarction. J. Am. Coll Cardiol. 2020;76:1934–1943. doi: 10.1016/j.jacc.2020.08.057. [DOI] [PubMed] [Google Scholar]
- 19.Gheeraert P.J., De Buyzere M.L., Taeymans Y.M., Gillebert T.C., Henriques J.P., De Backer G., De Bacquer D. Risk factors for primary ventricular fibrillation during acute myocardial infarction: A systematic review and meta-analysis. Eur. Heart J. 2006;27:2499–2510. doi: 10.1093/eurheartj/ehl218. [DOI] [PubMed] [Google Scholar]
- 20.Jabbari R., Engstrøm T., Glinge C., Risgaard B., Jabbari J., Winkel B.G., Terkelsen C.J., Tilsted H.H., Jensen L.O., Hougaard M., et al. Incidence and risk factors of ventricular fibrillation before primary angioplasty in patients with first ST-elevation myocardial infarction: A nationwide study in Denmark. J. Am. Heart Assoc. 2015;4:e001399. doi: 10.1161/JAHA.114.001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gheeraert P.J., Henriques J.P., De Buyzere M.L., Voet J., Calle P., Taeymans Y., Zijlstra F. Out-of-hospital ventricular fibrillation in patients with acute myocardial infarction: Coronary angiographic determinants. J. Am. Coll. Cardiol. 2000;35:144–150. doi: 10.1016/S0735-1097(99)00490-8. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz P.J. Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat. Rev. Cardiol. 2014;11:346–353. doi: 10.1038/nrcardio.2014.19. [DOI] [PubMed] [Google Scholar]
- 23.Janse M.J., Schwartz P.J., Wilms-Schopman F., Peters R.J., Durrer D. Effects of unilateral stellate ganglion stimulation and ablation on electrophysiologic changes induced by acute myocardial ischemia in dogs. Circulation. 1985;7:585–595. doi: 10.1161/01.CIR.72.3.585. [DOI] [PubMed] [Google Scholar]
- 24.Saia F., Marrozzini C., Ortolani P., Palmerini T., Guastaroba P., Cortesi P., Pavesi P.C., Gordini G., Pancaldi L.G., Taglieri N., et al. Optimisation of therapeutic strategies for ST-segment elevation acute myocardial infarction: The impact of a territorial network on reperfusion therapy and mortality. Heart. 2009;95:370–376. doi: 10.1136/hrt.2008.146738. [DOI] [PubMed] [Google Scholar]
- 25.Ravn Jacobsen M., Jabbari R., Glinge C., Kjær Stampe N., Butt J.H., Blanche P., Lønborg J., Wendelboe Nielsen O., Køber L., Torp-Pedersen C., et al. Potassium Disturbances and Risk of Ventricular Fibrillation Among Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020;9:e014160. doi: 10.1161/JAHA.119.014160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seghieri C., Mimmi S., Lenzi J., Fantini M.P. 30-day in-hospital mortality after acute myocardial infarction in Tuscany (Italy): An observational study using hospital discharge data. BMC Med. Res. Methodol. 2012;12:170. doi: 10.1186/1471-2288-12-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh R.W., Sidney S., Chandra M., Sorel M., Selby J.V., Go A.S. Population trends in the incidence and outcomes of acute myocardial infarction. N. Engl. J. Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 28.Krumholz H.M., Wang Y., Chen J., Drye E.E., Spertus J.A., Ross J.S., Curtis J.P., Nallamothu B.K., Lichtman J.H., Havranek E.P., et al. Reduction in acute myocardial infarction mortality in the United States: Risk-standardized mortality rates from 1995–2006. JAMA. 2009;302:767–773. doi: 10.1001/jama.2009.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jernberg T., Johanson P., Held C., Svennblad B., Lindbäck J., Wallentin L., SWEDEHEART/RIKS-HIA Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA. 2011;305:1677–1684. doi: 10.1001/jama.2011.522. [DOI] [PubMed] [Google Scholar]
- 30.Gale C.P., Cattle B.A., Moore J., Dawe H., Greenwood D.C., West R.M. Impact of missing data on standardised mortality ratios for acute myocardial infarction: Evidence from the Myocardial Ischaemia National Audit Project (MINAP) 2004–2007. Heart. 2011;97:1926–1931. doi: 10.1136/hrt.2010.204883. [DOI] [PubMed] [Google Scholar]
- 31.Ng V.G., Lansky A.J., Meller S., Witzenbichler B., Guagliumi G., Peruga J.Z., Brodie B., Shah R., Mehran R., Stone G.W. The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction: The Horizons-AMI trial. Eur. Heart J. Acute Cardiovasc. Care. 2014;3:67–77. doi: 10.1177/2048872613507149. [DOI] [PMC free article] [PubMed] [Google Scholar]