Abstract
Purpose: NK-5962 is a key component of photoelectric dye-coupled polyethylene film, designated Okayama University type-retinal prosthesis (OUReP™). Previously, we found that NK-5962 solution could reduce the number of apoptotic photoreceptors in the eyes of the Royal College of Surgeons (RCS) rats by intravitreal injection under a 12 h light/dark cycle. This study aimed to explore possible molecular mechanisms underlying the anti-apoptotic effect of NK-5962 in the retina of RCS rats. Methods: RCS rats received intravitreal injections of NK-5962 solution in the left eye at the age of 3 and 4 weeks, before the age of 5 weeks when the speed in the apoptotic degeneration of photoreceptors reaches its peak. The vehicle-treated right eyes served as controls. All rats were housed under a 12 h light/dark cycle, and the retinas were dissected out at the age of 5 weeks for RNA sequence (RNA-seq) analysis. For the functional annotation of differentially expressed genes (DEGs), the Metascape and DAVID databases were used. Results: In total, 55 up-regulated DEGs, and one down-regulated gene (LYVE1) were found to be common among samples treated with NK-5962. These DEGs were analyzed using Gene Ontology (GO) term enrichment, Kyoto Encyclopedia of Genes and Genomes (KEGG), and Reactome pathway analyses. We focused on the up-regulated DEGs that were enriched in extracellular matrix organization, extracellular exosome, and PI3K–Akt signaling pathways. These terms and pathways may relate to mechanisms to protect photoreceptor cells. Moreover, our analyses suggest that SERPINF1, which encodes pigment epithelium-derived factor (PEDF), is one of the key regulatory genes involved in the anti-apoptotic effect of NK-5962 in RCS rat retinas. Conclusions: Our findings suggest that photoelectric dye NK-5962 may delay apoptotic death of photoreceptor cells in RCS rats by up-regulating genes related to extracellular matrix organization, extracellular exosome, and PI3K–Akt signaling pathways. Overall, our RNA-seq and bioinformatics analyses provide insights in the transcriptome responses in the dystrophic RCS rat retinas that were induced by NK-5962 intravitreal injection and offer potential target genes for developing new therapeutic strategies for patients with retinitis pigmentosa.
Keywords: apoptosis, drug, retina, photoreceptors, retinitis pigmentosa, extracellular exosome, extracellular matrix organization, PI3K–Akt signaling pathway, SERPINF1, pigment epithelium-derived factor (PEDF)
1. Introduction
Retinitis pigmentosa (RP) is a hereditary disease that causes blindness due to the loss of retinal photoreceptor cells. Patients with RP experience slowly progressive loss in the peripheral visual field, finally leading to blindness in later decades [1]. Nowadays, many treatments including neurotrophic factors [2,3], antioxidants [4,5,6], retinal prostheses [7,8,9,10,11,12], and gene therapies [13] are used to rescue retinal degeneration and improve the visual function.
RCS rats were used as an animal model of RP in many previous studies. In the RCS rat, a 409 bp deletion in the receptor tyrosine kinase MERTK gene mutation leads to reduced phagocytic function of the retinal pigment epithelial (RPE) cells and causes accumulation of photoreceptor outer segment debris in the subretinal space. Later, this debris blocks efficient oxygen and nutrient transport to photoreceptor cells and then leads to progressive photoreceptor degeneration and subsequent vison decline [14,15,16]. Photoreceptor cells in the RCS rats begin to degenerate on postnatal day (P) 22. Apoptosis of photoreceptors reaches its peak on P32, and then it gradually decreases [17].
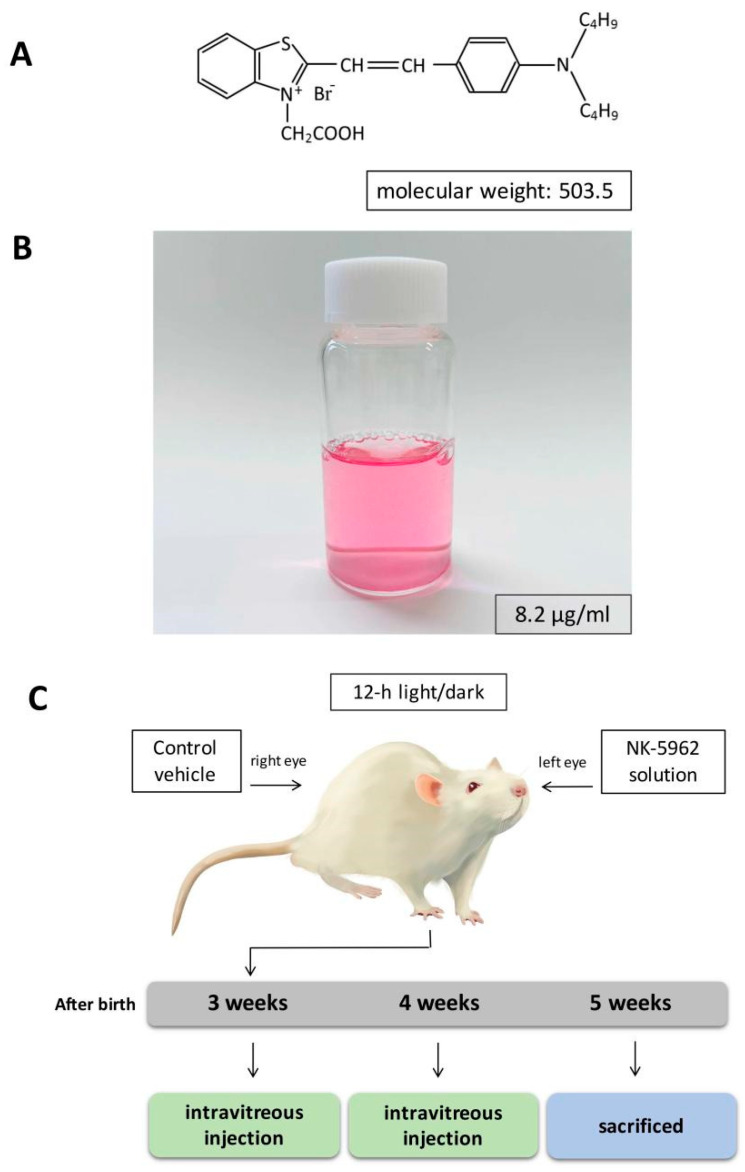
The photoelectric dye NK-5962 (Figure 1A), 2-[2-[4-(dibutylamino)phenyl]ethenyl]-3-carboxymethylbenzothiazolium bromide, generates electric potential in response to light [18,19]. We previously developed an Okayama University-type retinal prosthesis (OUReP™), which is composed of NK-5962-coupled polyethylene thin films, and showed that OUReP™ evokes neuronal response by light stimulation [20,21]. We found that the NK-5962 molecule itself protected both neural retinal cells and RPE cells from apoptosis through the primary mixed culture of retinal cells, NK-5962 coupled film transplanted into the eyes of RCS rats, and intravitreal injection of NK-5962 solution in RCS rats [22,23,24]. We recently demonstrated that NK-5962 shows low levels of reactive oxygen species (ROS) generation and that its phototoxicity is very low. These findings suggest that NK-5962 is a good candidate for the treatment of RP [25].
Figure 1.
NK-5962 and experimental design. (A) Chemical structure of NK-5962. (B) NK-5962 solution (8.2 μg/mL). (C) Experimental schedule.
In this study, we aimed to explore the mechanisms involved in the anti-apoptotic effect of intravitreal injection of NK-5962 in RCS rats by RNA-seq and bioinformatics analyses [26].
2. Results
2.1. Screening of DEGs in the Eyes Injected with NK-5962
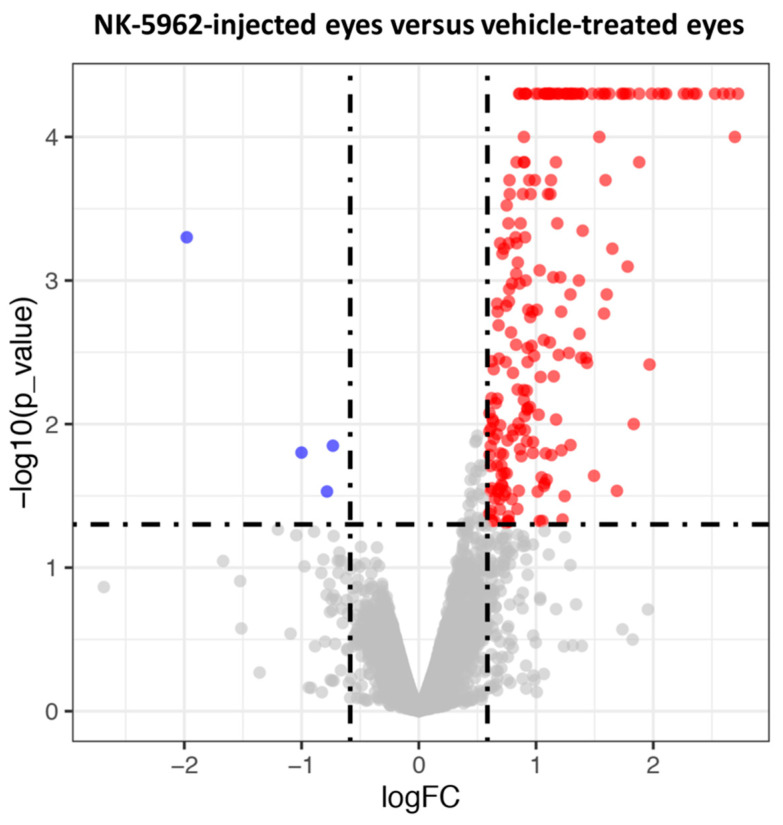
In order to reveal the mechanism of NK-5962 in attenuating retinal cell apoptosis, we examined the changes in gene expression between NK-5962-treated and control groups by RNA-seq analysis. The total number of reads per sample ranged from 46.2 million to 68.6 million. We only focused on the genes with FPKM (fragments per kilobase million) >0.1 in each group to avoid genes with low expression. Genes with log(FC) ≥ 0.672 and a p value < 0.05 were selected for follow-up studies. Volcano plots show the global transcriptional changes in NK-5962-injected eyes versus vehicle-treated eyes at the age of 5 weeks (Figure 2). Totally, 55 genes (Table 1) were chosen as up-regulated DEGs in the eyes treated with NK-5962. According to the p values and log(FC) values, Serpin Family F member 1 (SERPINF1) was found to be the most significantly up-regulated gene in NK-5962-treated retinas compared with the controls (Table 1). By contrast, we found a gene—the LYVE1 gene—that was commonly down-regulated among samples treated with NK-5962 (Table 2).
Figure 2.
The volcano plot shows the distribution of the fold changes of each mRNA transcript in NK-5962-injected eyes versus vehicle-treated eyes. Genes that pass a threshold of log(FC) > 0.585, p value < 0.05 are highlighted by red (up-regulated) and blue (down-regulated), respectively. Only one gene (LYVE1) was commonly down-regulated among samples treated with NK-5962. FC: fold change.
Table 1.
Up-regulated genes in NK-5962-treated retinas.
| Gene Name | Description | Locus | Log2(Fold_Change) | p_Value | q_Value | References |
|---|---|---|---|---|---|---|
| SERPINF1 | Serpin Family F Member 1 | chr10:62713440-62739444 | 2.722 | 5.00 × 10−5 | 0.012 | [27,28] |
| COL4A1 | Collagen Type IV Alpha 1 Chain | chr16:83045182-83157835 | 2.651 | 5.00 × 10−5 | 0.012 | [29] |
| CRYAB | Crystallin Alpha B | chr8:54107289-54111502 | 2.368 | 5.00 × 10−5 | 0.012 | [30] |
| COL4A2 | Collagen Type IV Alpha 2 Chain | chr16:82899293-83045155 | 2.293 | 5.00 × 10−5 | 0.012 | [31] |
| HSPG2 | Heparan Sulfate Proteoglycan 2 | chr5:156226988-156328912 | 2.089 | 5.00 × 10−5 | 0.012 | [32] |
| AQP1 | Aquaporin 1 | chr4:84098345-84110524 | 2.043 | 5.00 × 10−5 | 0.012 | [33] |
| ANXA1 | Annexin A1 | chr1:223478435-223494455 | 1.798 | 5.00 × 10−5 | 0.012 | [34] |
| Ecrg4 | ECRG4 augurin precursor | chr9:42930953-42950605 | 1.575 | 5.00 × 10−5 | 0.012 | [35] |
| WLS | Wnt Ligand Secretion Mediator | chr2:258014377-258128180 | 1.392 | 5.00 × 10−5 | 0.012 | [36] |
| SLC22A8 | Solute Carrier Family 22 Member 8 | chr1:211269365-211287596 | 1.388 | 5.00 × 10−5 | 0.012 | [37] |
| SOD3 | Superoxide dismutase 3 | chr14:63381446-63387180 | 1.328 | 5.00 × 10−5 | 0.012 | [38,39] |
| FBLN2 | Fibulin 2 | chr4:125380499-125441075 | 1.296 | 5.00 × 10−5 | 0.012 | [40] |
| OPTC | Opticin | chr13:46846755-46858100 | 1.292 | 5.00 × 10−5 | 0.012 | [41] |
| SLC13A4 | Solute Carrier Family 13 Member 4 | chr4:62679592-62724547 | 1.265 | 5.00 × 10−5 | 0.012 | [42] |
| FGFR2 | Fibroblast Growth Factor Receptor 2 | chr1:189482974-189589279 | 1.243 | 5.00 × 10−5 | 0.012 | [43] |
| FBLN1 | Fibulin 1 | chr7:123208153-123287289 | 1.194 | 5.00 × 10−5 | 0.012 | [44] |
| TYRP1 | Tyrosinase-Related Protein 1 | chr5:99518305-99537289 | 1.190 | 5.00 × 10−5 | 0.012 | [45] |
| OGN | Osteoglycin | chr17:20969065-21145330 | 1.160 | 5.00 × 10−5 | 0.012 | [46] |
| GJA1 | Gap Junction Protein Alpha 1 | chr20:35409814-35422259 | 1.117 | 5.00 × 10−5 | 0.012 | [47] |
| WFDC1 | WAP Four-Disulfide Core Domain 1 | chr19:49924309-49943113 | 1.116 | 5.00 × 10−5 | 0.012 | [48] |
| LTBP2 | Latent Transforming Growth Factor Beta Binding Protein 2 | chr6:108826438-108924895 | 1.112 | 5.00 × 10−5 | 0.012 | [49] |
| COL4A5 | Collagen Type IV Alpha 5 Chain | chrX:36918650-37130562 | 1.105 | 5.00 × 10−5 | 0.012 | [50] |
| DAPL1 | Death-Associated Protein Like 1 | chr3:41187966-41207910 | 1.070 | 5.00 × 10−5 | 0.012 | [51] |
| ENPP2 | Ectonucleotide Pyrophosphatase/Phosphodiesterase 2 | chr7:91295814-91377947 | 0.997 | 5.00 × 10−5 | 0.012 | [52] |
| SLC13A3 | Solute Carrier Family 13 Member 3 | chr3:156447899-156510620 | 0.914 | 5.00 × 10−5 | 0.012 | [53] |
| MXRA8 | Matrix Remodeling Associated 8 | chr5:172698112-172702607 | 0.899 | 5.00 × 10−5 | 0.012 | [54] |
| COL9A1 | Collagen Type IX Alpha 1 Chain | chr9:22907067-22990836 | 0.855 | 5.00 × 10−5 | 0.012 | [55] |
| COL8A1 | Collagen Type VIII Alpha 1 Chain | chr11:43604973-43737050 | 1.879 | 1.50 × 10−4 | 0.029 | [56] |
| MFRP | Membrane Frizzled-Related Protein | chr8:47084055-47089218 | 1.169 | 1.50 × 10−4 | 0.029 | [57] |
| COL5A1 | Collagen Type V Alpha 1 Chain | chr3:6825780-6973521 | 0.901 | 1.50 × 10−4 | 0.029 | [58] |
| FBN1 | Fibrillin 1 | chr3:112607811-112804951 | 0.895 | 1.50 × 10−4 | 0.029 | [59] |
| COL18A1 | Collagen alpha-1(XVIII) chain | chr20:11872458-11982466 | 0.834 | 1.50 × 10−4 | 0.029 | [60] |
| SLC6A13 | Solute Carrier Family 6 Member 13 | chr4:157736263-157771945 | 0.942 | 2.00 × 10−4 | 0.036 | [61] |
| ABI3BP | ABI Family Member 3 Binding Protein | chr11:44853363-45072422 | 1.122 | 2.50 × 10−4 | 0.041 | [62] |
| CPXM1 | Carboxypeptidase X, M14 Family Member 1 | chr3:118000979-118007777 | 1.102 | 2.50 × 10−4 | 0.041 | [63] |
| FMOD | Fibromodulin | chr13:46987713-46998331 | 0.887 | 2.50 × 10−4 | 0.041 | [64] |
| VCAN | Versican | chr2:19712628-19812592 | 0.868 | 4.00 × 10−4 | 0.061 | [44] |
| SERPINH1 | Serpin Family H Member 1 | chr1:156666873-156674336 | 0.765 | 4.00 × 10−4 | 0.061 | [65] |
| PCOLCE | Procollagen C-Endopeptidase Enhancer | chr12:19672504-19690374 | 1.398 | 4.50 × 10−4 | 0.068 | [66] |
| SLC26A4 | Solute Carrier Family 26 Member | chr6:49389211-49427000 | 0.835 | 5.50 × 10−4 | 0.078 | [67] |
| FSTL1 | Follistatin Like 1 | chr11:64680819-64735683 | 0.694 | 5.50 × 10−4 | 0.078 | [68] |
| OLFML2A | Olfactomedin Like 2A | chr3:18731164-18751940 | 0.713 | 6.50 × 10−4 | 0.089 | [69] |
| MRC2 | Mannose Receptor C Type 2 | chr10:94689060-94753073 | 0.831 | 9.00 × 10−4 | 0.117 | [70] |
| GSTM2 | Glutathione S-Transferase Mu 2 | chr2:203549021-203553380 | 1.207 | 9.50 × 10−4 | 0.120 | [71,72] |
| COL6A2 | Collagen Type VI Alpha 2 Chain | chr20:12436782-12464512 | 0.859 | 1.05 × 10−3 | 0.127 | [73] |
| COL9A2 | Collagen Type IX Alpha 2 Chain | chr5:141623364-141640224 | 0.770 | 1.15 × 10−3 | 0.137 | [74] |
| NID2 | nidogen-2 | chr15:4801182-4856895 | 0.769 | 1.40 × 10−3 | 0.163 | [75,76] |
| F5 | Coagulation Factor V | chr13:79934955-79997282 | 0.745 | 1.50 × 10−3 | 0.171 | [77] |
| SNED1 | Sushi, Nidogen, and EGF-Like Domains 1 | chr9:92509498-92568597 | 0.672 | 1.65 × 10−3 | 0.181 | [78] |
| COLEC12 | Collectin Subfamily Member 12 | chr18:996296-1188288 | 0.951 | 1.80 × 10−3 | 0.192 | [79] |
| COL1A2 | Collagen Type I Alpha 2 Chain | chr4:29393502-29429101 | 1.066 | 2.60 × 10−3 | 0.264 | [80] |
| SLC16A12 | Solute Carrier Family 16 Member 12 | chr1:238643039-238665699 | 0.962 | 2.85 × 10−3 | 0.281 | [81] |
| CLDN19 | Claudin 19 | chr5:139838013-139842711 | 0.896 | 5.80 × 10−3 | 0.480 | [82] |
| MYO5C | Myosin VC | chr8:80042255-80118773 | 0.921 | 5.85 × 10−3 | 0.481 | [83] |
| PMEL | Premelanosome Protein | chr7:2007881-2045336 | 1.294 | 1.40 × 10−2 | 0.941 | [84] |
Table 2.
Down-regulated genes in NK-5962-treated retinas.
| Gene Name | Description | Locus | Log2(Fold_Change) | p_Value | q_Value | Reference |
|---|---|---|---|---|---|---|
| LYVE1 | Lymphatic Vessel Endothelial Hyaluronan Receptor 1 | chr1:168601459-168622234 | −1.001 | 1.58 × 10−2 | 0.999 | [85] |
2.2. Bioinformatics Analysis of DEGs in the Eyes Injected with NK-5962
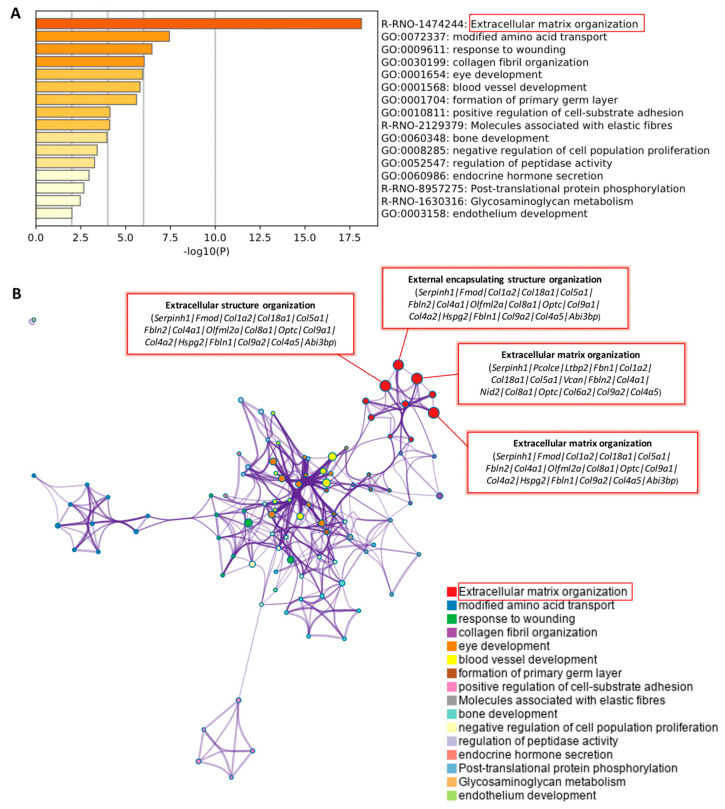
The functional annotation and pathway enrichment analysis of 55 up-regulated DEGs (Table 1) were explored by using GO terms, KEGG pathway, and Reactome pathway analyses in the Metascape database (Figure 3A,B). All GO terms and pathways can be seen online (See Supplementary Table S1 online). Then, we checked the relevant literature to find GO terms and pathways related to anti-apoptosis in the eyes treated with NK-5962. As shown in Figure 3A, enrichment analysis by Metascape showed that most of the DEGs were significantly enriched in the extracellular matrix organization pathway (red box and Table 3).
Figure 3.
The enrichment analysis of 55 significant up-regulated genes was performed by Metascape. (A) Metascape bar graph for viewing the top enriched clusters, where each cluster uses a discrete color to indicate statistical significance. (B) Metascape visualization of the interactome network formed by all 55 genes from the Table 1, where the MCODE compounds are colored according to their identities. The most interesting enriched terms in the category were extracellular matrix organization (red box).
Table 3.
Top Reactome pathways significantly enriched in DEGs related to anti-apoptosis in NK-5962-treated retinas (Metascape).
| Category | Term | Description | LogP | InTerm_ InList |
Genes |
|---|---|---|---|---|---|
| Reactome Gene Sets | R-RNO- 1474244 |
Extracellular matrix organization | −18.264 | 16/198 | Serpinh1, Pcolce, Ltbp2, Fbn1, Col1a2, Col18a1, Col5a1, Vcan, Fbln2, Col4a1, Nid2, Col8a1, Optc, Col6a2, Col9a2, Col4a5, Fmod, Olfml2a, Col9a1, Col4a2, Hspg2, Fbln1, Abi3bp, Fgfr2 |
In addition, the network was visualized by Cytoscape, where each node means an enriched term. A red box shows extracellular matrix-related pathways and genes, such as extracellular matrix organization, extracellular structure organization, and external encapsulating structure organization (Figure 3B, Table 4).
Table 4.
Top enriched GO terms significantly enriched in DEGs related to anti-apoptosis in NK-5962-treated retinas (Metascape).
| Category | Term | Description | LogP | InTerm_ InList |
Genes |
|---|---|---|---|---|---|
| GO Biological Processes | GO:0030198 | extracellular matrix organization |
−16.615 | 17/308 |
Serpinh1, Fmod, Col1a2, Col18a1, Col5a1, Fbln2, Col4a1, Olfml2a, Col8a1, Optc, Col9a1, Col4a2, Hspg2, Fbln1, Col9a2, Col4a5, Abi3bp |
| GO Biological Processes | GO:0043062 | extracellular structure organization |
−16.591 | 17/309 |
Serpinh1, Fmod, Col1a2, Col18a1, Col5a1, Fbln2, Col4a1, Olfml2a, Col8a1, Optc, Col9a1, Col4a2, Hspg2, Fbln1, Col9a2, Col4a5, Abi3bp |
| GO Biological Processes | GO:0045229 | external encapsulating structure organization |
−16.567 | 17/310 |
Serpinh1, Fmod, Col1a2, Col18a1, Col5a1, Fbln2, Col4a1, Olfml2a, Col8a1, Optc, Col9a1, Col4a2, Hspg2, Fbln1, Col9a2, Col4a5, Abi3bp |
The results of the KEGG pathway analysis (Metascape) showed that the up-regulated DEGs were significantly enriched in the ECM-receptor interaction and PI3K–Akt signaling pathway (Table 5).
Table 5.
Top KEGG pathways significantly enriched in DEGs related to anti-apoptosis in NK-5962-treated retinas (Metascape).
| Category | Term | Description | LogP | InTerm_InList | Genes |
|---|---|---|---|---|---|
| KEGG Pathway | ko04512, rno04512 |
ECM-receptor interaction |
−9.901 | 8/81 | Col1a2, Col4a1, Col9a1, Col4a2, Hspg2, Col6a2, Col9a2, Col4a5 |
| KEGG Pathway | ko04151, rno04151 |
PI3K–Akt signaling pathway | −5.166 | 8/329 | Fgfr2, Col1a2, Col4a1, Col9a1, Col4a2, Col6a2, Col9a2, Col4a5 |
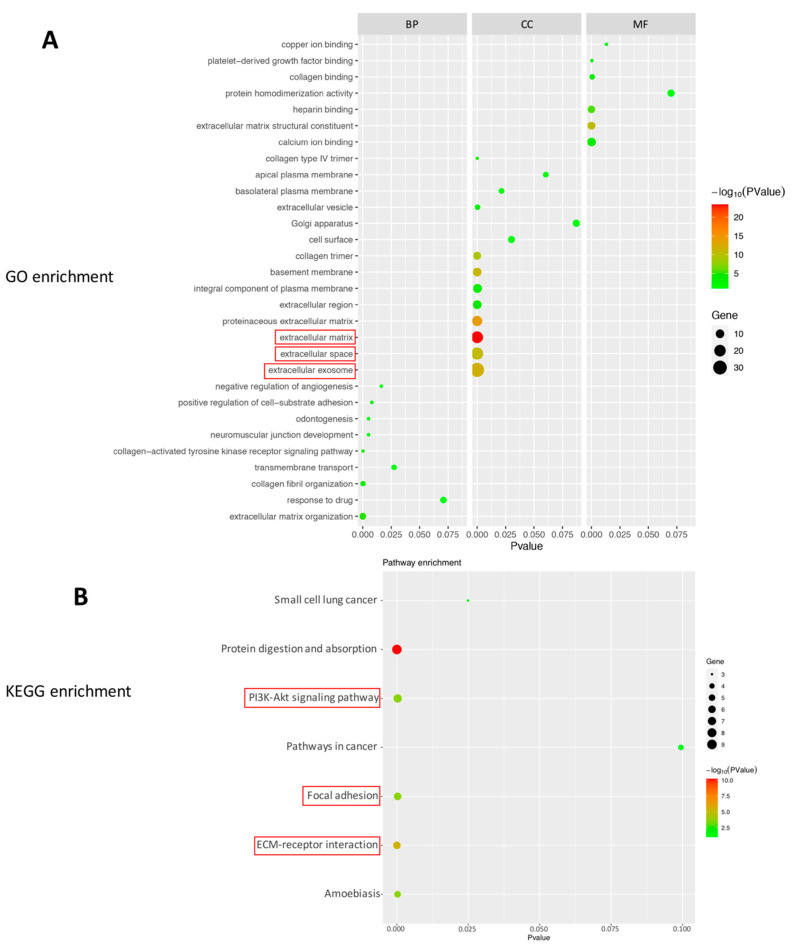
We also uploaded the 55 DEGs (Table 1) into DAVID bioinformation resources for functional annotation analysis. Based on smaller p values and greater number of genes contained therein, the up-regulated genes indicated that the proteins of biological process (BP) were associated with extracellular matrix organization. With regard to the cellular component (CC), the majority of proteins contained extracellular exosome (including 31 genes, p value = 8.19 × 10−13), extracellular space (including 22 genes, p value = 2.30 × 10−11), and extracellular matrix (including 21 genes, p value = 3.45 × 10−24). With regard to molecular function (MF), the majority of proteins were involved in processes such as, extracellular matrix structural constituent (Figure 4A, Table 6).
Figure 4.
Enrichment analysis of top 55 up-regulated genes based on DAVID bioinformation resources. (A) Bubble plot of the enriched GO terms: cellular component terms (CC), molecular function terms (MF), biological process terms (BP). The first three pathways with the most genes (smaller p value), and which may be related to protection of photoreceptor cells are as follows: extracellular exosome, extracellular space, and extracellular matrix (red box). (B) Bubble plot of the enriched KEGG pathways. The pathways which may be related to protection of photoreceptor cells are as follows: PI3K–Akt signaling pathway. In addition, there are PI3K–Akt signaling pathway-related pathways: focal adhesion, ECM-receptor interaction (according to the map of PI3K–Akt signaling pathway, https://www.genome.jp/kegg-bin/show_pathway?rno04151 (accessed on 18 June 2021). The colors of the nodes are illustrated from red to green in descending order of –log10 (p value). X-axis: signaling pathway or function; Y-axis: percentage of the number of DEGs assigned to a term among the total number of DEGs annotated in the network; Bubble size: number of DEGs assigned to a pathway or function; Color: enriched p value.
Table 6.
Top three GO terms significantly enriched in DEGs related to anti-apoptosis in NK-5962-treated retinas (DAVID).
| Category | Term | Count | % | p Value | Genes |
|---|---|---|---|---|---|
| GOTERM_ CC_DIRECT |
GO:0070062~ extracellular exosome |
31 | 56.3 | 8.19 × 10−13 | COLEC12, COL18A1, SNED1, LTBP2, FBLN1, FBLN2, FSTL1, NID2, AQP1, GJA1, SERPINH1, SLC13A3, GSTM2, ANXA1, SERPINF1, SLC6A13, PCOLCE, SOD3, HSPG2, COL1A2, COL4A2, COL5A1, COL6A2, OGN, MYO5C, MXRA8, COL8A1, SLC26A4, SLC22A8, CRYAB, FBN1 |
| GOTERM_ CC_DIRECT |
GO:0005615~ extracellular space |
22 | 40.0 | 2.30 × 10−11 | COL18A1, ANXA1, SERPINF1, RGD1305645, WFDC1, PCOLCE, LTBP2, FBLN1, SOD3, FSTL1, HSPG2, F5, VCAN, COL1A2, ABI3BP, COL6A2, OGN, SERPINH1, ENPP2, CPXM1, FMOD, FBN1 |
| GOTERM_ CC_DIRECT |
GO:0031012~ extracellular matrix |
21 | 38.1 | 3.45 × 10−24 | COL18A1, SERPINF1, PCOLCE, LTBP2, FBLN1, SOD3, NID2, HSPG2, FBLN2, VCAN, COL1A2, COL4A2, COL5A1, COL4A1, ABI3BP, COL6A2, OGN, COL8A1, FMOD, FGFR2, FBN1 |
Additionally, the up-regulated 55 genes were enriched in five KEGG pathways (DAVID), including the PI3K–Akt signaling pathway, ECM-receptor interaction, focal adhesion, protein digestion and absorption, and amoebiasis (Figure 4B, Table 7). The first three pathways are related to anti-apoptosis mechanisms.
Table 7.
Top three KEGG pathways significantly enriched in DEGs related to anti-apoptosis in NK-5962-treated retinas (DAVID).
| Category | Term | Count | % | p Value | Genes |
|---|---|---|---|---|---|
| KEGG_PATHWAY | rno04151:PI3K–Akt signaling pathway | 7 | 12.7 | 2.76 × 10−4 |
COL1A2, COL4A2, COL5A1, COL4A1, COL6A2, COL4A5, FGFR2 |
| KEGG_PATHWAY | rno04512:ECM- receptor interaction |
6 | 10.9 | 4.03 × 10−6 | COL1A2, COL4A2, COL5A1, COL4A1, COL6A2, COL4A5 |
| KEGG_PATHWAY | rno04510:Focal adhesion |
6 | 10.9 | 2.52 × 10−4 | COL1A2, COL4A2, COL5A1, COL4A1, COL6A2, COL4A5 |
3. Discussion
This study aimed to investigate the mechanisms of photoelectric dye NK-5962 in delaying the apoptosis of retinal neurons. We used RCS rats as a retinitis pigmentosa model, which show progressive photoreceptor degeneration as the consequence of MERTK mutation in the RPE cells [86]. Our results show that NK-5962 produces an effect on the expression of a variety of genes. These include genes involved in regulating the PI3K–Akt signaling pathway and inhibiting the apoptosis of photoreceptor cells in RCS rats.
First, we found that both Metascape and DAVID analyses showed a lot of extracellular matrix (ECM)-related terms in NK-5962-injected eyes. The ECM of the retina is divided into two separate entities: the interphotoreceptor matrix (IPM) and the retinal ECM. During retinal degeneration, the ECM structure is destroyed, leading to an acceleration of the retinal degeneration process. These changes would lead to an increase in the space between the cells and a reduction in the ECM materials that were required to support the retina. In turn, it would change the delivery of oxygen, growth factors, and nutrients from the retinal supply to the photoreceptor cells [87]. The effectiveness of drug treatment would be based on healthy retinal ECM so that neurotrophic factors may play the role in protecting photoreceptor cells [88]. We speculate that NK-5962 maybe postpone retinal cell degeneration by up-regulating ECM-related pathways to support the RPE-photoreceptor microenvironment and to provide an optimal microenvironment for viability of neurons.
Second, the extracellular exosome term that contained the highest number of genes in GO analysis using DAVID in this study was one of the subtypes of extracellular vesicles (EVs). EVs can reach injured and degenerative neural cells quickly and transfer biologically active substances directly into cells [89,90]. The recent research found that inhibited synthesis of extracellular exosomes leads to exacerbation of retinal degeneration. In mice that are depleted of extracellular exosomes, inflammation and cell death increases, and retinal function decreases after photo-oxidative damage occurs [91]. We speculate that the anti-apoptotic effect of NK-5962 in the retina of RCS may be mediated by extracellular exosomes, which release neurotrophic factors, lipids, and proteins, including PEDF and SOD3, promoting the survival of photoreceptors and maintaining the homeostasis of the retinal microenvironment.
Furthermore, in our study, the PI3K–Akt signaling pathway, focal adhesion pathway, and ECM-related pathways were up-regulated by NK-5962 in the KEGG pathway analysis using DAVID. According to the KEGG pathway map of PI3K–Akt signaling pathway–Norway rats (Rattus norvegicus), NK-5962 maybe activate PI3K–Akt signaling pathway through focal adhesion and ECM-receptor interaction pathway. Previous reports showed that PI3K–Akt pathway protected the survival of cone photoreceptors [92]. Additionally, we noticed that the genes involved in the PI3K–Akt signaling pathway were collagen genes and the FGFR2 gene (Table 7). The FGFR2 gene is a factor that mediates the rescue of photoreceptors in the rat and has an effect on anti-apoptotic and neurite repair [93,94]. These results indicate that the delivery of NK-5962 maybe protect photoreceptors from apoptosis in RCS rat through up-regulated FGFR2 gene by activating the PI3K–Akt signaling pathway. All of these possibilities need to be clarified through further research.
On the basis of p values and fold change values, the first gene to be noticed is SERPINF1, which encodes PEDF. PEDF is a multifunctional protein that has neurotrophic [95] and antioxidant properties [96] as well as an anti-inflammatory role [97]. PEDF is also known to protect photoreceptors from injury in rd10 mouse models of retinal degeneration [27,98]. The other reviews showed that molecular pathways of retinal survival activity triggered by PEDF are involved in PI3K–Akt [99]. The other gene we focused on is SOD3, which was up-regulated after injection of NK-5962. In recent studies, it has been shown that SOD3 is important in protecting the ECM from oxidative damage [100]. Whether the translation of these genes has also been changed remains to be verified.
This study showed the potential mechanism of NK-5962, with a protective effect at the early stage of photoreceptor degeneration in RCS rats by RNA-seq. In the next step, to locate the position of up-regulated genes in NK-5962-treated eyes, we will perform RT-PCR and multicolor immunostaining experiments to screen out important genes.
4. Methods
4.1. Chemicals and Preparations
NK-5962 was obtained from Hayashibara, Inc. (Okayama City, Japan) (Figure 1A), and was dissolved in distilled deionized water at a concentration of 8.2 μg/mL (16 μM) (Figure 1B).
4.2. Animals
All experiments were performed in compliance with the ARVO statement for the “Use of Animals in Ophthalmology and Vision Research” and were approved by the Animal Care and Use Committee at Okayama University (Identifier OKU-2019196). Eight male pink-eyed RCS (Jcl-rdy/rdy, p-) rats were obtained from CLEA Japan, Inc. (Tokyo, Japan), and reared under a 12 h light/dark cycle. All intravitreal injections were performed as described previously [6]. At the age of 3 and 4 weeks, the rats were anesthetized by intraperitoneal injection of ketamine (87 mg/kg body weight, Daiichi Sankyo, Tokyo, Japan) and xylasine (13 mg/kg, Bayer Japan, Osaka, Japan), and received an intravitreal injection of 5 μL of NK-5962 solution at 8.2 μg/mL (16 μM) in the left eye, and saline (0.9% sodium chloride) as a vehicle control in the right eye, with a 30-gauge needle-attached Hamilton syringe (50 μL 1705 LT SYR; Hamilton Company, Reno, NV, USA) under a dissecting microscope. All rats were sacrificed at the age of 5 weeks (Figure 1C).
4.3. RNA Extraction
Neural retinal tissue was dissected free from the other tissues of the eye and stored in an RNAlater RNA Stabilization Reagent (Cat# 74104, Qiagen, Germany). Total RNA was extracted from the dissected retinal tissue using an RNeasy Mini Kit (Cat# 74104, Qiagen, Germany) combined with a QIAshredder kit and RNase-free DNase Set (Qiagen) as per the manufacturer’s instructions.
4.4. RNA Sequencing
Total RNA samples were submitted to Macrogen Japan (Tokyo) and Riken Genesis (Tokyo) for bioanalyzer quality control analysis (QC), Illumina next-generation sequencing (NGS), and differential expressed gene (DEG) analysis. All submitted samples had an RNA integrity number (RIN) > 9 and were proceeded for library construction. The sequencing library was prepared from poly-A selected RNA from each sample with TruSeq Stranded mRNA Library Prep Kit (Illumina). On the platform of Novaseq 6000 System (Illumina) and HiSeq 2500 (Illumina), transcriptome sequencing was performed (100 bp paired-end sequencing). Adaptor sequences and low-quality bases from paired-reads were removed by Cutadapt (version 2.4). Filtered paired end reads were mapped to the rat reference genome (UCSU rn4) by HISAT2 (version 2.1.0), and then transcript assembly was performed by Cufflinks (v2.1.1) using a previously defined rat gene annotation [101]. Cuffdiff in the Cufflinks package was used to identify DEGs. RNA-seq was performed on three independent sample sets, and genes that showed reproducible changes in three experiments were used for bioinformatics analysis. The p values were calculated by combining the reads of the three experiments. A cutoff fold-change (FC) ≥ 1.3 and p value < 0.05 were assumed to identify genes significantly changed by NK-5962 treatment.
4.5. Bioinformatics Analysis
Identified DEGs were uploaded to Metascape (https://metascape.org/, accessed on 8 June 2021), which facilitates comparative analyses of multiple datasets, gene ontology (GO) annotation, Kyoto Encyclopedia of Genes and Genomes (KEGG), and Reactome pathway enrichment analyses. The database for Annotation, Visualization, and Integrated Discovery (DAVID, v6.8) bioinformatics tool (https://david.ncifcrf.gov, accessed on 8 June 2021) was also used for validating the results. GO and KEGG bioinformatic analyses were conducted in R 3.6.3 (https://cran.r-project.org/ (accessed on 28 June 2021). Volcano plots were created using the R-package ggplot2 (https://cran.r-project.org/ (accessed on 28 June 2021).
4.6. Data Availability
The datasets presented in this study can be found in online repositories. The raw data obtained in this study are available from DDBJ Read Archive (https://ddbj.nig.ac.jp//DRASearch/ (accessed on 9 December 2021) under accession numbers of (DRA013172) for RNA-seq.
5. Conclusions
We found that NK-5962 up-regulated several genes involved in extracellular matrix organization, extracellular exosome, and PI3K–Akt signaling pathways in RCS rats. Additionally, we observed the up-regulation of PEDF, which has been reported to prevent photoreceptor cells death. In order to further elucidate the molecular mechanisms of the anti-apoptotic properties of NK-5962 in a rat model of RP, more in-depth research is needed. These are very important for the development of new therapeutic agents for patients with retinal degenerative diseases.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms222413276/s1.
Author Contributions
Conceptualization, T.M.; methodology, T.M., S.L., M.M. and O.H.; software, M.M., S.L. and O.H.; validation, S.L., M.M. and O.H.; formal analysis, S.L., M.M. and O.H.; investigation, S.L., M.M. and O.H.; resources, T.M.; data curation, S.L., M.M. and O.H.; writing—original draft preparation, S.L.; writing—review and editing, O.H., M.M., S.L. and T.M.; visualization, S.L.; supervision, T.M.; project administration, T.M. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant for the Practical Research Program for Rare/Intractable Diseases (18950217, 2018, Toshihiko Matsuo) from the Japan Agency for Medical Research and Development (AMED), and also supported by Science and Technology Promotion grants (2018–2020) in Okayama Prefecture, Japan.
Institutional Review Board Statement
This study was approved by the Animal Care and Use Committee at the Okayama University (protocol code OKU-2016267 approved on 29 June 2016; protocol code OKU-2019196 approved on 1 April 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The raw data obtained in this study are available from DDBJ Read Archive (https://ddbj.nig.ac.jp//DRASearch/, accessed on 9 December 2021) under the accession number (DRA013172) for RNA-seq.
Conflicts of Interest
The authors declare that they have no competing financial interests in this study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hamel C. Retinitis pigmentosa. Orphanet. J. Rare Dis. 2006;1:40. doi: 10.1186/1750-1172-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faktorovich E.G., Steinberg R.H., Yasumura D., Matthes M.T., Lavail M.M. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347:83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- 3.LaVail M.M., Unoki K., Yasumura D., Matthes M.T., Yancopoulos G.D., Steinberg R.H. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the dam-aging effects of constant light. Proc. Nat. Acad. Sci. USA. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezaie T., McKercher S.R., Kosaka K., Seki M., Wheeler L., Viswanath V., Chun T., Joshi R., Valencia M., Sasaki S., et al. Protective effect of carnosic acid, a pro-electrophilic compound, in models of oxidative stress and light-induced retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2012;53:7847–7854. doi: 10.1167/iovs.12-10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanz M., Johnson L., Ahuja S., Ekström P., Romero J., van Veen T. Significant photoreceptor rescue by treatment with a combination of antioxidants in an animal model for retinal degeneration. Neuroscience. 2007;145:1120–1129. doi: 10.1016/j.neuroscience.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Liu S., Matsuo T., Miyaji M., Hosoya O. The Effect of Cyanine Dye NK-4 on Photoreceptor Degeneration in a Rat Model of Early-Stage Retinitis Pigmentosa. Pharmaceuticals. 2021;14:694. doi: 10.3390/ph14070694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J., Poulaki V., Kim S.-J., Eldred W.D., Kane S., Gingerich M., Shire D.B., Jensen R., DeWalt G., Kaplan H.J., et al. Implantation and Extraction of Penetrating Electrode Arrays in Minipig Retinas. Transl. Vis. Sci. Technol. 2020;9:19. doi: 10.1167/tvst.9.5.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuo T., Uchida T., Sakurai J., Yamashita K., Matsuo C., Araki T., Yamashita Y., Kamikawa K. Visual Evoked Potential Recovery by Subretinal Implantation of Photoelectric Dye-Coupled Thin Film Retinal Prosthesis in Monkey Eyes With Macular Degeneration. Artif. Organs. 2018;42:E186–E203. doi: 10.1111/aor.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo Y., da Cruz L. The Argus(®) II retinal prosthesis system. Prog. Retin. Eye Res. 2016;50:89–107. doi: 10.1016/j.preteyeres.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Maya-Vetencourt J.F., Manfredi G., Mete M., Colombo E., Bramini M., Di Marco S., Shmal D., Mantero G., Dipalo M., Rocchi A., et al. Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat. Nanotechnol. 2020;15:698–708. doi: 10.1038/s41565-020-0696-3. [DOI] [PubMed] [Google Scholar]
- 11.Maya-Vetencourt J.F., Di Marco S., Mete M., Di Paolo M., Ventrella D., Barone F., Elmi A., Manfredi G., Desii A., Sannita W.G., et al. Biocompatibility of a Conjugated Polymer Retinal Prosthesis in the Do-mestic Pig. Front. Bioeng. Biotechnol. 2020;15:579141. doi: 10.3389/fbioe.2020.579141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maya-Vetencourt J.F., Ghezzi D., Antognazza M.R., Colombo E., Mete M., Feyen P., Desii A., Buschiazzo A., Di Paolo M., Di Marco S., et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 2017;16:681–689. doi: 10.1038/nmat4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cehajic-Kapetanovic J., Xue K., Martinez-Fernandez de la Camara C., Nanda A., Davies A., Wood L.J., Salvetti A.P., Fischer M.D., Aylward J.W., Barnard A.R., et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pig-mentosa caused by mutations in RPGR. Nat. Med. 2020;26:354–359. doi: 10.1038/s41591-020-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourne M.C., Campbell D.A., Tansley K. Hereditary degeneration of the rat retina. Br. J. Ophthalmol. 1938;22:613–623. doi: 10.1136/bjo.22.10.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bok D., Hall M.O. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J. Cell Biol. 1971;49:664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullen R.J., LaVail M.M. Inherited retinal dystrophy: Primary defect in pigment epithelium determined with ex-perimental rat chimeras. Science. 1976;192:799–801. doi: 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- 17.Dowling J.E., Sidman R.L. Inherited retinal dystrophy in the rat. J. Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo T. A simple method for screening photoelectric dyes towards their use for retinal prosthese. Acta Med. Okayama. 2003;57:257–260. doi: 10.18926/AMO/32824. [DOI] [PubMed] [Google Scholar]
- 19.Matsuo T., Dan-oh Y., Suga S. Agent for Inducing Receptor Potential. US7,101,533 B2. U.S. Patent. 2006 September 5;
- 20.Matsuo T., Sakurai M., Terada K., Uchida T., Yamashita K., Tanaka T., Takarabe K. Photoelectric Dye-Coupled Polyethylene Film: Photoresponsive Properties Evaluated by Kelvin Probe and In Vitro Biological Response Detected in Dystrophic Retinal Tissue of Rats. Adv. Biomed. Eng. 2019;8:137–144. doi: 10.14326/abe.8.137. [DOI] [Google Scholar]
- 21.Matsuo T., Terada K., Sakurai M., Liu S., Yamashita K., Uchida T. Step-by-step procedure to test photoelectric dye-coupled polyethylene film as retinal prosthesis to induce light-evoked spikes in isolated retinal dystrophic tissue of rd1 mice. Clin Surg. 2020;5:2903. [Google Scholar]
- 22.Okamoto K., Matsuo T., Tamaki T., Uji A., Ohtsuki H. Short-term biological safety of a photoelectric dye used as a component of retinal prostheses. J. Artif. Organs. 2008;11:45–51. doi: 10.1007/s10047-008-0403-x. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo T., Hosoya O., Tsutsui K.M., Uchida T. Vision maintenance and retinal apoptosis reduction in RCS rats with Okayama University-type retinal prosthesis (OUReP™) implantation. J. Artif. Organs. 2015;18:264–271. doi: 10.1007/s10047-015-0825-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu S., Matsuo T., Hosoya O., Uchida T. Photoelectric Dye Used for Okayama University-Type Retinal Prosthesis Reduces the Apoptosis of Photoreceptor Cells. J. Ocul. Pharmacol. Ther. 2017;33:149–160. doi: 10.1089/jop.2016.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuo T., Liu S., Uchida T., Onoue S., Nakagawa S., Ishii M., Kanamitsu K. Photoelectric Dye, NK-5962, as a Potential Drug for Preventing Retinal Neurons from Apoptosis: Pharmacokinetic Studies Based on Review of the Evidence. Life. 2021;11:591. doi: 10.3390/life11060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wacker S., Houghtaling B.R., Elemento O., Kapoor T.M. Using transcriptome sequencing to identify mechanisms of drug action and resistance. Nat. Chem. Biol. 2012;8:235–237. doi: 10.1038/nchembio.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixit S., Polato F., Samardzija M., Abu-Asab M., Grimm C., Crawford S.E., Becerra S.P. PEDF deficiency increases the susceptibility of rd10 mice to retinal degeneration. Exp. Eye Res. 2020;198:108121. doi: 10.1016/j.exer.2020.108121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valiente-Soriano F.J., Di Pierdomenico J., García-Ayuso D., Ortín-Martínez A., De Imperial-Ollero J.A.M., Gallego-Ortega A., Jiménez-López M., Villegas-Pérez M.P., Becerra S.P., Vidal-Sanz M. Pigment Epithelium-Derived Factor (PEDF) Fragments Prevent Mouse Cone Photoreceptor Cell Loss Induced by Focal Phototoxicity In Vivo. Int. J. Mol. Sci. 2020;21:7242. doi: 10.3390/ijms21197242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould D.B., Marchant J.K., Savinova O.V., Smith R.S., John S.W. Col4a1 mutation causes endoplasmic reticulum stress and genetically modifiable ocular dysgenesis. Hum. Mol. Genet. 2007;16:798–807. doi: 10.1093/hmg/ddm024. [DOI] [PubMed] [Google Scholar]
- 30.Templeton J.P., Wang X., Freeman N.E., Ma Z., Lu A., Hejtmancik F., Geisert E.E. A crystallin gene network in the mouse retina. Exp. Eye Res. 2013;116:129–140. doi: 10.1016/j.exer.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo D.S., Labelle-Dumais C., Gould D.B. COL4A1 and COL4A2 mutations and disease: Insights into pathogenic mechanisms and potential therapeutic targets. Hum. Mol. Genet. 2012;21:R97–R110. doi: 10.1093/hmg/dds346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi M., Morita H., Sormunen R., Airenne S., Kreivi M., Wang L., Fukai N., Olsen B.R., Tryggvason K., Soininen R. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22:236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamer W.D., Bok D., Hu J., Jaffe G.J., McKay B.S. Aquaporin-1 channels in human retinal pigment epithelium: Role in tran-sepithelial water movement. Investig. Ophthalmol. Vis. Sci. 2003;44:2803–2808. doi: 10.1167/iovs.03-0001. [DOI] [PubMed] [Google Scholar]
- 34.Perucci L.O., Sugimoto M.A., Gomes K.B., Dusse L., Teixeira M.M., Sousa L.P. Annexin A1 and specialized proresolving lipid mediators: Promoting resolution as a therapeutic strategy in human inflammatory diseases. Expert. Opin. Ther. Targets. 2017;21:879–896. doi: 10.1080/14728222.2017.1364363. [DOI] [PubMed] [Google Scholar]
- 35.Porzionato A., Rucinski M., Macchi V., Sarasin G., Malendowicz L., De Caro R. ECRG4 Expression in Normal Rat Tissues: Expression Study and Literature Review. Eur. J. Histochem. 2015;59:2458. doi: 10.4081/ejh.2015.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capowski E.E., Wright L.S., Liang K., Phillips M.J., Wallace K., Petelinsek A., Hagstrom A., Pinilla I., Borys K., Lien J., et al. Regulation of WNT Signaling by VSX2 During Optic Vesicle Patterning in Human Induced Pluripotent Stem Cells. STEM CELLS. 2016;34:2625–2634. doi: 10.1002/stem.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahlin A., Geier E., Stocker S.L., Cropp C.D., Grigorenko E., Bloomer M., Siegenthaler J., Xu L., Basile A.S., Tang-Liu D.D.-S., et al. Gene Expression Profiling of Transporters in the Solute Carrier and ATP-Binding Cassette Superfamilies in Human Eye Substructures. Mol. Pharm. 2013;10:650–663. doi: 10.1021/mp300429e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishizu M., Murakami Y., Fujiwara K., Funatsu J., Shimokawa S., Nakatake S., Tachibana T., Hisatomi T., Koyanagi Y., Akiyama M., et al. Relationships Between Serum Antioxidant and Oxidant Statuses and Visual Function in Retinitis Pigmentosa. Investig. Opthalmology Vis. Sci. 2019;60:4462–4468. doi: 10.1167/iovs.19-26927. [DOI] [PubMed] [Google Scholar]
- 39.Martínez-Fernández de la Cámara C., Salom D., Sequedo M.D., Hervás D., Marín-Lambíes C., Aller E., Jaijo T., Díaz-LLopis M., Millán J.M., Rodrigo R. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLoS ONE. 2013;8:e74223. doi: 10.1371/journal.pone.0074223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanan Y., Brobst D., Han Z., Naash M.I., Al-Ubaidi M.R. Fibulin 2, a tyrosine O-sulfated protein, is up-regulated following retinal detachment. J. Biol. Chem. 2014;289:13419–13433. doi: 10.1074/jbc.M114.562157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedman J.S., Faucher M., Hiscott P., Biron V.L., Malenfant M., Turcotte P., Raymond V., Walter M.A. Protein localization in the human eye and genetic screen of opticin. Hum. Mol. Genet. 2002;11:1333–1342. doi: 10.1093/hmg/11.11.1333. [DOI] [PubMed] [Google Scholar]
- 42.Barnes S.K., Eiby Y., Lee S., Lingwood B.E., Dawson P.A. Structure, organization and tissue expression of the pig SLC13A1 and SLC13A4 sulfate transporter genes. Biochem. Biophys. Rep. 2017;10:215–223. doi: 10.1016/j.bbrep.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hochmann S., Kaslin J., Hans S., Weber A., Machate A., Geffarth M., Funk R.H.W., Brand M. Fgf Signaling is Required for Photoreceptor Maintenance in the Adult Zebrafish Retina. PLoS ONE. 2012;7:e30365. doi: 10.1371/journal.pone.0030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y.J., La Pierre D.P., Wu J., Yee A.J., Yang B.B. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 45.Qiu C., Li P., Bi J., Wu Q., Lu L., Qian G., Jia R., Jia R. Differential expression of TYRP1 in adult human retinal pigment epithelium and uveal melanoma cells. Oncol. Lett. 2016;11:2379–2383. doi: 10.3892/ol.2016.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radeke M.J., Peterson K.E., Johnson L.V., Anderson D.H. Disease susceptibility of the human macula: Differential gene transcription in the retinal pigmented epithelium/choroid. Exp. Eye Res. 2007;85:366–380. doi: 10.1016/j.exer.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Kerr N.M., Johnson C.S., de Souza C.F., Chee K.-S., Good W.R., Green C.R., Danesh-Meyer H.V. Immunolocalization of Gap Junction Protein Connexin43 (GJA1) in the Human Retina and Optic Nerve. Investig. Opthalmology Vis. Sci. 2010;51:4028–4034. doi: 10.1167/iovs.09-4847. [DOI] [PubMed] [Google Scholar]
- 48.Abbasi A.R., Khalaj M., Tsuji T., Tanahara M., Uchida K., Sugimoto Y., Kunieda T. A mutation of the WFDC1 gene is responsible for multiple ocular defects in cattle. Genomics. 2009;94:55–62. doi: 10.1016/j.ygeno.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Shi Y., Jones W., Beatty W., Tan Q., Mecham R., Kumra H., Reinhardt D., Gibson M., Reilly M., Rodriguez J., et al. Latent-transforming growth factor beta-binding protein-2 (LTBP-2) is required for longevity but not for development of zonular fibers. Matrix Biol. 2020;95:15–31. doi: 10.1016/j.matbio.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daga S., Donati F., Capitani K., Croci S., Tita R., Giliberti A., Valentino F., Benetti E., Fallerini C., Niccheri F., et al. New frontiers to cure Alport syndrome: COL4A3 and COL4A5 gene editing in podocyte-lineage cells. Eur. J. Hum. Genet. 2019;28:480–490. doi: 10.1038/s41431-019-0537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma X., Li H., Wang Y., Wang J., Zheng Q., Hua J., Yang J., Pan L., Lu F., Qu J., et al. DAPL1, a susceptibility locus for age-related macular degeneration, acts as a novel suppressor of cell proliferation in the retinal pigment epithelium. Hum. Mol. Genet. 2017;26:1612–1621. doi: 10.1093/hmg/ddx063. [DOI] [PubMed] [Google Scholar]
- 52.Reigada D., Lu W., Zhang X., Friedman C., Pendrak K., McGlinn A., Stone R.A., Laties A.M., Mitchell C.H. Degradation of extracellular ATP by the retinal pigment epithelium. Am. J. Physiol. Physiol. 2005;289:C617–C624. doi: 10.1152/ajpcell.00542.2004. [DOI] [PubMed] [Google Scholar]
- 53.Strungaru M.H., Footz T., Liu Y., Berry F.B., Belleau P., Semina E.V., Raymond V., Walter M.A. PITX2 Is Involved in Stress Response in Cultured Human Trabecular Meshwork Cells through Regulation of SLC13A3. Investig. Opthalmology Vis. Sci. 2011;52:7625–7633. doi: 10.1167/iovs.10-6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang R., Kim A.S., Fox J.M., Nair S., Basore K., Klimstra W.B., Rimkunas R., Fong R.H., Lin H., Poddar S. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature. 2018;557:570–574. doi: 10.1038/s41586-018-0121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C.-Y., Olsen B.R., Kao W.W.-Y. Developmental patterns of two α1(IX) collagen mRNA isoforms in mouse. Dev. Dyn. 1993;198:150–157. doi: 10.1002/aja.1001980208. [DOI] [PubMed] [Google Scholar]
- 56.Bilbao-Malavé V., Recalde S., Bezunartea J., Hernandez-Sanchez M., González-Zamora J., Maestre-Rellan L., Ruiz-Moreno J.M., Araiz-Iribarren J., Arias L., Ruiz-Medrano J., et al. Genetic and environmental factors related to the development of myopic maculopathy in Spanish patients. PLoS ONE. 2020;15:e0236071. doi: 10.1371/journal.pone.0236071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kameya S., Hawes N.L., Chang B., Heckenlively J.R., Naggert J.K., Nishina P.M. Mfrp, a gene encoding a frizzled related protein, is mutated in the mouse retinal degeneration 6. Hum. Mol. Genet. 2002;11:1879–1886. doi: 10.1093/hmg/11.16.1879. [DOI] [PubMed] [Google Scholar]
- 58.Vijayasarathy C., Zeng Y., Brooks M.J., Fariss R.N., Sieving P.A. Genetic Rescue of X-Linked Retinoschisis Mouse (Rs1-/y) Retina Induces Quiescence of the Retinal Microglial Inflammatory State Following AAV8-RS1 Gene Transfer and Identifies Gene Networks Underlying Retinal Recovery. Hum. Gene Ther. 2020;32:667–681. doi: 10.1089/hum.2020.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Handford P. Fibrillin-1, a calcium binding protein of extracellular matrix. Biochim. Biophys. Acta (BBA)-Bioenerg. 2000;1498:84–90. doi: 10.1016/S0167-4889(00)00085-9. [DOI] [PubMed] [Google Scholar]
- 60.Marneros A.G., Keene U.R., Hansen U., Fukai N., Moulton K., Goletz P.L., Moiseyev G., Pawlyk B.S., Halfter W., Dong S., et al. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 2003;23:89–99. doi: 10.1038/sj.emboj.7600014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan W., Laboulaye M.A., Tran N.M., Whitney I.E., Benhar I., Sanes J.R. Mouse Retinal Cell Atlas: Molecular Identification of over Sixty Amacrine Cell Types. J. Neurosci. 2020;40:5177–5195. doi: 10.1523/JNEUROSCI.0471-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hodgkinson C.P., Naidoo V., Patti K.G., Gomez J.A., Schmeckpeper J., Zhang Z., Davis B., Pratt R.E., Mirotsou M., Dzau V.J. Abi3bp is a multifunctional autocrine/paracrine factor that regulates mesen-chymal stem cell biology. Stem Cells. 2013;31:1669–1682. doi: 10.1002/stem.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitmore S.S., Wagner A.H., DeLuca A.P., Drack A.V., Stone E.M., Tucker B.A., Zeng S., Braun T.A., Mullins R.F., Scheetz T.E. Transcriptomic analysis across nasal, temporal, and macular regions of human neural retina and RPE/choroid by RNA-Seq. Exp. Eye Res. 2014;129:93–106. doi: 10.1016/j.exer.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu H., Li S., Li J., Huang C., Zhou F., Zhao L., Yu W., Qin X. Knockdown of Fibromodulin Inhibits Proliferation and Migration of RPE Cell via the VEGFR2-AKT Pathway. J. Ophthalmol. 2018;2018:5708537. doi: 10.1155/2018/5708537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu L., Liu Y., Wei C., Jin H., Mei L., Wu C. SERPINH1, Targeted by miR-29b, Modulated Proliferation and Migration of Human Retinal Endothelial Cells Under High Glucose Conditions. Diabetes Metab. Syndr. Obes. 2021;14:3471–3483. doi: 10.2147/DMSO.S307771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pollard R.D., Blesso C.N., Zabalawi M., Fulp B., Gerelus M., Zhu X., Lyons E.W., Nuradin N., Francone O.L., Li X.-A., et al. Procollagen C-endopeptidase Enhancer Protein 2 (PCPE2) Reduces Athero-sclerosis in Mice by Enhancing Scavenger Receptor Class B1 (SR-BI)-mediated High-density Lipoprotein (HDL)-Cholesteryl Ester Uptake. J. Biol. Chem. 2015;290:15496–15511. doi: 10.1074/jbc.M115.646240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao X., Soleimani M., Hughes B.A. SLC26A7 constitutes the thiocyanate-selective anion conductance of the basolateral membrane of the retinal pigment epithelium. Am. J. Physiol. Physiol. 2020;319:C641–C656. doi: 10.1152/ajpcell.00027.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niu R., Nie Z.-T., Liu L., Chang Y.-W., Shen J.-Q., Chen Q., Dong L.-J., Hu B.-J. Follistatin-like protein 1 functions as a potential target of gene therapy in proliferative diabetic retinopathy. Aging. 2021;13:8643–8664. doi: 10.18632/aging.202678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pérez-Ibave D.C., González-Alvarez R., Martinez-Fierro M.D.L.L., Ruiz-Ayma G., Luna-Muñoz M., Martínez-De-Villarreal L.E., Garza-Rodríguez M.D.L., Reséndez-Pérez D., Mohamed-Noriega J., Garza-Guajardo R., et al. Olfactomedin-like 2 A and B (OLFML2A and OLFML2B) expression profile in primates (human and baboon) Biol. Res. 2016;49:44. doi: 10.1186/s40659-016-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Obermann J., Priglinger C.S., Merl-Pham J., Geerlof A., Priglinger S., Götz M., Hauck S.M. Proteome-wide Identification of Glycosylation-dependent Interactors of Galectin-1 and Galectin-3 on Mesenchymal Retinal Pigment Epithelial (RPE) Cells. Mol. Cell. Proteom. 2017;16:1528–1546. doi: 10.1074/mcp.M116.066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mirzaei M., Gupta V.B., Chick J.M., Greco T.M., Wu Y., Chitranshi N., Wall R.V., Hone E., Deng L., Dheer Y., et al. Age-related neurodegenerative disease associated pathways identified in retinal and vitreous proteome from human glaucoma eyes. Sci. Rep. 2017;7:12685. doi: 10.1038/s41598-017-12858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lim J., Luderer U. Oxidative Damage Increases and Antioxidant Gene Expression Decreases with Aging in the Mouse Ovary. Biol. Reprod. 2011;84:775–782. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J., Clermont A.C., Gao B.-B., Feener E.P. Intraocular Hemorrhage Causes Retinal Vascular Dysfunction via Plasma Kallikrein. Investig. Opthalmology Vis. Sci. 2013;54:1086–1094. doi: 10.1167/iovs.12-10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baker S., Booth C., Fillman C., Shapiro M., Blair M.P., Hyland J.C., Ala-Kokko L. A loss of function mutation in the COL9A2 gene causes autosomal recessive Stickler syndrome. Am. J. Med Genet. Part A. 2011;155:1668–1672. doi: 10.1002/ajmg.a.34071. [DOI] [PubMed] [Google Scholar]
- 75.Giblin M., Penn J.S. Cytokine-induced ECM alterations in DR pathogenesis. Investig. Ophthalmol. Vis. Sci. 2020;61:1766. [Google Scholar]
- 76.Tangeman J., Luz-Madrigal A., Sreeskandarajan S., Grajales-Esquivel E., Liu L., Liang C., Tsonis P., Del Rio-Tsonis K. Transcriptome Profiling of Embryonic Retinal Pigment Epithelium Reprogramming. Genes. 2021;12:840. doi: 10.3390/genes12060840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hubens W.H., Breddels E.M., Walid Y., Ramdas W.D., Webers C.A., Gorgels T.G. Mapping mRNA Expression of Glaucoma Genes in the Healthy Mouse Eye. Curr. Eye Res. 2019;44:1006–1017. doi: 10.1080/02713683.2019.1607392. [DOI] [PubMed] [Google Scholar]
- 78.Recchia F.M., Xu L., Penn J.S., Boone B., Dexheimer P. Identification of Genes and Pathways Involved in Retinal Neovascularization by Microarray Analysis of Two Animal Models of Retinal Angiogenesis. Investig. Opthalmology Vis. Sci. 2010;51:1098–1105. doi: 10.1167/iovs.09-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fu Y.-P., Hallman D.M., Gonzalez V.H., Klein B.E.K., Klein R., Hayes M.G., Cox N.J., Bell G.I., Hanis C.L. Identification of Diabetic Retinopathy Genes through a Genome-Wide Association Study among Mexican-Americans from Starr County, Texas. J. Ophthalmol. 2010;2010:861291. doi: 10.1155/2010/861291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahram D.F., Cook A.C., Kecova H., Grozdanic S.D., Kuehn M.H. Identification of genetic loci associated with primary angle-closure glaucoma in the basset hound. Mol. Vis. 2014;20:497–510. [PMC free article] [PubMed] [Google Scholar]
- 81.Kole C., Berdugo N., DA Silva C., Aït-Ali N., Millet-Puel G., Pagan D., Blond F., Poidevin L., Ripp R., Fontaine V., et al. Identification of an Alternative Splicing Product of the Otx2 Gene Expressed in the Neural Retina and Retinal Pigmented Epithelial Cells. PLoS ONE. 2016;11:e0150758. doi: 10.1371/journal.pone.0150758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang S.B., Xu T., Peng S., Singh D., Ghiassi-Nejad M., Adelman R.A., Rizzolo L.J. Disease-associated mutations of claudin-19 disrupt retinal neurogenesis and visual function. Commun. Biol. 2019;2:113. doi: 10.1038/s42003-019-0355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rudolf R., Bittins C.M., Gerdes H.-H. The role of myosin V in exocytosis and synaptic plasticity. J. Neurochem. 2010;116:177–191. doi: 10.1111/j.1471-4159.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- 84.Burgoyne T., O’Connor M.N., Seabra M.C., Cutler D.F., Futter C.E. Regulation of melanosome number, shape and movement in the zebrafish retinal pigment epithelium by OA1 and PMEL. J. Cell Sci. 2015;128:1400–1407. doi: 10.1242/jcs.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu H., Chen M., Reid D.M., Forrester J.V. LYVE-1–Positive Macrophages Are Present in Normal Murine Eyes. Investig. Opthalmology Vis. Sci. 2007;48:2162–2171. doi: 10.1167/iovs.06-0783. [DOI] [PubMed] [Google Scholar]
- 86.Roddy G.W., Rosa R.H., Jr., Oh J.Y., Ylostalo J.H., Bartosh Y.J., Jr., Choi H., Lee R.H., Yasumura D., Ahern K., Nielsen G., et al. Stanniocalcin-1 rescued photoreceptor degeneration in two rat models of inherited retinal degeneration. Mol. Ther. 2012;20:788–797. doi: 10.1038/mt.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Ubaidi M.R., Naash M.I., Conley S.M. A Perspective on the Role of the Extracellular Matrix in Progressive Retinal De-generative Disorders. Investig. Ophthalmol. Vis. Sci. 2013;54:8119–8124. doi: 10.1167/iovs.13-13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lillien L.E., Sendtner M., Raff M.C. Extracellular matrix-associated molecules collaborate with ciliary neu- rotrophic factor to induce type-2 astrocyte development. J, Cell Biol. 1990;111:635–644. doi: 10.1083/jcb.111.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zaborowski M.P., Balaj L., Breakefield X.O., Lai C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience. 2015;65:783–797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andaloussi S., Mäger I., Breakefield X.O., Wood M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 91.Wooff Y., Cioanca A.V., Chu-Tan J.A., Aggio-Bruce R., Schumann U., Natoli R. Small-Medium Extracellular Vesicles and Their miRNA Cargo in Retinal Health and De-generation: Mediators of Homeostasis, and Vehicles for Targeted Gene Therapy. Front. Cell Neurosci. 2020;14:160. doi: 10.3389/fncel.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barber A.J., Nakamura M., Wolpert E.B., Reiter C.E.N., Seigel G.M., Antonetti D.A., Gardner T. Insulin Rescues Retinal Neurons from Apoptosis by a Phosphatidylinositol 3-Kinase/Akt-mediated Mechanism That Reduces the Activation of Caspase-3. J. Biol. Chem. 2001;276:32814–32821. doi: 10.1074/jbc.M104738200. [DOI] [PubMed] [Google Scholar]
- 93.Green E.S., Rendahl K.G., Zhou S., Ladner M., Coyne M., Srivastava R., Manning W.C., Flannery J.G. Two Animal Models of Retinal Degeneration Are Rescued by Recombinant Adeno-associated Virus-Mediated Production of FGF-5 and FGF-18. Mol. Ther. 2001;3:507–515. doi: 10.1006/mthe.2001.0289. [DOI] [PubMed] [Google Scholar]
- 94.Chen J., Wang Z., Zheng Z., Chen Y., Khor S., Shi K., He Z., Wang Q., Zhao Y., Zhang H., et al. Neuron and microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K-Akt signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent neuroinflammation to improve functional recovery after spinal cord injury. Cell Death Dis. 2017;8:e3090. doi: 10.1038/cddis.2017.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fudalej E., Justyniarska M., Kasarełło K., Dziedziak J., Szaflik J.P., Cudnoch-Jędrzejewska A. Neuroprotective Factors of the Retina and Their Role in Promoting Survival of Retinal Ganglion Cells: A Review. Ophthalmic. Res. 2021;64:345–355. doi: 10.1159/000514441. [DOI] [PubMed] [Google Scholar]
- 96.Liu X., Liu H., Lu X., Tombran-Tink J., Zhao S. PEDF Attenuates Ocular Surface Damage in Diabetic Mice Model through Its Antioxidant Properties. Curr. Eye Res. 2020;46:302–308. doi: 10.1080/02713683.2020.1805770. [DOI] [PubMed] [Google Scholar]
- 97.Ma B., Zhou Y., Liu R., Zhang K., Yang T., Hu C., Gao Y., Lan Q., Liu Y., Yang X., et al. Pigment epithelium-derived factor (PEDF) plays anti-inflammatory roles in the pathogenesis of dry eye disease. Ocul. Surf. 2021;20:70–85. doi: 10.1016/j.jtos.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 98.Hernández-Pinto A., Polato F., Subramanian P., de la Rocha-Muñoz A., Vitale S., de la Rosa E.J., Becerra S.P. PEDF peptides promote photoreceptor survival in rd10 retina models. Exp. Eye Res. 2019;184:24–29. doi: 10.1016/j.exer.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pagan-Mercado G., Becerra S.P. Signaling Mechanisms Involved in PEDF-Mediated Retinoprotection. Retin. Degener. Dis. 2019;1185:445–449. doi: 10.1007/978-3-030-27378-1_73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ikelle L., Naash M.I., Al-Ubaidi M.R. Modulation of SOD3 Levels Is Detrimental to Retinal Homeostasis. Antioxidants. 2021;10:1595. doi: 10.3390/antiox10101595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miyaji M., Furuta R., Hosoya O., Sano K., Hara N., Kuwano R., Kang J., Tateno M., Tsutsui K.M., Tsutsui K. Topoisomerase IIβ targets DNA crossovers formed between distant homologous sites to induce chromatin opening. Sci. Rep. 2020;10:18550. doi: 10.1038/s41598-020-75004-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The raw data obtained in this study are available from DDBJ Read Archive (https://ddbj.nig.ac.jp//DRASearch/, accessed on 9 December 2021) under the accession number (DRA013172) for RNA-seq.