Abstract
Curcumin, a yellow-colored molecule derived from the rhizome of Curcuma longa, has been identified as the bioactive compound responsible for numerous pharmacological activities of turmeric, including anticancer, antimicrobial, anti-inflammatory, antioxidant, antidiabetic, etc. Nevertheless, the clinical application of curcumin is inadequate due to its low solubility, poor absorption, rapid metabolism and elimination. Advancements in recent research have shown several components and techniques to increase the bioavailability of curcumin. Combining with adjuvants, encapsulating in carriers and formulating in nanoforms, in combination with other bioactive agents, synthetic derivatives and structural analogs of curcumin, have shown increased efficiency and bioavailability, thereby augmenting the range of applications of curcumin. The scope for incorporating biotechnology and nanotechnology in amending the current drawbacks would help in expanding the biomedical applications and clinical efficacy of curcumin. Therefore, in this review, we provide a comprehensive overview of the plethora of therapeutic potentials of curcumin, their drawbacks in efficient clinical applications and the recent advancements in improving curcumin’s bioavailability for effective use in various biomedical applications.
Keywords: curcumin, bioavailability, antibiotics, anticancer agents, nanomedicine, pharmaceutical formulations
1. Introduction
Bioactive compounds with therapeutic value are found in a huge spectrum of natural resources. Among them, phytochemicals from plants have gained greater interest for mankind ever since ancient times. Various research groups have analyzed diverse groups of plant-based bioactive compounds to discover their pharmaceutical efficiency in treating various kinds of human illness [1]. In traditional Indian and Chinese medications, the turmeric plant, Curcuma longa Linn., has been widely used, owing to its therapeutic magnitude [2]. One of the major bioactive components of the rhizome extract of turmeric is curcumin, a yellow-colored hydrophobic polyphenol. Chemically, curcumin is known as 1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione or, precisely, diferuloylmethane [3]. Aroma and color are the primary characteristics of curcumin in the diet. Though no nutraceutical value was found, numerous research articles have shown the therapeutic potential of curcumin, which includes but is not limited to being antioxidant, anticarcinogenic, antimicrobial, anti-inflammatory, hypoglycemic, hepato- and neuroprotective [4,5,6], etc. Due to its broad spectrum of therapeutic efficiencies, researchers are engrossed in unearthing the molecular targets and their encoding mechanisms of action of curcumin that underlie these biological activities. The nontoxic nature of curcumin makes it an ideal therapeutic agent, yet the limited bioavailability is a major concern in clinical application [7]. Due to its poor absorption, rapid metabolism and rapid elimination, the concentration of curcumin available in plasma and target tissue becomes low. In recent times, several studies have devised methods to increase the bioavailability of curcumin. Curcumin complex with the following adjuvants, such as nanoparticles, micelles, phospholipid complexes, liposomes, etc., have been used to increase the bioavailability. For instance, piperine, a major alkaloid from black pepper, is known to enhance the bioavailability of curcumin by 2000% [5]. Advanced research on adjuvants has shown enhancements in bioavailability which, in the near future, could bring promising clinical outcomes for curcumin treatment. Encapsulation of curcumin in nanostructures has shown enhanced therapeutic efficiency by extending the half-life of the drug, enhancing the solubility and sustained release of the drug [8]. Curcumin encapsulation into carriers such as liposomes, micelles, polymeric nanoparticles, mesoporous silica nanoparticles or nanoformulations has been used for a variety of applications, such as cancer therapy [9], wound healing application [10], diabetic complications [11], antimicrobial agents [12], neuroprotection [13] and so on. This review provides a comprehensive overview of the structure and the recent advancements in the biomedical applications of curcumin, use of nanotechnology to produce nanoencapsulated curcumin, significance of curcumin bioavailability and current trends in improving bioavailability.
2. Biosynthesis Pathway and Structure of Curcumin
Curcumin (diferuloylmethane) (C21H20O6), an orange-yellow component of turmeric, is a polyphenol natural product extracted from C. longa. Significantly, this natural polyphenol is commonly called the “wonder drug of life” [14]. Curcumin has been used in various forms for centuries around the world for a variety of potential health benefits, as well as a food-coloring agent [15]. The chemical composition of curcumin has been well known since the eighteenth century. According to the current IUPAC rules, the name of curcumin is 1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione [3,16]. The molecular formula and weight of the curcumin are C21H20O6 and 368.38 g/mol and other physicochemical properties of curcumin, such as lipophilicity (ADME: cLogP, cLogS), pharmacokinetics (gastrointestinal absorption, blood–brain barrier (BBB)) and druglikeness (bioavailability), are given in Table 1 [17]. The - curcumin has three different chemical substances, such as two aromatic ring systems: o-methoxy phenolic groups, linked by a seven-carbon connector comprising an α, β-unsaturated β-diketone moiety [18]. In general, curcumin and its chemical structure make it less soluble in water at both acidic and neutral pH. On the other hand, curcumin is soluble in alkali, acetic acid, acetone, chloroform, DMSO, ethanol and ketone [19].
Table 1.
Physicochemical properties of curcumin.
| Formula | C21H20O6 |
| Molecular weight | 368.38 g/mol |
| Num. heavy atoms | 27 |
| Num. arom. heavy atoms | 12 |
| Fraction Csp3 | 0.14 |
| Num. rotatable bonds | 8 |
| Num. H-bond acceptors | 6 |
| Num. H-bond donors | 2 |
| Molar refractivity | 102.80 |
| Topological polar surface area | 93.06 Å2 |
| Nviolation | 0 |
| Melting temperature | 176–177 °C |
| cLogP | 3.2 |
| cLogS | −3.94 |
| Bioavailability score | 0.55 |
| Gastrointestinal absorption | High |
| Blood–brain barrier (BBB) permeant | No |
The turmeric rhizome contains a complex of curcuminoids, and it has various methoxy groups: bisdeshydroxybisdesmethoxycurcumin, bisdemethoxycurcumin and demethoxycurcumin, and curcumins are significantly responsible for several biological and pharmacological activities that are significant in treating many complex disorders/diseases.
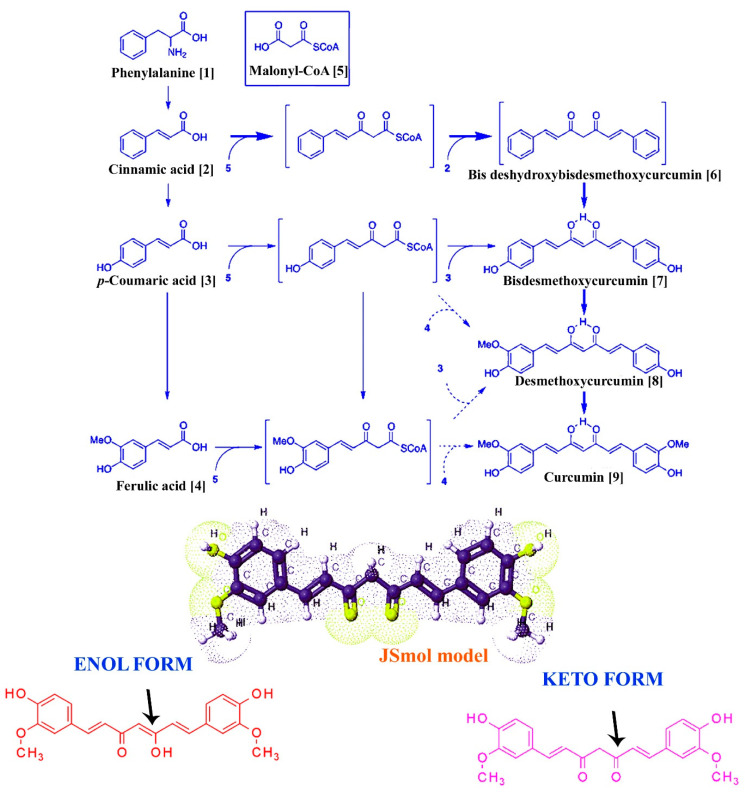
In particular, curcuminoids are synthesized by type III polyketide synthases (PK-S) in plants (Figure 1) and contain two phenylpropanoid molecules which are chemically derived from the aromatic amino acid, namely, phenylalanine (1), and interact by a central carbon unit originally derived from malonyl coenzyme A (malonyl-CoA (5) is also known as an extender substrate, providing sequential cyclization of the PK chain [20]). Further, cinnamic acid (2) is produced from phenylalanine (1) with the help of catalyzing enzyme phenylalanine ammonia lyase (PAL) and converted into p-coumaric acid (3) by cinnamate-4-hydroxylase (C4H) and 4-coumarate-CoA ligase (4CL). Then, 4CL converts the p-coumaric acid to coumaroyl-CoA, p-coumaroyl shikimate transferase (CST), caffeoyl-CoA O-methyltransferase (CCoAOMT) and p-coumaroyl 5-O-shikimate 3-hydroxylase (CS3=H) convert it to ferulic acid (4). Then, a combination of CoAs (i.e., coumaroyl-CoA and feruloyl-CoA) are formally converted to diketide-CoAs with the help of diketide-CoA synthase (DCS) by condensation of malonyl-CoA. At the end of the biosynthetic process, curcumin synthases (CURSs) catalyze the formation of curcuminoids via condensing the diketide-CoAs in combination with coumaroyl-CoA and feruloyl-CoA. Based on the combination, types of curcuminoids are synthesized/produced, namely, bisdeshydroxybisdesmethoxycurcumin (6), bisdemethoxycurcumin (7), demethoxycurcumin (8) and curcumin (9).
Figure 1.
Basic structure and biosynthesis of curcumin. The figure represents the partial biosynthesis pathway of curcumin in turmeric: the JSMol model, enol and keto form of curcumin.
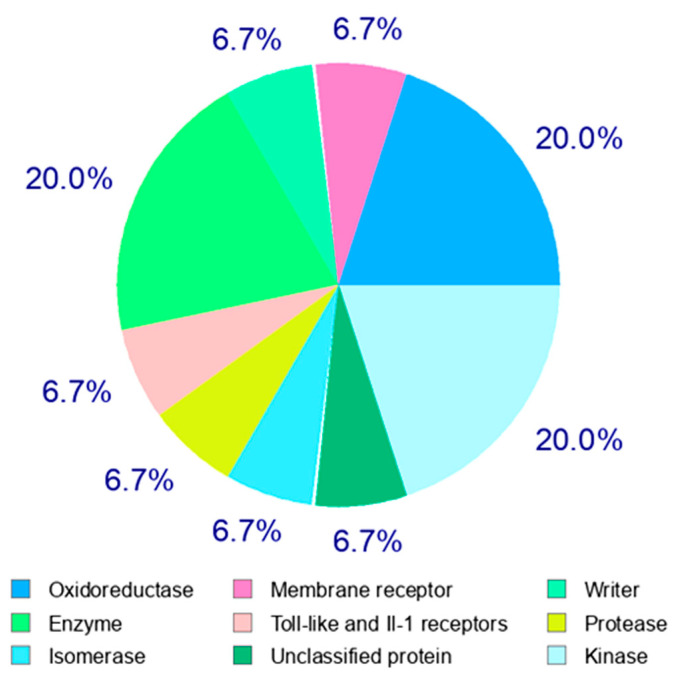
Curcumin has a high potential ability to bind and alter the regulation of different proteins, growth factors, transcription factors, enzymes, receptors and other regulatory genes directly/indirectly [21]. So far, several researchers have comprehensively investigated the targets of curcumin in human beings and identified the direct targets as including protein kinases/reductases, proteosomes, inflammatory molecules, carrier proteins, DNA methyltransferases and metal ions. Enzymes, transcription factors, growth factors, receptors, inflammation mediators, cell cycles and adhesion molecules are considered as indirect targets as well [15]. Curcumin is a pleiotropic molecule that can interact with a variety of molecular targets. The classes of interactions mediated by curcumin were predicted by the SwissTargetPrediction tool and are summarized in Figure 2 and Supplementary Table S1.
Figure 2.
Target classes of curcumin. Among the various classes of enzymes, curcumin majorly targets oxidoreductases, kinases and several other enzymes, as stated in the image.
3. Biological Properties and Biomedical Applications
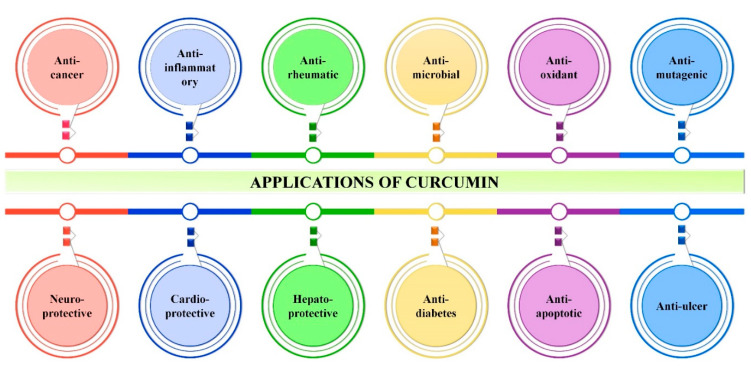
The medicinal usage of curcumin was started more than 2500 years ago in Asia, especially by the native population of India. In the scientific community, the medicinal use of curcumin was first reported to be against chronic cholecystitis and allied diseases in 1937. To date, there are more than 8000 studies/articles available in the PubMed database demonstrating the various biological properties of curcumin, such as antioxidant, antibacterial, antifungal, antiviral, anti-inflammatory, anticancer, antiproliferative, proapoptotic and anti-atherosclerotic effects, and its medicinal benefits against various forms of human illnesses, including neurodegenerative diseases, arthritis, allergy, inflammatory bowel disease, nephrotoxicity, diabetes, multiple sclerosis, cardiovascular disease, lung fibrosis [3,18,22], AIDS [23] and psoriasis [24] (Figure 3). As stated, curcumin has various biological applications for which the molecular mechanisms of these properties are presented in Table 2.
Figure 3.
Overall applications of curcumin. Image depicts various biological applications of curcumin irrespective to its bioavailability.
Table 2.
Biological activities and their molecular mechanisms of curcumin.
| S. No. | Biological Activities | Molecular Mechanisms | References |
|---|---|---|---|
| 1 | Antioxidant | Scavenges the free radicals (ROS, RNS); Obstructs the enzyme (GSH, catalase, GPx and SOD) activity to optimize the free radicals; Alters the ROS-producing enzymes such as lipoxygenase (LOX)/cyclooxygenase (COX), xanthine oxidase. |
[25,26,27,28] |
| 2 | Anticancer | STAT3 and NF-κB signaling pathways play a crucial role in cancer growth. Curcumin eradicates or obstructs its growth. It controls the migration and invasion by directing the expression of Sp-1 and housekeeping genes. | [29,30,31] |
| 3 | Antimicrobial | Interacts with FtsZ, which is a significant player in cell division initiating proteins. | [32,33,34] |
| 4 | Anti-inflammatory | Regulates and controls the inflammatory response via decreasing the enzymatic activity of phospholipases A2 (PLA2s), lipoxygenase (LOX) and cyclooxygenase-2(COX-2), triggering the nitric oxide synthase (iNOS) enzymatic pathways that can modulate prostaglandin synthesis and essential inflammatory response mediators. | [35,36,37] |
| 5 | Antifibrosis | Prevents the migration and proliferation abilities of fibroblast and collagen production via altering the expression of transforming growth factor (TGF)-β and angiotensin signaling (Ang). | [38] |
| 6 | Antiamyloid | Regulates amyloid beta (Aβ) metabolism; Inhibits Aβ aggregation; Disaggregates to form fibrillar Aβ16, Aβ40 and Aβ42 to produce strong anti-amyloidogenic effects. |
[39,40] |
| 7 | Antidiabetic | Suppresses the advanced glycation end products (AGEs) formation; Stimulates peroxisome proliferator-activated receptor gamma (PPARg) activity; Increases glutathione synthesis; Increases secretion of insulin from pancreatic cells, as well as reduces insulin resistance. |
[41,42] |
3.1. Anticancer Effect
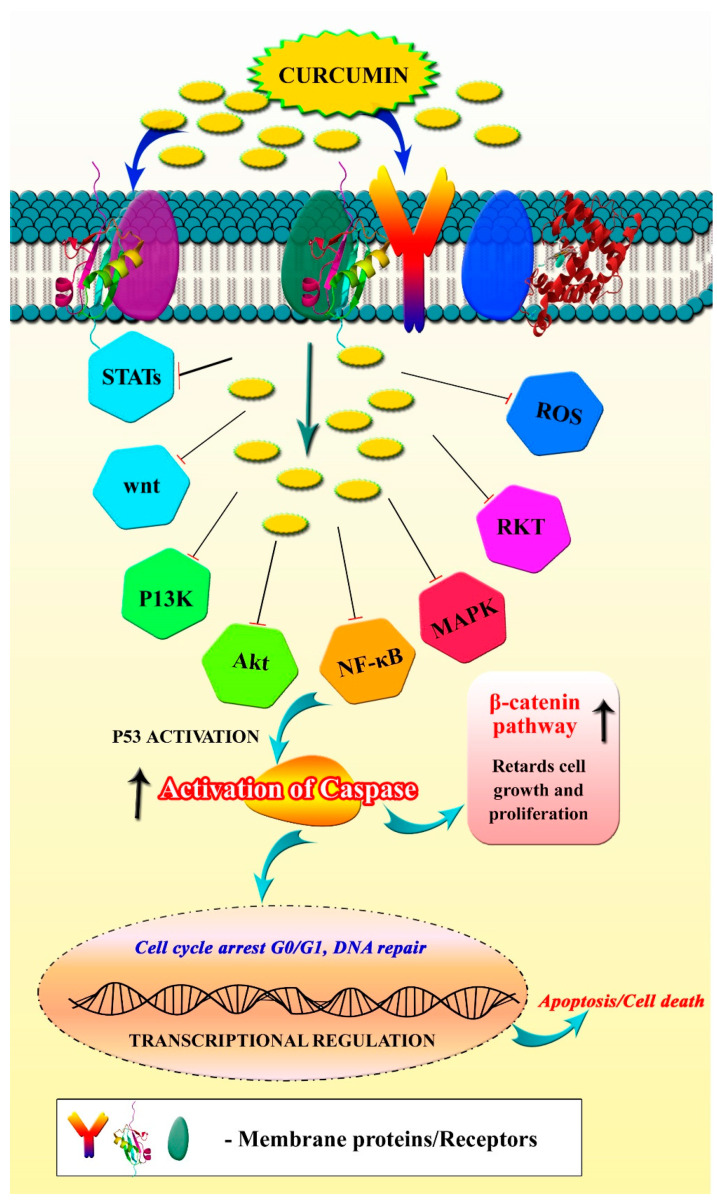
Though curcumin has been reported for its inhibitory efficacy against a variety of diseases, its anticancer effect is capturing the attention of cancer researchers. A plethora of cancer prevention research studies have demonstrated the efficacy of curcumin in both preventing and treating cancer, as well as described its various mechanisms of action [43]. According to several research studies, curcumin hinders the proliferation of cancer cells by the following mechanisms: (i) downregulation of the NF-B/STAT3 signaling pathway [44], (ii) inhibition of different regulatory protein kinases [45], (iii) regulation of apoptosis and other cancer-related pathways [46], (iv) promoting cell cycle arrest [47], (v) suppression of metastasis factors and (vi) promoting ROS-mediated cancer cell death. The inhibitory effect and mode of action of curcumin on cancer cells are illustrated in Figure 4.
Figure 4.
Anticancer mechanism of curcumin. Image shows the regulatory targets/pathways of curcumin in anticancer activity.
3.1.1. Downregulation of NF-κB/STAT3 Signaling Pathways
The transcriptional factor NF-κB is responsible for the regulation of certain genes that promote cancer cell proliferation, including cyclin D1, and the cyclin-dependent kinase inhibitors p21 and MMP-1 and MMP-3 genes [48]. The continuous activation of NF-κB can promote cancer cell proliferation and development via suppressing apoptosis. A study by Liu et al. [49] demonstrated that the anticancer activity of curcumin against breast cancer is mainly via downregulating the NF-κB-inducing genes. It was believed that downregulation of NF-κB genes by curcumin could plausibly suppress other downstream genes that may be responsible for cancer cell proliferation.
Similarly, curcumin was also found to inhibit the pathways that are mediated by another transcriptional factor, namely, signal transducer and activator of transcription 3 (STAT3), which is solely responsible for cancer cell survival, angiogenesis and metastasis. Curcumin demonstrated antivasculogenic mimicry (VM) activity in hepatocellular carcinoma cells by inhibiting/suppressing the STAT3 and AKT signaling pathways [50]. In addition, curcumin has been shown to hamper the STAT3 phosphorylation mediated by IL-6 in myeloma cells [31,51].
3.1.2. Inhibition of Protein Kinases
Kinases are a group of enzymes whose function is to transfer the phosphate group to the target/substrate protein via the process called “phosphorylation”. Though phosphorylation is a reversible process, activated kinases can aid the cancer cell by inducing angiogenesis, metastasis and cell multiplication and also thwart the event of apoptosis. Curcumin has been shown to suppress several cancer-related kinases, including AMP-kinase, S-phase kinase-associated protein 2 (Skp2) pathway, mitogen-activated protein kinase (MAPK) (p38, ERK) and AKT/protein kinase B (PKB).
A study by Chen et al. [52] revealed that curcumin has the VM effect by downregulating the expression of the erythropoietin-producing hepatocellular receptor tyrosine kinase class A2 (EphA2)/PI3K/MMP signaling pathway in a murine choroidal melanoma model. Another research study revealed the anticancer mode of action of curcumin against human epithelial cancer cells (A431) and unveiled the fact that curcumin manifestation could inhibit the EGFR tyrosine kinase by promoting autophosphorylation [53]. Previous reports revealed that the antiproliferative and antimetastatic effects of curcumin were mediated by inducing the phosphorylation of AMP-kinase in breast cancer cells [54].
3.1.3. Regulation of Apoptosis
Apoptosis is the fundamental, natural mechanism in which unhealthy/unwanted cells undergo programmed cell death. This natural phenomenon is vital to balancing inner cell homeostasis. Any apoptosis misbehavior can result in abnormal cell growth and contribute to the progression of cancer [55]. Although curcumin has been shown to target different molecular pathways, in most of the research studies it has been demonstrated that the anticancer mechanism of curcumin is mainly via apoptosis. A myriad of research studies has reinforced the fact that curcumin can balance apoptosis either through increasing the expression of proapoptotic genes or by downregulating certain antiapoptotic genes. For instance, curcumin has been identified as possessing anticancer activity through promoting apoptosis in scleroderma lung fibroblasts [56]. Furthermore, numerous studies have shown that curcumin modulates important apoptotic proteins such as BAX, BCL-2, C-MYC, BCL-XL and p53 and, as a result, induces apoptosis in human hepatoma SMMC-7721 cells [57], mammary epithelial and breast carcinoma cell lines [58].
Furthermore, previous studies revealed the ability of curcumin to modulate various apoptotic signaling pathways that, ultimately, result in the downregulation of certain antiapoptosis genes (Bcl-2) or upregulation of proapoptosis genes (Bax, PUMA and caspase cascades) [46]. In addition, curcumin has been found to activate the JNK and ER stress apoptotic pathways, which are mainly mediated through the accumulation of reactive oxygen species (ROS) in gastric cancer cells [59]. Some more studies revealed that curcumin is able to downregulate the expression of several genes, such as protein tyrosine kinase, protein kinase C, c-myc mRNA expression and bcl-2 mRNA expression, that lead to the induction of apoptosis in cancer cells [30].
3.1.4. Promoting Cell Cycle Arrest
In mammals, the cell cycle is coordinated by five distinct phases that include G1, G2 (nucleic acid and protein synthesis phase), S (DNA-replicating phase), M (mitosis phase) and G0 (resting phase) [60]. Any malfunction in the cell cycle regulatory check points can lead to tumor formation. Derangements in the regulatory control of the cell cycle can result in the formation of tumor cells in which growth and proliferation proceed unchecked. According to Wu et al. [61], curcumin induced G2/M cell cycle arrest by inhibiting protein kinase 1 (DAPK1), which resulted in the inhibition of STAT3 and NF-B, which further activated caspase-3. Research studies also documented that the modulation of the miR-21 gene by curcumin could cause G2/M phase cell cycle arrest in HCT 116 colorectal cancer cells [62]. It has also been observed that the downregulation of certain cancer-associated genes such as tubulin and p53 by curcumin could also cause cell cycle arrest at the G2/M phase in the treatment of colon cancer [63]. In addition, curcumin similarly acted on Aurora-A SiRNA (small interfering RNA), leading to monopolar spindle formation, S and G2/M arrest and cell division reduction in MCF-7 cells.
3.1.5. Suppression of Metastasis Factors
Metastasis is a process whereby cancer cells can spread from the original site to new potential locations, mostly carried out through the circulation of the bloodstream or lymph system [64]. Curcumin has the potential to inhibit cancer cell metastasis through the regulation of various cancer-associated factors, such as inflammatory cytokines, growth factors and growth factors’ receptors, enzymes, adhesion molecules, apoptosis and cell-cycle-related proteins [65]. In particular, curcumin has been reported to inhibit metastasis by blocking the activation of STAT3 (which is associated with cancer cell metastasis). Curcumin hinders the signal transduction needed for the phosphorylation and activation of STAT3 via binding to the Janus-activated kinase (JAK) activation loop [66]. Curcumin also blocks metastasis through the inhibition of focal adhesion kinase (FAK) activity. A study by Chen et al. [52] demonstrated the metastasis inhibitory efficacy of curcumin in both in vitro and in vivo conditions. From the study, they conclude that exposure to curcumin could potentially downregulate the expression of one of the tumor adhesive molecules (CD24), resulting in the suppression of FAK phosphorylation [52].
3.1.6. Promoting ROS-Mediated Cancer Cell Death
In cancer cells, ROS showcased double-sided effects; it can trigger or suppress the cancer cell expression. Several studies have shown that the anticancer mechanism of curcumin is either through scavenging or induction of ROS generation. Khan et al. [31] demonstrated the ROS-mediated apoptosis in T-cell lymphoma (HuT-78) cells. They suggested that rapid generation of ROS by curcumin could potentially activate the caspase activity, alter the mitochondrial membrane potential and downregulate the expression of antiapoptotic proteins c-FLIP and Bcl-xL [31,67]. Evidence suggested that ROS-mediated upregulation of DR5 and activation of TRAIL-induced apoptosis were observed in renal cancer cells and colon cancer cells that were manifested with curcumin [68,69]. In gastric tumor cells, curcumin induced the rapid ROS generation and, thereby, reduces the cellular bioenergetics, i.e., decreased mtDNA content and DNA polymerase γ (POLG), resulting in cancer cell death [70]. It has also been reported that curcumin induces ROS generation to the threshold level and suppresses the ROS multiple enzymes to inhibit the cancer cell growth in human erythroleukemic K562 cells [71].
3.2. Antioxidant and Radical Scavenging Properties
ROS is continuously produced by all aerobic organisms during respiration and some physiological events. If ROS is not scavenged, it could be responsible for developing more than 100 diseases. To avoid these issues, every aerobic microorganism has a specific mechanism to protect itself from ROS (antioxidant property) [72]. Numerous research studies have shown the potential antioxidant and radical scavenging properties of curcumin in both in vivo and in vitro models. Most of the studies confirm that the pharmacological/therapeutic activity of curcumin is mainly attributed to its ability to scavenge reactive free radicals. Previous studies showed the antioxidant property of curcumin and its derivatives (demethyoxycurcumin, bisdemethoxycurcumin and diacetylcurcumin) and, from the results, they concluded that curcumin protects hemoglobin oxidation through its antioxidant property in macrophages [25,26]. Another study found that curcumin can be a “superb antioxidant” agent by donating its H-bond. They also suggested using curcumin in various pharmaceutical products to inhibit lipid oxidation and to improve the medicinal quality of products [73]. Curcumin administered orally to male Wistar rats for 10 days could reduce iron-induced hepatic damage by reducing ROS-induced lipid peroxidation [74]. It was highly controversial whether the antioxidant property of curcumin was attributable to the phenolic or the central methylenic hydrogen. Many researchers have been trying to find the solution. In both acidic and neutral environments, Jovanovic et al. [75] found that the H atom was donated from the central methylenic group of curcumin. Contrary to this result, Barclay et al. suggested that the H atom in curcumin was donated from its two phenolic groups and not from enol [76]. In agreement with this point, another study claimed that the phenolic group of curcumin is mainly responsible for its free radical scavenging property [77].
3.3. Anti-Inflammatory Properties
Though inflammatory responses by immunological cells are considered a necessary step in protecting against infections, undesired inflammation may lead to the development of disease, e.g., rheumatoid arthritis. Curcumin has been shown to modulate the different mediators that are solely responsible for uncontrolled inflammation [4]. The most accepted anti-inflammatory response of curcumin is mediated through (1) downregulation of cyclooxygenase-2 (COX-2), lipoxygenase and inducible nitric oxide synthase (iNOS), (2) inhibition of cytokine production and (3) reduction of transcriptional factors [22]. COX-2 is an enzyme that mediates the conversion of prostaglandins (PGs) from arachidonic acid. The overexpression of PGs may induce the overgrowth of tumor cells in both human beings and experimental animals [78]. It has been reported that constant upregulation of COX could potentially be responsible for various tumor developments such as colon, rectum, breast, head and neck, lung, pancreas, stomach and prostate cancer cells [79]. Several studies have shown that curcumin possesses anti-inflammatory activity by blocking the overexpression of COX-2 in different cell lines and animal models [80]. Topical application of curcumin has been shown to reduce the expression of PGs by blocking the lipopolysaccharide-mediated COX-2 expression [36,80]. As discussed previously, the transcriptional factor NF-κB plays a vital role in cancer cell proliferation, oncogenesis and cell transformation via mediating inflammatory responses. An experimental study has shown that oxidative stress mediates NF-κB inhibition of curcumin [81]. Singh et al. [82] explained that the curcumin mediated the NF-κB activation pathway inhibition a step before I kappa B-alpha phosphorylation but after the convergence of various stimuli.
3.4. Antimicrobial and Antibiofilm Activities of Curcumin
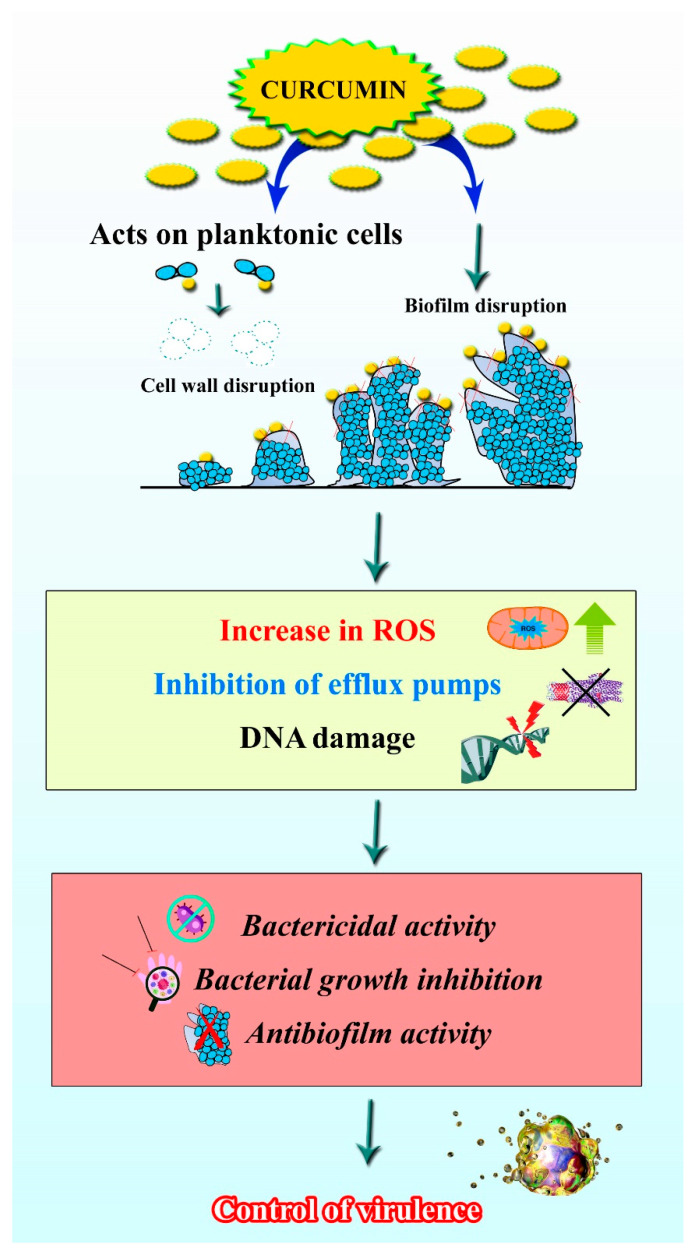
The rhizome of C. longa has been traditionally used as an antimicrobial agent [83]. Curcumin has been used for a variety of purposes, and topical application of curcumin on the skin can help with antimicrobial care for wounds, acne, blisters, parasitic infections and other conditions [84]. For common colds, liver infections and urinary tract infections, oral administration was preferred [85]. Due to its comprehensive antimicrobial activity and innocuous nature, even at higher dosage concentrations, the structure of curcumin is being used as a prototype to design new antimicrobials and synthetic derivatives of curcumin with increased efficiency [85]. The structure of curcumin has conjugates such as two methoxylated phenols and a β-diketone in its enol form, which enables additional inter- and intra-hydrogen bonds. However, the presence of hydroxyl and methoxyl groups is considered to be accountable for antimicrobial activity [86]. The ability to inhibit the growth of various microscopic organisms has paved the way for curcumin’s numerous applications, including in the textile industry for the fabrication of antimicrobial clothes, wound dressing, photodynamic therapy in dental applications, nanoformulations for burn wounds, etc. [87,88]. In this context, curcumin targets the ROS mechanism of the microorganism, efflux pumps and causes DNA damage to control cell growth, as shown in Figure 5.
Figure 5.
Antibiofilm activity of curcumin. Image shows that curcumin can act on the planktonic cells, as well biofilms of certain bacteria by increasing the ROS, inhibiting the efflux pumps and causing DNA damage.
3.4.1. Antimicrobial Fabric
Among the indoor microbial contaminations of several industries, the textile industry is more susceptible to the growth of pathogenic organisms. Factors such as large surface area, moisture content, etc., provide a favorable environment for the microbial colonization, which often leads to deterioration in the quality of the fabric and an unpleasant odor that affects the product as well as those who wear it [89]. To overcome these shortcomings, a variety of novel approaches have been implemented. One such is the use of natural ingredients as antimicrobial agents as a final glaze for fabrics. Curcumin is commonly used for this purpose due to its dual function, viz., natural dye and antimicrobial property. Numerous studies have reported that wool fabric treated with curcumin exhibits increased antimicrobial activity even after prolonged wash cycles [87,90]. Certain researchers have synthesized novel dyes based on curcumin. Gaffer et al. [91] synthesized curcumin containing sulphadiazine and sulphathiazole dyes for application in antimicrobial silk fabrication. Antibacterial medical textiles with curcumin were also fabricated as a curcumin-based water-dispersal polyurethane textile finish [92]. Several investigations have described the proficiency of curcumin in antimicrobial wound dressing applications as well [36,93,94,95].
3.4.2. Antimicrobial Photodynamic Therapy
Photodynamic therapy (PDT) has been a useful therapeutic option to treat various solid tumors for decades with the administration of photosensitizing agents and light of specific wavelengths [96]. Due to the constant increase in antimicrobial resistance and the decline in the effectiveness of antibiotics, antimicrobial photodynamic therapy (aPDT) has been used as one of the alternative approaches to eradicate infections caused by resistant pathogens [97]. aPDT has been used as a treatment for various infections caused by bacteria, fungus and viruses [98]. Curcumin has been identified as a potential photosensitizing agent [99] since the absorption maximum of curcumin is in the blue wavelength range (420–430 nm) and the penetration depth is comparably lower than that of red light. Thus, curcumin as a photosensitizer in PDT is limited to treatment for topical infections [100]. Curcumin-mediated aPDT has been demonstrated as an effectual therapy against oral infections such as dental plaque [101], periodontitis [102] and oral candidiasis [103]. Dosage concentration, incubation time and broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacteria, fungi, etc., enhance the efficiency of curcumin in aPDT significantly [104].
3.4.3. Antimicrobial Combinations with Curcumin
Among various strategies to combat antimicrobial resistance, combinatorial drug therapy has gained more attention due to the benefits of reduced toxicity, improved efficacy and lack of antibiotic resistance with greater potential than individual drug candidates [105]. Curcumin has shown excellent synergistic activity with various antibiotics, as well as other natural bioactives. The combination of curcumin and polymyxins has demonstrated promising antimicrobial effects against multidrug-resistant pathogens. In addition to increased antimicrobial activity, curcumin–polymyxin combination therapy has reduced the polymyxin-induced nephron and neurotoxicity [106]. Another study found that the interaction of quercetin and curcumin exhibited synergistic antimicrobial activity against MRSA, as well as improved anti-inflammatory activity [107]. Several other in vitro studies have shown that various active compounds have successful antimicrobial combinatorial proficiency against a wide range of multidrug-resistant pathogens [108,109].
3.4.4. Antimicrobial Activity of Curcumin in Nanoformulations
In recent decades, nanomedicine has been rising as a promising field. Nanoscale bioactive structures have shown increased physical and chemical properties and thus are utilized in numerous diagnostic and therapeutic applications [18]. Due to the hydrophobic nature of curcumin, its modification to nanoform has shown increased therapeutic efficiency, bioavailability and stability [110]. The most common forms of curcumin nanoformulations include nanoparticles, nanoencapsulation, micelles and liposomes [17]. Curcumin nanoencapsulations and nanocrystals have demonstrated antimicrobial activity against several Gram-positive and Gram-negative pathogens, including S. aureus, E. coli, P. aeruginosa, L. monocytogenes, S. epidermidis, etc. [111,112,113]. Similarly, the nanogel form of curcumin has been used as a topical antimicrobial for wound burns [114]. It is not only limited to antibacterial activity; it possesses a broad spectrum of antimicrobial activity when it is formulated using nanotechnology, by which it can act against fungi [115], parasites [116] and also viruses [117].
3.4.5. Antibiofilm Activity
Biofilm is the most common form of microbial lifestyle, wherein organisms are associated with various kinds of surfaces. Pathogens are protected from host defense and environmental factors by encasing them within the self-produced matrix of exopolymeric substances [118]. Biofilm formation has a significant impact on the rise of antimicrobial resistance. Hence, antibiofilm molecules which can arrest or disrupt the biofilms of pathogenic organisms are part of the current trend [119]. In addition to all the therapeutic efficiencies, curcumin has been shown to possess antibiofilm activity against several organisms. Sethupathy et al. [120] displayed the efficacy of curcumin in inhibiting the virulence attributes and biofilm formation of P. aeruginosa and its clinical isolates without affecting the growth. The study also reported that the mechanism underlying the antibiofilm potential of curcumin against P. aeruginosa is through iron acquisition and interference in the metabolic intermediates of virulence factor production. Similar antibiofilm activities have been reported for Acinetobacter baumannii [121], L. monocytogenes [122], Enterococcus fecalis [123] and other organisms. Additionally, curcumin exerted antibiofilm activity against mixed species biofilms of C. albicans and S. aureus [124]. These reports suggest that curcumin is a potential antibiofilm agent in treating biofilm-associated infections.
3.5. Bioactive Efficacy of Curcumin against Human Diseases and Metabolic Disorders
In recent decades, curcumin has been well recognized for its versatile therapeutic properties. Curcumin has also been shown to have promising pharmacological activities against common human diseases such as arthritis, neuroprotective activity, hepatoprotective effect, cardioprotective activity and metabolic syndrome [125]. The influence of curcumin on various human diseases is briefly discussed below.
3.5.1. Influence of Curcumin on Neurodegenerative Disorders
Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, spinal muscular atrophy, motor neuron disease and Friedreich’s ataxia are very common neurodegenerative disorders found worldwide. Neurodegenerative disorders are serious and life-threatening to the human population, and they affect most of the regular activities of humans, such as movement, talking, breathing and other biological functions [126,127]. Most of the neurodegenerative disorders are caused by genetic factors and other human activities, including alcoholism, prolonged medication, toxic substances, smoking, etc. Most of the available treatments for neurodegenerative disorders may relieve pain and reduce the impact of disease. There is no complete recovery treatment against most of the neurodegenerative disorders [128]. Alzheimer’s disease is a progressive neurodegenerative disorder that continuously abolishes human memory power and regular physical activity. Amyloid-β plaques formation is the main histopathological reason for Alzheimer’s disease. Alzheimer’s disease occurs due to formation of amyloidal plaques in the nerve cells owing to cleavage of amyloid precursor proteins into amyloid-beta (Aβ) peptides through proteolytic processing using secretase enzymes including β and γ-secretase. Also, neuroinflammation, neurofibrillary tangle formation, accumulation of hyperphosphorylated tau proteins, vascular abnormalities, oxidative stress and mitochondrial damage are the reasons for Alzheimer’s disease [129]. Currently, pharmacological-based therapy to prevent or cure Alzheimer’s disease is very meagre. However, clinically approved drugs are available for the treatment of Alzheimer’s disease, such as donepezil, maypostpone and galantamine. These drugs have shown temporary efficacy against Alzheimer’s disease and have also been reported for their side effects [130]. Several reports have revealed that bioactive compounds from natural sources could show potential activity against Alzheimer’s disease. For instance, Utomo et al. [131] developed a curcumin-based SAR matrix that has a great inhibitory effect on amyloid-β aggregation to treat Alzheimer’s disease. This study also unveiled the non-cytotoxicity effect of a curcumin-based SAR matrix and reduced Aβ-induced cytotoxicity. Finally, their study conclude that a curcumin-based SAR matrix has the ability to prevent the neurotoxicity which causes Alzheimer’s disease [131]. Kim et al. [132] reported that curcumin impedes oxidative stress generated due to Aβ and it helps to decrease the number of oxidized proteins and inflammatory cytokines.
Parkinson’s disease is one of the notable neurodegenerative disorders and it is clinically categorized as a movement disorder associated with age and genetic factors of the human population. This disease arises due to the degeneration of particular dopaminergic neurons in the substantia nigra of the ventral midbrain, through which it reduces the level of dopamine in the striatum [133]. All the emerging treatments for Parkinson’s disease are intended to increase the dopamine level. Further, drugs used for the treatment of Parkinson’s disease do not show strong neuroprotective effects. Subsequently, novel studies have revealed that natural molecules have neuroprotective activity in the treatment of Parkinson’s disease [134]. Curcumin is one of the natural compounds identified with neuroprotective activity. Numerous studies have been conducted to investigate the clinical application, therapeutic potential and potent activity of curcumin against Parkinson’s disease [135]. Nguyen et al. [136] reported that curcumin treatment improves locomotive abilities and induces less severe neurodegeneration in the Drosophila melanogaster model (dUCH knockdown) for Parkinson’s disease. Furthermore, Sharma and Nehru [137] evaluated the inhibitory potential of curcumin against alpha-synuclein (one of the crucial proteins responsible for Parkinson’s disease) by generating oxidative stresses, including hydrogen peroxide, dopamine oxidation, nitric oxide synthase, glutathione depletion and NADPH oxidase activation. Surprisingly, epidemiological reports stated that the frequency of Alzheimer’s disease and Parkinson’s disease is very low in the Indian population when compared to the Europeans and this may be due to the enormous consumption of turmeric and curcumin by Indians [138].
3.5.2. Hepatoprotective Activity of Curcumin
Globally, hepatic diseases are one of main causes of mortality and morbidity among the human population. There are several factors that induce hepatic dysfunctions, such as drugs, toxic substances, microbial infections, medications, oxidative stress, inflammation, chronic alcoholism and human modern habits [139]. There are numerous plant-associated compounds explored with potent hepatoprotective activities, among which curcumin is one of the well-known plant-derived bioactive compounds that can be used as a significant compound source for the development of hepatoprotective drugs [140]. A study by Girish and Pradhan determines the ability of curcumin to improve liver cell regeneration in mice by restoring the cytochrome P450 enzyme system and reducing oxidative stress [141]. Further, El Swefy et al. [142] demonstrated that curcumin enhanced biochemical and structural properties of liver cells affected by bile duct ligation-induced fibrosis. Generally, the hepatoprotective efficacy of curcumin is observed through inhibiting COX-2, NF-κB, lipid peroxidation and reduced glutathione. Thus, the mechanisms driving the hepatoprotective activity of curcumin were through the inhibition of oxidative stress and inflammation in the liver. Curcumin, as a result, plays a significant therapeutic role in the treatment of liver diseases [143].
3.5.3. Efficacy of Curcumin against Arthritis
Arthritis is one of the autoimmune disorders caused by hyperplasia of the cartilage lining. It is observed due to the accumulation of inflammatory cells in synovial tissue, which leads to joint damage, significant pain and disability. Several cytokines are responsible for arthritis, such as IL-1b, TNF-α, IL-1β, IL-6 and IFN-γ of dendritic cells, T cells and macrophages, respectively [144]. Thus, inhibition of these pro-inflammatory cytokines may be a strong therapeutic strategy for the treatment of arthritis. The current pharmaceutical drugs for treating arthritis are analgesics, ion nonsteroidal anti-inflammatory drugs, steroids, disease-modifying antirheumatic drugs and nonsteroidal anti-inflammatory drugs. They have the potential to reduce the symptoms of severe pain and inflammation. However, these drugs are responsible for causing numerous serious side-effects, including insufficient pain relief, gastrointestinal disorders, immune system disturbances and cardiovascular adverse effects [145,146]. Among the explored bioactives, curcumin is one of the notable bioactive compounds that could be used against arthritis due to its anti-inflammatory property. Moon et al. [147] reported that curcumin treatment improves recovery of collagen-induced arthritis in mice and IL-1β-induced activation in fibroblast-like synoviocytes. In addition, arthritis-associated factors, such as TNF-α, IL-1β, NF-κB transcription activity, prostaglandin E2, matrix metalloproteinases and COX-2 expression were inhibited by curcumin treatment. Several studies investigated the therapeutic potential of curcumin against arthritis by targeting the pro-inflammatory cytokine responsible for arthritis [148,149,150].
3.5.4. Attenuation of Metabolic Syndrome by Curcumin
Metabolic syndrome is a disease caused by multiple factors such as aging, genetics and human lifestyle, including physical inactivity, obesity, inappropriate nutrition, etc. It is assessed through the following criteria: high-density lipoprotein cholesterol, fasting plasma glucose, triglycerides, blood pressure and obesity [151]. Curcumin has attenuated the various traits of metabolic syndrome by reducing high-density lipoprotein cholesterol, inflammation, oxidative stress, blood pressure and improving insulin sensitivity [152]. Furthermore, there are reports available for curcumin altering the lipoprotein metabolism, which is responsible for plasma triglycerides, cholesterol and high-density lipoprotein cholesterol quantity, thereby reducing the obesity-associated metabolic syndrome [153]. Pro-inflammatory cytokines have been linked to metabolic syndrome, which includes obesity, diabetes and cardiovascular disease. There, curcumin helps recovery from various metabolic syndrome-associated pro-inflammatory cytokines [5]. A study reported that curcumin supplementation to patients with metabolic syndrome-related disorders reduced weight and leptin levels while increasing adiponectin levels [154].
3.6. Wound Dressing Application
A wound is an injury that damages the physical structure of the skin and results in fluid loss and pain. The healing of wounds is a natural phenomenon that involves various cellular and molecular processes. Numerous biological properties, such as anti-inflammatory, antioxidant and antibacterial activity, make curcumin a potential wound healing agent. Oxidative stress is the major cause of delayed wound healing and makes the wound chronic. A previous study proved that reduction of ROS at the site of injury accelerates the healing process. Hence, targeting ROS was speculated to be the mechanism of curcumin to enhance wound healing [155]. As an antioxidant agent, curcumin has effectively enhanced the wound healing process by eradicating ROS, as demonstrated by in vitro and in vivo experiments. In addition, curcumin also decreases the expression of inflammatory factors to reduce the inflammatory damage of local tissue and thereby supports wound healing [156].
Curcumin has been widely explored for wound healing applications not only because of its therapeutic activities, but also for its non-toxic nature. Curcumin is designated as a “generally regarded as safe” (GRAS) compound by the U.S. Food and Drug Administration (FDA). Hence, it is not surprising that curcumin is globally employed for biomedical applications [15]. However, the hydrophobic nature of curcumin results in poor solubility in the aqueous phase and limits the bioavailability [157]. Many polymer-based wound dressings are being tested to overcome these limiting factors. Topical application of curcumin is predicted to be the best way of administration to avoid the bioavailability issues associated with systemic mode. Table 3 summarizes the various formulations loaded with curcumin for wound dressing application.
Table 3.
Various formulations loaded with curcumin for wound healing application.
| Type of Formulation | Composition | Reference |
|---|---|---|
| Films | Methoxy PEG-g-chitosan composite films encapsulated with curcumin | [158] |
| PLA/chitosan nanofilms loaded with curcumin | [159] | |
| Antimicrobial cellulose nanocrystals film loaded with curcumin | [160] | |
| Carboxymethylated guar gum-g-gelatin film encapsulated with curcumin | [161] | |
| Cellulose/chitosan microcrystals antimicrobial films loaded with curcumin | [162] | |
| Hydrogel | Cellulose nanofiber-PVA hydrogels incorporated with curcumin | [163] |
| Thermal-responsive hydrogels from chitosan–Pluronic P123 loaded with curcumin and gelatin | [164] | |
| Cellulose–halloysite nanotube hydrogel loaded with curcumin |
[165] | |
| Nanosilver nanohydrogels of polymethacrylic acid blended with a PEO/PVA/carboxymethyl cellulose matrix and loaded with curcumin and aloe vera | [166] | |
| PEG-poly (ε-caprolactone) (PCL)-PEG copolymer-based in situ gel-forming hydrogels loaded with curcumin | [167] | |
| Thermosensitive β-glycerophosphate/chitosan hydrogels encapsulated with β-cyclodextrin-curcumin | [168] | |
| Bandages | Oleic acid-based chitosan–alginate bandages loaded with curcumin | [169] |
| Chitosan–alginate bandages co-encapsulated with curcumin | [170] | |
| Nanofibers | PCL-based nanofibers loaded with curcumin | [171] |
| Curcumin-loaded PLA nanofibers | [172] | |
| Curcumin-loaded PLA/PEG nanofibers | [173] | |
| PCL-PEG nanofibers incorporated with curcumin | [174] | |
| Electrospun poly (3-hydroxybutyric acid-co-3-hydroxyvaleric acid) (PHBV)-based nanofibers loaded with curcumin | [175] |
4. Bioavailability of Curcumin: Problems and Prospects
Regardless of its potential advantages, curcumin has not been efficient in treating a variety of ailments due to its low bioavailability, which is characteristic of a weak and impractical drug. Low solubility, limited gastrointestinal tract absorption, quick metabolism, rapid systemic clearance and restricted blood–brain barrier (BBB) penetration are all major factors that contribute to the low bioavailability [176]. Orally consumed curcumin is rapidly conjugated in the small intestine, liver and kidneys into curcumin glucuronide, curcumin sulphate and methylated curcumins, which are rapidly excreted in the urine and feces [177,178]. Similarly, if administered systemically, it turns into tetrahydrocurcumin, hexahydrocurcumin and octahydrocurcumin [179]. Curcumin is largely found in the blood as physiologically and pharmacologically inert conjugates, with comparatively little free bioactive curcumin, comparable to other polyphenol compounds. Furthermore, when compared to other known drugs, various researchers have revealed that curcumin has unfavorable pharmacokinetics in terms of absorption, distribution, metabolism, excretion and toxicity (ADMET) properties [180]. It is also unstable in aqueous solutions due to its hydrophobic nature, and it degrades promptly at physiological pH [181].
However, the most challenging factor is low water solubility (0.4 μg/mL at normal pH). Furthermore, in a clinical trial study of curcumin on Alzheimer’s disease (AD), no difference in absorption was found between the 1 g and 4 g doses. As per a previous report, curcumin and its metabolites were not discovered in the blood after administration due to its restricted tissue dispersion [182]. In a report of a clinical trial, patients with an advanced stage of colorectal cancer were given 440–2200 mg/day of oral curcumin extract for 29 days. Curcumin and its metabolites were not detected in the plasma or urine of patients [183]. Similarly, following an oral dose of 8.0 g of curcumin per day, only 22–41 ng/mL of curcumin was measured in plasma in a Phase II trial of curcumin therapy for patients with advanced pancreatic cancer [184].
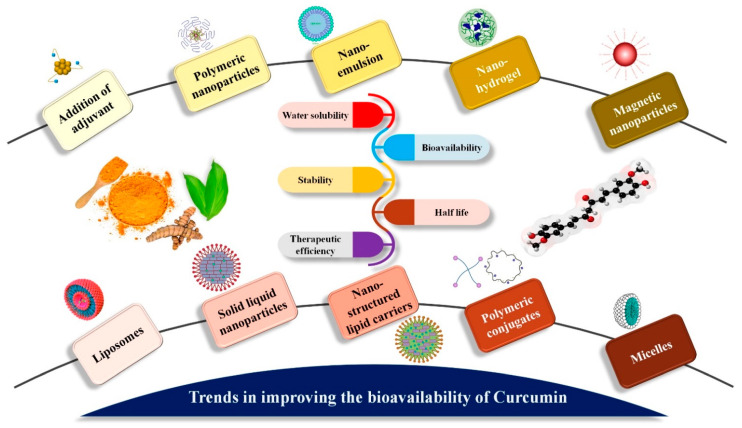
Regardless, curcumin’s substantial limitations have not stopped scientists from researching and enhancing its potential. Rather, they have created greater scope for the development of innovative solutions to address issues with the native form. Curcumin formulations have come about because of research to improve bioavailability, permeability, circulation and half-life, as well as withstand metabolic processes. These formulations admit chemical curcumin derivatives and analogs with metabolic adjuvants, nanoparticles, liposomes, micelles, nanostructured lipid carriers (NLC) and phospholipid complexes [180] (Figure 6). Phospholipid complexes appear to have been effectively released into the worldwide market among the formulations that emerged (MerivaTM and LongvidaTM). Longvida® (Verdure Sciences, Noblesville, IN, USA), the bioavailability of a solid lipid formulation containing roughly 80 mg of curcumin, was observed to be four times better than that of curcumin alone. In an aged population, daily therapy with 400 mg of Longvida® for four weeks resulted in better cognitive capabilities [185]. Meriva® (Indena, Milan, Italy), another proprietary curcumin formulation from Indena, is used to treat osteoarthritis, diabetes mellitus and microangiopathy in a 1:2 weight ratio of curcumin and soy lecithin. In comparison to Meriva, normal curcuminoid combination absorption was 29-fold lower in clinical studies [186]. When compared to C95, which contained 95% of curcuminoid powder (curcumin, demethoxycurcumin and bisdemethoxycurcumin) and micronized curcuminoids, plus turmeric oil, showed five times improvement in bioavailability [187]. The CCC (curcumin cyclodextrin complex) is said to have a 45-fold higher bioavailability than C95 [188]. CPC (curcuminoid phospholipid complex) demonstrated a 20-fold better bioavailability when compared to curcumin alone and 30-fold higher than total curcuminoids [187]. Another study by Zeng et al. [189] examined the effect of piperine pre-administration on oral curcumin bioavailability. In this investigation, rats were given 20 mg/kg piperine first, followed by 200 mg/kg curcumin at intervals of 0.5 to 8 h after piperine treatment. Piperine pre-treatment before curcumin administration resulted in a significant increase in curcumin oral bioavailability in all tested rats. Curcumin permeability rose 1.85-fold when quercetin and resveratrol were combined. This suggested that resveratrol and quercetin had a cumulative impact on curcumin absorption [190].
Figure 6.
Trends in enhancing the oral bioavailability of curcumin. As shown in the image, various methods of nanotechnology have been used to enhance bioavailability of curcumin.
5. Recent Trends to Improve Oral Bioavailability of Curcumin
5.1. Use of Adjuvants
Though curcumin has a lot of advantages, a large number of preclinical studies evidenced that it cannot be readily used to treat any diseases in human beings owing to its incredibly poor bioavailability [191]. One of the important strategies to improve the oral bioavailability of curcumin is using the adjuvants to neutralize detoxification enzymes demonstrated in the curcumin metabolism. Among the adjuvants, piperine (a compound from black pepper) is found to be one of the greatest enhancers of curcumin bioavailability [192]. Serum concentration was reported to be increased by the concomitant administration of curcumin with piperine in humans/animals (more than a thousandfold) [193]. Additionally, epigallocatechin-3-gallate (EGCG) was used as an adjuvant to curcumin which could improve the curcumin bioavailability to many folds [194]. The adjuvants are not limited to these above-mentioned compounds only, however. A number of adjuvants have been used to increase the oral bioavailability of curcumin for various therapeutic applications, including cancer. A recent article reported that oral administration of curcumin along with piperine for COVID-19 treatment (symptomatic) could significantly decrease mortality and morbidity [195]. Also, the article represents that curcumin, along with piperine, may be a natural therapeutic and safe choice to prevent post-COVID thromboembolic measures as well. One more research article emphasized that sustained topical delivery and enhanced bioavailability of curcumin was achieved with curcumin nanoparticle, notably the potential for skin staining. A murine model organism was used to investigate the skin inflammation with or without ultraviolet-B radiation exposure along with encapsulated/unencapsulated curcumin in coconut oil. After 24 h incubation, the experimental setup treated with encapsulated curcumin showed less skin reddening than those treated with only curcumin. Also, inflammation cytokine analysis and histology of the encapsulated curcumin-exposed skin exhibited less skin cell damage and decreased inflammation (markers) when compared to control groups and nonencapsulated groups [196]. Recently, evidence suggested that nanocapsulated/unencapsulated forms of curcumin functions as an effective bio molecule for restoring the host–microbial relationships and healthy gut homeostasis [197,198]. However, it is suggested that detailed research investigation at the preclinical/clinical stage is mandatory to explore the full potential of the adjuvant role of curcumin and other bioactive components in various applications, including cancer prevention and treatment [199].
5.2. Nanoformulation of Curcumin: Nanoparticle, Nanocomposite, Hydrogels, etc.
The nanoformulation of curcumin has been demonstrated with vast clinical applicability to utilize the real potential of the ultimate drug candidate, curcumin. The major nanoformulations of curcumin include nanoemulsions, solid lipid nanoparticles, nanocomposite, nanosuspension, nanoparticles, liposomes, micelles, polymeric nanoparticles, hydrogels, etc. [200]. Liposomes are vesicular structures with one or more phospholipid bilayers capable of delivering potential drug molecules to the cells of interest. Liposomes bind to the target cell’s lipid membrane and release the liposomal substance into the cytoplasm. Liposomes may entrap both lipophilic and hydrophilic molecules, ensuring optimal effectiveness and safety in the delivery of the target-specific medication [201]. Micelles are core–shell nanostructures with a lipophilic core and a hydrophilic outer layer that make up the shell. They are made by amphiphilic co-polymers self-assembling at a crucial micellar concentration. The hydrophobic core serves as a good carrier for drugs that are hydrophobic [202]. Nanosuspensions are water-insoluble pharmaceuticals dispersed in aqueous solutions without the need for a carrier. The medications have a colloidal size range of less than 1 µm and surfactants and other substances incorporated as stabilizers. The combination of tiny particle size (PS) and a large surface area, as well as high thermodynamic energy, favors fast drug dispersion [167]. Solid lipid nanoparticles (SLNs) are biocompatible and biodegradable colloidal lipid carriers (50–1000 nm) composed of biological lipids. Unlike liposomes, SLNs are hard particles that are only suited for loading hydrophobic compounds such as curcumin. High drug-loading capacity, strong stability, outstanding biocompatibility and increased bioavailability are all prominent attributes of SLNs. Being hydrophobic, they are effective nanocarriers for controlled release and site/cell-directed medication administration to the reticuloendothelial system [203].
Nanoemulsions (NEs) are clear or translucent dispersions of oil, emulsifier and water with particle sizes within 100 nm that are kinetically stable. In contrast to microemulsions, NEs do not develop spontaneously; generating NEs requires a significant amount of energy due to the low surfactant content in them. As they are emulsions, they allow for the inclusion of both hydrophobic and hydrophilic therapeutics, as well as the bio-enhancement of hydrophobic pharmaceuticals due to very small vesicle size [204].
Nano-sized quantum dots, manganese phosphate nanoparticles, noble metals, carbon nanotubes, silica nanoparticles and magnetic nanoparticles are examples of inorganic nanoparticles. They have unique physical characteristics that are size-dependent, including optical and electrical effects, efficient contrasting effect and magnetism. Additionally, they are resistant to microbes and have strong storage characteristics. Curcumin inorganic NPs have the potential to be employed in a variety of essential bio-applications [205]. Polymeric nanoparticles are granular colloids with a diameter of up to 1000 nm that are made from natural or synthetic polymers that are biodegradable or not. They are classified as either nanocapsules (where the medication is contained within a cavity covered by a polymer covering) or nanospheres (where the medication is distributed throughout the polymer). When compared to liposomes, polymeric NPs have more reactivity, surface area, sensitivity and stability. They are engaging drug carriers because of their high membrane permeability (due to their small size) and their ability to target specific organs [206]. A list of previously published curcumin nanoformulations with various medical applications is shown in Table 4.
Table 4.
Name list of the nanoformulations and their corresponding therapeutic applications.
| S. No. | Name of the Nanoformulation | Formulation Ingredients and Method | Application | References |
|---|---|---|---|---|
| 1 | Cur@CRN | Nanocomposites containing Cur and ι-Carrageenan using emulsion technique | Enhanced stability, oral bioavailability, antioxidant and anti-inflammatory effects | [207] |
| 2 | CUR-Gel-HAp | Encapsulation of curcumin into the hydrophilic network of gelatin (Gel) and hydroxyapatite (HAp) by colloidal suspension | Enhanced cytotoxicity towards human lung cancer cells (A549) | [9] |
| 3 | Nanocurcumin | Nanomicelle with an average size of 50 nm containing curcumin | Enhanced antioxidant and anti-inflammatory effects against LPS-induced coliform mastitis in rat model | [208] |
| 4 | Curcumin nanoparticle | Curcumin loaded in poly lactic-co-glycolic acid nanoparticles with an average size of ~90 ± 10 nm by green surfactant-based synthesis | Enhanced solubility, photo-stability, antibiofilm activity and cytotoxicity towards HepG2 cells | [209] |
| 5 | CUR-loaded NC | Curcumin-loaded polymeric nanocapsules | Enhanced antiangiogenic, non-teratogenic and antioxidant effects in chick embryo model. | [210] |
| 6 | MSN-HA-C | Curcumin loaded in nanohybrid composed of mesoporous silica nanoparticle (MSN) and hyaluronic acid (HA) with an average size of ~75 nm | In vivo and in vitro anti-breast cancer activity | [211] |
| 7 | Fe@Au-CU-CS-FA NP’s | Nanoencapsulation comprised of folic acid, gold, curcumin, chitosan and iron synthesised by microemulsion method | Cytotoxic effect on lung cancer cells | [212] |
| 8 | SD-CUR | A core–shell solid dispersion composed of curcumin-loaded micelles self-assembled from disodium glycyrrhizin (Na2GA), coated with pectin and tannic acid | Enhanced bioavailability and antihyperlipidemic activity | [213] |
| 9 | SNLYZ-Cur | The nanoconjugate SNLYZ-Cur composed of curcumin surface-conjugated on self-assembled lysozyme nanoparticle with an average size of ~120 nm | Antibacterial, antioxidant and anticancer activity against multiple cancer cell lines such as HeLa, MCF-7, MDAMB-231 and MG 63 | [214] |
| 10 | Cur-CasNPs | Encapsulation of curcumin in casein nanoparticles | Enhanced bioavailability and anticancer activity against MCF-7 cell lines | [215] |
| 11 | CDD-CANPs | Encapsulation of curcumin diethyl disuccinate (CDD) in chitosan–alginate nanoparticles (CANPs) | High chemical stability upon UV light exposure, fivefold enhanced bioavailability and anticancer activity against HepG2 cell lines | [216] |
| 12 | Cur NE-CLA-n-3FA | Nanoemulsions containing curcumin stabilized with mono- and diacylglycerols structured with conjugated linoleic acid (CLA) and n-3 fatty acids (n-3FA) | Greater bioavailability | [217] |
| 13 | Cur-C3-CNPs | Nanoparticle composed of curcumin-C3 complex (curcumin, demethoxycurcumin and isdemethoxycurcumin) encapsulated in chitosan | Antioxidant and antibacterial activity | [12] |
| 14 | CLL | Nanoliposomes (formulated from salmon, rapeseed and soya lecithin) comprising of curcumin encapsulated in chitosan | Increased bioavailability and anticancer activity against MCF-7 breast cancer cell lines | [218] |
| 15 | Cur-BR liposome | Nanoliposome containing curcumin and bromocriptine synthesized by thin-film method | In vitro anticancer activity against lung cancer cells by induction of apoptosis | [219] |
| 16 | LRA-CS-CUR hydrogel | A composite hydrogel containing lotus root amylopectin (LRA)-chitosan (CS) in the ratio of 3:2 at pH 4.0 with an average size of 410.3 nm | Enhanced stability in stomach and sustained release in small intestine | [220] |
| 17 | Chitosan-PEG-Cur-hydrogel | Hydrogel comprising of chitosan, polyethylene glycol and curcumin using microwave technology at frequency of 2450 MHz, 500 Watt and 120 s time. | In vivo open incision wound healing activity | [221] |
Nanocomposites are non-homogenous materials made at the nanoscale level by combining polymers with inorganic solids. Their frameworks have been discovered to be more complex than microcomposites. Individual property structure, composition, interfacial interactions and components have a significant impact on them. The method of in situ growth and polymerization of biopolymer and inorganic matrix is the most common way to create nanocomposites [222].
Hydrogels are three-dimensional (3D) polymeric structures that are extremely swollen, hydrophilic and capable of absorbing huge volumes of water insoluble in water, due to cross-linked polymers, enmeshment or crystalline areas in its composition. Natural, synthetic or hybrid polymers can be used to make hydrogels. Biopolymer-based hydrogels have attracted a lot of attention as promising options for medicinal applications, including therapeutics delivery [223]. As a result, nanotechnology has been shown to be a very effective tool to increase the limits of native curcumin for the improvement of its therapeutic potential in combination with some key features such as high cellular uptake, bio-distribution, dissociation rates, stability in serum and sustained drug release at the target site.
5.3. Structural Analogs
Typically, curcumin analogs are divided into three groups, namely, natural derivatives from turmeric, curcumin analogs from mother nature and synthetic analogs [224] (Table 5). Curcumin, cyclocurcumin, bisdemethoxycurcumin and demethoxycurcumin (generally referred as curcuminoids) are the three major analogs originated from turmeric. Bioactive compounds that occur naturally and also possess structural similarity to curcumin are defined as curcumin analogs from mother nature. Synthetic analogs of curcumin compounds are produced by modifying the basic structure of curcumin using various chemical reactions. The major possibilities to modify curcumin structurally are making changes in the dike to functionality, aryl side chain, active methylene functionality, double bond and metal complexes [225]. Some of the synthetic analogs are EF24 [226], (1E, 6Z) 1,7-bis(13-fluoro-9-ethylcoumarin-8-yl)-5-hydroxy3-oxohepta-1,4,6-triene [227].
Table 5.
List of natural derivatives and analogs of curcumin.
| Compound Name | Origin | PubChem CID | Molecular Formula | References | |
|---|---|---|---|---|---|
| Scientific Name | Common Name | ||||
| Curcumin natural derivatives from turmeric | |||||
| Curcumin | Curcuma longa | Turmeric | 969516 | C21H20O6 | [228] |
| Bisdemethoxycurcumin | Curcuma longa | Turmeric | 5315472 | C19H16O4 | [229] |
| Cyclocurcumin | Curcuma longa | Turmeric | 69879809 | C21H20O6 | [230] |
| Demethoxycurcumin | Curcuma longa | Turmeric | 5469424 | C20H18O5 | [231] |
| Curcumin natural analogs from mother nature | |||||
| Dehydrozingerone | Zingiber officinale Roscoe | Ginger | 5354238 | C11H12O3 | [232] |
| Cassumunin B | Zingiber cassumunar | Ginger | 10054109 | C34H36O9 | [224] |
| Cassumunin A | Zingiber cassumunar | Ginger | 10460395 | C33H34O8 | [224] |
| 6-shogaol | Zingiber officinale | Ginger | 5281794 | C17H24O3 | [233] |
| 6-paradol | Zingiber officinale Roscoe | Ginger | 94378 | C17H26O3 | [234] |
| 6-gingerol | Zingiber officinale Roscoe | Ginger | 442793 | C17H26O4 | [235] |
5.4. Liposomal Curcumin
Curcumin is likely to be metabolized quickly with less photo-stability, making it an ineffective therapeutic agent in its natural form [236]. In this context, various reports suggest that these restrictions can be reverted by solubilizing the curcumin in a phospholipid bilayer, liposomes, etc., which can facilitate the delivery/bioavailability of curcumin with ease [237]. It is well known that nanoparticles (liposomes, micelles, etc.) have been considered to improve the intracellular and targeted drug delivery of curcumin, which can cross the inaccessible anatomical and physiological barriers. Thus, liposomes must be a successful method to increase the bioavailability and stability of curcumin [238]. Major preparation methods to produce curcumin liposomes include an injection method, a reversed-phase evaporation method, thin-film method, freeze–thawing method, etc., [239]. Basically, curcumin encapsulation including liposomes for any type of cancer/disease should be of enhanced bioavailability when compared to freely available curcumin. At the same time, it must be non-toxic in nature to normal cells in the resulting environment. In this context, the synthesis of liposomes can be done using a solvent-free method as well (since solvents may be toxic). For instance, De Leo et al. [240] reported the encapsulation method of curcumin liposomes for drug delivery in pH-responsive polymers using an organic free method. It is reported that liposomal curcumin was found to have enhanced pharmacological and antitumor activities put forth by improving the pharmacodynamics and pharmacokinetics. Especially, liposomal curcumin was prepared with diverse conjugates including vitamin A, polyethylene glycol, hyaluronic acid, silica and folic acid. Besides, liposomal nanoparticles encapsulated with drug combinations could sensitize cancer cells in the OS cell line (human osteosarcoma cell line) [241]. However, with the constant progress of various liposomes, curcumin liposomes should be more standardized for other diseases as well as cancer.
5.5. Curcumin Phospholipid Complex
Several studies have implied the crucial roles of phospholipids in improving the therapeutic efficiency of small molecules for those who have poor oral bioavailability [242]. Generally, amphipathic phospholipid complexes act as bioactive components to aid in passing them through the gastrointestinal cells to the blood eventually [85]. Theoretically, phospholipid complexes are explained as appropriate strategies for any bioactive small molecule. The curcumin molecule is found to have high affinity towards biological membranes and tends to penetrate them rapidly to form dimeric biological complexes. Despite being a phenolic and poorly soluble compound, curcumin can link with phospholipids (particularly phosphatidylcholine) by forming non-covalent adducts. At last, formation of these curcumin–phospholipid complexes could enhance the curcumin pharmacokinetics by stabilizing at intestinal pH values and shielding curcumin in terms of retro-Claisen hydrolysis [243,244]. Maiti et al. [245] reported that a curcumin–phospholipid complex enhanced the oral bioavailability of curcumin when compared with curcumin suspension to five folds. In another study, results of ex vivo absorption of a phospholipid–curcumin complex in Wistar rats showed that the bioavailability was significantly more enhanced than the kinetically free curcumin. In addition, pharmacokinetic study results revealed that a phospholipid–curcumin complex implied significantly high plasma concentrations and was found to be more stable when compared to natural curcumin [246]. There are various research findings that suggested the phospholipid–curcumin complex is one of the most precious methods for making the curcumin more stable and improving its bioavailability [191,247].
6. Conclusions
Curcumin, the natural polyphenol of the turmeric rhizome, is a food additive, food colorant and natural dye that is also known for its huge spectrum of therapeutic applications and has been practically used in traditional medication for decades. In addition, a relatively low dose of curcumin can provide various health benefits for people diagnosed with any health condition. In the recent past, several studies have been involved in increasing the therapeutic efficiency and clinical applications of curcumin in order to improve the treatment of various diseases and disorders. Yet, due to poor solubility and low bioavailability, the direct usage of curcumin in clinical applications is challenging. With the advent of new technologies and the introduction of various alternative therapeutic approaches, these drawbacks have also been addressed. As of today, synthetic derivatives and various nanoforms of curcumin are being utilized in several biomedical applications, large scale production, stability, bioavailability, quality control of drug loading, etc. This review illustrated the role of curcumin in terms of structure and biochemical properties, as well as mechanisms of action, in order to aid in understanding its significant therapeutic applications and valuable health benefits. Further, we elaborated on the hurdles, recent trends and future perspectives on improving the bioavailability of curcumin for the effective utilization of curcumin in biomedical applications.
7. Future Perspectives
Additional validation studies are enormously needed to address and investigate the clinical effectiveness of both free curcumin and its analogs in various human diseases, including cancer, neurodegenerative disease, etc. The important gaps identified in using curcumin for therapeutic applications and its effectiveness include a complete safety/toxicity profile and data-dependent analysis. Additionally, there is less information available relating to the nanocarriers and a suitable delivery system for curcumin for increased targeted and therapeutic applications. More research needs to be undertaken to identify the appropriate formulation and delivery system for each of the biomedical application. Furthermore, clinical trials need to be conducted to analyze the increased therapeutic efficiency of the nanoform of curcumin, structural analogs and their delivery techniques. Following research to fill these knowledge gaps can improve the magnitude and competence of curcumin in various clinical applications. In addition to this, identifying the molecular targets of curcumin and their mechanisms of action against various human diseases will help in developing more specific structural molecules that can exert precise remedial activity against the disease under study. That said, improved oral bioavailability and convincing preclinical/clinical trials of curcumin are more likely to bring this promising natural small molecule to the cutting edge by creating a “super curcumin molecule” quite soon.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pharmaceutics13122102/s1, Table S1: Molecular targets of Curcumin and its details.
Author Contributions
Conceptualization: S.-I.S., A.P. and S.P.; Writing—original draft preparation: A.P., B.B., P.M., C.S., A.S., A.V., R.J. and S.P.; Writing—review and editing: A.P., B.B., P.M. and S.P.; Supervision: S.-I.S. and S.P.; Project administration and funding acquisition: S.-I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out with the support of New Breeding Technologies Development Program (Project No. PJ014929022021), Rural Development Administration and 2021 Post-Doctoral Fellowship Program (Project No. PJ014943012021), National Institute of Agricultural Sciences, Rural Development Administration, Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dillard C.J., German J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000;80:1744–1756. doi: 10.1002/1097-0010(20000915)80:12<1744::AID-JSFA725>3.0.CO;2-W. [DOI] [Google Scholar]
- 2.Hsu C.H., Cheng A.L. Clinical Studies with Curcumin. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer; Berlin/Heidelberg, Germany: 2007. pp. 471–480. [Google Scholar]
- 3.Sharma R., Gescher A., Steward W. Curcumin: The story so far. Eur. J. Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Joe B., Vijaykumar M., Lokesh B.R. Biological Properties of Curcumin-Cellular and Molecular Mechanisms of Action. Crit. Rev. Food Sci. Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 5.Hewlings S.J., Kalman D.S. Curcumin: A Review of Its Effects on Human Health. Foods. 2017;6:92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidari S., Mahdiani S., Hashemi M., Kalalinia F. Recent advances in neurogenic and neuroprotective effects of curcumin through the induction of neural stem cells. Biotechnol. Appl. Biochem. 2020;67:430–441. doi: 10.1002/bab.1891. [DOI] [PubMed] [Google Scholar]
- 7.Vitaglione P., Lumaga R.B., Ferracane R., Radetsky I., Mennella I., Schettino R., Koder S., Shimoni E., Fogliano V. Curcumin Bioavailability from Enriched Bread: The Effect of Microencapsulated Ingredients. J. Agric. Food Chem. 2012;60:3357–3366. doi: 10.1021/jf204517k. [DOI] [PubMed] [Google Scholar]
- 8.Jafari S., McClements D. Nanotechnology Approaches for Increasing Nutrient Bioavailability. Adv. Food Nutr. Res. 2017;81:1–30. doi: 10.1016/bs.afnr.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Verma G., Gajipara A., Shelar S.B., Priyadarsini K., Hassan P. Development of water-dispersible gelatin stabilized hydroxyapatite nanoformulation for curcumin delivery. J. Drug Deliv. Sci. Technol. 2021;66:102769. doi: 10.1016/j.jddst.2021.102769. [DOI] [Google Scholar]
- 10.Ahmad N., Ahmad R., Al-Qudaihi A., Alaseel S.E., Fita I.Z., Khalid M.S., Pottoo F.H. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019;9:20192–20206. doi: 10.1039/C9RA03102B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousefi A., Yousefi R., Panahi F., Sarikhani S., Zolghadr A.R., Bahaoddini A., Khalafi-Nezhad A. Novel curcumin-based pyrano [2, 3-d] pyrimidine anti-oxidant inhibitors for α-amylase and α-glucosidase: Implications for their pleiotropic effects against diabetes complications. Int. J. Biol. Macromol. 2015;78:46–55. doi: 10.1016/j.ijbiomac.2015.03.060. [DOI] [PubMed] [Google Scholar]
- 12.Reddy D.N., Huang F.-Y., Wang S.-P., Kumar R. Synergistic Antioxidant and Antibacterial Activity of Curcumin-C3 Encapsulated Chitosan Nanoparticles. Curr. Pharm. Des. 2020;26:5021–5029. doi: 10.2174/1381612826666200609164830. [DOI] [PubMed] [Google Scholar]
- 13.Chhunchha B., Fatma N., Kubo E., Rai P., Singh S.P., Singh D.P. Curcumin abates hypoxia-induced oxidative stress based-ER stress-mediated cell death in mouse hippocampal cells (HT22) by controlling Prdx6 and NF-κB regulation. Am. J. Physiol.-Cell Physiol. 2013;304:C636–C655. doi: 10.1152/ajpcell.00345.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gera M., Sharma N., Ghosh M., Huynh D.L., Lee S.J., Min T., Kwon T., Jeong D.K. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget. 2017;8:66680–66698. doi: 10.18632/oncotarget.19164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milobedeska J., Kostanecki V., Lampe V. Structure of curcumin. Ber. Dtsch. Chem. Ges. 1910;43:2163–2170. [Google Scholar]
- 17.Daina A., Michielin O., Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karthikeyan A., Senthil N., Min T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020;11:487. doi: 10.3389/fphar.2020.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mošovská S., Petáková P., Kaliňák M., Mikulajová A. Antioxidant properties of curcuminoids isolated from Curcuma longa L. Acta Chim. Slovaca. 2016;9:130–135. doi: 10.1515/acs-2016-0022. [DOI] [Google Scholar]
- 20.Katsuyama Y., Kita T., Horinouchi S. Identification and characterization of multiple curcumin synthases from the herb Curcuma longa. FEBS Lett. 2009;583:2799–2803. doi: 10.1016/j.febslet.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Menon V.P., Sudheer A.R. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer; Berlin/Heidelberg, Germany: 2007. Antioxidant and anti-inflammatory properties of curcumin; pp. 105–125. [DOI] [PubMed] [Google Scholar]
- 23.Prasad S., Tyagi A.K. Curcumin and its analogues: A potential natural compound against HIV infection and AIDS. Food Funct. 2015;6:3412–3419. doi: 10.1039/C5FO00485C. [DOI] [PubMed] [Google Scholar]
- 24.Kang D., Li B., Luo L., Jiang W., Lu Q., Rong M., Lai R. Curcumin shows excellent therapeutic effect on psoriasis in mouse model. Biochimie. 2016;123:73–80. doi: 10.1016/j.biochi.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Lourestanpour P., Babaahmadi-Rezaei H., Shahanipour K. Curcumin as an Environmental Potent Antioxidant Decreases Risk of Arthrosclerosis. Arch. Hyg. Sci. 2017;6:105–110. doi: 10.29252/ArchHygSci.6.1.105. [DOI] [Google Scholar]
- 26.Abrahams S., Haylett W.L., Johnson G., Carr J.A., Bardien S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience. 2019;406:1–21. doi: 10.1016/j.neuroscience.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Fakhri S., Shakeryan S., Alizadeh A., Shahryari A. Effect of 6 Weeks of High Intensity Interval Training with Nano curcumin Supplement on Antioxidant Defense and Lipid Peroxidation in Overweight Girls- Clinical Trial. Iran. J. Diabetes Obes. 2019;11:173–180. doi: 10.18502/ijdo.v11i3.2606. [DOI] [Google Scholar]
- 28.Sharifi-Rad J., El Rayess Y., Rizk A.A., Sadaka C., Zgheib R., Zam W., Sestito S., Rapposelli S., Neffe-Skocińska K., Zielińska D., et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020;11:01021. doi: 10.3389/fphar.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung S.S., Dutta P., Chard N., Wu Y., Chen Q.-H., Chen G., Vadgama J. A novel curcumin analog inhibits canonical and non-canonical functions of telomerase through STAT3 and NF-κB inactivation in colorectal cancer cells. Oncotarget. 2019;10:4516–4531. doi: 10.18632/oncotarget.27000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong K.E., Ngai S.C., Chan K.-G., Lee L.-H., Goh B.H., Chuah L.-H. Curcumin Nanoformulations for Colorectal Cancer: A Review. Front. Pharmacol. 2019;10:152. doi: 10.3389/fphar.2019.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan A.Q., Ahmed E.I., Elareer N., Fathima H., Prabhu K.S., Siveen K.S., Kulinski M., Azizi F., Dermime S., Ahmad A., et al. Curcumin-Mediated Apoptotic Cell Death in Papillary Thyroid Cancer and Cancer Stem-Like Cells through Targeting of the JAK/STAT3 Signaling Pathway. Int. J. Mol. Sci. 2020;21:438. doi: 10.3390/ijms21020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groundwater P.W., Narlawar R., Liao V.W.Y., Bhattacharya A., Srivastava S., Kunal K., Doddareddy M., Oza P.M., Mamidi R., Marrs E.C.L., et al. A Carbocyclic Curcumin Inhibits Proliferation of Gram-Positive Bacteria by Targeting FtsZ. Biochemistry. 2017;56:514–524. doi: 10.1021/acs.biochem.6b00879. [DOI] [PubMed] [Google Scholar]
- 33.Da Silva A.C., De Freitas Santos P.D., Do Prado Silva J.T., Leimann F.V., Bracht L., Gonçalves O.H. Impact of curcumin nanoformulation on its antimicrobial activity. Trends Food Sci. Technol. 2018;72:74–82. doi: 10.1016/j.tifs.2017.12.004. [DOI] [Google Scholar]
- 34.Rai M., Ingle A.P., Pandit R., Paralikar P., Anasane N., Dos Santos C.A. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Expert Rev. Anti-Infect. Ther. 2020;18:367–379. doi: 10.1080/14787210.2020.1730815. [DOI] [PubMed] [Google Scholar]
- 35.Farhood B., Mortezaee K., Goradel N.H., Khanlarkhani N., Salehi E., Nashtaei M.S., Najafi M., Sahebkar A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2018;234:5728–5740. doi: 10.1002/jcp.27442. [DOI] [PubMed] [Google Scholar]
- 36.Kumari A., Singh D.K., Dash D., Singh R. Intranasal curcumin protects against LPS-induced airway remodeling by modulating toll-like receptor-4 (TLR-4) and matrixmetalloproteinase-9 (MMP-9) expression via affecting MAP kinases in mouse model. Inflammopharmacology. 2018;27:731–748. doi: 10.1007/s10787-018-0544-3. [DOI] [PubMed] [Google Scholar]
- 37.Lee S.E., Park H.R., Jeon S., Han D., Park Y.S. Curcumin Attenuates Acrolein-induced COX-2 Expression and Prostaglandin Production in Human Umbilical Vein Endothelial Cells. J. Lipid Atheroscler. 2020;9:184–194. doi: 10.12997/jla.2020.9.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X., Chen X., Shi X., Gao Z., Guo Z. Curcumin attenuates endothelial cell fibrosis through inhibiting endothelial–interstitial transformation. Clin. Exp. Pharmacol. Physiol. 2020;47:1182–1192. doi: 10.1111/1440-1681.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brahmkhatri V.P., Sharma N., Sunanda P., D’Souza A., Raghothama S., Atreya H.S. Curcumin nanoconjugate inhibits aggregation of N-terminal region (Aβ-16) of an amyloid beta peptide. New J. Chem. 2018;42:19881–19892. doi: 10.1039/C8NJ03541E. [DOI] [Google Scholar]
- 40.Hotsumi M., Tajiri M., Nikaido Y., Sato T., Makabe K., Konno H. Design, synthesis, and evaluation of a water soluble C5-monoketone type curcumin analogue as a potent amyloid β aggregation inhibitor. Bioorg. Med. Chem. Lett. 2019;29:2157–2161. doi: 10.1016/j.bmcl.2019.06.052. [DOI] [PubMed] [Google Scholar]
- 41.Gutierres V.O., Assis R.P., Arcaro C.A., Oliveira J.O., de Lima T.F.O., Beretta A.L.R.Z., Costa P.I., Baviera A.M., Brunetti I.L. Curcumin improves the effect of a reduced insulin dose on glycemic control and oxidative stress in streptozotocin-diabetic rats. Phytother. Res. 2019;33:976–988. doi: 10.1002/ptr.6291. [DOI] [PubMed] [Google Scholar]
- 42.Den Hartogh D.J., Gabriel A., Tsiani E. Antidiabetic Properties of Curcumin I: Evidence from In Vitro Studies. Nutrients. 2020;12:118. doi: 10.3390/nu12010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomeh M.A., Hadianamrei R., Zhao X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019;20:1033. doi: 10.3390/ijms20051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghasemi F., Shafiee M., Banikazemi Z., Pourhanifeh M.H., Khanbabaei H., Shamshirian A., Moghadam S.A., ArefNezhad R., Sahebkar A., Avan A., et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol.-Res. Pract. 2019;215:152556. doi: 10.1016/j.prp.2019.152556. [DOI] [PubMed] [Google Scholar]
- 45.Liu J.-Y., Lin S.-J., Lin J.-K. Inhibitory effects of curcumin on protein kinase C activity induced by 12-O-tetradecanoyl-phorbol-13-acetate in NIH 3T3 cells. Carcinogenesis. 1993;14:857–861. doi: 10.1093/carcin/14.5.857. [DOI] [PubMed] [Google Scholar]
- 46.Mortezaee K., Salehi E., Mirtavoos-Mahyari H., Motevaseli E., Najafi M., Farhood B., Rosengren R.J., Sahebkar A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 2019;234:12537–12550. doi: 10.1002/jcp.28122. [DOI] [PubMed] [Google Scholar]
- 47.Liu E., Wu J., Cao W., Zhang J., Liu W., Jiang X., Zhang X. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J. Neuro-Oncol. 2007;85:263–270. doi: 10.1007/s11060-007-9421-4. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Yu J., Cui R., Lin J., Ding X. Curcumin in Treating Breast Cancer: A Review. J. Lab. Autom. 2016;21:723–731. doi: 10.1177/2211068216655524. [DOI] [PubMed] [Google Scholar]
- 49.Liu Q., Loo W.T., Sze SC W., Tong Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFκB, cyclinD and MMP-1 transcription. Phytomedicine. 2009;16:916–922. doi: 10.1016/j.phymed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Chiablaem K., Lirdprapamongkol K., Keeratichamroen S., Surarit R., Svasti J. Curcumin suppresses vasculogenic mimicry capacity of hepatocellular carcinoma cells through STAT3 and PI3K/AKT inhibition. Anticancer. Res. 2014;34:1857–1864. [PubMed] [Google Scholar]
- 51.Ghandadi M., Sahebkar A. Curcumin: An Effective Inhibitor of Interleukin-6. Curr. Pharm. Des. 2017;23:921–931. doi: 10.2174/1381612822666161006151605. [DOI] [PubMed] [Google Scholar]
- 52.Chen L.X., He Y.J., Zhao S.Z., Wu J.G., Wang J.T., Zhu L.M., Lin T.T., Sun B.C., Li X.R. Inhibition of tumor growth and vasculogenic mimicry by curcumin through down-regulation of the EphA2/PI3K/MMP pathway in a murine choroidal melanoma model. Cancer Biol. Ther. 2011;11:229–235. doi: 10.4161/cbt.11.2.13842. [DOI] [PubMed] [Google Scholar]
- 53.Starok M., Preira P., Vayssade M., Haupt K., Salomé L., Rossi C. EGFR Inhibition by Curcumin in Cancer Cells: A Dual Mode of Action. Biomacromolecules. 2015;16:1634–1642. doi: 10.1021/acs.biomac.5b00229. [DOI] [PubMed] [Google Scholar]
- 54.Pavan A.R., Da Silva G.D.B., Jornada D.H., Chiba D.E., Fernandes G.F.D.S., Chin C.M., Dos Santos J.L. Unraveling the Anticancer Effect of Curcumin and Resveratrol. Nutrients. 2016;8:628. doi: 10.3390/nu8110628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahmani A.H., Al Zohairy M.A., Aly S.M., Khan M.A. Curcumin: A Potential Candidate in Prevention of Cancer via Modulation of Molecular Pathways. BioMed Res. Int. 2014;2014:761608. doi: 10.1155/2014/761608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tourkina E., Gooz P., Oates J.C., Ludwicka-Bradley A., Silver R.M., Hoffman S. Curcumin-induced apoptosis in scleroderma lung fibroblasts: Role of protein kinase Cε. Am. J. Respir. Cell Mol. Biol. 2004;31:28–35. doi: 10.1165/rcmb.2003-0354OC. [DOI] [PubMed] [Google Scholar]
- 57.Yu J., Zhou X., He X., Dai M., Zhang Q. Curcumin induces apoptosis involving bax/bcl-2 in human hepatoma SMMC-7721 cells. Asian Pac. J. Cancer Prev. 2011;12:1925–1929. [PubMed] [Google Scholar]
- 58.Ramachandran C., You W. Differential sensitivity of human mammary epithelial and breast carcinoma cell lines to curcumin. Breast Cancer Res. Treat. 1999;54:269–278. doi: 10.1023/A:1006170224414. [DOI] [PubMed] [Google Scholar]
- 59.Zou P., Zhang J., Xia Y., Kanchana K., Guo G., Chen W., Huang Y., Wang Z., Yang S., Liang G. ROS generation mediates the anti-cancer effects of WZ35 via activating JNK and ER stress apoptotic pathways in gastric cancer. Oncotarget. 2015;6:5860–5876. doi: 10.18632/oncotarget.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harper J.V., Brooks G. Cell Cycle Control. Springer; Berlin/Heidelberg, Germany: 2005. The mammalian cell cycle; pp. 113–153. [Google Scholar]
- 61.Wu B., Yao H., Wang S., Xu R. DAPK1 modulates a curcumin-induced G2/M arrest and apoptosis by regulating STAT3, NF-κB, and caspase-3 activation. Biochem. Biophys. Res. Commun. 2013;434:75–80. doi: 10.1016/j.bbrc.2013.03.063. [DOI] [PubMed] [Google Scholar]
- 62.Mudduluru G., George-William J.N., Muppala S., Asangani I.A., Kumarswamy R., Nelson L.D., Allgayer H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011;31:185–197. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 63.Van Erk M.J., Teuling E., Staal Y.C., Huybers S., Van Bladeren P.J., Aarts J.M., Van Ommen B. Time- and dose-dependent effects of curcumin on gene expression in human colon cancer cells. J. Carcinog. 2004;3:8. doi: 10.1186/1477-3163-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fares J., Fares M.Y., Khachfe H.H., Salhab H.A., Fares Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng Y., Verron E., Rohanizadeh R. Molecular Mechanisms of Anti-metastatic Activity of Curcumin. Anticancer Res. 2016;36:5639–5648. doi: 10.21873/anticanres.11147. [DOI] [PubMed] [Google Scholar]
- 66.Yang C.-L., Liu Y.-Y., Ma Y.-G., Xue Y.-X., Liu D.-G., Ren Y., Liu X.-B., Li Y., Li Z. Curcumin Blocks Small Cell Lung Cancer Cells Migration, Invasion, Angiogenesis, Cell Cycle and Neoplasia through Janus Kinase-STAT3 Signalling Pathway. PLoS ONE. 2012;7:e37960. doi: 10.1371/journal.pone.0037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khan M.A., Gahlot S., Majumdar S. Oxidative Stress Induced by Curcumin Promotes the Death of Cutaneous T-cell Lymphoma (HuT-78) by Disrupting the Function of Several Molecular Targets. Mol. Cancer Ther. 2012;11:1873–1883. doi: 10.1158/1535-7163.MCT-12-0141. [DOI] [PubMed] [Google Scholar]
- 68.Jung E.M., Lim J.H., Lee T.J., Park J.-W., Choi K.S., Kwon T.K. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5) Carcinogenesis. 2005;26:1905–1913. doi: 10.1093/carcin/bgi167. [DOI] [PubMed] [Google Scholar]
- 69.Yang X., Li Z., Wu Q., Chen S., Yi C., Gong C. TRAIL and curcumin codelivery nanoparticles enhance TRAIL-induced apoptosis through upregulation of death receptors. Drug Deliv. 2017;24:1526–1536. doi: 10.1080/10717544.2017.1384863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L., Chen X., Du Z., Li G., Chen M., Chen X., Liang G., Chen T. Curcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase γ depletion disrupting cellular bioenergetics. J. Exp. Clin. Cancer Res. 2017;36:1–14. doi: 10.1186/s13046-017-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larasati Y., Yoneda-Kato N., Nakamae I., Yokoyama T., Meiyanto E., Kato J.-Y. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci. Rep. 2018;8:2039. doi: 10.1038/s41598-018-20179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halliwell B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 73.Ak T., Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 74.Reddy A., Lokesh B. Effect of curcumin and eugenol on iron-induced hepatic toxicity in rats. Toxicology. 1996;107:39–45. doi: 10.1016/0300-483X(95)03199-P. [DOI] [PubMed] [Google Scholar]
- 75.Jovanovic S.V., Steenken S., Boone A.C.W., Simic M.G. H-Atom Transfer Is a Preferred Antioxidant Mechanism of Curcumin. J. Am. Chem. Soc. 1999;121:9677–9681. doi: 10.1021/ja991446m. [DOI] [Google Scholar]
- 76.Barclay L.R.C., Vinqvist M.R., Mukai K., Goto H., Hashimoto Y., Tokunaga A., Uno H. On the Antioxidant Mechanism of Curcumin: Classical Methods Are Needed To Determine Antioxidant Mechanism and Activity. Org. Lett. 2000;2:2841–2843. doi: 10.1021/ol000173t. [DOI] [PubMed] [Google Scholar]
- 77.Priyadarsini K., Maity D.K., Naik G., Kumar M., Unnikrishnan M., Satav J., Mohan H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free. Radic. Biol. Med. 2003;35:475–484. doi: 10.1016/S0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- 78.Qiao L., Kozoni V., Tsioulias G.J., Koutsos M.I., Hanif R., Shiff S.J., Rigas B. Selected eicosanoids increase the proliferation rate of human colon carcinoma cell lines and mouse colonocytes in vivo. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1995;1258:215–223. doi: 10.1016/0005-2760(95)00100-Q. [DOI] [PubMed] [Google Scholar]
- 79.Fang H., Lin T.-S., Lin J.-P., Wu Y., Chow K.-C., Wang L.-S. Cyclooxygenase-2 in human non-small cell lung cancer. Eur. J. Surg. Oncol. EJSO. 2003;29:171–177. doi: 10.1053/ejso.2002.1316. [DOI] [PubMed] [Google Scholar]
- 80.Surh Y.J., Chun K.S. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer; Berlin/Heidelberg, Germany: 2007. Cancer chemopreventive effects of curcumin; pp. 149–172. [DOI] [PubMed] [Google Scholar]
- 81.Sarkar F.H., Li Y. Cell signaling pathways altered by natural chemopreventive agents. Mutat. Res./Fundam. Mol. Mech. Mutagenesis. 2004;555:53–64. doi: 10.1016/j.mrfmmm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 82.Singh S., Aggarwal B.B. Activation of Transcription Factor NF-κB Is Suppressed by Curcumin (Diferuloylmethane)(*) J. Biol. Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 83.Rudrappa T., Bais H.P. Curcumin, a Known Phenolic from Curcuma longa, Attenuates the Virulence of Pseudomonas aeruginosa PAO1 in Whole Plant and Animal Pathogenicity Models. J. Agric. Food Chem. 2008;56:1955–1962. doi: 10.1021/jf072591j. [DOI] [PubMed] [Google Scholar]
- 84.Mohammed N.A., Habil N.Y. Evaluation of antimicrobial activity of curcumin against two oral bacteria. Autom. Control Intell. Syst. 2015;3:18. doi: 10.11648/j.acis.s.2015030201.14. [DOI] [Google Scholar]
- 85.Anand P., Thomas S.G., Kunnumakkara A.B., Sundaram C., Harikumar K.B., Sung B., Tharakan S.T., Misra K., Priyadarsini I.K., Rajasekharan K.N., et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 86.Krishnankutty K., Venugopalan P. Metal chelates of curcuminoids. Synth. React. Inorg. Met.-Org. Chem. 1998;28:1313–1325. doi: 10.1080/00945719809349407. [DOI] [Google Scholar]
- 87.Han S., Yang Y. Antimicrobial activity of wool fabric treated with curcumin. Dye. Pigment. 2005;64:157–161. doi: 10.1016/j.dyepig.2004.05.008. [DOI] [Google Scholar]
- 88.Varaprasad K., Mohan Y.M., Vimala K., Raju K.M. Synthesis and characterization of hydrogel-silver nanoparticle-curcumin composites for wound dressing and antibacterial application. J. Appl. Polym. Sci. 2011;121:784–796. doi: 10.1002/app.33508. [DOI] [Google Scholar]
- 89.Reshma A., Priyadarisini V., Amutha K. Sustainable antimicrobial finishing of fabrics using natural bioactive agents—A review. Int. J. Life Sci. Pharma Res. 2018;8:10–20. doi: 10.22376/ijpbs/lpr.2018.8.4.L10-20. [DOI] [Google Scholar]
- 90.Reddy N., Han S., Zhao Y., Yang Y. Antimicrobial activity of cotton fabrics treated with curcumin. J. Appl. Polym. Sci. 2012;127:2698–2702. doi: 10.1002/app.37613. [DOI] [Google Scholar]
- 91.Gaffer H., Mashaly H., Abdel-Rhman S., Hammouda M. Synthesis of novel dyes based on curcumin for the creation of antibacterial silk fabrics. Pigment. Resin Technol. 2017;46:478–484. doi: 10.1108/PRT-11-2016-0112. [DOI] [Google Scholar]
- 92.Arshad N., Zia K.M., Hussain M.T., Zuber M., Arshad M.M. Synthesis of novel curcumin-based aqueous polyurethane dispersions for medical textile diligences with potential of antibacterial activities. Polym. Bull. 2021:1–17. doi: 10.1007/s00289-021-03871-y. [DOI] [Google Scholar]
- 93.Varaprasad K., Vimala K., Ravindra S., Reddy N.N., Reddy G.V.S., Raju K.M. Fabrication of silver nanocomposite films impregnated with curcumin for superior antibacterial applications. J. Mater. Sci. Mater. Med. 2011;22:1863–1872. doi: 10.1007/s10856-011-4369-5. [DOI] [PubMed] [Google Scholar]
- 94.Bajpai S., Ahuja S., Chand N., Bajpai M. Nano cellulose dispersed chitosan film with Ag NPs/Curcumin: An in vivo study on Albino Rats for wound dressing. Int. J. Biol. Macromol. 2017;104:1012–1019. doi: 10.1016/j.ijbiomac.2017.06.096. [DOI] [PubMed] [Google Scholar]
- 95.Kamel N.A., Soliman A.A., Rozik N.N., Abd-Elmessieh S.L. Biophysical investigation of curcumin based nanocomposite for wound dressing application. J. Appl. Pharm. Sci. 2018;8:35–44. [Google Scholar]
- 96.Agostinis P., Berg K., Cengel K.A., Foster T.H., Girotti A.W., Gollnick S.O., Hahn S.M., Hamblin M.R., Juzeniene A., Kessel D., et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cieplik F., Deng D., Crielaard W., Buchalla W., Hellwig E., Al-Ahmad A., Maisch T. Antimicrobial photodynamic therapy–what we know and what we don’t. Crit. Rev. Microbiol. 2018;44:571–589. doi: 10.1080/1040841X.2018.1467876. [DOI] [PubMed] [Google Scholar]
- 98.Hamblin M.R., Hasan T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hegge A.B., Nielsen T.T., Larsen K.L., Bruzell E., Tønnesen H.H. Impact of Curcumin Supersaturation in Antibacterial Photodynamic Therapy—Effect of Cyclodextrin Type and Amount: Studies on Curcumin and Curcuminoides XLV. J. Pharm. Sci. 2012;101:1524–1537. doi: 10.1002/jps.23046. [DOI] [PubMed] [Google Scholar]
- 100.Wikene K., Hegge A.B., Bruzell E., Tønnesen H.H. Formulation and characterization of lyophilized curcumin solid dispersions for antimicrobial photodynamic therapy (aPDT): Studies on curcumin and curcuminoids LII. Drug Dev. Ind. Pharm. 2014;41:969–977. doi: 10.3109/03639045.2014.919315. [DOI] [PubMed] [Google Scholar]
- 101.Manoil D., Filieri A., Gameiro C., Lange N., Schrenzel J., Wataha J.C., Bouillaguet S. Flow cytometric assessment of Streptococcus mutans viability after exposure to blue light-activated curcumin. Photodiagnosis Photodyn. Ther. 2014;11:372–379. doi: 10.1016/j.pdpdt.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Najafi S., Khayamzadeh M., Paknejad M., Poursepanj G., Fard M.J.K., Bahador A. An In Vitro Comparison of Antimicrobial Effects of Curcumin-Based Photodynamic Therapy and Chlorhexidine, on Aggregatibacter actinomycetemcomitans. J. Lasers Med Sci. 2016;7:21–25. doi: 10.15171/jlms.2016.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sakima V.T., Barbugli P.A., Cerri P.S., Chorilli M., Carmello J.C., Pavarina A.C., Mima E.G.D.O. Antimicrobial Photodynamic Therapy Mediated by Curcumin-Loaded Polymeric Nanoparticles in a Murine Model of Oral Candidiasis. Molecules. 2018;23:2075. doi: 10.3390/molecules23082075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dias L.D., Blanco K.C., Mfouo-Tynga I.S., Inada N.M., Bagnato V.S. Curcumin as a photosensitizer: From molecular structure to recent advances in antimicrobial photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020;45:100384. doi: 10.1016/j.jphotochemrev.2020.100384. [DOI] [Google Scholar]
- 105.Chanda S., Rakholiya K. Combination therapy: Synergism between natural plant extracts and antibiotics against infectious diseases. Microbiol Book Ser. 2011;1:520–529. [Google Scholar]
- 106.Dai C., Wang Y., Sharma G., Shen J., Velkov T., Xiao X. Polymyxins–Curcumin Combination Antimicrobial Therapy: Safety Implications and Efficacy for Infection Treatment. Antioxidants. 2020;9:506. doi: 10.3390/antiox9060506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Güran M., Şanlıtürk G., Kerküklü N.R., Altundağ E.M., Yalçın A.S. Combined effects of quercetin and curcumin on anti-inflammatory and antimicrobial parameters in vitro. Eur. J. Pharmacol. 2019;859:172486. doi: 10.1016/j.ejphar.2019.172486. [DOI] [PubMed] [Google Scholar]
- 108.Betts J.W., Wareham D.W. In vitro activity of curcumin in combination with epigallocatechin gallate (EGCG) versus multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 2014;14:172. doi: 10.1186/1471-2180-14-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bahari S., Zeighami H., Mirshahabi H., Roudashti S., Haghi F. Inhibition of Pseudomonas aeruginosa quorum sensing by subinhibitory concentrations of curcumin with gentamicin and azithromycin. J. Glob. Antimicrob. Resist. 2017;10:21–28. doi: 10.1016/j.jgar.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 110.Basniwal R.K., Buttar H.S., Jain V.K., Jain N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011;59:2056–2061. doi: 10.1021/jf104402t. [DOI] [PubMed] [Google Scholar]
- 111.Hatamie S., Nouri M., Karandikar S., Kulkarni A., Dhole S., Phase D., Kale S. Complexes of cobalt nanoparticles and polyfunctional curcumin as antimicrobial agents. Mater. Sci. Eng. C. 2012;32:92–97. doi: 10.1016/j.msec.2011.10.002. [DOI] [Google Scholar]
- 112.Krausz A.E., Adler B.L., Cabral V., Navati M., Doerner J., Charafeddine R.A., Chandra D., Liang H., Gunther L., Clendaniel A., et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomed. Nanotechnol. Biol. Med. 2015;11:195–206. doi: 10.1016/j.nano.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shlar I., Poverenov E., Vinokur Y., Horev B., Droby S., Rodov V. High-Throughput Screening of Nanoparticle-Stabilizing Ligands: Application to Preparing Antimicrobial Curcumin Nanoparticles by Antisolvent Precipitation. Nano-Micro Lett. 2014;7:68–79. doi: 10.1007/s40820-014-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aghamoosa M., Arbabi-Bidgoli S., Ghafari S., Sabokbar A., Harzandi N. Preclinical Evaluation of Silver-Curcumin Nano-gel: A complete assessment on a new topical antimicrobial product for burn. J. Nanoanal. 2020;7:62–72. [Google Scholar]
- 115.Martins C.V.B., Da Silva D.L., Neres A.T.M., Magalhaes T.F.F., Watanabe G.A., Modolo L., Sabino A., De Fatima A., De Resende M.A. Curcumin as a promising antifungal of clinical interest. J. Antimicrob. Chemother. 2008;63:337–339. doi: 10.1093/jac/dkn488. [DOI] [PubMed] [Google Scholar]
- 116.Cheraghipour K., Marzban A., Ezatpour B., Khanizadeh S., Koshki J., Service K.L.P.V. Antiparasitic properties of curcumin: A review. AIMS Agric. Food. 2018;4:1–18. doi: 10.3934/agrfood.2018.4.561. [DOI] [Google Scholar]
- 117.Mathew D., Hsu W.-L. Antiviral potential of curcumin. J. Funct. Foods. 2017;40:692–699. doi: 10.1016/j.jff.2017.12.017. [DOI] [Google Scholar]
- 118.Watnick P., Kolter R. Biofilm, city of microbes. J. Bacteriol. 2000;182:2675–2679. doi: 10.1128/JB.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Priya A., Pandian S.K. Piperine Impedes Biofilm Formation and Hyphal Morphogenesis of Candida albicans. Front. Microbiol. 2020;11:756. doi: 10.3389/fmicb.2020.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sethupathy S., Prasath K.G., Ananthi S., Mahalingam S., Balan S.Y., Pandian S.K. Proteomic analysis reveals modulation of iron homeostasis and oxidative stress response in Pseudomonas aeruginosa PAO1 by curcumin inhibiting quorum sensing regulated virulence factors and biofilm production. J. Proteom. 2016;145:112–126. doi: 10.1016/j.jprot.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 121.Raorane C.J., Lee J.-H., Kim Y.-G., Rajasekharan S.K., García-Contreras R., Lee J. Antibiofilm and Antivirulence Efficacies of Flavonoids and Curcumin Against Acinetobacter baumannii. Front. Microbiol. 2019;10:990. doi: 10.3389/fmicb.2019.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang J., Chen B., Li H., Zeng Q.-H., Wang J.J., Liu H., Pan Y., Zhao Y. Enhanced antibacterial and antibiofilm functions of the curcumin-mediated photodynamic inactivation against Listeria monocytogenes. Food Control. 2020;108:106886. doi: 10.1016/j.foodcont.2019.106886. [DOI] [Google Scholar]
- 123.Devaraj S., Jagannathan N., Neelakantan P. Antibiofilm efficacy of photoactivated curcumin, triple and double antibiotic paste, 2% chlorhexidine and calcium hydroxide against Enterococcus fecalis in vitro. Sci. Rep. 2016;6:24797. doi: 10.1038/srep24797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tan Y., Leonhard M., Moser D., Ma S., Schneider-Stickler B. Antibiofilm efficacy of curcumin in combination with 2-aminobenzimidazole against single- and mixed-species biofilms of Candida albicans and Staphylococcus aureus. Colloids Surfaces B Biointerfaces. 2018;174:28–34. doi: 10.1016/j.colsurfb.2018.10.079. [DOI] [PubMed] [Google Scholar]
- 125.Rahmani A.H., Alsahli M.A., Aly S.M., Khan M.A., Aldebasi Y.H. Role of Curcumin in Disease Prevention and Treatment. Adv. Biomed. Res. 2018;7:38. doi: 10.4103/abr.abr_147_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brettschneider J., Del Tredici K., Lee V.M.-Y., Trojanowski J.Q. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat. Rev. Neurosci. 2015;16:109–120. doi: 10.1038/nrn3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gitler A.D., Dhillon P., Shorter J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017;10:499–502. doi: 10.1242/dmm.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mattson M.P. Infectious agents and age-related neurodegenerative disorders. Ageing Res. Rev. 2004;3:105–120. doi: 10.1016/j.arr.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bondi M.W., Edmonds E.C., Salmon D.P. Alzheimer’s disease: Past, present, and future. J. Int. Neuropsychol. Soc. 2017;23:818–831. doi: 10.1017/S135561771700100X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim S.H., Kandiah N., Hsu J.L., Suthisisang C., Udommongkol C., Dash A. Beyond symptomatic effects: Potential of donepezil as a neuroprotective agent and disease modifier in Alzheimer’s disease. Br. J. Pharmacol. 2017;174:4224–4232. doi: 10.1111/bph.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Utomo R.Y., Asawa Y., Okada S., Ban H.S., Yoshimori A., Bajorath J., Nakamura H. Development of curcumin-based amyloid β aggregation inhibitors for Alzheimer’s disease using the SAR matrix approach. Bioorg. Med. Chem. 2021;46:116357. doi: 10.1016/j.bmc.2021.116357. [DOI] [PubMed] [Google Scholar]
- 132.Kim H.S., Lee J.H., Lee J.P., Kim E.M., Chang K.A., Park C.H., Jeong S.J., Wittendorp M.C., Seo J.H., Choi S.H., et al. Amyloid β peptide induces cytochrome c release from isolated mitochondria. Neuroreport. 2002;13:1989–1993. doi: 10.1097/00001756-200210280-00032. [DOI] [PubMed] [Google Scholar]
- 133.DeMaagd G., Philip A. Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharm. Ther. 2015;40:504. [PMC free article] [PubMed] [Google Scholar]
- 134.Rizek P., Kumar N., Jog M.S. An update on the diagnosis and treatment of Parkinson disease. Can. Med. Assoc. J. 2016;188:1157–1165. doi: 10.1503/cmaj.151179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mythri R.B., Srinivas Bharath M.M. Curcumin: A potential neuroprotective agent in Parkinson’s disease. Curr. Pharm. Des. 2012;18:91–99. doi: 10.2174/138161212798918995. [DOI] [PubMed] [Google Scholar]
- 136.Nguyen T.T., Vuu M.D., Huynh M.A., Yamaguchi M., Tran L.T., Dang T.P.T. Curcumin Effectively Rescued Parkinson’s Disease-Like Phenotypes in a Novel Drosophila melanogaster Model with dUCH Knockdown. Oxidative Med. Cell. Longev. 2018;2018:2038267. doi: 10.1155/2018/2038267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sharma N., Nehru B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology. 2017;26:349–360. doi: 10.1007/s10787-017-0402-8. [DOI] [PubMed] [Google Scholar]
- 138.Yao E.C., Xue L. Therapeutic Effects of Curcumin on Alzheimer’s Disease. Adv. Alzheimer’s Dis. 2014;03:145–159. doi: 10.4236/aad.2014.34014. [DOI] [Google Scholar]
- 139.Heidelbauch J., Bruderly M. Cirrhosis and chronic liver failure: Part I Diagnosis and evaluation. Am. Fam. Physician. 2006;74:756–762. [PubMed] [Google Scholar]
- 140.Khan H., Ullah H., Nabavi S.M. Mechanistic insights of hepatoprotective effects of curcumin: Therapeutic updates and future prospects. Food Chem. Toxicol. 2018;124:182–191. doi: 10.1016/j.fct.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 141.Girish C., Pradhan S.C. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride-induced liver toxicity in mice. J. Pharmacol. Pharmacother. 2012;3:149–155. doi: 10.4103/0976-500X.95515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.El Swefy S., Hasan R.A., Ibrahim A.A.S., Mahmoud M.F. Curcumin and hemopressin treatment attenuates cholestasis-induced liver fibrosis in rats: Role of CB1 receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015;389:103–116. doi: 10.1007/s00210-015-1181-7. [DOI] [PubMed] [Google Scholar]
- 143.Kyung E.J., Kim H.B., Hwang E.S., Lee S., Choi B.K., Kim J.W., Kim H.J., Lim S.M., Kwon O.I., Woo E.J. Evaluation of Hepatoprotective Effect of Curcumin on Liver Cirrhosis Using a Combination of Biochemical Analysis and Magnetic Resonance-Based Electrical Conductivity Imaging. Mediat. Inflamm. 2018;2018:5491797. doi: 10.1155/2018/5491797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luty J., Ruckemann-Dziurdzińska K., Witkowski J.M., Bryl E. Immunological aspects of autoimmune thyroid disease—Complex interplay between cells and cytokines. Cytokine. 2019;116:128–133. doi: 10.1016/j.cyto.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 145.Daily J.W., Yang M., Park S. Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food. 2016;19:717–729. doi: 10.1089/jmf.2016.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Amalraj A., Varma K., Ac K.V., Divya C., Kunnumakkara A.B., Stohs S.J., Gopi S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food. 2017;20:1022–1030. doi: 10.1089/jmf.2017.3930. [DOI] [PubMed] [Google Scholar]
- 147.Moon D.-O., Kim M.-O., Choi Y.H., Park Y.-M., Kim G.-Y. Curcumin attenuates inflammatory response in IL-1β-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int. Immunopharmacol. 2010;10:605–610. doi: 10.1016/j.intimp.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 148.Huang G., Xu Z., Huang Y., Duan X., Gong W., Zhang Y., Fan J., He F. Curcumin Protects Against Collagen-Induced Arthritis via Suppression of BAFF Production. J. Clin. Immunol. 2012;33:550–557. doi: 10.1007/s10875-012-9839-0. [DOI] [PubMed] [Google Scholar]
- 149.Cai H., Zheng Z., Sun Y., Liu Z., Zhang M., Li C. The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des. Dev. Ther. 2015;9:4931–4942. doi: 10.2147/DDDT.S90147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fan Z., Li J., Liu J., Jiao H., Liu B. Anti-Inflammation and Joint Lubrication Dual Effects of a Novel Hyaluronic Acid/Curcumin Nanomicelle Improve the Efficacy of Rheumatoid Arthritis Therapy. ACS Appl. Mater. Interfaces. 2018;10:23595–23604. doi: 10.1021/acsami.8b06236. [DOI] [PubMed] [Google Scholar]
- 151.Azhdari M., Karandish M., Mansoori A. Metabolic benefits of curcumin supplementation in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Phytotherapy Res. 2019;33:1289–1301. doi: 10.1002/ptr.6323. [DOI] [PubMed] [Google Scholar]
- 152.Rezaianzadeh A., Namayandeh S.-M., Sadr S.-M. National Cholesterol Education Program Adult Treatment Panel III Versus International Diabetic Federation Definition of Metabolic Syndrome, Which One is Associated with Diabetes Mellitus and Coronary Artery Disease? Int. J. Prev. Med. 2012;3:552–558. [PMC free article] [PubMed] [Google Scholar]
- 153.Sahebkar A. Curcuminoids for the management of hypertriglyceridaemia. Nat. Rev. Cardiol. 2014;11:123. doi: 10.1038/nrcardio.2013.140-c1. [DOI] [PubMed] [Google Scholar]
- 154.Akbari M., Lankarani K.B., Tabrizi R., Ghayour-Mobarhan M., Peymani P., Ferns G., Ghaderi A., Asemi Z. The Effects of Curcumin on Weight Loss among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2019;10:649. doi: 10.3389/fphar.2019.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Akbik D., Ghadiri M., Chrzanowski W., Rohanizadeh R. Curcumin as a wound healing agent. Life Sci. 2014;116:1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 156.Emiroglu G., Coskun Z.O., Kalkan Y., Erdivanli O.C., Tumkaya L., Terzi S., Özgür A., Demirci M., Dursun E. The Effects of Curcumin on Wound Healing in a Rat Model of Nasal Mucosal Trauma. Evid.-Based Complement. Altern. Med. 2017;2017:9452392. doi: 10.1155/2017/9452392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mohanty C., Sahoo S.K. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31:6597–6611. doi: 10.1016/j.biomaterials.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 158.Li X., Nan K., Li L., Zhang Z., Chen H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly(ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr. Polym. 2011;88:84–90. doi: 10.1016/j.carbpol.2011.11.068. [DOI] [Google Scholar]
- 159.Dhurai B., Saraswathy N., Maheswaran R., Sethupathi P., Vanitha P., Vigneshwaran S., Rameshbabu V. Electrospinning of curcumin loaded chitosan/poly (lactic acid) nanofilm and evaluation of its medicinal characteristics. Front. Mater. Sci. 2013;7:350–361. doi: 10.1007/s11706-013-0222-8. [DOI] [Google Scholar]
- 160.Tong W.Y., bin Abdullah A.Y.K., bin Wahid M.I.A., Hossain M., Ring L.C., Lazim Y., Tan W.N. Antimicrobial wound dressing film utilizing cellulose nanocrystal as drug delivery system for curcumin. Cellulose. 2018;25:631–638. doi: 10.1007/s10570-017-1562-9. [DOI] [Google Scholar]
- 161.Manna P.J., Mitra T., Pramanik N., Kavitha V., Gnanamani A., Kundu P. Potential use of curcumin loaded carboxymethylated guar gum grafted gelatin film for biomedical applications. Int. J. Biol. Macromol. 2015;75:437–446. doi: 10.1016/j.ijbiomac.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 162.Bajpai S., Chand N., Ahuja S. Investigation of curcumin release from chitosan/cellulose micro crystals (CMC) antimicrobial films. Int. J. Biol. Macromol. 2015;79:440–448. doi: 10.1016/j.ijbiomac.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 163.Shefa A.A., Sultana T., Park M.K., Lee S.Y., Gwon J.-G., Lee B.-T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2019;186:108313. doi: 10.1016/j.matdes.2019.108313. [DOI] [Google Scholar]
- 164.Pham L., Dang L.H., Truong M.D., Nguyen T.H., Le L., Le V.T., Nam N.D., Bach L.G., Nguyen V.T., Tran N.Q. A dual synergistic of curcumin and gelatin on thermal-responsive hydrogel based on Chitosan-P123 in wound healing application. Biomed. Pharmacother. 2019;117:109183. doi: 10.1016/j.biopha.2019.109183. [DOI] [PubMed] [Google Scholar]
- 165.Huang B., Liu M., Zhou C. Cellulose–halloysite nanotube composite hydrogels for curcumin delivery. Cellulose. 2017;24:2861–2875. doi: 10.1007/s10570-017-1316-8. [DOI] [Google Scholar]
- 166.Anjum S., Gupta A., Sharma D., Gautam D., Bhan S., Sharma A., Kapil A., Gupta B. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater. Sci. Eng. C. 2016;64:157–166. doi: 10.1016/j.msec.2016.03.069. [DOI] [PubMed] [Google Scholar]
- 167.Gong C., Wu Q., Wang Y., Zhang D., Luo F., Zhao X., Wei Y., Qian Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials. 2013;34:6377–6387. doi: 10.1016/j.biomaterials.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 168.Zhao Y., Liu J.G., Chen W.M., Yu A.X. Efficacy of thermosensitive chitosan/β glycerophosphate hydrogel loaded with β cyclodextrin curcumin for the treatment of cutaneous wound infection in rats. Exp. Ther. Med. 2018;15:1304–1313. doi: 10.3892/etm.2017.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mohanty C., Das M., Sahoo S.K. Sustained Wound Healing Activity of Curcumin Loaded Oleic Acid Based Polymeric Bandage in a Rat Model. Mol. Pharm. 2012;9:2801–2811. doi: 10.1021/mp300075u. [DOI] [PubMed] [Google Scholar]
- 170.Mohanty C., Pradhan J. A human epidermal growth factor-curcumin bandage bioconjugate loaded with mesenchymal stem cell for in vivo diabetic wound healing. Mater. Sci. Eng. C. 2020;111:110751. doi: 10.1016/j.msec.2020.110751. [DOI] [PubMed] [Google Scholar]
- 171.Merrell J.G., McLaughlin S.W., Tie L., Laurencin C.T., Chen A.F., Nair L.S. Curcumin loaded poly (ε-caprolactone) nanofibers: Diabetic wound dressing with antioxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009;36:1149. doi: 10.1111/j.1440-1681.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nguyen T.T.T., Ghosh C., Hwang S.-G., Tran L.D., Park J.S. Characteristics of curcumin-loaded poly (lactic acid) nanofibers for wound healing. J. Mater. Sci. 2013;48:7125–7133. doi: 10.1007/s10853-013-7527-y. [DOI] [Google Scholar]
- 173.Moradkhannejhad L., Abdouss M., Nikfarjam N., Shahriari M.H., Heidary V. The effect of molecular weight and content of PEG on in vitro drug release of electrospun curcumin loaded PLA/PEG nanofibers. J. Drug Deliv. Sci. Technol. 2020;56:101554. doi: 10.1016/j.jddst.2020.101554. [DOI] [Google Scholar]
- 174.Bui H.T., Chung O.H., Cruz J.D., Park J.S. Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol. Res. 2014;22:1288–1296. doi: 10.1007/s13233-014-2179-6. [DOI] [Google Scholar]
- 175.Mutlu G., Calamak S., Ulubayram K., Güven E. Curcumin-loaded electrospun PHBV nanofibers as potential wound-dressing material. J. Drug Deliv. Sci. Technol. 2018;43:185–193. doi: 10.1016/j.jddst.2017.09.017. [DOI] [Google Scholar]
- 176.Ullah F., Liang A., Rangel A., Gyengesi E., Niedermayer G., Münch G. High bioavailability curcumin: An anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch. Toxicol. 2017;91:1623–1634. doi: 10.1007/s00204-017-1939-4. [DOI] [PubMed] [Google Scholar]
- 177.Prasad S., Tyagi A.K., Aggarwal B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014;46:2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stohs S.J., Chen C.Y.O., Preuss H.G., Ray S.D., Bucci L.R., Ji J., Ruff K.J. The fallacy of enzymatic hydrolysis for the determination of bioactive curcumin in plasma samples as an indication of bioavailability: A comparative study. BMC Complement. Altern. Med. 2019;19:293–298. doi: 10.1186/s12906-019-2699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Garcea G., Berry D.P., Jones D.J.L., Singh R., Dennison A.R., Farmer P.B., Sharma R.A., Steward W.P., Gescher A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Prev. Biomark. 2005;14:120–125. [PubMed] [Google Scholar]
- 180.Olotu F., Agoni C., Soremekun O., Soliman M.E.S. An Update on the Pharmacological Usage of Curcumin: Has it Failed in the Drug Discovery Pipeline? Cell Biophys. 2020;78:267–289. doi: 10.1007/s12013-020-00922-5. [DOI] [PubMed] [Google Scholar]
- 181.Wang Y.-J., Pan M.-H., Cheng A.-L., Lin L.-I., Ho Y.-S., Hsieh C.-Y., Lin J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997;15:1867–1876. doi: 10.1016/S0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 182.Aggarwal B.B., Sundaram C., Malani N., Ichikawa H. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer; Berlin/Heidelberg, Germany: 2007. Curcumin: The Indian solid gold; pp. 1–75. [DOI] [PubMed] [Google Scholar]
- 183.Sharma R.A., McLelland H.R., Hill K.A., Ireson C.R., Euden S.A., Manson M.M., Pirmohamed M., Marnett L.J., Gescher A.J., Steward W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 184.Dhillon N., Aggarwal B.B., Newman R.A., Wolff R.A., Kunnumakkara A.B., Abbruzzese J.L., Ng C.S., Badmaev V., Kurzrock R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 185.Cox K.H.M., Pipingas A., Scholey A. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2014;29:642–651. doi: 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- 186.Cuomo J., Appendino G., Dern A.S., Schneider E., McKinnon T.P., Brown M.J., Togni S., Dixon B.M. Comparative Absorption of a Standardized Curcuminoid Mixture and Its Lecithin Formulation. J. Nat. Prod. 2011;74:664–669. doi: 10.1021/np1007262. [DOI] [PubMed] [Google Scholar]
- 187.Antony B., Merina B., Iyer V.S., Judy N., Lennertz K., Joyal S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J. Pharm. Sci. 2008;70:445–449. doi: 10.4103/0250-474X.44591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Singh R., Tønnesen H.H., Vogensen S.B., Loftsson T., Másson M. Studies of curcumin and curcuminoids. XXXVI. The stoichiometry and complexation constants of cyclodextrin complexes as determined by the phase-solubility method and UV–Vis titration. J. Incl. Phenom. Macrocycl. Chem. 2010;66:335–348. doi: 10.1007/s10847-009-9651-5. [DOI] [Google Scholar]
- 189.Zeng Z., Shen Z.L., Zhai S., Xu J.L., Liang H., Shen Q., Li Q.Y. Transport of curcumin derivatives in Caco-2 cell monolayers. Eur. J. Pharm. Biopharm. 2017;117:123–131. doi: 10.1016/j.ejpb.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 190.Lund K.C., Pantuso T. Combination Effects of Quercetin, Resveratrol and Curcumin on In Vitro Intestinal Absorption. J. Restor. Med. 2014;3:112–120. doi: 10.14200/jrm.2014.3.0108. [DOI] [Google Scholar]
- 191.Kunnumakkara A.B., Harsha C., Banik K., Vikkurthi R., Sailo B.L., Bordoloi D., Gupta S.C., Aggarwal B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019;15:705–733. doi: 10.1080/17425255.2019.1650914. [DOI] [PubMed] [Google Scholar]
- 192.Cai D., Chen Q., Han J., Song Y., Meng Z., Zheng Y., Shen H. Effects of curcumin on the bioavailability of dioxin-like pollutants in rats. Sci. Rep. 2021;11:12461. doi: 10.1038/s41598-021-92085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hatab H.M., Hamid F.F.A., Soliman A.F., Al-Shafie T.A., Ismail Y.M., El-Houseini M.E. A combined treatment of curcumin, piperine, and taurine alters the circulating levels of IL-10 and miR-21 in hepatocellular carcinoma patients: A pilot study. J. Gastrointest. Oncol. 2019;10:766–776. doi: 10.21037/jgo.2019.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ruzicka J., Urdzikova L.M., Svobodova B., Amin A.G., Karova K., Dubisova J., Zaviskova K., Kubinova S., Schmidt M., Jhanwar-Uniyal M., et al. Does combined therapy of curcumin and epigallocatechin gallate have a synergistic neuroprotective effect against spinal cord injury? Neural Regen. Res. 2018;13:119–127. doi: 10.4103/1673-5374.224379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pawar K.S., Mastud R.N., Pawar S.K., Pawar S.S., Bhoite R.R., Bhoite R.R., Kulkarni M.V., Deshpande A.R. Oral Curcumin with Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial. Front. Pharmacol. 2021;12:1056. doi: 10.3389/fphar.2021.669362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Adusumilli N.C., Mordorski B., Nosanchuk J., Friedman J.M., Friedman A.J. Curcumin nanoparticles as a photoprotective adjuvant. Exp. Dermatol. 2021;30:705–709. doi: 10.1111/exd.14282. [DOI] [PubMed] [Google Scholar]
- 197.Chan E.D., Oberley-Deegan R., Bai A., Ovrutsky A., Kinney W., Weaver M., Honda J., Pott G., Shapiro L., Bai X. B47 HOST PATHOGEN INTERACTIONS II: Curcumin Enhances Macrophage Control of Mycobacterium Tuberculosis Infection. Am. J. Respir. Crit. Care Med. 2015;191:1. [Google Scholar]
- 198.Zhong Y.-B., Kang Z.-P., Wang M.-X., Long J., Wang H.-Y., Huang J.-Q., Wei S.-Y., Zhou W., Zhao H.-M., Liu D.-Y. Curcumin ameliorated dextran sulfate sodium-induced colitis via regulating the homeostasis of DCs and Treg and improving the composition of the gut microbiota. J. Funct. Foods. 2021;86:104716. doi: 10.1016/j.jff.2021.104716. [DOI] [Google Scholar]
- 199.Lau C.B.S., Yue G.G.L. Natural Products for Cancer Chemoprevention. Springer; Cham, Switzerland: 2020. Adjuvant value of turmeric extract (containing curcumin) in colorectal cancer management; pp. 209–239. [DOI] [Google Scholar]
- 200.Ipar V.S., Dsouza A., Devarajan P.V. Enhancing Curcumin Oral Bioavailability through Nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019;44:459–480. doi: 10.1007/s13318-019-00545-z. [DOI] [PubMed] [Google Scholar]
- 201.McClements D.J. Design of Nano-Laminated Coatings to Control Bioavailability of Lipophilic Food Components. J. Food Sci. 2010;75:R30–R42. doi: 10.1111/j.1750-3841.2009.01452.x. [DOI] [PubMed] [Google Scholar]
- 202.Ahmad Z., Shah A., Siddiq M., Kraatz H.-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014;4:17028–17038. doi: 10.1039/C3RA47370H. [DOI] [Google Scholar]
- 203.Mukherjee S., Ray S., Thakur R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009;71:349–358. doi: 10.4103/0250-474X.57282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sharma N., Mishra S., Sharma S., Deshpande R.D., Sharma R.K. Preparation and optimization of nanoemulsions for targeting drug delivery. Int. J. Drug Dev. Res. 2013;5:37–48. [Google Scholar]
- 205.Pandey P., Dahiya M. A brief review on inorganic nanoparticles. J. Crit. Rev. 2016;3:18–26. [Google Scholar]
- 206.Mallakpour S., Behranvand V. Polymeric nanoparticles: Recent development in synthesis and application. Express Polym. Lett. 2016;10:895. doi: 10.3144/expresspolymlett.2016.84. [DOI] [Google Scholar]
- 207.Lee J.Y., Lee S., Choi J.H., Na K. ι-Carrageenan nanocomposites for enhanced stability and oral bioavailability of curcumin. Biomater. Res. 2021;25:32. doi: 10.1186/s40824-021-00236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lebda M.A., Elmassry I.H., Taha N.M., Elfeky M.S. Nanocurcumin alleviates inflammation and oxidative stress in LPS-induced mastitis via activation of Nrf2 and suppressing TLR4-mediated NF-κB and HMGB1 signaling pathways in rats. Environ. Sci. Pollut. Res. 2021 doi: 10.1007/s11356-021-16309-9. [DOI] [PubMed] [Google Scholar]
- 209.Kumari A., Guliani A., Shukla A.K., Kumar S., Acharya A. Green surfactant based synthesis of curcumin loaded poly lactic-co-glycolic acid nanoparticles with enhanced solubility, photo-stability and anti-biofilm activity. J. Drug Deliv. Sci. Technol. 2020;59:101884. doi: 10.1016/j.jddst.2020.101884. [DOI] [Google Scholar]
- 210.de Carvalho F.B., de Gomes M.G., Savall A.S.P., Fidelis E.M., Pinton S., Ribeiro A.C.F., Munieweg F.R., Oelke C.A., Haas S.E. Evaluation of curcumin-loaded polymeric nanocapsules with different coatings in chick embryo model: Influence on angiogenesis, teratogenesis and oxidative stress. Pharmacol. Rep. 2021;73:563–573. doi: 10.1007/s43440-021-00218-2. [DOI] [PubMed] [Google Scholar]
- 211.Ghosh S., Dutta S., Sarkar A., Kundu M., Sil P.C. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surfaces B Biointerfaces. 2020;197:111404. doi: 10.1016/j.colsurfb.2020.111404. [DOI] [PubMed] [Google Scholar]
- 212.Al-Kinani M.A., Haider A.J., Al-Musawi S. Design, Construction and Characterization of Intelligence Polymer Coated Core–Shell Nanocarrier for Curcumin Drug Encapsulation and Delivery in Lung Cancer Therapy Purposes. J. Inorg. Organomet. Polym. Mater. 2020;31:70–79. doi: 10.1007/s10904-020-01672-w. [DOI] [Google Scholar]
- 213.Zhang Q., Wang H., Feng Z., Lu Z., Su C., Zhao Y., Yu J., Dushkin A.V., Su W. Preparation of pectin-tannic acid coated core-shell nanoparticle for enhanced bioavailability and antihyperlipidemic activity of curcumin. Food Hydrocoll. 2021;119:106858. doi: 10.1016/j.foodhyd.2021.106858. [DOI] [Google Scholar]
- 214.Somu P., Paul S. Surface conjugation of curcumin with self-assembled lysozyme nanoparticle enhanced its bioavailability and therapeutic efficacy in multiple cancer cells. J. Mol. Liq. 2021;338:116623. doi: 10.1016/j.molliq.2021.116623. [DOI] [Google Scholar]
- 215.Barick K., Tripathi A., Dutta B., Shelar S.B., Hassan P. Curcumin Encapsulated Casein Nanoparticles: Enhanced Bioavailability and Anticancer Efficacy. J. Pharm. Sci. 2020;110:2114–2120. doi: 10.1016/j.xphs.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 216.Sorasitthiyanukarn F.N., Muangnoi C., Rojsitthisak P., Rojsitthisak P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2020;256:117426. doi: 10.1016/j.carbpol.2020.117426. [DOI] [PubMed] [Google Scholar]
- 217.Esperón-Rojas A.A., Baeza-Jiménez R., Santos-Luna D., Velasco-Rodríguez L.D.C., Ochoa-Rodríguez L.R., García H.S. Bioavailability of curcumin in nanoemulsions stabilized with mono- and diacylglycerols structured with conjugated linoleic acid and n-3 fatty acids. Biocatal. Agric. Biotechnol. 2020;26:101638. doi: 10.1016/j.bcab.2020.101638. [DOI] [Google Scholar]
- 218.Reddy S.-P.W.A.F.-Y.H.D.N.K., Kumar R., Wang S.-P., Huang F.-Y. Curcumin-C3 Complexed with α-, β-cyclodextrin Exhibits Antibacterial and Antioxidant Properties Suitable for Cancer Treatments. Curr. Drug Metab. 2020;20:988–1001. doi: 10.2174/1389200220666191001104834. [DOI] [PubMed] [Google Scholar]
- 219.Hasan M., ElKhoury K., Belhaj N., Kahn C., Tamayol A., Barberi-Heyob M., Arab-Tehrany E., Linder M. Growth-Inhibitory Effect of Chitosan-Coated Liposomes Encapsulating Curcumin on MCF-7 Breast Cancer Cells. Mar. Drugs. 2020;18:217. doi: 10.3390/md18040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sheikhpour M., Sadeghizadeh M., Yazdian F., Mansoori A., Asadi H., Movafagh A., Shahraeini S.S. Co-Administration of Curcumin and Bromocriptine Nano-liposomes for Induction of Apoptosis in Lung Cancer Cells. Iran. Biomed. J. 2020;24:24–29. doi: 10.29252/ibj.24.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu Z., Smart J.D., Pannala A.S. Recent developments in formulation design for improving oral bioavailability of curcumin: A review. J. Drug Deliv. Sci. Technol. 2020;60:102082. doi: 10.1016/j.jddst.2020.102082. [DOI] [Google Scholar]
- 222.Wang Z., Zhao Z., Khan N.R., Hua Z., Huo J., Li Y. Microwave assisted chitosan-polyethylene glycol hydrogel membrane synthesis of curcumin for open incision wound healing. Pharmazie. 2020;75:118–123. doi: 10.1691/ph.2020.9179. [DOI] [PubMed] [Google Scholar]
- 223.Desai P.P., Patravale V.B. Curcumin Cocrystal Micelles—Multifunctional Nanocomposites for Management of Neurodegenerative Ailments. J. Pharm. Sci. 2018;107:1143–1156. doi: 10.1016/j.xphs.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 224.Gunathilake T.M.S.U., Ching Y.C., Chuah C.H. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers. 2017;9:64. doi: 10.3390/polym9020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ullah A., Johora F.T., Sarkar B., Araf Y., Rahman H. Curcumin analogs as the inhibitors of TLR4 pathway in inflammation and their drug like potentialities: A computer-based study. J. Recept. Signal Transduct. 2020;40:324–338. doi: 10.1080/10799893.2020.1742741. [DOI] [PubMed] [Google Scholar]
- 226.Vyas A., Dandawate P., Padhye S., Ahmad A., Sarkar F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr. Pharm. Des. 2013;19:2047–2069. [PMC free article] [PubMed] [Google Scholar]
- 227.He Y., Li W., Hu G., Sun H., Kong Q. Bioactivities of EF24, a Novel Curcumin Analog: A Review. Front. Oncol. 2018;8:614. doi: 10.3389/fonc.2018.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Oglah M.K., Mustafa Y.F. Curcumin analogs: Synthesis and biological activities. Med. Chem. Res. 2019;29:479–486. doi: 10.1007/s00044-019-02497-0. [DOI] [Google Scholar]
- 229.Lopes-Rodrigues V., Sousa M.E., Vasconcelos M.H. Curcumin as a Modulator of P-Glycoprotein in Cancer: Challenges and Perspectives. Pharmaceuticals. 2016;9:71. doi: 10.3390/ph9040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Park S.Y., Kim D.S. Discovery of natural products from Curcuma l onga that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer’s disease. J. Nat. Prod. 2002;65:1227–1231. doi: 10.1021/np010039x. [DOI] [PubMed] [Google Scholar]
- 231.Fu M., Chen L., Zhang L., Yu X., Yang Q. Cyclocurcumin, a curcumin derivative, exhibits immune-modulating ability and is a potential compound for the treatment of rheumatoid arthritis as predicted by the MM-PBSA method. Int. J. Mol. Med. 2017;39:1164–1172. doi: 10.3892/ijmm.2017.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jayaprakasha G.K., Jagan Mohan Rao L., Sakariah K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006;98:720–724. doi: 10.1016/j.foodchem.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 233.Mapoung S., Suzuki S., Fuji S., Naiki-Ito A., Kato H., Yodkeeree S., Sakorn N., Ovatlarnporn C., Takahashi S., (Dejkriengkraikul) P.L. Dehydrozingerone, a Curcumin Analog, as a Potential Anti-Prostate Cancer Inhibitor In Vitro and In Vivo. Molecules. 2020;25:2737. doi: 10.3390/molecules25122737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nkambeu B., Ben Salem J., Beaudry F. Capsaicin and Its Analogues Impede Nocifensive Response of Caenorhabditis elegans to Noxious Heat. Neurochem. Res. 2020;45:1851–1859. doi: 10.1007/s11064-020-03049-4. [DOI] [PubMed] [Google Scholar]
- 235.Singh A., Duggal S., Singh J., Katekhaye S. Experimental advances in pharmacology of gingerol and analogues. Pharm. Glob. Int. J. Compr. Pharm. 2010;2:1–5. [Google Scholar]
- 236.Zhao S., Pi C., Ye Y., Zhao L., Wei Y. Recent advances of analogues of curcumin for treatment of cancer. Eur. J. Med. Chem. 2019;180:524–535. doi: 10.1016/j.ejmech.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 237.Chang M., Wu M., Li H. Antitumor activities of novel glycyrrhetinic acid-modified curcumin-loaded cationic liposomes in vitro and in H22 tumor-bearing mice. Drug Deliv. 2018;25:1984–1995. doi: 10.1080/10717544.2018.1526227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sinjari B., Pizzicannella J., D’Aurora M., Zappacosta R., Gatta V., Fontana A., Trubiani O., Diomede F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-inflammatory Activities via NFkB/ERK/pERK Pathway in Human Dental Pulp Treated With 2-HydroxyEthyl MethAcrylate (HEMA) Front. Physiol. 2019;10:633. doi: 10.3389/fphys.2019.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bulboacă A.E., Boarescu P.M., Bolboacă S.D., Blidaru M., Feștilă D., Dogaru G., Nicula C.A. Comparative effect of curcumin versus liposomal curcumin on systemic pro-inflammatory cytokines profile, MCP-1 and RANTES in experimental diabetes mellitus. Int. J. Nanomed. 2019;14:8961. doi: 10.2147/IJN.S226790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.De Leo V., Milano F., Mancini E., Comparelli R., Giotta L., Nacci A., Longobardi F., Garbetta A., Agostiano A., Catucci L. Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a pH-responsive polymer cluster using a pH-driven and organic solvent-free process. Molecules. 2018;23:739. doi: 10.3390/molecules23040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Feng T., Wei Y., Lee R.J., Zhao L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017;12:6027–6044. doi: 10.2147/IJN.S132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chauhan M., Saha S., Roy A. Curcumin: A review. J. Appl. Pharm. Res. 2014;2:18–28. [Google Scholar]
- 243.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 244.Belcaro G., Cesarone M.R., Dugall M., Pellegrini L., Ledda A., Grossi M.G., Togni S., Appendino G. Product-evaluation registry of Meriva®, a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010;52:55–62. [PubMed] [Google Scholar]
- 245.Maiti K., Mukherjee K., Gantait A., Saha B.P., Mukherjee P.K. Curcumin–phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007;330:155–163. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 246.Liu W., Zhai Y., Heng X., Che F.Y., Chen W., Sun D., Zhai G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016;24:694–702. doi: 10.3109/1061186X.2016.1157883. [DOI] [PubMed] [Google Scholar]
- 247.Slika L., Patra D. A short review on chemical properties, stability and nano-technological advances for curcumin delivery. Expert Opin. Drug Deliv. 2019;17:61–75. doi: 10.1080/17425247.2020.1702644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.